Burkholderia†,‡
Abstract
Burk.hol.de' ri.a. M.L. fem. n. Burkholderia named after W.H. Burkholder, American bacteriologist who discovered the etiological agent of onion rot.
Proteobacteria / Betaproteobacteria / Burkholderiales / Burkholderiaceae / Burkholderia
Cells single or in pairs, straight or curved rods, but not helical. Dimensions, generally 0.5–1 × 1.5–4 µm. Motile by means of one or, more commonly, several polar flagella. One species (Burkholderia mallei) lacks flagella and is nonmotile. Do not produce sheaths or prosthecae. No resting stages are known. Gram negative. Most species accumulate poly-β-hydroxybutyrate (PHB) as carbon reserve material. Chemoorganotrophs. Have a strictly respiratory type of metabolism with oxygen as the terminal electron acceptor. Some species can exhibit anaerobic respiration with nitrate. Strains of some of the species (B. cepacia, B. vietnamiensis) are able to fix N2. Catalase positive. A wide variety of organic compounds can be used as sources of carbon and energy for growth. Although hydroxylated fatty acids are present in the lipids of members of other genera of aerobic pseudomonads, species of Burkholderia are characterized by the presence of hydroxy fatty acids of 14, 16, and 18 carbon atoms (C14:0 3OH and C16:0, and C16:0 2OH, C16:1, and C18:1). The most characteristic of these acids is the C16:0 3OH. Two different ornithine lipids are present in strains of some of the species. Over one-half of the species are pathogenic for plants or animals (including humans). The genus belongs to the ribosomal RNA similarity group II, which can be differentiated from other groups of aerobic pseudomonads by rRNA/DNA hybridization experiments or by rDNA sequencing.
The mol% G + C of the DNA is: 59–69.6.
Type species: Burkholderia cepacia (Palleroni and Holmes 1981) Yabuuchi, Kosako, Oyaizu, Yano, Hotta, Hashimoto, Ezaki and Arakawa 1993, 398 (Effective publication: Yabuuchi, Kosako, Oyaizu, Yano, Hotta, Hashimoto, Ezaki and Arakawa 1992, 1271) (Pseudomonas cepacia Palleroni and Holmes 1981, 479.)
Cells single or in pairs, straight or curved rods, but not helical. Dimensions, generally 0.5–1 × 1.5–4 µm. Motile by means of one or, more commonly, several polar flagella. One species (Burkholderia mallei) lacks flagella and is nonmotile. Do not produce sheaths or prosthecae. No resting stages are known. Gram negative. Most species accumulate poly-β-hydroxybutyrate (PHB) as carbon reserve material. Chemoorganotrophs. Have a strictly respiratory type of metabolism with oxygen as the terminal electron acceptor. Some species can exhibit anaerobic respiration with nitrate. Strains of some of the species (B. cepacia, B. vietnamiensis) are able to fix N2. Catalase positive. A wide variety of organic compounds can be used as sources of carbon and energy for growth. Although hydroxylated fatty acids are present in the lipids of members of other genera of aerobic pseudomonads, species of Burkholderia are characterized by the presence of hydroxy fatty acids of 14, 16, and 18 carbon atoms (C14:0 3OH and C16:0, and C16:0 2OH, C16:1, and C18:1). The most characteristic of these acids is the C16:0 3OH. Two different ornithine lipids are present in strains of some of the species. Over one-half of the species are pathogenic for plants or animals (including humans). The genus belongs to the ribosomal RNA similarity group II, which can be differentiated from other groups of aerobic pseudomonads by rRNA/DNA hybridization experiments or by rDNA sequencing.
The mol% G + C of the DNA is: 59–69.6.
Type species: Burkholderia cepacia (Palleroni and Holmes 1981) Yabuuchi, Kosako, Oyaizu, Yano, Hotta, Hashimoto, Ezaki and Arakawa 1993, 398 (Effective publication: Yabuuchi, Kosako, Oyaizu, Yano, Hotta, Hashimoto, Ezaki and Arakawa 1992, 1271) (Pseudomonas cepacia Palleroni and Holmes 1981, 479.)
Number of validated species: 19
Further descriptive information
Cell morphology
The cells of the genus Burkholderia correspond in their general characteristics to those of other aerobic pseudomonads: Gram-negative rods, straight or slightly curved, with rounded ends, usually motile when suspended in liquid. Motility is due to several polar flagella, but a single flagellum per cell has been reported for B. andropogonis, B. glathei, and B. norimbergensis.* The single flagellum of B. andropogonis is sheathed (Fuerst and Hayward, 1969a). One species, B. mallei, is nonmotile and lacks flagella (Redfearn et al., 1966).
Intracellular granules
The cells of species of the genus accumulate granules of carbon reserve material (poly-β-hydroxybutyrate, PHB, which may be part of a copolymer with poly-β-hydroxyvalerate, PHA). Proteins (“phasins”) have been found to be associated with the granules (Wieczorek et al., 1996). Only B. pseudomallei and some strains of B. mallei use extracellular PHB for growth (Ramsay et al., 1990). All species able to accumulate PHB can use the intracellular polymer when needed; however, in the description of some species, the statement “can use PHB” has been included, without indicating whether the PHB was endo- or extracellular.
The colonies of most species of the genus are smooth, but those of the human pathogen B. pseudomallei often have a rough surface.
Lipids
The first detailed analysis of the fatty acids of aerobic pseudomonads, using both saprophytic and phytopathogenic strains, demonstrated the possibility of establishing a correlation with the phylogenetic subdivision of the genus Pseudomonas, as classically defined, on the basis of rRNA–DNA hybridization (Oyaizu and Komagata, 1983). Years later, the results of this survey were confirmed and extended (Stead, 1992). The results of these investigations firmly established the fact that members of rRNA similarity group II of Palleroni et al. (1973), which includes the genus Burkholderia, have a fatty acid composition containing C14:0 3OH and C16:0, and C18:1 2OH. Most strains also contained C16:0 2OH and C16:1. Even though hydroxylated fatty acids are present in the lipids of members of other groups, group II including Burkholderia is the only one having hydroxylated fatty acids of 14, 16, and 18 carbon atoms (Table 1). A recent evaluation of the taxonomic significance of fatty acid composition emphasizes the diagnostic value of the above results (Vancanneyt et al., 1996), confirming earlier findings on this approach for the characterization of major phylogenetic groups within the pseudomonads (Wollenweber and Rietschel, 1990).
| Fatty acids | Ribosomal RNA groups | ||||
|---|---|---|---|---|---|
| I | II | III | IV | V | |
| 3-OH | |||||
| C10:0 | + | + | + | ||
| C11:0 | + | ||||
| C11:0 iso | + | ||||
| C12:0 | + | + | + | ||
| C12:0 iso | + | ||||
| C13:0 iso | + | ||||
| C14:0 | + | + | |||
| C16:1 | + | ||||
| 2-OH | |||||
| C12:0 | (+) | ||||
| C16:0 | (+) | ||||
| C16:1 | (+) | ||||
| C18:1 | + | ||||
| Ubiquinones | Q-9 | Q-8 | Q-8 | Q-10 | Q-8 |
Ornithine-containing lipids in Pseudomonas aeruginosa, P. putida, and B. cepacia represent from 2–15% of the total of extractable lipids. The amino acid was not found in the phospholipids that amount to more than 80% of all the extractable lipids (Kawai et al., 1988). An analysis of the polar lipids and fatty acids of B. cepacia has shown that the only significant phospholipids in this species are phosphatidyl-ethanolamine and bis(phosphatidyl)-glycerol. These characteristics, taken together with the unusual lipid profiles of B. cepacia, can be used as markers of chemotaxonomic importance (Cox and Wilkinson, 1989a).
A striking feature of the cellular composition of B. cepacia is the range of polar lipids, which include two forms (with and without 2-OH fatty acids) of phosphatidyl-ethanolamine and ornithine amide lipids. Variations in the lipid composition, as well as in pigmentation and flagellation, were observed as the consequence of changes in growth temperature and limiting oxygen, carbon, phosphorus, and magnesium supplies in the medium. Phosphorus limitation appears to be the only nutritional factor that results in a composition with polar lipids represented only by ornithine amide lipids (Taylor et al., 1998).
Interestingly, the 3-hydroxylated fatty acid of 10 carbon atoms is a component of the lipids of B. gladioli but not of those of B. cepacia, as indicated by lipid analysis performed on B. gladioli strains isolated from respiratory tract infections in cystic fibrosis patients (Christenson et al., 1989).
Differentiation of the plant pathogenic species of Burkholderia can be done by a direct colony thin-layer chromatographic method. Only minor uncertainties have been noticed with respect to the composition of aminolipids (Matsuyama, 1995).
The general qualitative profile of hydroxylated fatty acids indicated above is constant for a given species, although at least in one case (B. glumae) a subdivision of strains into two types is possible based on differences in composition. One of the subgroups, which included the type strain, had a composition that was similar to the bulk of rRNA similarity group II. The other was represented by strains that had significant amounts of the C10:0 3OH fatty acid, and was unique in rRNA similarity group II in having the C12:0 3OH fatty acid (Stead, 1992). For some of the components of the fatty acid profile, significant quantitative variations can be observed among the strains of different species of Burkholderia.
In contrast to the hydroxylated fatty acids, which have their origin in lipid A, the more abundant, nonhydroxylated fatty acids are mainly located in the cytoplasmic membrane. Their value as taxonomic markers is significant at the species level and less at the higher level of the RNA similarity groups. All strains of group II have C16:0, C16:1 cis, and C18:1 cis nonhydroxylated fatty acids (Stead, 1992). The investigations of Komagata and his collaborators have established that Q-8 is the quinone characteristic of group II (Oyaizu and Komagata, 1983).
Hopanes have been detected in the composition of cells of B. cepacia (Rohmer et al., 1979), but the value of these compounds as chemotaxonomic markers is not known because later studies apparently did not include strains of other species of the group. This point perhaps warrants further attention.
Flagella
Motility can be observed in young cultures of strains of all species of Burkholderia, with the exception of B. mallei, which lacks flagella. Cells of the latter species do not even show twitching motility on the surface of solid media (Henrichsen, 1975a). Motility in liquid is due to one or, more commonly, several polar flagella. A single flagellum per cell has been reported for B. andropogonis, B. glathei, and B. norimbergensis (see descriptions in the list of species at the end of this chapter). The best-known example is that of B. andropogonis, whose single flagellum is sheathed (Fuerst and Hayward, 1969a) (Fig. 1).

SDS-polyacrylamide gel electrophoresis has been used for the characterization of the flagellins of different species of aerobic pseudomonads. Based on flagellin composition, B. cepacia strains have been divided into two groups. Group I has flagellin of molecular weight 31,000, whereas group II flagellin ranges from 44,000–46,000. Type I was serologically uniform, while group II was heterologous. The flagellin types of B. cepacia appear to be analogous to the two major flagellin types of P. aeruginosa, and they could be used as molecular epidemiological tools (Montie and Stover, 1983).
The methodology for the isolation of B. pseudomallei flagellin and its characterization has been described. Electrophoretic analysis under denaturing conditions results in monomer protein bands with an estimated Mr of 43,000 (Brett et al., 1994). O-polysaccharide-flagellin conjugates in this species have been described with respect to their structural and immunological characteristics (Brett and Woods, 1996).
Pili (fimbriae)
Peritrichous pili have been identified years ago in B. cepacia (Fuerst and Hayward, 1969b). They are thought to facilitate adherence to mucosal epithelial surfaces (Kuehn et al., 1992; Sajjan and Forstner, 1993). Twitching motility is correlated with the presence of polar fimbriae, a correlation that is supported by the fact that this type of motility is absent from B. cepacia, which has no polar fimbriae (Henrichsen, 1975a). No fimbriae have been observed in B. andropogonis (Fuerst and Hayward, 1969b).
One or more of five morphologically distinct classes of pili can be present in the cells of B. cepacia. Some of the types have been identified in cells of epidemically transmitted strains, and others in environmental isolates (Goldstein et al., 1995; Sajjan et al., 1995). The role of a 22-kDa pilin in binding B. cepacia to the mouth epithelial cells has been described (Sajjan and Forstner, 1993). Further details on fimbriae will be given in the section on pathogenesis for humans and animals.
Composition of cell envelope
The earliest data on the cell wall composition of B. cepacia were obtained in S. Wilkinson's laboratory, where it was found that the major components, myristic, 3-hydroxymyristic, and 3-hydroxypalmitic acids, were indications that members of this group had a different composition than other species of aerobic pseudomonads. These results are in agreement with those of fatty composition of whole cells (Samuels et al., 1973). The core polysaccharide contains glucose, rhamnose, and heptoses, but at most a very low phosphorus content. The lipopolysaccharide (LPS) has a low content of 3-deoxy-D-manno-2-octulosonic acid (KDO), and the side chain is basically a mannan. One added peculiar feature is the presence of an acid-labile amino sugar phosphate presumably associated with the lipid A.
The results have been confirmed, at least in part, by Manniello et al. (1979). Analyses of B. cepacia performed by these workers revealed the presence of rhamnose, glucose, heptose, and hexosamine, but no KDO, and the phosphorus content was found to be about one-third of that of P. aeruginosa. The presence of KDO in the LPS of B. cepacia, however, was later confirmed (Straus et al., 1990).
A method of extraction of LPS from B. pseudomallei has been described. As in the case with B. cepacia, the link between the inner core and lipid A is stable to acid hydrolysis (Kawahara et al., 1992). The LPS of clinical strains of this last species was composed of two polymers made of different repeating units, but both polymers contained D-rhamnose and D-galactose residues (Cerantola and Montrozier, 1997).
Five major outer membrane proteins have been isolated from several strains of B. pseudomallei, with Mr values ranging from 17,000–70,000. One of the proteins associated with the peptidoglycan acts as a porin through which small saccharides may diffuse (Gotoh et al., 1994b).
Outer membrane proteins of B. cepacia and B. pseudomallei that are inducible by phosphate starvation appear to be similar to the E. coli PhoE porin protein. The latter does not have the binding sites for anions and phosphate that are present in the analogous proteins from Pseudomonas species (Poole and Hancock, 1986).
One porin of B. cepacia is an oligomer composed of two proteins. The purified 81-kDa whole protein (OpcPO) upon heating gives a major 36-kDa protein (OpcP1) and a minor one (OpcP2) of 27 kDa. The association of these two components is noncovalent (Gotoh et al., 1994a). Recently, the major porin protein, (OpcP1), was partially sequenced, and the information was used for cloning the gene. Its sequence showed an open reading frame of a length in agreement with that of OpcP1 (Tsujimoto et al., 1997).
Additional information about porins and other components of the cell envelopes may be found in the sections on antibiotic susceptibility and antigenic structure.
Pigmentation
Pigmentation is by no means a universal character of Burkholderia. Some B. cepacia strains are not pigmented, whereas others produce phenazine pigments of a bewildering variety of colors when grown on solid chemically defined media containing different carbon sources. Pigmented strains of the species can be subdivided into two types on the basis of their pigmentation: some are yellow on glucose yeast extract peptone agar and others are various shades of brown, red, violet, and purple (Morris and Roberts, 1959). Morris and Roberts isolated the pigment from a purple-pigmented strain and demonstrated that its basic structure was that of a phenazine. In fact, two phenazine pigments—one yellow and the other purple—may be synthesized, both of which are water-soluble under neutral or alkaline conditions. A single strain can produce both types, only one type, or none. Because the pigments are soluble in water, both the colonies and the medium appear pigmented. Growth on King A medium2 often enhances pigment production.
In the author's experience, pigment production in Burkholderia is, as in many other cases, a striking but not very reliable taxonomic character, because pigment biosynthesis often requires conditions that cannot be precisely controlled.
Nutrition and growth conditions
The strains of Burkholderia species grow in media of minimal composition, without the addition of organic growth factors. Occasionally, strains are isolated from nature that grow extremely slowly but are stimulated by addition of complex organic mixtures such as yeast extract. Some of the fluorescent plant pathogens of the genus Pseudomonas fall in this category, and the phenomenon has also been observed for some B. caryophylli strains (Palleroni, 1984).
The ability to grow in media of very simple and chemically defined composition stimulated research on the nutritional versatility of the aerobic pseudomonads—among them, species later to be assigned to the genus Burkholderia. These studies have revealed a remarkable variety of organic compounds that can serve individually as carbon and energy sources for strains of some species (B. cepacia, B. pseudomallei). The types of compounds used for growth are the basis of the vast phenotypic information now available for many strains (Redfearn et al., 1966; Stanier et al., 1966; Ballard et al., 1970). One of the simplest chemically defined media used for nutritional studies is the one recommended for the hydrogen pseudomonads (Palleroni and Doudoroff, 1972).3
The nutritional investigations revealed that some strains of B. cepacia could utilize any of a list of 100 organic compounds (two-thirds of the list of tested substrates) (Stanier et al., 1966). Later work performed in various laboratories has enlarged the list considerably. This remarkable metabolic versatility of the species was unknown to plant pathologists, and its discovery rapidly converted B. cepacia into a fascinating subject for biochemical research. A sample of the nutritional properties of Burkholderia species is presented in Table 2, and some of these properties, together with general characteristics of taxonomic importance, are summarized in Table 3. Comparisons of nutritional properties of some related species are also to be found in later sections (Tables 5 and BXII.β.8). To the remarkable metabolic versatility of B. cepacia we have to add the ability to fix N2 that is exhibited by some of the strains (Bevivino et al., 1994; Tabacchioni et al., 1995). This ability is also present in a closely related taxon, B. vietnamiensis (Gillis et al., 1995).
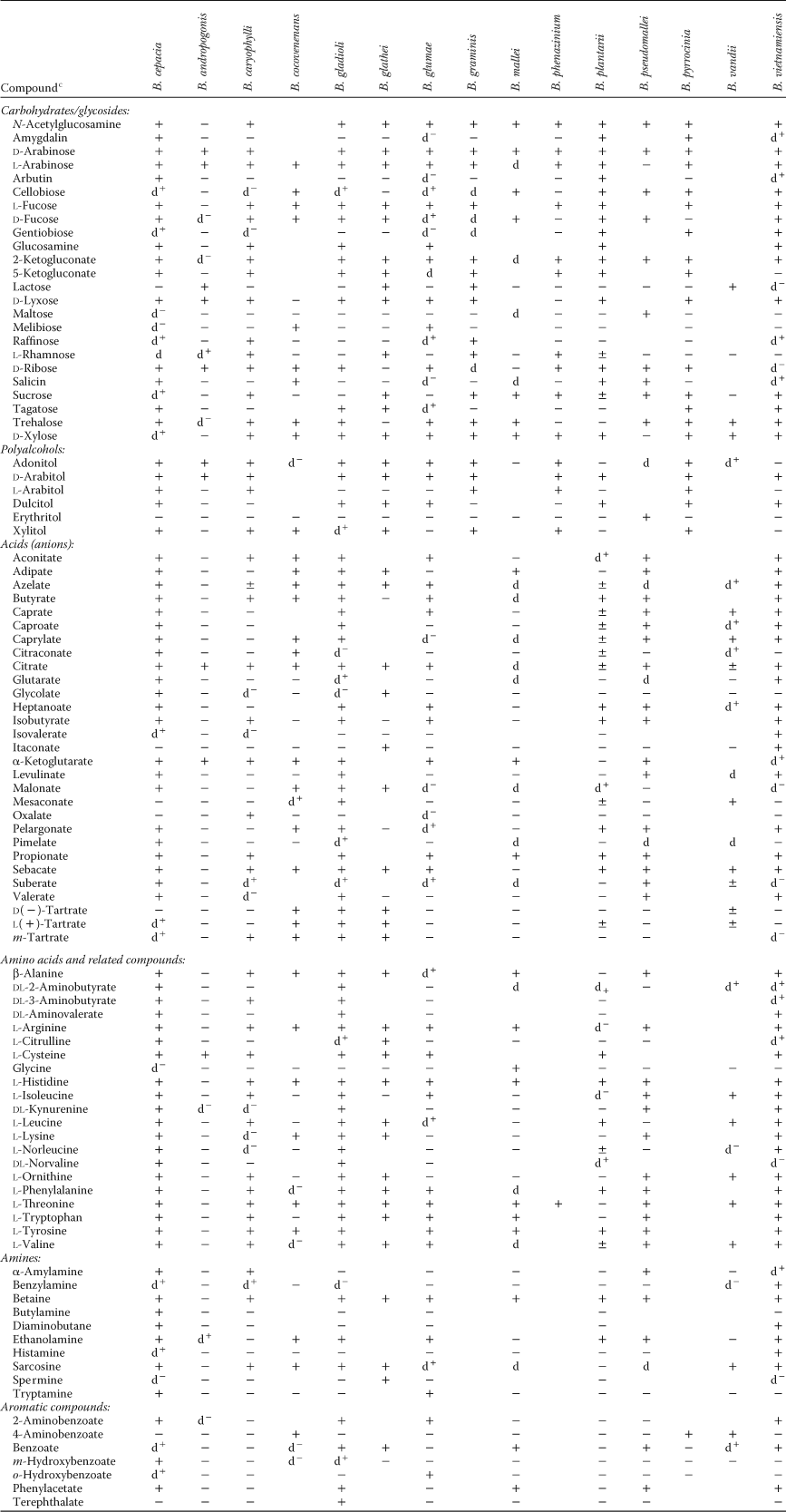
- a For symbols see standard definitions; ±, slow. The + or − superscripts of the d symbol refer to the result obtained with the type strain, when known.
- b Data from Gillis et al. (1995), Viallard et al. (1998), and Palleroni (1984).
- c Carbon compounds used by all strains (with few exceptions; see Gillis et al., 1995) are fructose, fumarate, galactose, gluconate, glucose, mannose, glycerol, m-inositol, mannitol, sorbitol, acetate, dl-glycerate, dl-lactate, l-malate, pyruvate, succinate, d-α-alanine, l-α-alanine, dl-aminobutyrate, l-aspartate, l-glutamate, l-serine, l-proline, p-hydroxybenzoate. Carbon compounds not used by any strain: glycogen, methyl-mannoside, d-melezitose, inulin, starch, d-turanose, aesculin, creatine, 3-aminobenzoate, d-mandelate, phthalate, isophthalate.
| Characteristic | B. cepacia | B. andropogonis | B. caryophylli | B. cocovenenans | B. gladioli | B. glathei | B. glumae | B. mallei | B. plantarii | B. pseudomallei | B. vandii | B. vietnamiensis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flagellar number | >1 | 1 | >1 | >1 | >1 | 1 | >1 | 0 | >1 | >1 | >1 | |
| Diffusible pigmentsb | + | − | + | + | + | − | + | − | + | − | − | − |
| Arginine dihydrolase | − | − | + | − | − | − | − | + | − | + | − | |
| Denitrification | − | − | + | − | − | − | + | + | + | + | + | − |
| Growth at 40°C | + | + | + | + | + | + | + | − | + | − | + | |
| Gelatin hydrolysis | d | − | − | + | + | − | + | + | + | + | + | + |
| Starch hydrolysis | − | w | − | − | − | − | − | d | − | + | − | − |
| Extracellular PHB hydrolysis | − | − | − | d | + | |||||||
| Oxidase reaction | d | − | − | − | d | + | d | + | + | + | + | + |
| Growth on: | ||||||||||||
| Adonitol | + | + | + | d− | + | d | + | − | − | d | d+ | − |
| α-Amylamine | + | − | − | − | − | − | − | + | − | d+ | ||
| Citraconate | + | − | − | d+ | + | − | w | − | d+ | − | ||
| Erythritol | − | − | − | − | − | − | − | + | ||||
| m-Hydroxybenzoate | + | − | − | d− | d− | − | − | − | − | − | − | − |
| Levulinate | + | − | − | − | + | − | − | − | + | d− | + | |
| Mesaconate | − | − | − | d+ | + | − | − | w | − | + | − | |
| l-Rhamnose | d | d+ | + | − | − | + | − | − | w | − | − | − |
| l-Ribose | + | + | + | + | + | + | + | − | + | + | d− | |
| d(− )-Tartrate | − | − | − | + | + | + | − | − | − | − | w | − |
| meso-Tartrate | d+ | − | + | + | + | + | − | − | − | − | d− | |
| Tryptamine | + | − | − | − | − | + | − | − | − | − | − | |
| d-Xylose | d+ | − | + | + | + | + | + | + | + | − | + | + |
- a For the symbols and abbreviations see Table BXII.β.1. Data for B. graminis, B. multivorans, B. norimbergensis, B. phenazinium, B. pyrrocinia, and B. thailandensis have not been included since, aside from the general phenotypic features, for most of the characters in the table there is no information in the original sources.
- b Strains of B. cepacia may produce nonfluorescent pigments of various colors; strains of B. gladioli and of B. caryophylli may excrete yellow-green nonfluorescent pigments; strains of B. cocovenenans produce greenish-yellow diffusible pigments.
Temperature for growth
Usually a temperature of 30°C is used for growth of all strains of the genus. Many of them, however, can grow well at 37°C and even at 40°C. Additional information on temperature relationships will be given in the section dealing with the description of individual species.
Oxygen relationships
All strains grow well under aerobic conditions. Some species (B. mallei, B. pseudomallei, B. caryophylli, B. plantarii, B. vandii) can also use nitrate as the terminal electron acceptor under anaerobic conditions. The original description of B. vietnamiensis (Gillis et al., 1995) indicates that the strains are able to reduce nitrate to nitrite, suggesting that this is the final stage of the reduction process. However, B. vietnamiensis is described elsewhere as a denitrifier. In view of this, the species was omitted from Table 4, which presents the general properties of denitrifying aerobic pseudomonads. In spite of this, denitrification was included among the general properties useful as differential characteristics for Burkholderia species.
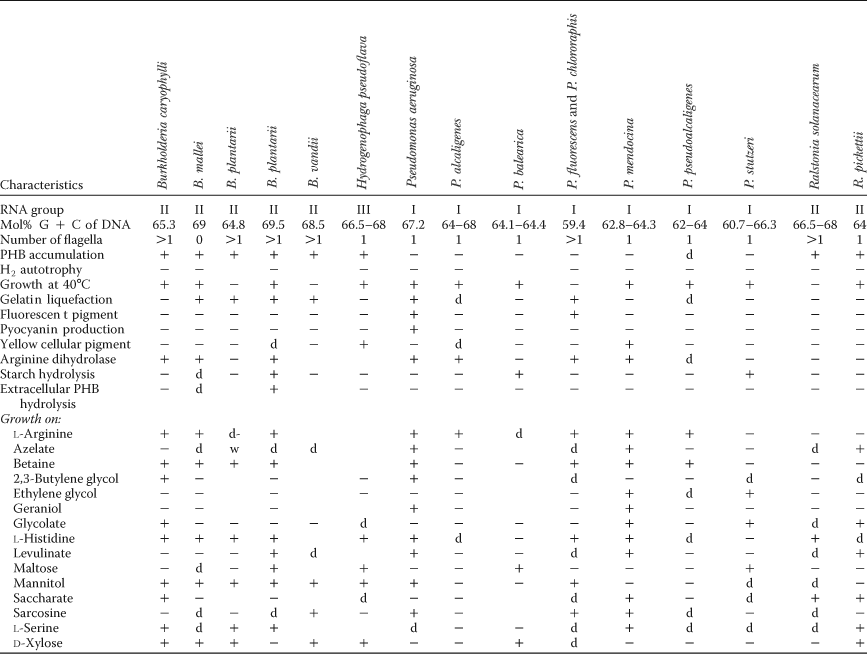
- a For symbols see standard definitions. The denitrifying pseudomonads P. azotoformans, P. mucidolens, and P. nitroreducens have not been included in this table.
Metabolism and metabolic pathways
Knowledge of many areas of the general metabolism of species of the genus Burkholderia is scanty and fragmentary. The following refers to some aspects of the general metabolism of the genus, with the exclusion of the catabolism of aromatic compounds, which will be treated in a second part.
Extensive nutritional information is available on a large number of carbon substrates used individually as sole sources of carbon and energy for B. cepacia, B. gladioli, B. caryophylli, B. pseudomallei, and B. mallei (Redfearn et al., 1966; Stanier et al., 1966; Ballard et al., 1970; Palleroni and Holmes, 1981; Palleroni, 1984). In some of these reports, the information refers to names that are synonyms of some of the above (“Pseudomonas marginata” for B. gladioli, and “Pseudomonas multivorans” for some of the strains of B. cepacia). The information has been summarized in several tables in this chapter.
For B. vietnamiensis and B. andropogonis, a source of information on nutritional spectra useful for a comparison with other species of the genus, is given by Gillis et al. (1995). Less extensive surveys have been performed with B. glumae, B. vandii, and B. cocovenenans (Azegami et al., 1987; Urakami et al., 1994; Zhao et al., 1995).
As mentioned in the section on nutrition, some of the Burkholderia species are extremely versatile from the metabolic standpoint. This is particularly true of B. cepacia, the most versatile of all known aerobic pseudomonads, but B. pseudomallei and B. gladioli are similarly remarkable for their capacity of living at the expense of any of a long list of organic compounds as sole source of carbon and energy. Unfortunately, these species also have an infamous reputation as direct or opportunistic human and animal pathogens, and investigations on the saprophytic activities of some of the species are sparse because of the danger of handling the organisms in the laboratory.
All species of the genus Burkholderia that have been examined are able to accumulate PHB as a carbon reserve material, which they can degrade when nutrients in the medium become exhausted. However, use of exogenous PHB is an uncommon property among the aerobic pseudomonads. In Burkholderia, only B. pseudomallei and some strains of B. mallei are capable of degrading extracellular PHB (Redfearn et al., 1966; Stanier et al., 1966).
Table 5 summarizes data on arginine utilization and the occurrence of arginine deiminase in some representative members of the various rRNA similarity groups of aerobic pseudomonads. Interestingly, B. cepacia—a member of RNA group II that is notorious for its nutritional versatility and for the diversity of its catabolic pathways—can degrade arginine only through the use of the succinyl transferase pathway, although it can use 2-ketoarginine and agmatine, the products of arginine oxidase and arginine decarboxylase, respectively (Stalon and Mercenier, 1984; Vander Wauven and Stalon, 1985).
| Organisms | RNA group | Arginine utilization | Arginine deiminase |
|---|---|---|---|
| Fluorescent saprophytic Pseudomonas species | I | + | + |
| Burkholderia cepacia, B. gladioli | II | + | − |
| B. mallei, B. pseudomallei | II | + | + |
| Ralstonia solanacearum, R. pickettii | II | − | − |
| Comamonas, Hydrogenophaga, Acidovorax | III | − | − |
| Brevundimonas | IV | − | − |
| Stenotrophomonas, Xanthomonas | V | − | − |
| Stenotrophomonas, Xanthomonas | V | − | − |
In a survey of lysine catabolic pathways in the pseudomonads, it has been reported that B. cepacia and P. aeruginosa use the pipecolate pathway and not the so-called oxygenase pathway. P. aeruginosa can also use the cadaverine pathway, but this is not operative in B. cepacia (Fothergill and Guest, 1977; Palleroni, 1984). These facts are summarized in Table 1 (BXII.γ.108, p. 334) of the genus Pseudomonas in Volume 2, Part B.
N2 fixation has been detected in strains of B. cepacia isolated from plant rhizospheres (Bevivino et al., 1994; Tabacchioni et al., 1995). A different set of nitrogen fixing strains studied in another laboratory was found to be closely related to this species, and was assigned the new species name B. vietnamiensis (Gillis et al., 1995).
Some miscellaneous activities of interest of the lesser-known species of the genus include the following. An α-terpineol dehydratase—capable of converting the citrus compound limonene to α-terpineol—was partially solubilized from a particulate fraction obtained from B. gladioli cells (Cadwallader et al., 1992). A lipase from B. glumae has been purified and some of its properties have been described (Deveer et al., 1991; Cleasby et al., 1992). Cloning and sequencing of a lipase gene of B. cepacia (lipA) have been performed, and its expression is dependent on a second gene (limA) (Jorgensen et al., 1991).
In cells of B. caryophylli, a d-threo-aldolase dehydrogenase that catalyzes the oxidation of l-fucose and the “unnatural” sugars l-glucose, l-xylose, and d-arabinose, has been purified and characterized. It is inhibited by d-glucose and other natural aldoses (Sasajima and Sinskey, 1979). Some enzymatic activities of species other than B. cepacia may be of environmental importance. Thus, B. pseudomallei is capable of breaking the C-P bond in the utilization of the herbicide N-(phosphonomethyl)-glycine, which is known by the empirical name glyphosate. The genes controlling this activity have been cloned and sequenced (Peñaloza-Vazquez et al., 1995). Similarly, the phytopathogen and human opportunistic pathogen B. gladioli was found to be able to form and cleave C-P bonds (Nakashita et al., 1991; Nakashita and Seto, 1991). In a more general way, it has been suggested that B. gladioli probably participates in the degradation of some xenobiotic compounds in the environment (Cadwallader et al., 1992).
Several siderophores are produced by species of Burkholderia. In iron-deficient media, Pseudomonas aeruginosa, P. fluorescens, and B. cepacia synthesize salicylic acid, a compound of particular interest because of its siderophore capacity (Visca et al., 1993) and the fact that it is a precursor of another siderophore, pyochelin. Interestingly, salicylate is used as a source of carbon and energy by many strains of Burkholderia and of many other aerobic pseudomonads. An iron-binding compound produced by the great majority of B. cepacia strains isolated from the respiratory tract was named azurechelin and is capable of releasing Fe from transferrins (Sokol et al., 1992). Later, however, azurechelin was found to be salicylic acid (Visca et al., 1993).
Pyochelin production was found in half of 43 strains of B. cepacia isolated from cystic fibrosis patients. The siderophore has in its structure one molecule of salicylic acid and two molecules of cysteine (Cox et al., 1981). A siderophore of a linear hydroxamate/hydroxycarboxylate type, was discovered in B. cepacia and also found in B. vietnamiensis. It was named ornibactin and functions as a specific iron-transport system equivalent to that of the pyoverdin system of fluorescent pseudomonads. In the composition of ornibactins, there is a peptide, an amine, and acyl groups of different lengths (Stephan et al., 1993a, b).
In nature, these siderophores are often successful in competing for iron with siderophores of various other sources (Yang et al., 1993). Thus, pyochelin allows B. cepacia to grow in the presence of transferrin (Sokol, 1986).
In low Fe-content medium, B. cepacia excretes both pyochelin and a low molecular weight compound (1-hydroxy-5-methoxy-6-methyl-2(1H)-pyridinone), which received the name of cepabactin. The structure resembles that of a cyclic hydroxamate, and it can also be considered a heterocyclic analogue of catechol (Meyer et al., 1989). The compound is related to synthetic hydroxy-pyridinones. Cepabactin had already been described as chelator (Winkler et al., 1986) and was known to have antibiotic properties (Itoh et al., 1979).
At least three different siderophores—ornibactins, pyochelin, and cepabactin—can be produced by a single B. cepacia strain. Other strains produce either two siderophores—ornibactins plus pyochelin or ornibactins plus cepabactin—and still other strains produce only pyochelin. In a survey of strains from a collection, 88% of the strains produced ornibactin, 50% pyochelin, and 14% cepabactin (J.M. Meyer, personal communication).
A recently identified member of the siderophore group produced by a strain of B. cepacia is cepaciachelin, a catecholate compound. The producing strain appears to be a unique example of a pseudomonad able to synthesize both hydroxamate and catecholate siderophores (Barelmann et al., 1996).
In summary, the variety of siderophores that B. cepacia is able to synthesize demonstrates once again the remarkable biochemical versatility of strains of this species.
All 84 strains of B. pseudomallei included in a study were found to produce a siderophore of approximately 1000 molecular weight. The compound, called malleobactin (Yang et al., 1991a), permitted cell growth in the presence of EDTA and of transferrin, and can trap iron from transferrin (at all pH values tested) and from lactoferrin. B. cepacia can use malleobactin as an iron-scavenging compound. However, pyochelin and azurechelin (salicylic acid) are more effective in trapping cell-derived iron as well as protein-bound iron (Yang et al., 1993).
Media with limiting phosphate concentrations enhance the production of a particular OM protein (OprP) of Pseudomonas aeruginosa. This protein is believed to be involved in phosphate transport and is only produced by species of Pseudomonas. Under similar conditions, both B. cepacia and B. pseudomallei produce proteins of a size similar to the PhoE protein of Escherichia coli and other enteric bacteria (Poole and Hancock, 1986).
Metabolism of aromatic and halogenated compounds
For many years this subject has attracted the attention of biochemists because of the striking ability of prokaryotes to degrade aromatic compounds that are resistant to chemical attack and are not readily metabolized by other organisms. In recent years, this interest has increased due to concern for chemically polluted environments and the obvious convenience of favoring these degradative activities as part of bioremediation strategies.
The first edition of this Manual included information on basic catabolic activities of the aerobic pseudomonads on simple aromatic compounds because of their obvious taxonomic implications (Palleroni, 1984). Much research has since been done on the catabolism of many aromatic compounds that include benzoate and derivatives, polycyclic aromatic hydrocarbons, biphenyl (particularly the halogenated derivatives), metabolic intermediates such as the halogenated catechols, and some important herbicides (24D and 245T). This field of research now contains a multitude of references, of which only a selected minority will be discussed here.
The investigations of Ornston and his collaborators on the biochemistry of degradation of aromatic compounds are of particular interest because the corresponding pathways were also analyzed for their phylogenetic implications. The β-ketoadipate pathway has taken center stage in these investigations. Natural selection has adopted many permutations in the distribution of its components, their regulation, and the genetic makeup, all of which have been highlighted in an excellent review (Harwood and Parales, 1996). Immunological cross-reaction was observed between the enzymes of one of the species (Pseudomonas putida) and the corresponding enzymes of other fluorescent Pseudomonas species. Cross-reaction, however, was also detected between the γ-carboxymuconolactone decarboxylases of P. putida and B. cepacia (Patel and Ornston, 1976). Although the results suggested that the interspecific transfer of the structural gene for the enzyme was not common among pseudomonads, it nevertheless seemed to have occurred between these two distantly related species. The cross-reaction did not extend to other enzymes of the two species, including the salicylate hydroxylases, which are structurally different, and for which an explanation based on convergent evolution has been proposed (Kim and Tu, 1989).
As mentioned in a description of the metabolism of para-hydroxybenzoate (POB), B. cepacia is able to convert meta-hydroxybenzoate (MOB) to gentisate by the action of a 6-hydroxylase (Yu et al., 1987). Interestingly, this finding relates to an earlier report on the formation of gentisate from MOB by Comamonas acidovorans, which is a member of the Betaproteobacteria in which Burkholderia is located (Wheelis et al., 1967). A description of the induction of the hydroxylase from B. cepacia has been published (Wang et al., 1987).
The genetic organization and sequence of genes of the α and β subunits of protocatechuate 3,4-dioxygenase of B. cepacia has been investigated, and there is extensive similarity to genes of other Pseudomonas species, although this similarity does not extend to the promoter sequences (Zylstra et al., 1989b). The pattern of induction of this and other enzymes of the POB metabolism has been examined (Zylstra et al., 1989a).
As a member of a microbial community, B. cepacia was the most competitive member among other aerobic pseudomonads in the degradation of toluene (Duetz et al., 1994). A novel pathway of toluene catabolism was described for this organism, with the participation of toluene monooxygenase, which is able to hydroxylate toluene, phenol, and cresol, and also to catalyze the degradation of trichloroethylene (TCE) (Shields et al., 1991). Cometabolism of TCE and toluene has been described (Landa et al., 1994). A constitutive strain was selected for the degradation of TCE (Shields and Reagin, 1992). Degradation of TCE by B. cepacia using the toluene monooxygenase system is expressed at higher capacity than the toluene dioxygenase systems present in other organisms (for instance, P. putida) (Leahy et al., 1996).
The toluene 2-monooxygenase from B. cepacia is a three-component system capable of oxidizing toluene to o-cresol and this to 3-methylcatechol. The catabolic features of this system resemble those of soluble methane monooxygenase from methanotrophic bacteria (Newman and Wackett, 1995). For the activity of toluene 2-monooxygenase on TCE, all its protein components and NADH are required. All protein components were modified during TCE oxidation, but reducing compounds such as cysteine protected the enzyme (Newman and Wackett, 1997).
Phthalate oxygenase is an enzyme specific for phthalate and closely related compounds. The system of B. cepacia, which requires the contribution of phthalate oxygenase reductase for efficient catalytic activity, is similar to other bacterial oxygenase systems. The B. cepacia enzyme can be isolated in large quantities and its stability is higher than that from other sources (Batie et al., 1987).
B. cepacia strains grow on fluorene and degrade this compound by a mechanism analogous to naphthalene catabolism. The system has wide specificity, and the range of substrates includes many other polycyclic aromatic compounds (Grifoll et al., 1995). But in spite of the remarkable catabolic versatility of the species, there are some limitations to the range of susceptible substrates. Thus, strains isolated on phenanthrene from polyaromatic hydrocarbon (PAH)-contaminated soils had limited capacity to use higher PAHs (Mueller et al., 1997).
For more than a decade, the degradation of the halogenated herbicides 2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid (245T) by B. cepacia has been the object of attention by researchers, among them, A. Chakrabarty and his group (Haugland et al., 1990). In fact, B. cepacia grows luxuriantly on 245T. Although initially this species is not able to use phenoxyacetate, it can acquire this property by long selective pressure. Gene activation in the mutants seems to be due to translocation of insertion elements (Ghadi and Sangodkar, 1994). Polychlorinated phenols are produced in the degradation, and they are further metabolized by B. cepacia to the corresponding hydroquinones (Tomasi et al., 1995). The cloning, mapping, and expression of genes controlling the degradation by strains of this species have been under investigation (Sangodkar et al., 1988).
The study of spontaneous B. cepacia mutants unable to degrade 245T has permitted the identification of insertion sequences that facilitate or are required for growth at the expense of 245T (Haugland et al., 1991). The rapid evolution of the degradative pathway for this herbicide probably has been possible by insertion elements (such as IS1490) that play a central role in the transcription of 245T genes in B. cepacia (Hubner and Hendrickson, 1997). A 1477 bp sequence was repeated several times in a B. cepacia strain chromosome, and by the location it was concluded that genes involved in 245T degradation were actually recruited from foreign sources (Tomasek et al., 1989). Originally, foreign gene recruitment such as the mechanism postulated by Lessie and Gaffney (1986), may have been responsible for the acquisition of 245T degradation capacity. Better knowledge of the evolution of these pathways may facilitate the job of developing strains with enhanced degradative capacity (Daubaras et al., 1996a).
B. cepacia competes effectively in the microflora for the degradation of the herbicide 2,4-dichlorophenoxyacetic acid (24D) (Ka et al., 1994a). The enzyme that cleaves 3,5-dichlorocatechol in this pathway has been purified (Bhat et al., 1993). To monitor a strain in the degradation of 24D, a reporter gene system containing luxAB and lacZY was integrated into the chromosome of the strain, which thus could be readily identified in the community (Masson et al., 1993).
Oxygenase TftAB, capable of converting 245T to 2,4,5-chlorophenol, is an enzyme of wide specificity, since it can give phenolic derivatives of several other related compounds (Danganan et al., 1995). Two proteins, TftA1 and TftA2, characterized in the pathway, were sequenced and found to have similarity to BenA and BenB of the benzoate 1,2-dioxygenase system of Acinetobacter calcoaceticus, and to XylX and XylY from the equivalent system in Pseudomonas putida (Danganan et al., 1994). The B. cepacia enzyme that degrades 2,4,5-trichlorophenol, the intermediate in 245T catabolism, has been characterized and found to consist of two components (Xun, 1996).
1,2,4-trihydroxybenzene, another intermediate in 245T degradation, is a substrate for the enzyme hydroxyquinol 1,2-dioxygenase. The enzyme is specific, and it is a dimeric protein of 68 kDa (Daubaras et al., 1996b).
Environmental strains of B. cepacia are active in the degradation of polychlorinated biphenyls. As in other examples of aerobic metabolism of haloaromatic compounds, dehalogenation often occurs after ring cleavage (Arensdorf and Focht, 1995). In vitro constructed hybrids of B. cepacia could carry out total degradation of 2-Cl, 3-Cl, 2,4-dichloro-, and 3,5-dichlorobiphenyl (Havel and Reineke, 1991).
Chlorocatechols are key intermediates in the metabolism of haloaromatic compounds, and they are metabolized through reactions resembling those for catechol. A 2-halobenzoate 1,2-dioxygenase, a two-component system of B. cepacia strain 2CBS has a very broad specificity (Fetzner et al., 1992). However, in B. cepacia isolated from enrichment on 2-chlorobenzoate and in mutants blocked in different steps of the pathway, the 2,3-dioxygenase of the meta pathway predominated over the 1,2-dioxygenase (or ortho) system (Fetzner et al., 1989).
The dioxygenases and the cycloisomerases involved in the modified ortho pathways resemble the enzymes of pathways for nonhalogenated compounds, while the diene-lactone hydrolases needed in a later step are quite different (Schlomann et al., 1993). The results of work done on systems isolated from Alcaligenes and B. cepacia suggest that the hydrolases of the modified pathway may have been recruited from a different preexisting pathway, which was already operating before the start of industrial synthesis of halogenated compounds (Schlomann, 1994). When plasmid TOL is introduced into B. cepacia strains, these strains are able to grow with 3,4- and 3,5-dichlorotoluene, thus bypassing dead-end routes of chlorocatechols degradation (Brinkmann and Reineke, 1992).
The results of the experiments that have been briefly discussed in the preceding paragraphs often point to the high similarity among some components of peripheral metabolic pathways among organisms that are rather distantly related, suggesting horizontal transfer of the corresponding genetic determinants. One additional case is the high similarity between the genes coding for the cis-biphenyl dihydrodiol dehydrogenases of P. putida (gene bphB) and that of B. cepacia (Khan et al., 1997), and also between the bphC genes of both species, which code for the 2,3-dihydroxybiphenyl 1,2-dioxygenase (Khan et al., 1996b). On the other hand, in spite of having almost identical amino acid sequences, the biphenyl dioxygenase of B. cepacia (gene bphA1) and that of Pseudomonas pseudoalcaligenes have markedly different substrate ranges, the one for B. cepacia being wider (Kimura et al., 1997).
Additional details on the metabolism of aromatic compounds may be found in the section on plasmids.
Genetic characteristics
The genome of a strain of B. cepacia (ATCC 17616) consists of three replicons whose respective sizes are 3.4, 2.5, and 0.9 Mb, which, together with the 170-kb cryptic plasmid present in the strain, gives an overall estimate for genome size of approximately 7 Mb (Cheng and Lessie, 1994). The three large replicons had ribosomal RNA genes, as well as the insertion elements previously described by Lessie and collaborators. Studies on mutants and the associated reductions in size of the replicons provide a convenient framework for genetic analysis of this strain.
The genome of another strain of B. cepacia (ATCC 25416) was analyzed and found to contain four circular replicons of sizes 3.65, 3.17, 1.07, and 0.2 Mb (Rodley et al., 1995). The values total 8.1 Mb. Again the interpretation is that the genome is made of three chromosomes and a large plasmid, because of the presence of ribosomal RNA genes only in the three large replicons. An interesting additional observation is the fact that multiple chromosomes are not confined to B. cepacia but also are found in other members of rRNA similarity group II (B. glumae, Ralstonia pickettii, and R. solanacearum) (Rodley et al., 1995). A very useful review that highlights the remarkable genomic complexity and plasticity of B. cepacia has been published (Lessie et al., 1996).
A recA gene has been identified in B. cepacia that is able to complement a recA mutation in E. coli, also restoring UV and methylmethane sulfonate resistance and proficiency in recombination (Nakazawa et al., 1990). Additionally, an SOS box related to LexA-regulated promoters and −10 and −35 consensus sequences have been detected in B. cepacia. The predicted RecA protein sequence shows 72% similarity with that of P. aeruginosa.
Transposable elements that activate gene expression in B. cepacia have been identified by Lessie and his collaborators (Scordilis et al., 1987). This opened a new horizon on insertional activation and on the significance of a high frequency of genomic rearrangements that presumably are related to the remarkable versatility of the species (Gaffney and Lessie, 1987; Lessie et al., 1996).
In spite of all these developments, at present there is incomplete knowledge of gene expression and of regulatory mechanisms in species of Burkholderia because of the lack of an appropriate gene exchange system. In the absence of a conventional bacterial genetic system, some alternatives have been developed. One of the proposed methods of genetic analysis is based on transposon mutagenesis and complementation of mutations by means of cloned genes. In a particular application, a shuttle plasmid was constructed that could be used for cloning genes of B. cepacia involved in protease production (Abe et al., 1996).
Studies on population genetics have been carried out on a population of B. cepacia isolated from a southwestern stream in the United States, to examine the allelic variation in a group of loci using multilocus enzyme electrophoresis. The studies showed a low degree of association between the loci, or extensive genetic mixing. This evidence of frequent recombination (and consequent low levels of linkage disequilibrium) indicates that the structure of the population was not clonal (Wise et al., 1995).
The topology of a 23S rRNA phylogenetic gene tree agrees with the 16S rRNA tree (Höpfl et al., 1989).
Plasmids
A cryptic 170-kb plasmid that was discovered in B. cepacia ATCC 17616 has been the subject of much research. Derivatives of this strain carried versions of the plasmid containing various insertion sequences in different combinations. These elements, inserted in the broad host range plasmid RP1, served as probes to examine the extent of the reiteration of the various components in the genome of the organism. The results indicated a high frequency of genomic rearrangements, mainly the result of replicon fusions promoted by the insertion elements, that could help explain the remarkable biochemical versatility of the species (Barsomian and Lessie, 1986; Gaffney and Lessie, 1987).
Derivatives of a nonconjugative Pseudomonas plasmid (pVS1), carrying genes for mercury and sulfonamide resistance as well as segments required for stability and for mobilization by plasmid RP1, have been established in B. cepacia (Itoh et al., 1984). Some further constructions based on pVS1 could be used as cloning vectors (Itoh and Haas, 1985).
In many instances, the degradation of toxic compounds and environmental pollutants is controlled by genes located in plasmids. Some of them already have been mentioned, and only brief reference to some additional instances will be made here. A 50-kb plasmid is responsible for the degradation of para-nitrophenol by B. cepacia, following an oxidative route with the production of hydroquinone and nitrite. The plasmid can be conjugationally transferred (Prakash et al., 1996). A 70-kb plasmid in B. cepacia strain 2CBS carries a gene cluster with the determinant of an enzyme able to catalyze double hydroxylation of 2-halobenzoates with release of halogenide and CO2 and producing catechol (Haak et al., 1995). A catabolic plasmid involved in the degradation of 4-methyl-o-phthalate was described and named MOP (Saint and Ribbons, 1990). Finally, a fragment of a plasmid involved in the catabolism of 4-methylphthalate in B. cepacia was sequenced and two open-reading frames were discovered, one of which encoded a permease that belongs to a group of symport proteins found in both pro- and eucaryotes. Information on this system could be used to improve the degradative capability for bioremediation purposes (Saint and Romas, 1996).
The novel pathway of toluene degradation mentioned in the section on metabolism of aromatic compounds is inducible and the corresponding genes are located in a plasmid. A strain of B. cepacia was found to carry two plasmids, one of 108 kb (named TOM) containing the genes for a toluene monooxygenase pathway that was expressed constitutively. The same strain also contained a small plasmid of less than 70 kb (Shields et al., 1995).
A new plasmid (pMAB1) with genes controlling the degradation of 24D in B. cepacia was characterized, and spontaneous negative mutants were isolated under nonselective conditions. Instead of the original 90-kb plasmid, these mutants had a smaller one (70-kb) or had lost it altogether. The activity could be regained by reintroducing the larger plasmid by electroporation. The 70-kb plasmid lacked a region that included the gene tfdC encoding the 3,5-dichlorocatechol 1,2-dioxygenase, whose sequence was identical with that of a well-characterized 24D degradative plasmid (pJP4) of Alcaligenes eutrophus. The similarity did not extend to the rest of the plasmids (Bhat et al., 1994). Another B. cepacia plasmid (pBS1502) was found to be able to control the early dehalogenation of 2,4-dichlorobenzoate (Zaitsev et al., 1991). There is also a report of the presence of a catabolic plasmid named MOP carrying genes involved in the catabolism of phthalate derivatives (Saint and Ribbons, 1990), and a small (2-kb) plasmid was implicated in the degradation of phenylcarbamate herbicides (Gaubier et al., 1992).
Plasmid analyses in combination with other typing techniques have been proposed for epidemiological studies on nosocomial infections by B. cepacia (Yamagishi et al., 1993). Early studies based on agarose gel electrophoresis of B. cepacia extracts demonstrated the presence of one or more plasmids in several strains of B. cepacia of plant and clinical origin. The plasmid composition, together with bacteriocin production and sensitivity, and pectolytic activity, could have applications in epidemiological studies (González and Vidaver, 1979).
Strains of B. cepacia from clinical and pharmaceutical origin carried large plasmids (146–222 kb) containing antibiotic resistance genes (Lennon and DeCicco, 1991). The nonconjugative B. cepacia plasmid pVS1 contained Hg and sulfonamide resistance genes, and a segment required for mobilization by RP1 (Itoh et al., 1984).
Bacteriophages
Most cultures of B. pseudomallei from collections have shown spontaneous phage production (Denisov and Kapliev, 1991). From the strains from a collection, 14 pure lines of bacteriophages belonging to two morphological types were isolated. The specificity of these phages was studied, and it was found that some strains of the host undergo poly-lysogeny, which was inferred from the fact that phages of different morphological types could be isolated from single strains (Denisov and Kapliev, 1995).
A generalized transducing phage was isolated from a lysogenic strain of B. cepacia, and half of more than 100 strains of the species were sensitive to it (Matsumoto et al., 1986).
Strain Berkeley 249 (ATCC 17616) of B. cepacia, which has been studied very intensively in Lessie's laboratory, carries an organic solvent-sensitive phage (Cihlar et al., 1978). Its sensitivity is attributed to alteration of a tail component provoked by the solvent. Results obtained by using other B. cepacia strains as hosts imply the occurrence of host restriction and modification systems. The phage has a head of 55 nm in diameter, a broad contractile tail of 15 × 145 nm, and double-stranded DNA of a molecular weight of about 3 × 107.
Many years ago a bacteriophage lytic for a wide range of aerobic pseudomonads was isolated and tested against strains of different species, among which was “Pseudomonas multivorans” (later identified as a synonym of B. cepacia) (Kelln and Warren, 1971). B. cepacia was insensitive; the phage was lytic only for species of rRNA similarity group I (Pseudomonas sensu stricto) and not for members of other rRNA groups.
Bacteriocins
Early work performed on B. cepacia strains isolated from plants and from clinical specimens showed that the two groups could be differentiated by bacteriocin production patterns, onion maceration tests, and hydrolysis of pectate at low pH, thus suggesting the usefulness of these characteristics in epidemiological studies (González and Vidaver, 1979). Additional differences between strains from the two sources have been recorded (Bevivino et al., 1994) and will be discussed below (see Ecology, Habitats, and Niches).
A number of B. cepacia bacteriocins (“cepaciacins”) were defined in work done on a collection of 34 strains isolated from plant rhizospheres and human patients (Dodatko et al., 1989a). One of the cepaciacins consisted of protein and carbohydrate in a 3:1 molecular ratio. The bacteriocin was thermolabile, stable within a narrow range of pH values, and it was destroyed by proteolytic action. UV irradiation or mitomycin C stimulated its biosynthesis (Dodatko et al., 1989b).
A typing scheme has been described based on bacteriocin susceptibility and production by B. cepacia strains using six producer strains and a set of eight indicator strains (Govan and Harris, 1985). The majority of strains of a large collection were typed into a total of 44 combinations, and the typing scheme was found to be useful for the possibility of its application to epidemiological studies.
Antigenic structure
The LPS structure of different B. pseudomallei strains is quite homogeneous. Antibodies prepared with material from one strain react with all others (Pitt et al., 1992). In agreement with the degree of their phylogenetic relationships, cross-reactions are observed with B. mallei and, to a lesser extent, with B. cepacia.
Many features of the biological activity of LPS isolated from B. pseudomallei cells have been described, and the strong mitogenic activity that it has toward murine splenocytes has been attributed to unusual chemical structures in the inner core attached to lipid A (Matsuura et al., 1996). In addition, information is available about the identification, isolation, and purification of an exopolysaccharide of this species. As in the instance described above, the compound having a molecular mass of >150 kDa did not show cross-reactivity with any of the species of all the Pseudomonas rRNA similarity groups, with the exception of the closely related species B. mallei (Steinmetz et al., 1995).
Purification to homogeneity of flagellin from several B. pseudomallei strains gave monomer flagellin bands of Mr 43,400 Da. Passive immunization studies showed that a specific antiserum could protect animals from challenge by a B. pseudomallei strain of different origin (Brett et al., 1994).
Two monoclonal antibodies were found to be highly specific for B. pseudomallei when tested by indirect enzyme-linked immunosorbent assay and immunoblotting against whole-cell extracts of other Burkholderia species, fluorescent pseudomonads, and E. coli. One of the antibodies could agglutinate all 42 B. pseudomallei strains included in the study, thus providing a tool for rapid identification of the species using primary bacterial cultures from clinical specimens (Pongsunk et al., 1996).
Of the serological typing schemes devised for B. cepacia, the one most widely used is that proposed by Werneburg and Monteil (1989). Originally, the scheme described procedures for the preparation, adsorption, and titration of O and H rabbit sera, and it could define seven O (O1 to O7) and five H antigens (H1, H3, H5, H6, and H7) for the slide agglutination test and the agglutination and immobilization test, respectively (Heidt et al., 1983). The scheme later was supplemented with new serotypes, using strains of a different geographical origin, to make a total of 9 O and 7 H antigens (Werneburg and Monteil, 1989). Other immunological typing schemes have been proposed (Nakamura et al., 1986).
Wilkinson and his collaborators have studied the composition of the O-specific polymers from the LPS of B. cepacia strains belonging to groups O1 (Cox and Wilkinson, 1990b), O3, O5 (Cox and Wilkinson, 1989b), O7 (Cox and Wilkinson, 1990a), and O9 (Taylor et al., 1994a). The O9 group has repeating units that are also present in Serratia marcescens. In the same laboratory it has been discovered that the O antigen of the LPS of B. cepacia serotype E (O2) is composed of two different trisaccharide repeating units in a 2:1 ratio (Beynon et al., 1995), and that the same O specific polymer is found in the two related species B. cepacia and B. vietnamiensis.
Interestingly, the lipopolysaccharides extracted from B. cepacia and B. gladioli have a higher endotoxic activity and provoke a higher cytokine response than that from Pseudomonas aeruginosa (Shaw et al., 1995). This adds to the importance to the human pathogenic propensities of P. gladioli, an example of a plant pathogen of medical importance similar to that of B. cepacia. Plant-associated B. gladioli can be differentiated from other pathogenic and symbiotic bacterial species by a rapid slide agglutination test using polyclonal antisera conjugated to protein-rich Staphylococcus aureus whole cells (Lyons and Taylor, 1990).
Cross-reactivity of P. aeruginosa antipilin monoclonal antibodies with heterogeneous strains of P. aeruginosa and B. cepacia has been reported (Saiman et al., 1989).
Antibiotic susceptibility
For obvious reasons, most information on antibiotic susceptibility of species of the genus Burkholderia refers to only a few species of medical importance (B. pseudomallei, B. cepacia, B. gladioli).
All strains of B. cepacia that have been tested are sensitive to sulfonamides and novobiocin. Most are also sensitive to trimethoprim plus sulfamethoxazole, and to minocycline and chloramphenicol (Santos Ferreira et al., 1985). Both B. cepacia and B. gladioli are resistant to a wide variety of antibiotics (ticarcillin by itself or mixed with clavulanic acid; cefsulodin, imipenem, aminoglycosides, colistin, and fosfomycin) (Baxter et al., 1997). A catechol-containing monobactam (BMS-180680) was quite active (MIC90, 1 µg/ml; MIC90 is a minimum concentration which inhibits 90% of the strains tested) (Fung-Tomc et al., 1997).
Eighty percent of a collection of strains of B. cepacia was tested for inhibition by ceftazidime (Tabe and Igari, 1994). A number of quinolone analogs and derivatives (trovafloxacin, ciprofloxacin, ofloxacin, levofloxacin, sparfloxacin, clinafloxacin, ceftazidime) were active on several Gram-negative, nonfermentative species, including B. cepacia. In comparison, the MIC for imipenem on B. cepacia and on Stenotrophomonas maltophilia was very high (Visalli et al., 1997).
In a comparison of the activity of many antibiotics against B. pseudomallei, a quinolone (tosufloxacine) and a tetracycline derivative (minocycline) appeared to be the most active (Yamamoto et al., 1990).
For years, the oral maintenance treatment of melioidosis has depended on the combined action of amoxicillin and the β-lactamase inhibitor clavulanic acid (Suputtamongkol et al., 1991). Biapenem, one of several carbapenem antibiotics tested against B. pseudomallei, was the most active against strains that showed a diminished susceptibility to third-generation cephalosporins (Smith et al., 1996).
Several resistance mechanisms operate in different species of Burkholderia. Resistance to cationic antibiotics in B. cepacia has been attributed to their ineffective binding to the outer membrane as a consequence of the low number of phosphate and carboxylate groups in the LPS, and the presence of protonated aminodeoxypentose (Cox and Wilkinson, 1991). The involvement of the outer membrane of B. cepacia in the resistance to polymyxin and aminoglycosides may also be related to a particular arrangement in the structure of the outer membrane, in which cation-binding sites on LPS are protected from polycations (Moore and Hancock, 1986).
An important factor in the antibiotic resistance in these organisms is porin permeability (Parr et al., 1987; Burns et al., 1996). However, a different interpretation is that resistance may not be a direct consequence of permeability of the porins, but instead may be related to the low number of porins per cell. A β-lactam-resistant mutant of B. cepacia and resistant strains of this species isolated from cystic fibrosis cases owe their resistance to a low porin content. These strains had reduced amounts of a 36-kDa outer membrane protein and did not express a 27-kDa outer membrane protein that can be a major porin or a major component of the porin complex of the cells (Aronoff, 1988). According to this view, the most common resistance mechanism in B. cepacia is the low porin-mediated outer membrane permeability, combined with multiple drug resistance due to an efflux pump system (Burns et al., 1996).
It may be of interest to mention here that growth in the presence of salicylate or other weak acids can induce resistance to antibiotics in B. cepacia, because these compounds have been found to be inhibitors of porin formation (Burns and Clark, 1992).
A number of β-lactams are susceptible to hydrolysis by a β-lactamase of B. cepacia, a metalloenzyme of type I that is induced by imipenem (Baxter and Lambert, 1994). A significant degree of similarity was found between the chromosomal β-lactamase of B. cepacia strain 249 and the enzymes of Pseudomonas aeruginosa and E. coli. Interestingly, in spite of differences in the mol% G + C content of the DNA of these organisms, the codon usage in B. cepacia resembled that of E. coli (Proenca et al., 1993).
The multiple resistance gene oprM of P. aeruginosa is part of a highly conserved efflux system. A gene homologous to oprM was identified in B. cepacia (Burns et al., 1996). Moreover, an intragenic probe hybridized the genomic DNA of several fluorescent pseudomonads and, in addition, the DNA of B. pseudomallei (Bianco et al., 1997). A lucid review is available on the participation of multidrug efflux pumps in the resistance of Gram-negative organisms to antibiotics (Nikaido, 1996).
The fusaric acid resistance gene of B. cepacia has been cloned and sequenced (Utsumi et al., 1991).
Antibiotic production
Antifungal antibiotics have been identified in strains of B. cepacia. Cepacidine A, composed of two related forms, cepacidine A1 and A2, is a cyclic peptide and xylose connected to a 5,7-dihydroxy-3,9-diaminooctadecanoic acid (Lee et al., 1994a; Lim et al., 1994). Two antibiotics previously discovered and described under the names cepacin A and B are not related to the cepacidines. The cepacins showed good antistaphylococcal activities (MICs of 0.2 and 0.05 µg/ml for cepacin A and B, respectively), but no significant activity against Gram-negative bacteria (Parker et al., 1984).
Another antifungal antibiotic is pyrrolnitrin (Jayaswal et al., 1993). The producing organism was named Pseudomonas pyrrocinia (Imanaka et al., 1965), now Burkholderia pyrrocinia. The antibiotic is also produced by B. cepacia and by some strains of Pseudomonas chlororaphis (Elander et al., 1968). A study by Burkhead et al. (1994) has examined the conditions of pyrrolnitrin by B. cepacia in culture and in the wounded areas of potatoes colonized by the organism.
Some compounds related to pyrrolnitrin (amino-pyrrolnitrin, and monochloroamino-pyrrolnitrine) also have antifungal properties (McLoughlin et al., 1992). The antibiotic activity of B. cepacia has been reported to antagonize the pathogenic activity of the sunflower wilt fungus (McLoughlin et al., 1992) and to be a suppressor of maize soil-borne disease (Hebbar et al., 1992). The influence of some environmental factors on the antagonism of B. cepacia toward Trichoderma viride has been analyzed (Upadhyay et al., 1991), as well as some morphological alterations and inhibition of conidiation of plant pathogenic fungi (Upadhyay and Jayaswal, 1992). A compound having both hemolytic activity and antifungal action was characterized and given the name cepalycin (Abe and Nakazawa, 1994).
Transposon mutagenesis could eliminate pyrrolnitrin production ability. However, the mutation failed to be complemented by the cloned gene because of difficulties encountered in mobilizing the carrier cosmids from E. coli to B. cepacia mutants (Jayaswal et al., 1992).
A group of eight cyclic peptides of antifungal activity, the xylocandins, has been isolated from B. cepacia and characterized (Bisacchi et al., 1987). A mixture of two of the forms (A1 and A2) showed potent anticandidal and antidermatophytic activities in vitro (Meyers et al., 1987).
The antagonistic activity of B. cepacia is not limited to antifungal activity. Of practical importance is the finding that metabolites produced by strains of this species can antagonize plant pathogenic agents such as Ralstonia solanacearum (Aoki et al., 1991).
Toxoflavin, an azapteridine antibiotic produced by B. cocovenenans, was identified more than 30 years ago (Lauquin et al., 1976). The production of toxoflavin is inhibited by the combined action of 2% NaCl and acidity (pH 4.5) in the medium (Buckle and Kartadarma, 1990). A monobactam antibiotic (MM 42842) is also produced by B. cocovenenans. It is related to a previously described antibiotic named sulfazecin. In addition, the B. cocovenenans strain synthesizes bulgecin, an antibiotic also produced by other aerobic pseudomonads (Box et al., 1988; Gwynn et al., 1988).
Tropolone, which is known to have antibacterial and antifungal activities (Lindberg, 1981), is produced by B. plantarii cultures (Azegami et al., 1987).
Plant pathogenicity
Many species of the genus Burkholderia are pathogenic for animals or plants. Phytopathogenic pseudomonads are located in three of the five RNA similarity groups. One of them is rRNA group II, in which Burkholderia and Ralstonia are allocated. The various symptoms produced in plants by the phytopathogenic pseudomonads are tumorous outgrowth, rot, blight or chlorosis, and necrosis, which are caused by alteration of the normal metabolism of plant cells by pathogenicity factors excreted by the bacteria. These factors include enzymes capable of degrading components of plant tissues, toxins, and plant hormones.
The phytopathogenic species of the genus Burkholderia mainly produce rots, due to active pectinolytic enzymes and cellulases (Gehring, 1962; Hildebrand, personal communication). Further details on symptoms and on the list of hosts attacked by each species will be given in the section of species descriptions at the end of this chapter.
Aside from acting as agents of diseases, members of the genus also participate in producing beneficial effects by antagonizing other phytopathogenic organisms, mainly fungi. This effect has been reported in the literature for B. cepacia in a number of instances (Kawamoto and Lorbeer, 1976; Fantino and Bazzi, 1982; Janisiewicz and Roitman, 1988; Homma et al., 1989; Jayaswal et al., 1990; Parke et al., 1991).
Pathogenicity for humans and animals
The most serious animal and human pathogenic species of the genus Burkholderia are B. mallei and B. pseudomallei, the agents of glanders or farcy of equids, and of melioidosis in humans, respectively. Detailed descriptions of these organisms and the diseases that they cause are available (Redfearn et al., 1966; Redfearn and Palleroni, 1975). As a free-living species present in the warm regions of the planet, B. pseudomallei has not spread far from the equator, although in China it has reached a northern latitude of at least 25.5 degrees (Yang et al., 1995b).
A useful updated treatment of the bacteriology of glanders and melioidosis is available (Pitt, 1998). Many years ago, melioidosis was recognized as a glanders-like disease (Whitmore, 1913), and subsequently the organism was assigned to several different genera. In fact, this has also been true of B. mallei, which has spent much of its scientific career in search of a proper generic allocation.
Two different biotypes have been recognized among various strains of B. pseudomallei isolated from patients and from soil in Thailand. However, all of the strains were recognized by using a specific polyclonal antibody. One of the biotypes may have low virulence or it may represent a different species altogether, based on the distribution of these phenotypes and the respective incidence of melioidosis in different areas (Wuthiekanun et al., 1996).
Strains of B. pseudomallei isolated in Australia were examined for their genomic relationships using random amplification of polymorphic DNA and multilocus enzyme electrophoresis. The strains could be divided into two groups that correlated with the clinical presentation and not with the geographic origin; in other words, there was a correlation between the clinical manifestation of the disease and the molecular characteristics of the pathogen (Norton et al., 1998).
Some of the human pathogens are “opportunistic pathogens” that create major medical problems in patients with reduced levels of natural resistance. This situation emerges because of an increasing use of instrumentation or drugs (including antibiotics) that reduce or bypass the level of natural resistance and/or the specific immune mechanisms (Spaulding, 1974).
In reference to B. cepacia, it seems appropriate here to highlight the main points of the abstract of an excellent article published more than a decade ago (Goldmann and Klinger, 1986). From its original habitat as a plant pathogen, B. cepacia has invaded the hospital environment as an important pathogen of compromised human hosts. Many nosocomial infections have their source in contaminated medicaments and even disinfectants and antiseptics. Various conditions in hospital patients can be complicated by B. cepacia infections, but the properties that define the virulence of strains of this species are still poorly defined. The last addition to the long list of pathological conditions that are further deteriorated by infections of this pathogen is cystic fibrosis. As in other conditions, some patients may be simply colonized, while other patients' initial conditions can be very seriously complicated, to which the remarkable resistance of B. cepacia to a wide range of antibiotics contributes very effectively. This bleak panorama has become even worse in the intervening years, particularly with respect to pulmonary exacerbations in cystic fibrosis patients, in many cases ending in rapid and fatal deterioration of lung function.
Characteristics that occur more frequently among strains isolated from infections in cystic fibrosis patients include catalase, ornithine decarboxylase, valine amino-peptidase, lipase, alginase, trypsin, ability to reduce nitrate, hydrolysis of xanthine and urea, complete hemolysis of bovine red blood cells, cold-sensitive hemolysis of human red blood cells, and greening of horse and rabbit red blood cells. Although some of these properties are associated with pathogenicity in other bacteria, their relationships to cystic fibrosis-associated pulmonary disease are far from clear (Gessner and Mortensen, 1990). Acid phosphatase in Burkholderia species is a factor that may be related to pathogenicity. It is present in B. cepacia and B. pseudomallei, and in fact the activity in the latter species is remarkably high (Dejsirilert Butraporn et al., 1989).
In addition to these factors, as mentioned before in the section on antigenic structure, LPS preparations from clinical and environmental isolates of B. cepacia and from the closely related species B. gladioli exhibit a higher endotoxic activity and more pronounced cytokine response in vitro when compared to preparations of P. aeruginosa LPS. The latter species is also involved in infections in cystic fibrosis patients (Shaw et al., 1995).
B. gladioli, a species originally described as phytopathogenic, has been implicated in infections complicating cases of cystic fibrosis (Mortensen et al., 1988). In one study, the organism was isolated from respiratory tract specimens obtained from 11 cystic fibrosis patients and was identified by its biochemical properties, DNA hybridization, and fatty acid analysis. The authors recommend the inclusion of some of these criteria for the differentiation between B. gladioli and B. cepacia, two closely related species, and point out that most B. gladioli strains have C10:0 3OH fatty acid, which is absent from B. cepacia lipids (Christenson et al., 1989). Also based on the results of fatty acid analysis, a pseudomonad that was isolated from pleural fluid and pulmonary decortication tissue with granulomatous disease more closely resembled B. gladioli than B. cepacia (Trotter et al., 1990). Further information on the activity of B. gladioli as a human pathogen has been reported by Ross et al. (1995). A strain of this species was involved in empyema and bloodstream infection occurring after lung transplantation in a cystic fibrosis case (Khan et al., 1996c).
The pili of B. cepacia mediate adherence to mucous glycoproteins (Kuehn et al., 1992; Sajjan and Forstner, 1993) and epithelial cells in cystic fibrosis patients. Structural variant classes of pilus fibers have been identified in B. cepacia strains (Goldstein et al., 1995). One or more of five morphologically different types can be coexpressed. It has been noticed that, when present in the infective population, Pseudomonas aeruginosa cells enhance the adhesion of B. cepacia to epithelial cells (Saiman et al., 1990).
The major pilin subunit of B. cepacia corresponds to peritrichous fimbriae, which, based on their appearance as giant intertwined fibers, have been called “cable” (Cbl) pili. The cblA gene (the first pilin subunit gene to be identified in B. cepacia) has been detected in a DNA library (Sajjan et al., 1995).
One very infectious cystic fibrosis strain isolated as the agent of epidemics in England and Canada was found to have the cable pilin subunit gene. A conserved DNA marker in a 1.4-Kb fragment was present in epidemic strains, absent from the nonepidemic ones, and rare among the environmental strains. The presumed ORF was designated “epidemic marker regulator” (esmR) (Mahenthiralingam et al., 1997). Strains were recovered in cystic fibrosis centers in France. There was cross-colonization in 7 of 13 centers. The most chronically colonized patients harbored a single B. cepacia strain, which suggests a geographically clustered distribution of B. cepacia, with the exception of one genotype. This genotype was detected in four regions and proved to be different from the British-Canadian highly transmissible strain and was able to spread among cystic fibrosis units (Segonds et al., 1997).
Biochemical and genomic properties have been used for the typing of B. cepacia strains of nosocomial origin. In a first step, six enzymes and pigment production subdivided a collection of strains of this species into 12 groups, and the strains from one-third of the collection were further characterized by DNA fingerprinting, ribotyping, and plasmid analysis. By testing the typing scheme on strains isolated years later, the results showed the usefulness and consistency of the genomic typing, and the marked diversity of phenotypes among the strains of the species (Ouchi et al., 1995).
The amplified products of the internal transcribed spacers (ITS) separating the 16S and 23S rRNA genes in the DNA have been effective as tools for identification of reference strains of Pseudomonas aeruginosa and B. pseudomallei, but the primer pairs tested for B. cepacia have not provided much help in strain identification (Tyler et al., 1995). In contrast, PCR-ribotyping targeted on the 16S-23S intergenic spacer to determine the length heterogeneity of this region is a rapid and accurate method for B. cepacia strain typing (Dasen et al., 1994).
The internal diversity of B. cepacia strains is also manifested in differences in whole-cell protein profiles (Li and Hayward, 1994) and in the fatty acid composition of populations from various cystic fibrosis centers (Mukwaya and Welch, 1989).
A novel marker has been found to be associated with epidemic B. cepacia strains causing infections in cystic fibrosis patients. A highly infectious strain had both the cable pilin subunit gene (cblA) and a unique combination of insertion sequences. Although no specific marker was identified in common with other epidemic strains, a conserved DNA fragment among epidemic strains was detected. This fragment (called the “B. cepacia epidemic strain marker”) was absent from other strains infecting individual cystic fibrosis patients and rarely found in environmental strains (Mahenthiralingam et al., 1997).
In a related study on 97 clinical and 2 environmental strains of B. cepacia, a search for possible correlations was made with respect to parameters such as certain insertion sequence (IS) elements, the cblA gene for a pilin subunit, the electrophoretic type (ET), and the ribotype (RT) (Tyler et al., 1996). No linkage was found between the presence of each of five different IS elements and ET or RT. All strains of a given ET also possessed cblA. One IS element different from the five initially identified was present in 72% of all isolates, and in half of them the new IS element was inserted in one of the original five. This hybrid IS element only was found in epidemic strains from Ontario, Canada, and the UK.
Methods were developed to detect the presence of B. cepacia, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia in sputum, using primers based on 16S rRNA sequences. The results are in agreement with those of direct isolation of cultures from the samples (Campbell et al., 1995; Karpati and Jonasson, 1996).
Bongkreik acid (also named flavotoxin A), a product of fermentation by B. cocovenenans, is the cause of serious food poisoning outbreaks in China and other Far East countries (Hu et al., 1989). An isomer, isobongkreik acid, was later identified, and, like bongkreik acid, it acts as an uncompetitive inhibitor of ADP transport in mitochondria, although it is less active than bongkreik acid (Lauquin et al., 1976).
Ecology, habitats, and niches
The habitats from which Burkholderia species have been isolated are quite diverse: soils and rhizospheres (B. cepacia, B. vietnamiensis, B. pseudomallei, B. pyrrocinia, B. phenazinium, B. multivorans, B. thailandensis, B. glathei), water (B. norimbergensis), plants (B. cepacia, B. gladioli, B. caryophylli, B. glumae, B. plantarii, B. andropogonis, B. vandii), foods (B. cocovenenans), animals (B. mallei, B. pseudomallei), and clinical specimens (B. cepacia, B. vietnamiensis, B. gladioli, B. multivorans). The occurrence of B. pseudomallei mainly in soils of tropical regions has been discussed elsewhere (Redfearn et al., 1966).
As mentioned in the section on metabolic properties, some species of the genus are metabolically very versatile, and there is little doubt that those normally found in soils and water must contribute substantially to the mineralization of carbon compounds in nature.
B. cepacia is commonly found in natural materials, particularly soil, and methods have been developed for its detection. One such method of detection in the environment was based on amplification of sequences in genomic DNA using primers specific for repetitive extragenic palindromic segments, followed by cloning of the amplified fragments. Probes were constructed based on the strain-specific sequences (Matheson et al., 1997). A striking example of the power of molecular approaches was the report of the detection of a single B. cepacia cell in a soil sample containing a population of 1010 procaryotic cells (Steffan and Atlas, 1988). Estimates of the relative abundance of B. cepacia in stream bacterioplankton can be performed after collecting the cells by various procedures, of which the use of filters made of inorganic materials was found to give the highest recoveries (Lemke et al., 1997).
Strains of B. cepacia can be isolated from rhizosphere environments (Tabacchioni et al., 1995). This is also the habitat from which B. graminis was originally obtained (Viallard et al., 1998). Rhizosphere strains of B. cepacia differed in phenotypic characteristics from those isolated from clinical materials. Among the differences were nitrogen fixation, indole-acetic acid production, a wide temperature range, antibiosis vs. phytopathogenic fungi, and growth promotion of Cucumis sativus—all positive for rhizosphere strains. These properties were absent from clinical strains, which instead possessed characteristics such as adhesion to human cells, protease production, and synthesis of siderophores different from those found in the nonclinical strains (Bevivino et al., 1994). The character of N2 fixation is not clear-cut, since DNA preparations from clinical strains hybridized with the nifA gene probe from Klebsiella pneumoniae, and the DNA of one of the rhizosphere strains hybridized with the nifHDK from Azospirillum brasilense (Tabacchioni et al., 1995).
As far as animal pathogenicity is concerned, observations made in other laboratories seem to support somewhat different conclusions. Thus, strains isolated from plant or clinical materials did not differ in their lethality to mice, i.e., they have similar LD50 values (González and Vidaver, 1979). Indeed, strains of species known to be causal agents of human infections may be isolated from rhizosphere environments (Tabacchioni et al., 1995).
Although B. cepacia helps in the degradation of toxic compounds and has an inhibitory action on soil-borne plant pathogens, it can also behave as a serious opportunistic human pathogen. The key question of interest for its use in biotechnological projects is whether the two types of populations can interact to the point of transmission of the characteristics required for the pathogenic condition. Yohalem and Lorbeer (1994) state that “although there are (B. cepacia) strains with significant potential for the remediation of environmental toxins … and others with potential as biological controls of plant disease … environmental release of any strain may be prohibited because some strains of the nomenspecies have been implicated in nosocomial infections.” The same considerations apply to other versatile species of the genus that manifest pathogenic propensities.
Enrichment and Isolation Procedures
For the specific isolation of B. cepacia from environmental water samples or various aqueous solutions, a medium containing 9-chloro-9-(4-diethylaminophenyl)-10-phenylacridan (C-390) and polymyxin B sulfate (PBS) has been proposed.4 The two drugs inhibited all the organisms tested, with the exception of B. cepacia and Serratia marcescens (Wu and Thompson, 1984).
A medium proposed for the isolation of B. cepacia contains tryptamine and azelaic acid as sole sources of nitrogen and carbon, respectively, in addition to the antifungal compound chlorothalonil (Diamond Shamrock Corp., Cleveland, Ohio) (Burbage and Sasser, 1982). Later on it was found that the effectiveness of this medium was rather low, and a different one, medium (TB-T)5, based on the combined action of trypan blue (TB) and tetracycline (T) at a relatively low pH (5.5), was proposed. Using this medium, the efficiency of recovery was very variable among strains, and for some of them reached 76–86% (Hagedorn et al., 1987).
A medium for B. cepacia has been described in a patent (Lumsden and Sasser, 1986).
Three selective enrichment liquid media and four solid media were evaluated at two temperatures (35°C and room temperature) for their capacity of supporting growth of B. pseudomallei strains. Enrichment with trypticase soy broth with addition of 5 mg of crystal violet and 20 mg of colistin per liter and subculture in Ashdown medium6 gave the highest recovery rates and the greatest suppression of other members of the soil microflora. The results were comparable at the two temperatures (room temperatures ranged between 20°C and 32°C).
Differentiation of the genus Burkholderia from other genera
Similarities between Burkholderia and other Gram-negative bacteria
A sharp differentiation of members of Burkholderia and Pseudomonas is difficult because of many similarities between these organisms. In the course of biochemical studies carried out at Berkeley before the rRNA–DNA hybridization experiments showed that “P. multivorans” (B. cepacia) was not closely related to the fluorescent group, it was noticed that key steps of the metabolism of aromatic compounds were remarkably similar between the two groups. In the section on the metabolism of aromatic compounds, some comments have been made on the similarity of components of some of the enzymes involved in the degradation by distantly related organisms. In fact, many details of the metabolic constitution of the fluorescent pseudomonads are far closer to those found in some species of RNA similarity group II—in particular, B. cepacia—than to those characteristic of RNA group V (Stenotrophomonas and Xanthomonas), which is closer to RNA group I. Such is the case of the enzymes codified by genes bphB and bphC in P. putida and B. cepacia, and the same is true for the gamma-carboxymuconolactone decarboxylases of these species. Fluorescent organisms and B. cepacia are able to synthesize salicylic acid, which acts both as a siderophore and also as the precursor of another siderophore found in both groups of organisms, pyochelin. Members of the fluorescent group and B. pyrrocinia, a species related to B. cepacia, produce the antifungal antibiotic pyrrolnitrin, and similarities also have been observed in the pilin antigenic structures of P. aeruginosa and B. cepacia (Saiman et al., 1989).
Similarities have also been observed between B. cepacia and bacteria of groups other than the aerobic pseudomonads. With respect to the production of chaperonins (so named for their relationship with eucaryotic chaperones), an evolutionary homolog of the protein involved in plants in the assembly of ribulose-bisphosphate carboxylase-oxygenase (the key enzyme in CO2 fixation) was identified in E. coli. This protein, GroEL, is one of the chaperonins, a group widely present in procaryotes and organelles of procaryotic origin (chloroplasts and mitochondria) (Hemmingsen et al., 1988). Aside from E. coli and pseudomonads, chaperonins have been identified in species of Legionella, Bacillus, Borrelia, Treponema, Mycobacterium, and Coxiella (Kaijser, 1975; Houston et al., 1990). In work performed on the cloning and nucleotide sequencing of the groE operons of P. aeruginosa and B. cepacia, a high degree of similarity was found, which extended to the E. coli groEL. The level of similarity with the human protein was lower, but still considerable. Comparable results were obtained in the study of a second chaperonin, GroES (Jensen et al., 1995).
The above-cited findings on chaperonins indicate that the similarities between groups I and II (Pseudomonas and Burkholderia) in some cases go beyond the dispensable catabolic systems or the biosynthesis of secondary products.
Differentiation of Burkholderia from related genera
The examples of similarities mentioned in the previous section show that a sharp differentiation of members of Burkholderia and Pseudomonas is difficult. Studies on DNA–DNA hybridization (Ballard et al., 1970) and on rRNA–DNA hybridization (Palleroni et al., 1973) demonstrated that species of the genus were members of RNA similarity group II. They were eventually allocated to a newly proposed genus under the name Burkholderia (Yabuuchi et al., 1992), but two of the species (B. pickettii and B. solanacearum) were later transferred to the new genus Ralstonia, and the criteria for the differentiation of this genus from Burkholderia were defined (Yabuuchi et al., 1995). Unfortunately, as in the case of the proposal of the genus Burkholderia, the published differential characteristics were limited to those of a single strain of each species, which makes it impossible to estimate the intraspecies diversity.
In a polyphasic study of Burkholderia species, Gillis et al. (1995) redefined this genus. Among the characteristics of differential value, these authors mention the presence of C16:0 3OH in the cellular fatty acid composition. Most of the properties given in the definition of the genus Burkholderia apply equally well to other genera of aerobic pseudomonads, and, as in the case of the genus Pseudomonas, the fatty acid composition and the 16S rRNA characteristics remain among the few useful differential criteria.
Taxonomic Comments
In the 1960s, a project that focused on the construction of a rational system of classification of Pseudomonas species was organized at the Department of Bacteriology of the University of California at Berkeley. This project had its main justification in the highly unsatisfactory situation of the taxonomy of this genus to which were assigned several hundreds of species names, many of which could not be identified based on published descriptions, and their type strains had been lost.
A thorough phenotypic characterization of a large collection of strains resulted in a subdivision of the genus into species and species groups (Stanier et al., 1966). With time, species not included in the original project were subjected to the same analysis and located in the classification scheme. Eventually, the phenotypic characterization received confirmation from the results of DNA–DNA hybridization experiments. More significantly, the results of these experiments revealed a very wide range of DNA similarity values among the species assigned to the genus, suggesting a considerable degree of genomic heterogeneity among the members of the genus. This hypothesis was corroborated by rRNA–DNA hybridization experiments. They clearly showed that Pseudomonas, as classically described, could be subdivided into five RNA similarity groups representing at least five different genera (Palleroni et al., 1973).
This demonstration that the genus Pseudomonas was in fact a complex entity of suprageneric hierarchy was taken as the basis for proposals from various other laboratories to give different generic names to designate members of the five rRNA similarity groups. Of the five groups, rRNA group I retained the name Pseudomonas and the type species P. aeruginosa was originally proposed for the genus. For rRNA group II, the new genus name Burkholderia was introduced (Yabuuchi et al., 1992), comprising seven new combinations: B. cepacia (Palleroni and Holmes, 1981), B. mallei (Zopf, 1885), B. pseudomallei (Whitmore, 1913), B. caryophylli (Burkholder, 1942), B. gladioli (Severini, 1913), B. pickettii (Ralston et al., 1973), and B. solanacearum (Smith, 1896). Since the heterogeneity of the genus even extended to the rRNA similarity group level, some of the groups could still be further subdivided to include more than one bacterial genus. Thus, the last two of the above-mentioned species, were assigned to a newly created genus, Ralstonia (Yabuuchi et al., 1995). The overall similarity of Ralstonia solanacearum and R. pickettii already had been noticed in studies on their phenotypic characteristics and on DNA homologies (Ralston et al., 1973).
The present treatment refers to those species of rRNA group II assigned to the genus Burkholderia.
In their studies on the aerobic pseudomonads, Stanier et al. (1966) described a group of metabolically versatile organisms under the new species name “Pseudomonas multivorans”. In the plant pathology department of the University of California, David Sands applied the same methodology to the study of phytopathogenic pseudomonads. Soon his studies resulted in the unexpected finding that the properties of the above strains were virtually identical to those of a species that had been known to phytopathologists for almost two decades under the name of P. cepacia. The remarkable versatility of strains of this species had been totally overlooked by the early workers.
DNA–DNA hybridization experiments confirmed the synonymy soon thereafter (Ballard et al., 1970). The collection of strains of B. cepacia examined at the time could be differentiated from other species on the basis of the capacity to grow at the expense of d-arabinose, d-fucose, cellobiose, saccharate, mucate, 2,3-butylene glycol, sebacate, meso-tartrate, citraconate, o-hydroxybenzoate, m-hydroxybenzoate, l-threonine, dl-ornithine, and tryptamine. This set of characteristics sharply separated B. cepacia from all other members of the various phenotypic groups, as can be seen in Table 6 of the original reference (Stanier et al., 1966).
| Characteristic | H. flava | H. palleronii | H. pseudoflava | H. taeniospiralis |
|---|---|---|---|---|
| Tolerance to 20% oxygen | − | + | + | nd |
| Aerobic autotrophic growth with CO | nd | nd | d | − |
| Growth at: | ||||
| 37°C | nd | nd | + | +c |
| 40°C | nd | nd | + | + |
| 41°C | − | − | + | nd |
| 42°C | nd | nd | − | − |
| Use of ammonia as sole nitrogen source | + | + | + | nd |
| Nitrogen fixation | − | − | d | − |
| Hydrolysis of acetamide, casein, DNA | nd | nd | − | − |
| Hydrolysis of esculin | + | nd | −d | −d |
| Lysine and ornithine decarboxylase | nd | nd | − | − |
| Production of pyoverdins or phenazine pigments | − | − | − | nd |
| β-Galactosidase | nd | nd | + | + |
| Hemolysis | nd | nd | − | − |
| Growth on: | ||||
| Trehalose | + | − | + | +c |
| d-Turanose | + | − | +c | − |
| l-Rhamnose | + | − | v | + |
| l-Fucose | + | − | −e | + |
| d-Ribose, d-fucose | − | − | −e | − |
| d-Arabinose | −d, f | − | −e | − |
| d-Lyxose | nd | nd | d | − |
| d-Melibiose, d-raffinose, d-tagatose, l-sorbose, l-xylose, melezitose, β-gentiobiose, dulcitol, l-arabitol, xylitol, glycogen, α-methyl-d-glucoside, α-methyl-d-mannoside, α-methylxyloside | nd | nd | − | − |
| Salicin | −d, f | − | + | − |
| Amygdalin, arbutin, esculin | nd | nd | −e | − |
| Ethanol | + | + | vf | nd |
| n-Propanol | +g | + | ve | nd |
| n-Butanol | − | + | ve | nd |
| Isobutanol | − | + | nd | nd |
| Methanol, phenylethanediol | − | − | nd | nd |
| Ethylene glycol, 2,3-butylene glycol | − | − | − | nd |
| Propylene glycol | + | + | nd | nd |
| Phenol | − | + | + | nd |
| Testosterone | − | − | − | nd |
| Acetate | + | +c, g | +e | − |
| Propionate | − | −e, h | −f | − |
| Butyrate | −f | + | vd, f | −d, f |
| Isobutyrate | − | −f | − | − |
| Formate | − | −f | − | + |
| Valerate | − | −e, h | vf | − |
| Isovalerate | − | −d, f | vc | − |
| 2-Ketoglutarate | +c, g | +c, g | ve | + |
| dl-Glycerate | + | + | vd, f | + |
| d(−)-Tartrate | + | + | vc | − |
| l(+)-Tartrate | +c, g | +c, g | v | − |
| meso-Tartrate | − | + | −e | − |
| 3-Hydroxybutyrate | + | + | + | nd |
| Glycerate | + | + | nd | nd |
| Glycolate | −d | + | vf | − |
| Citrate | − | +c, g | +e | − |
| Aconitate | − | +c, g | +c, g | − |
| Levulinate | −d, f | − | + | + |
| m-Hydroxybenzoate | +g | + | d | − |
| Suberate | − | + | vd, f | + |
| Saccharate | − | + | v | nd |
| Adipate | − | d | +c | − |
| Caproate, oxalate, phthalate, 2-ketogluconate, citraconate, benzoate | − | − | −e | − |
| 5-Ketogluconate | nd | nd | −e | − |
| Quinate | − | − | + | nd |
| Kynurenate, mucate | − | + | nd | nd |
| Benzoylformate | − | d | nd | nd |
| Terephthalate | − | −h | − | − |
| Itaconate | d | − | − | |
| Pimelate | − | −e, h | vc, f | − |
| Sebacate | − | −d, f | + | + |
| Anthranilate, hippurate, hydroxymethylglutarate, nicotinate, pantothenate, poly-β-hydroxybutyrate | − | − | − | nd |
| Eicosanedioate | − | − | nd | nd |
| l-Phenylalanine | + | +e, h | + | + |
| l-Tyrosine | +e | +e, h | + | + |
| l-Alanine | +c, g | +e, h | +e | − |
| d-Alanine | − | +e, h | + | − |
| l-Leucine, l-tryptophan | − | +e, h | + | + |
| l-Isoleucine | −d, f | d | + | + |
| l-Histidine | −d, f | − | + | − |
| l-Threonine, citrulline | − | − | −e | − |
| l-Lysine | − | − | + | +c, g |
| l-Aspargine | − | + | + | nd |
| Glycine | − | − | vc, g | − |
| l-Arginine | − | − | v | + |
| l-Valine | − | − | v | − |
| β-Alanine | − | − | dc | − |
| dl-2-Aminobutyrate | − | − | d | − |
| dl-3-Aminobutyrate | − | − | −e | + |
| l-Serine, benzylamine | − | − | d | − |
| dl-Arginine, dl-citrulline, dl-ornithine, methylamine, dl-2-aminovalerate | − | − | nd | nd |
| l-Cysteine, l-methionine, dl-kynurenine, ethylamine, glucosamine, trigonelin, urea | nd | nd | − | − |
| l-Norleucine | nd | d | d | − |
| dl-Norvaline | nd | nd | d | − |
| Butylamine | + | − | dd | + |
| l-Kynurenine | − | − | − | nd |
| 3-Aminobenzoate, 4-aminobenzoate | − | − | − | − |
| 2-Aminobenzoate, phenylacetate | nd | nd | − | − |
| N-Acetyl glucosamine | nd | nd | −e | − |
| Amylamine | nd | nd | + | − |
| Diaminobutane | nd | nd | + | + |
| Cetrimide | nd | nd | − | − |
| Growth in the presence of: | ||||
| NaCl, 0.5% | nd | nd | + | − |
| NaCl (1.5%, 3%, 4.5%, 6.5%) | nd | nd | − | − |
| Aerobic acid production from: | ||||
| d-Glucose, maltose | + | − | +c | − |
| Adonitol | nd | nd | − | − |
| Saccharose, trehalose, cellobiose | + | − | + | nd |
| d-Fructose | + | − | +c | |
| Arabinose | + | − | + | − |
| Mannose, galactose, rhamnose | + | − | d | nd |
| Xylose | − | − | d | − |
| Fucose | − | − | − | nd |
| Growth inhibited by: | ||||
| SDS, 100 µg/ml | + | + | + | nd |
| SDS, 50 µg/ml | − | + | − | nd |
| 5% NaCl, 3% Glycine | + | + | + | nd |
| 2% Glycine | − | + | − | nd |
| 3% Tween 20 | + | + | + | nd |
| 3% Tween 40, 3% Tween 80 | + | + | − | nd |
| Sodium azide 100 mg/ml | + | + | + | nd |
| 2% Methionine | + | + | − | nd |
| Susceptibility to:i | ||||
| Chloramphenicol (30 µg), erythromycin (15 µg), neomycin (30 µg), novobiocin (30 µg), streptomycin (10 µg), tetracycline (30 µg) | + | + | + | nd |
| Ampicillin (10 µg), kanamycin (30 µg), methicillin (5 µg), polymyxin B (300 U) | + | d | + | nd |
| Penicillin (10 µg) | nd | nd | + | + |
- a For symbols see standard definitions; nd, not determined.
- b Data taken from Auling et al. (1978), Aragno and Schlegel (1992), Davis et al. (1969, 1970), Palleroni (1984), and Willems et al. (1989). All strains can grow at 30°C and use nitrate as sole nitrogen source and grow on the following substrates: d-malate, dl-β-hydroxybutyrate, fumarate, pyruvate, succinate, gluconate, lactate, glucose, glycerol, m-inositol, l-aspartate, l-glutamate, l-malate, and l-proline. None of the strains hydrolyzes starch or poly-β-hydroxybutyrate, possesses arginine dihydrolase or produces acid from ribose. All strains fail to grow on caprate, caprylate, heptanoate, maleate, malonate, mesaconate, o-hydroxybenzoate, pelargonate, d-mandelate, isophthalate, adonitol, erythritol, inulin, starch, d-tryptophan, creatine, betaine, histamine, acetamide, sarcosine, and tryptamine.
- c Negative according to Willems et al. (1989).
- d Positive according to Willems et al. (1989).
- e Mixed reaction reported by Aragno and Schlegel (1992).
- f Positive according to Aragno and Schlegel (1992).
- g Negative according to Aragno and Schlegel (1992).
- h Mixed reaction reported by Willems et al. (1989).
- i +, inhibition zone >5 mm; −, inhibition zone <2 mm; d, inhibition zone 2–5 mm.
The early observations that helped to uncover the remarkable metabolic versatility of B. cepacia and other species of the genus Burkholderia also contributed substantial lists of phenotypic characteristics for use in the identification of newly isolated strains. Some of the results are summarized in the tables included in this chapter. Substantial collections of strains of the species are now available, and studies on intraspecific diversity have been published. The interesting metabolic properties of members of this group of procaryotes stimulated the isolation of many strains, which has contributed to the recent proliferation of names for new species and for groups (biovars, genomovars) at the intraspecific level. On the one hand, the nutritional versatility of Burkholderia species—which includes the ability to degrade toxic environmental contaminants—suggests an important function in the carbon cycle in nature. However, research directed to the use of these characteristics in bioremediation projects has to take into account the fact that some of the species are serious pathogenic agents for plants and animals, and proper precautions should be taken to counteract these activities.
Early studies on B. cepacia and its synonym “Pseudomonas multivorans” (Stanier et al., 1966; Ballard et al., 1970) clearly indicated considerable intraspecific heterogeneity in phenotypic characteristics such as pigmentation and nutritional properties, and in the DNA similarity results of DNA–DNA hybridization experiments. One hundred strains of B. cepacia were examined, and a proposal for a subdivision of the species into biovars was suggested (Richard et al., 1981). In recent times, other comparative studies showed marked differences between B. cepacia strains isolated from the clinical environment and those found in plant rhizospheres (Bevivino et al., 1994; Tabacchioni et al., 1995). Excellent reviews are available on the intraspecific diversity of this species (Yohalem and Lorbeer, 1994) and on its genomic complexity and versatility (Lessie et al., 1996). All these reports have had a taxonomic impact, resulting in the proposal of converting some of the intraspecific groups into independent taxa at the species level, as discussed below.
The newly proposed species names include B. vietnamiensis and B. multivorans, related to B. cepacia, B. thailandensis, a relative of B. pseudomallei, and B. graminis, which is close to the group B. caryophylli–B. glathei–B. phenazinium. Some of the descriptions fail to create a precise circumscription of the proposed taxa, but it is to be expected that the interest in these organisms will eventually contribute to generate characterizations that are more comprehensive.
A genotypic analysis of 128 strains of Burkholderia, Ralstonia, and Pseudomonas was taken as the basis for a definition of the taxonomic structure of B. cepacia (Vandamme et al., 1997b). The strains isolated from cases of cystic fibrosis could be grouped into five so-called genomic species, very similar from the phenotypic standpoint. One of the genomic species of this “B. cepacia complex” corresponded to the previously described B. vietnamiensis, and a second one received the formal name B. multivorans, a revival of a synonym of B. cepacia used many years before (Stanier et al., 1966). The rest of the genomovars (I, III, and IV) have remained unnamed.
A description of B. multivorans is given in this chapter (see List of Species of the genus Burkholderia), and a more complete report of its properties can be found in the original description (Vandamme et al., 1997b). Four of the strains of genomovar II— now B. multivorans—exhibited a high level of relatedness by DNA–DNA hybridization. The organisms are phenotypically similar to other biovars of B. cepacia and cannot be readily differentiated from them.
The name B. thailandensis was recently proposed for a group of strains closely related to B. pseudomallei based on a high 16S rRNA sequence similarity (Brett et al., 1998). The organisms differ phenotypically from B. pseudomallei by relatively few characteristics, including an ability to use l-arabinose, 5-ketogluconate, adonitol, erythritol, and dulcitol. Differences in biochemical profile and virulence also occur. DNA–DNA hybridization studies of the two species have not been reported.
The position occupied by B. graminis in the phylogenetic tree of Fig. 2 indicates that this species is located in a cluster that includes B. glathei and B. phenazinium. This is further supported by a set of phenotypic properties of differential value and clear-cut differences in the DNA reassociation values, as can be seen in Tables 2 and 3 of the original publication (Viallard et al., 1998).
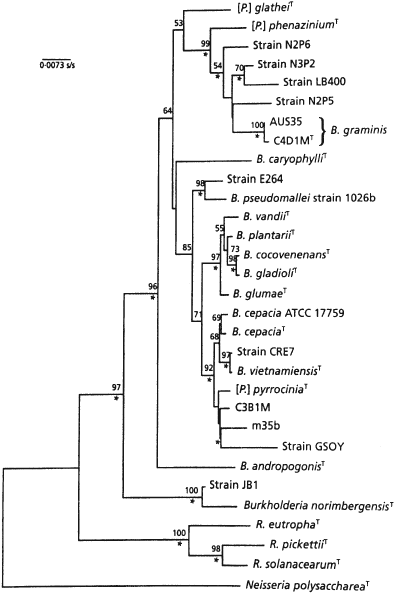
(Reproduced with permission from V. Viallard et al., International Journal of Systematic Bacteriology 48: 549–563, 1998, ©International Union of Microbiological Societies.)
Finally, a new species with rather unique properties, B. norimbergensis (Pandoraea norimbergensis), has been described for a strain isolated from an oxic water layer above a sulfide-containing sediment (Wittke et al., 1997). This organism oxidizes several inorganic sulfur compounds including sulfur and produces sulfate. The reported characteristics of the new species do not fit the organization of the tables in this chapter. The description is given in the list of species for the genus Pandoraea, together with some comments on its phylogenetic relationships. The 16S rRNA sequence of a new species of Burkholderia that has been named B. caribensis has been deposited in GenBank under the accession numbers Y17009, Y17010, and Y17011. The sequence places this species near B. graminis (V. Viallard and J. Balandreau, personal communication).
Following the recommendation formulated by Vandamme et al. (1997b), buttressed by the evidence reported by Viallard et al. (1998), three species previously assigned to Pseudomonas are now being included in the list of Burkholderia species as B. glathei, B. pyrrocinia, and B. phenazinium.
List of species of the genus Burkholderia
-
Burkholderia cepacia (Palleroni and Holmes 1981) Yabuuchi, Kosako, Oyaizu, Yano, Hotta, Hashimoto, Ezaki and Arakawa 1993, 398VP (Effective publication: Yabuuchi, Kosako, Oyaizu, Yano, Hotta, Hashimoto, Ezaki and Arakawa 1992, 1271) (Pseudomonas cepacia Palleroni and Holmes 1981, 479.)
ce.pa' ci.a. L. fem. n. caepa or cepa onion; M.L. fem. adj. cepacia of or like an onion.
Properties useful for differentiation from other species of the genus are given in Table 2. Characteristics of the species are presented in Table 3. As suggested in the description of “Pseudomonas multivorans” (Stanier et al., 1966), strains of B. cepacia can be differentiated from other species on the basis of their ability to grow at the expense of d-arabinose, d-fucose, cellobiose, saccharate, mucate, 2,3-butylene glycol, sebacate, m-tartrate, citraconate, o-hydroxybenzoate, m-hydroxybenzoate, l-threonine, dl-ornithine, and tryptamine. These characteristics, although not present in all strains, are useful for the identification of newly isolated strains. For further descriptive information see Ballard et al. (1970) and Palleroni and Holmes (1981). Optimal growth temperature, ∼30–35°C.
Many strains have been isolated from rotten onions, soils, various natural materials, and clinical specimens. The species is considered a serious opportunistic human pathogen, and it has been found associated with infections of nosocomial origin. A study by Vandamme et al. (1997b) on strains isolated from cystic fibrosis cases has resulted in the identification of multiple genomovars of B. cepacia, among which some (genomovar II) are being described under the new species name Burkholderia multivorans (see comments in the description of this species below).
The mol% G + C of the DNA is: 67.4 (Bd).
Type strain: ATCC 25416, Ballard 717, DSM 7288, ICPB 25, NCTC 10743.
-
Burkholderia andropogonis (Smith 1911) Gillis, Van, Bardin, Goor, Hebbar, Willems, Segers, Kersters, Heulin and Fernandez 1995, 287VP (Pseudomonas andropogonis (Smith 1911) Stapp 1928, 27; Pseudomonas andropogoni (sic) Smith 1911, 63.)
an.dro.po' go.nis. M.L. n. Andropogon genus of widely distributed grasses; M.L. gen. n. andropogonis of the genus Andropogon.
The following description is slightly modified from the one given by Palleroni (1984) in the first edition of this Manual.
Slender rods with rounded ends, 0.5–0.7 × 1–2 µm, with one or rarely two polar flagella. Colonies change from butyrous to viscid with age. No fluorescent pigment is produced. Most strains are oxidase negative. Gelatin liquefaction, nitrate reduction, lipolysis, and arginine dihydrolase reactions negative. Production of sheathed flagella (flagella surrounded by a membrane that is a continuation of the cell wall) was reported by Fuerst and Hayward (1969a).
Although the species had been tentatively assigned to the rRNA similarity group III based on its enzymatic properties, it is now considered a member of the genus Burkholderia (Gillis et al., 1995). Further details of its phenotypic properties are given by Palleroni (1984) and in Tables 2 and BXII.β.3. A comprehensive description is given in the Ph.D. thesis of Xiang Li (1993).
Recommendations to consider P. stizolobii and P. woodsii as synonyms of B. andropogonis were formulated by several workers (Goto and Starr, 1971; Hayward, 1972; Nishiyama et al., 1979; Shanks and Hale, 1984), and this opinion was reinforced by the demonstration of similar protein profiles (Vidaver and Carlson, 1978) and polyamine composition (Auling et al., 1991).
Pathogenic for sorghum, corn, clover, and velvet bean (Stizolobium deeringianum). To the host list should be added highbush blueberry, in which the pathogen causes leaf spot lesions in hardwood cuttings (Kobayashi et al., 1995). Perhaps the host specialization may justify creating two pathovars—pathovar andropogonis for the causal agent of the bacterial stripe of sorghum, and pathovar stizolobii for that of the bacterial leaf spot of velvet bean (Palleroni, 1984). As mentioned in a previous section, strains of B. andropogonis are able to synthesize rhizobitoxine, a phytotoxin capable of causing foliar chlorosis.
The mol% G + C of the DNA is: 59–61.3 (Tm).
Type strain: ATCC 23061, DSM 9511, LMG 2129.
GenBank accession number (16S rRNA): X67037.
-
Burkholderia caribensis Achouak, Christen, Barakat, Martel and Heulin 1999, 792VP
ca.ri.ben' sis. M.L. adj. caribensis pertaining to the Caribbean Islands, where the strains were isolated.
The description is taken from the original paper.
Short rods, 1–2 × 0.5 µm. Motile and pleomorphic in actively growing cultures in LB medium. In sugar-rich media (with 2% glucose, xylose, fructose, sorbitol, arabinose, mannitol, or sorbitol) it produces abundant exopolysaccharide.
Oxidase, catalase, urease, arginine dihydrolase, and β-galactosidase are produced. A list of organic compounds that have been tested as substrates of oxidative activities is given in the paper. Isolated from a vertisol fraction in the island of Martinique.
The mol% G + C of the DNA is: 63.1 (Tm).
Type strain: LMG 18531, MWAP 64.
GenBank accession number (16S rRNA): Y17009.
-
Burkholderia caryophylli (Burkholder 1942) Yabuuchi, Kosako, Oyaizu, Yano, Hotta, Hashimoto, Ezaki and Arakawa 1993, 398VP (Effective publication: Yabuuchi, Kosako, Oyaizu, Yano, Hotta, Hashimoto, Ezaki and Arakawa 1992, 1273) (Pseudomonas caryophylli (Burkholder 1942) Starr and Burkholder 1942, 601; Phytomonas caryophylli Burkholder 1942, 143.)
ca.ry.o' phyl.li. M.L. masc. n. caryophyllus specific epithet of Dianthus caryophyllus, carnation; M.L. gen. n. caryophylli of the carnation.
The general characteristics of the species are presented in Tables 2, 3, and 8. The main properties for differentiation from several other Burkholderia species and other denitrifying pseudomonads, are summarized in Table 7. Optimal growth temperature ∼30–33°C. For further descriptive information, see Ballard et al. (1970). Isolated from diseased carnations.
Table 7. Nutritional characteristics of Burkholderia cepacia, Burkholderia gladioli, and Burkholderia caryophyllia Characteristics B. cepacia B. gladioli B. caryophylli Utilization of: d-Ribose, d-arabinose, l-arabinose, d-fucose, d-glucose, N-acetylglucosamineb, d-mannose, d-galactose, d-fructose, sucrose, cellobiose, gluconate, 2-ketogluconate, sacchare, mucate, salicin, acetate, propionate, butyrate, isobutyrate, valerate, malonate, succinate, fumarate, d-malate, l-malate, m-tartrate, β-hydroxybutyrate, lactate, glycerate, hydroxymethyl-glutarate, citrate, α-ketoglutarate, pyruvate, aconitate, mannitol, sorbitol, m-inositol, adonitolb, glycerol, p-hydroxybenzoate, phenylacetate, quinate, l-alanine, β-alanine, l-serine, l-cysteineb, l-aspartate, l-glutamate, l-arginine, γ-aminobutyrate, l-histidine, l-proline, l-tyrosine, l-phenylalanine, l-tryptophan, betaine, hippurate + + + d-Xylose, n-propanol d + + l-Rhamnose, glycolate d − + Trehalose, l-threonine + + d Isovalerate, glutarate, citrulline, anthranilate, sarcosine + d − Heptanoate, caproate, caprylate, pelargonate, caprate, adipate, azelate, sebacate, citraconate, adonitol, dulcitolb, benzoate, d-alanine, ornithine, kynurenate, ethanolamine, 2-aminobenzoate + + − Lyxoseb, tagatoseb, 5-ketogluconateb, melibioseb, gentiobioseb, raffinoseb, amygdalinb, arbutinb, aesculinb, salicinb, l-arabitolb, pimelate, suberate, levulinate, m-hydroxybenzoate, δ-aminovalerate, putrescine, spermine, butylamine, tryptamine, α-amylamine, diaminobutaneb + − − d(−)-Tartrate, mesaconate − + − l(+)-Tartrate, ethanol, l-isoleucine, nicotinate, trigonelline d + − Itaconate, propylene glycol, glycine, norleucine, α-aminobutyrate, α-aminovalerate − d − l-Fucoseb, 2,3-butylene glycol, d-arabitolb, xylitolb + − + n-Butanol d d + Isobutanol d d − l-Mandelate, benzoylformate d − d o-Hydroxybenzoate, testosterone, benzylamine, histamine, acetamide d − − l-Leucine, l-valine d + d Dodecane, hexadecane d - a For symbols see standard definitions.
- b Data for the type strains, taken from Yabuuchi et al. (1992).
The mol% G + C of the DNA is: 65.3 (Bd).
Type strain: ATCC 25418, DSM 50341, ICPB PC113, NCPPB 2151, PDDCC 512.
GenBank accession number (16S rRNA): X67039.
-
Burkholderia cocovenenans (van Damme, Johannes, Cox and Berends 1960) Zhao, Qu, Wang and Chen 1995, 601VP (Pseudomonas cocovenenans van Damme, Johannes, Cox and Berends 1960, 255.)
co.co.ve' ne.nans. M.L. n. Cocos genus of coconut; L. v. veneno to poison; M.L. part. adj. cocovenenans coconut poisoning.
The following description is taken from the paper by Zhao et al. (1995). Rods 0.3–0.5 × 1.6–2.0 µm, occurring singly or in pairs. Motile by means of one to five polar flagella. No lipid soluble or fluorescent pigment is produced; however, a greenish-yellow diffusible pigment (toxoflavin) is formed after 1–2 d of incubation on potato dextrose agar or other media.
Growth temperature range goes from 6–41°C. Metabolism is respiratory and denitrification is negative, but nitrate is reduced to nitrite. Oxidase reaction is negative. Gelatinase, Tween 80 hydrolysis, and lecithinase reactions are all positive. Many organic compounds can be used as sole carbon and energy sources. Detailed information can be found in the original report (Zhao et al., 1995), in Gillis et al. (1995), and in Tables 2 and 3.
Strains have been isolated from fermented coconut food (bongkrek) in Indonesia, fermented cornmeal in China, deteriorated white fungus (Tremella fuciformis), and soil. DNA–DNA hybridization experiments have shown that this species is close to B. cepacia and B. gladioli (Zhao et al., 1995). The organism is not infectious to humans, but it is responsible for serious cases of food poisoning due to the production of a yellow poisonous compound, toxoflavin.
Recent studies by Coenye et al. (1999b) based on whole protein electrophoretic profiles, DNA–DNA hybridization, and comparison of many biochemical properties indicate that B. cocovenenans should be considered a junior synonym of B. gladioli.
The mol% G + C of the DNA is: 69 ± 0.5 (Tm).
Type strain: ATCC 33664, DSM 11318, LMG 11626, NCIB 9450.
GenBank accession number (16S rRNA): U96934.
-
Burkholderia gladioli (Severini 1913) Yabuuchi, Kosako, Oyaizu, Yano, Hotta, Hashimoto, Ezaki and Arakawa 1993, 398VP (Effective publication: Yabuuchi, Kosako, Oyaizu, Yano, Hotta, Hashimoto, Ezaki and Arakawa 1992, 1273) (Pseudomonas gladioli Severini 1913, 420.)
gla.di.o' li. L. n. gladiolus a small sword lily; M.L. masc. n. gladioli of gladiolus.
The characteristics of the species are summarized in Tables 2, BXII.β.3, and BXII.β.8. Optimal growth temperature, ∼30–35°C. Further descriptive information may be found in Ballard et al. (1970), where the species appears under the name “Pseudomonas marginata” (see also Hildebrand et al., 1973).
Isolated from decayed onions, Gladiolus spp, and Iris spp., for which the species is believed to be pathogenic. Two pathovar names have been proposed for the phytopathogenic strains, pathovar gladioli and pathovar aliicola (Young et al., 1978). To these, the new pathovar name pathovar agaricicola has been created to distinguish the B. gladioli strains producing rapid soft rot of cultivated mushrooms (Agaricus bitorquis) (Lincoln et al., 1991). B. gladioli was identified as the agent of leaf spot and blight of the bird's nest fern Asplenium nidus (Chase et al., 1984). As indicated in the section on pathogenicity for humans, strains of the species have been found to be serious opportunistic pathogens.
As a phytopathogen, B. gladioli pathovar alliicola behaves in a manner similar to that of B. cepacia, causing soft rot of onions (61-gl; 21-gl) (Tesoriero et al., 1982; Wright et al., 1993). It has also been identified as a probable agent of early blight in cherries (26-gl) (Li and Scholberg, 1992), and to cause rapid soft rot disease of the edible mushroom Agaricus bitorquis (38-gl; 29-gl) (Lincoln et al., 1991; Atkey et al., 1992). B. gladioli also contributes to postharvest diseases of fruits and vegetables, an activity in which the species has been identified by analyses of fatty acid composition (24-gl) (Wells et al., 1993).
The mol% G + C of the DNA is: 68.5 (Bd).
Type strain: ATCC 10248, DSM 4285, NCPPB 1891, PDDCC 3950.
GenBank accession number (16S rRNA): X67038.
Additional Remarks: This is also the reference strain for B. gladioli pathovar gladioli. The reference strain for B. gladioli pathovar alliicola is ATCC 19302 (PDDCC 2804; NCPPB 947). The reference strain for B. gladioli pathovar agaricicola is NCPPB 3580.
-
Burkholderia glathei (Zolg and Ottow 1975) Vandamme, Holmes, Vancanneyt, Coenye, Hoste, Coopman, Revets, Lauwers, Gillis, Kersters and Govan 1997b, 1199VP (Pseudomonas glathei Zolg and Ottow 1975, 296.)
gla' the.i. M.L. gen. glathei of Glathe, named after H. Glathe of Giessen, Germany.
The description is taken from Zolg and Ottow (1975). Rods to oval cocci, 0.5–0.7 × 1.5 µm, motile by a polar flagellum. Optimal growth temperature 30–37°C. Oxidase reaction positive. Negative for hydrolysis of starch, gelatin, lecithin (egg yolk reaction), esculin, and polypectate. Tributyrin, urea, and hippurate are hydrolyzed. Nitrate is reduced to nitrite. Denitrification and H2S production are negative. No growth factor requirement has been found. The organism is capable of growth in nitrogen-deficient media, but acetylene reduction by cells grown under those conditions is negative. Acid tolerant (pH 4.5).
From the extensive phenotypic characterization of B. glathei following the methodology described by Stanier et al. (1966), it has been found that at least 68 organic compounds can be utilized as sole carbon and energy sources for growth. These include aldoses, ketoses, deoxysugars, sugar-alcohols, and sugar-acids. Except for lactose, melibiose, and melezitose, no di-, tri-, and polysaccharides are utilized. The only amino acids that are not utilized are glycine, l-serine, l-isoleucine, l-methionine, and β-alanine. Of 28 aliphatic organic acids, 23 are utilized. The list of utilizable acids includes oxalate. Some properties are summarized in Table 3.
A 1,2-oxygenase responsible for the ortho cleavage of aromatic compounds is produced constitutively. This species differs in several important characteristics from other species of the genus. The main differences include the capacity for growing in nitrogen-deficient media, the acid tolerance, and the utilization of oxalate for growth. A study by Vandamme et al. (1997b), which includes fatty acid analysis, indicates that this species should be allocated to the genus Burkholderia, an opinion that has found confirmation by rRNA sequencing studies (Viallard et al., 1998).
The mol% G + C of the DNA is: 64.8 (Tm).
Type strain: N15, ATCC 29195, DSM 50014, LMG 14190.
-
Burkholderia glumae (Kurita and Tabei 1967) Urakami, Ito-Yoshida, Araki, Kijima, Suzuki and Komagata 1994, 242VP (Pseudomonas glumae Kurita and Tabei 1967, 111.)
glu' mae. L. n. gluma hull; L. gen. n. glumae of a husk.
The following description is taken from the original paper by Kurita and Tabei (1967). Rods, 0.5–0.7 × 1.5–2.5 µm, motile by means of two to four flagella. A fluorescent pigment is produced in potato agar. Nitrate reduction, starch hydrolysis, and H2S production are negative. Gelatin liquefaction was not reported in the original paper, but Urakami et al. (1994) found that the test was positive. Temperature limits for growth: 11–40°C; optimum: 30–35°C. Acid is produced from arabinose, glucose, fructose, galactose, mannose, xylose, glycerol, mannitol, and inositol. No acid is produced from rhamnose, sucrose, maltose, lactose, raffinose, dextrin, starch, inulin, or salicin. Milk is coagulated and peptonized. Pathogenic for the rice plant (Oryza sativa, fam. Gramineae).
To the above description, Urakami et al. (1994) have added a substantial amount of information on the nutritional spectrum of the type strain, and part of it has been summarized in Tables 2 and BXII.β.3. A peculiar property of this species is the production of fluorescent pigment, but no further details are available on its relationship to the pigment characteristic of the fluorescent species of the genus Pseudomonas. This is a point of interest for further investigation. As mentioned before, two subgroups of B. glumae strains can be distinguished based on their fatty acid composition (Stead, 1992).
The mol% G + C of the DNA is: 68.2 (reversed HPLC) (Urakami et al., 1994).
Type strain: ATCC 33617, DSM 7169, LMG 2196, NCPPB 2981, NIAES 1169.
GenBank accession number (16S rRNA): U96931.
-
Burkholderia graminis Viallard, Poirier, Cournoyer, Haurat, Wiebkin, Ophel-Keller and Balandreau 1998, 560VP
gra' mi.nis. M.L. adj. graminis referring to its isolation from the rhizosphere of grasses.
The description is taken from the original paper (Viallard et al., 1998). Rods, 0.3–0.8 × 1.0–1.5 µm; motile. The number of flagella per cell is not reported. Colonies on LB agar are thin, brownish-yellow, and translucent. On agar prepared with the special medium PCAT (Burbage and Sasser, 1982) after 3 d at 28°C, the colonies are white, somewhat opaque and creamy, with entire margin. Oxidase, catalase, urease, and arginine deiminase reactions are positive. Nitrates are reduced, but there is no denitrification. Characteristics in common with other Burkholderia species are the assimilation of glycerol, d- and l-arabinose, ribose, galactose, glucose, fructose, mannose, inositol, mannitol, sorbitol, d-arabitol, gluconate, and 2-ketogluconate, and the inability to use l-sorbose, methyl-α-d-xyloside, methyl-α-d-mannoside, methyl-α-d-glucoside, inulin, melezitose, starch, glycogen, or d-turanose. Properties not found in some of the other species include the incapacity of acid formation from glucose, to hydrolyze esculin or to produce gelatinase. In addition, the strains grow on l-xylose, lactose, rhamnose, trehalose, d-lyxose, l-arabitol, xylitol, and raffinose, but not on dulcitol or d-tagatose. Isolated from the rhizosphere of wheat, corn, and pasture grasses.
The mol% G + C of the DNA is: 62.5–63.0 (HPLC).
Type strain: C4D1M, ATCC 700544.
GenBank accession number (16S rRNA): U96939.
-
Burkholderia kururiensis Zhang, Hanada, Shigematsu, Shibuya, Kamagata, Kanagawa and Kurane 2000a, 747VP
ku.ru.ri.en' sis. M.L. adj. kururiensis referring to Kururi, Chiba Prefecture, Japan, where the strain was isolated.
The following description is taken from the original paper. The cells are Gram negative and ovoid or rod shaped. (1 × 1.2–1.5 µm), occurring singly or in pairs. Growth occurs between 15–42°C, with an optimum at 37°C. The optimal growth pH is 7.2. Under optimal conditions, the doubling time is about 1 h. Oxidase and catalase positive. Starch and gelatin are not hydrolyzed, but glycogen and Tween 80 are. The following organic compounds are degraded aerobically: arabinose, fructose, fucose, galactose, glucose, lactulose, maltose, mannose, psicose, rhamnose, adonitol, arabitol, glycerol, inositol, mannitol, sorbitol, xylitol, N-acetylgalactosamine, acetate, citrate, formate, galacturonate, gluconate, lactate, propionate, alanine, asparagine, aspartate, glutamate, glycine, histidine, leucine, phenylalanine, proline, serine, threonine, inosine, 2,3-butanediol, benzene, p-cresol, fluorobenzene, and phenol. The following compounds are not oxidized: cellobiose, lactose, melibiose, raffinose, sucrose, trehalose, dextrin, malonate, uridine, thymidine, glucose-1-phosphate, and glucose-6-phosphate. UQ-8 is the dominant respiratory quinone. Main cellular fatty acids are C16:0, cyclopropanic acids C17:0, cyclopropanic acid C19:0, C16:1, and C18:1. C13:1 and C17:1 are also present. The organism was isolated from an aquifer polluted with trichloroethylene (TCE) in Kururi, Chiba Prefecture, Japan, and shows degradation activity for this contaminant when the cells are grown in the presence of phenol.
The mol% G + C of the DNA is: 64.8 (HPLC).
Type strain: KP23, ATCC 700977, CIP 106643, DSM 13646, LMG 19447.
GenBank accession number (16S rRNA): AB024310.
This species has some properties that are not common in the genus Burkholderia. The cells are nonmotile, a characteristic shared only with B. mallei. The strain uses maltose, which is rarely used by strains of other species, and fails to use disaccharides used by the latter. No DNA–DNA hybridization data have been reported.
-
Burkholderia mallei (Zopf 1885) Yabuuchi, Kosako, Oyaizu, Yano, Hotta, Hashimoto, Ezaki and Arakawa 1993, 398VP (Effective publication: Yabuuchi, Kosako, Oyaizu, Yano, Hotta, Hashimoto, Ezaki and Arakawa 1992, 1271) (Pseudomonas mallei (Zopf 1885) Redfearn, Palleroni and Stanier 1966, 305; Bacillus mallei Zopf 1885, 89.)
mal' le.i. L. n. malleus the disease of glanders; L. gen. n. mallei of glanders.
Characteristics of the species are listed in Tables 2, 3, and 8, and those that are useful for the differentiation of the species from various other denitrifying pseudomonads are summarized in Table 4. Optimal growth temperature ∼37°C. For further descriptive information, see Redfearn et al. (1966) and Redfearn and Palleroni (1975). Parasitic on horses and donkeys, in which it causes glanders and farcy. The infection is transmissible to humans and to other animal species.
Table 8. Nutritional properties of Burkholderia mallei and Burkholderia pseudomalleia Characteristicsb B. mallei B. pseudomallei Utilization of: Acetate, N-acetylglucosaminec, adipate, d-alanine, l-alanine, β-alanine, γ-aminobutyrate, d-arabinose, l-arginine, l-aspartate, benzoate, betaine, cellobiose, d-fucose, fumarate, galactose, glucose, l-glutamate, glycerate, glycerol, hippurate, l-histamine, β-hydroxybutyrate, p-hydroxybenzoate, m-inositol, α-ketoglutarate, lactate, l-malate, mannitol, mannose, phenylacetate, poly-β-hydroxybutyrate, propionate, pyruvate, quinate, sorbitol, succinate, sucrose, l-threonine, trehalose, tryptamine, l-tyrosine + + Aconitate, 2-aminobenzoatec α-amylamine, d-arabitolc, benzoylformate, butylamine, caprate, caproate, l-cysteinec, dulcitolc, erythritol, ethanol, ethanolamine, l-fucosec, glycogenc, heptanoate, isobutyrate, l-isoleucine, isovalerate, kynurenate, kynurenine, levulinate, l-lysine, pelargonate, putrescine, ribose, sebacate, valerate − + Glycine, d-xylose + − α-Aminobutyrate, l-arabinose, malonate d − δ-Aminovalerate, anthranilate, butyrate, caprylate, citrate, fructose, gluconate, 2-ketogluconate, maltose, l-phenylalanine, l-proline, putrescine, salicin, l-serine, suberate, starch, l-valine d + Azelate, glutarate, d-malate, pimelate, sarcosine, trigonelline d d Adonitol, ethanol, hexadecane, l-mandelate, spermine − d - a For symbols see standard definitions.
- b The following compounds are not used by either species: acetamide, aesculinc, m-aminobenzoate, p-aminobenzoate, α-aminovalerate, amygdalinc, arbutinc, benzylamine, n-butanol, 2,3-butylene glycol, citraconate, l-citrulline, creatine, dodecane, ethylene glycol, gentiobiosec, geraniol, glycolate, histamine, m-hydroxybenzoate, o-hydroxybenzoate, hydroxymethylglutarate, inulin, isobutanol, isophthalate, isopropanol, 5-ketogluconate, lactose, l-leucine, lyxosec, maleate, d-mandelate, melibiosec, mesaconate, methanol, methylaminec, mucate, nicotinate, norleucine, l-ornithine, oxalatec, pantothenate, phenol, phenylethanediol, phthalate, n-propanol, propylene glycol, raffinosec, l-rhamnose, saccharate, salicinc, tagatosec, d(−)-tartrate, l(+)-tartrate, meso-tartrate, terephthalate, testosterone, tryptamine, d-tryptophan, xylitolc.
- c Data for the type strains, taken from Yabuuchi et al. (1992).
The mol% G + C of the DNA is: 69 (Bd).
Type strain: NBL 7, ATCC 23344.
GenBank accession number (16S rRNA): S55000.
-
Burkholderia multivorans Vandamme, Holmes, Vancanneyt, Coenye, Hoste, Coopman, Revets, Lauwers, Gillis, Kersters and Govan 1997b, 1198VP
mul.ti' vo.rans. L. adj. multus much; L. part. adj. vorans devouring, digesting; M.L. part. adj. multivorans digesting many compounds.
Rods, 0.6–0.9 × 1.0–2.0 µm. Motile at room temperature and at 37°C. Number of flagella per cell not reported. Able to grow at 42°C but not at 5°C. According to the original description, some strains grow at room temperature. No pigments are produced. Growth in some media used for enteric bacteria is reported. No information is given on utilization of carbon compounds for growth, but, instead, production of acids from some compounds is reported. Tolerance to cyanide is strain dependent. No fluorescence occurs in King's B medium. Tween 20 and 80 are hydrolyzed. Urease, catalase, oxidase, and lecithinase activities are all positive. Nitrate is reduced, but not nitrite. Gelatin liquefaction is negative. No hydrolysis of casein, starch, or esculin occurs. Arginine deiminase is negative. PHB is accumulated and, according to the description, is utilized. The report does not specifically state whether this refers to extracellular PHB.
The strains assigned to this species belonged to one of the genomovars (genomovar II) into which was subdivided a collection of B. cepacia isolated from cases of cystic fibrosis. Four strains of this genomovar shared high DNA sequence similarity as determined by hybridization methods, and the melting temperatures of rRNA–DNA hybrid molecules using B. cepacia as reference were lower than the homologous B. cepacia reassociated molecules. The phenotypic differences between B. multivorans and B. cepacia are limited to casein digestion, growth at 42°C, and acid production from sucrose and raffinose. In fact, these last two properties may be redundant, since the two saccharides may be hydrolyzed by the same enzyme, for instance, invertase. Acid may be produced from the resulting monosaccharides (glucose and fructose). Since the physiological properties used for the description of this species differ from those used for other species of the genus, it is not possible to use them in a more extensive comparison, and therefore the species has been excluded from the comparative Table 3.
The mol% G + C of the DNA is: 68–69 (method unknown).
Type strain: LMG 13010, ATCC BAA-247, CCUG 34080, CIP 105495, DSM 13243, NCTC 13007.
GenBank accession number (16S rRNA): AF14855.
-
Burkholderia phenazinium (Bell and Turner 1973) Viallard, Poirier, Cournoyer, Haurat, Wiebkin, Ophel-Keller and Balandreau 1998, 5618VP (Pseudomonas phenazinium Bell and Turner 1973, 753.)
phe.na.zi' ni.um. Orthography and etymology uncertain; possibly refers to iodinin, which is a phenazine pigment.
The original bacteriological information on this species is fragmentary. It is included in a paper describing the production of iodinin under various conditions (Bell and Turner, 1973). The strain was isolated from soil after an enrichment using l-threonine as the sole carbon source. This property is mentioned by Viallard et al. (1998) in their short description. It has no value whatsoever as a diagnostic character, since many aerobic pseudomonads use this amino acid and the property is usually shared by all the strains of a given species.
An unusual feature mentioned in the original description of the species is its acidophilic character. The strain was acidophilic, the optimal pH for growth being 5.0. It failed to grow at pH 7.0. This feature apparently has not been mentioned by later researchers who have worked with this strain. Colonies on agar media produced iodine crystals, and the presence of l-threonine or glycine in the medium seemed to enhance pigment synthesis. In experiments designed to test iodinin production, growth was observed with threonine, glycine, fumarate, glycerol, sucrose, malate, succinate, dl-lactate, citrate, glucose, glutamate, pyruvate, and serine. Growth was poor with aminopropanol, and no growth was observed with propionate. Additional information, taken from Viallard et al. (1998), is summarized in Table 2. The description does not mention specifically use of extracellular PHB as a growth substrate. Acid production from various sugars is given in the original paper.
The mol% G + C of the DNA is: (HPLC).
Type strain: ATCC 33666, DSM 10684, LMG 2247, NCIB 11027.
GenBank accession number (16S rRNA): U96936.
As a general comment, the phenotypic properties of the description for B. phenazinium do not clearly differentiate this species from B. cepacia, and no data on DNA similarity expressed by percent DNA hybridization are reported. Only the Tm(e) values of the DNA–rRNA hybrids obtained with 23S rRNA seem to differentiate this species from B. cepacia. For the moment, it is advisable to consider this taxon as a genomovar of B. cepacia until more evidence is available to decide on its independent species rank.
-
Burkholderia plantarii (Azegami, Nishiyama, Watanabe, Kadota, Ochuchi and Fukazawa 1987) Urakami, Ito-Yoshida, Araki, Kijima, Suzuki and Komagata 1994, 242VP (Pseudomonas plantarii Azegami, Nishiyama, Watanabe, Kadota, Ochuchi and Fukazawa 1987, 151.)
plan' tar.i.i. L. n. plantarium seedbed; L. gen. n. plantarii of seedbed.
The following description is summarized from that in the original paper by Azegami et al. (1987). Nonencapsulated straight rods (0.7–1.0 × 1.4–1.9 µm), motile with one to three polar flagella. They occur singly, in pairs, or in short chains. Colonies have a slightly yellow tint, and they produce a water-soluble reddish brown pigment depending on the conditions and the medium. Oxidase positive. Do not produce fluorescent pigment. Gelatinase, lecithinase, hydrolysis of Tween 80, and denitrification are all positive. Arginine dihydrolase negative. Acid production on a number of carbon compounds and nutritional properties are described in the original paper. Further details are summarized in Tables 2, 3, and 4. The species causes rice seedling blight, and strains have been isolated from rice seedlings and from bed soil in nursery boxes in Japan.
The mol% G + C of the DNA is: 64.8 (Bd).
Type strain: ATCC 43733, AZ 6201, DSM 9509, JCM 5492, LMG 9035, NIAES 1723.
GenBank accession number (16S rRNA): U96933.
-
Burkholderia pseudomallei (Whitmore 1913) Yabuuchi, Kosako, Oyaizu, Yano, Hotta, Hashimoto, Ezaki and Arakawa 1993, 398VP (Effective publication: Yabuuchi, Kosako, Oyaizu, Yano, Hotta, Hashimoto, Ezaki and Arakawa 1992, 1273) (Pseudomonas pseudomallei (Whitmore 1913) Haynes and Burkholder 1957, 100; Bacillus pseudomallei Whitmore 1913, 9.)
pseu.do.mal' le.i. Gr. adj. pseudes false; L. n. malleus the disease glanders; M.L. gen. n. pseudomallei of false glanders.
The general characteristics of the species and properties useful for differentiation from the related species B. mallei are given in Tables 2, 3, and 8. Colonies can range in structure from extremely rough to mucoid, and in color from cream to bright orange. Optimal growth temperature, ∼37°C. For further descriptive information see Redfearn et al. (1966) and Redfearn and Palleroni (1975). Isolated from human and animal cases of melioidosis and from soil and water in tropical regions, particularly Southeast Asia. Probably a soil organism and accidental pathogen, causing melioidosis.
The mol% G + C of the DNA is: 69.5 (Bd).
Type strain: ATCC 23343, NCTC 12939, WRAIR 286.
-
Burkholderia pyrrocinia (Imanaka, Kousaka, Tamura and Arima 1965) Vandamme, Holmes, Vancanneyt, Coenye, Hoste, Coopman, Revets, Lauwers, Gillis, Kersters and Govan 1997b, 1199VP (Pseudomonas pyrrocinia Imanaka, Kousaka, Tamura and Arima 1965, 205.)
pyr.ro.ci' ni.a. Etymology uncertain, possibly M.L. adj. “pyrrocin” referring to the antibiotic properties of pyrrolnitrin, which is produced by strains of this species.
The following description is taken from the original paper. Rods, 0.5–0.8 × 1.2–2.0 µm, occurring singly. Motile by means of polar flagella. No pigment is produced. Oxidase reaction, nitrate reduction, denitrification, and starch hydrolysis are all negative. H2S is produced. Optimal temperature for growth, 26–30°C. Growth is scanty at 37°C and negative at 42°C. Acid produced from glucose, galactose, lactose, sucrose, and glycerol, but not from maltose, trehalose, mannose, raffinose, starch, or inulin. 2-Ketogluconate is produced from gluconate.
Growth on glucose, gluconate, 2-ketogluconate, and p-hydroxybenzoate as sole carbon sources. 5-Ketogluconate, citrate, ethanol, phenol, succinate, benzoate, salicylate, m-hydroxybenzoate, protocatechuate, gentisate, anthranilate, and p-aminobenzoate are not utilized for growth. Some of the properties are summarized in Table 2. The strains produce the antibiotic pyrrolnitrin. This compound is also produced by some strains of Pseudomonas chlororaphis, P. aureofaciens, and Burkholderia cepacia (“Pseudomonas multivorans”) (Elander et al., 1968), indicating that the synthesis occurs in organisms of different branches of the Proteobacteria.
The first suggestion of allocation of B. pyrrocinia to the RNA similarity group II was made by Byng et al. (1980). In their proposal for the transfer of Pseudomonas pyrrocinia to the genus Burkholderia, Vandamme et al. (1997b) add to the above description, the characteristic fatty acid composition. Allocation to the genus Burkholderia also has been recommended by Viallard et al. (1998) based on nucleic acid sequencing studies.
The mol% G + C of the DNA is: 65.
Type strain: ATCC 15958, DSM 10685, LMG 14191.
GenBank accession number (16S rRNA): U96930.
-
Burkholderia thailandensis Brett, Deshazer and Woods 1998, 318VP
thai.lan.den' sis. M.L. adj. thailandensis pertaining to Thailand, where the organism was originally isolated.
Rods, motile due to the presence of two to four polar flagella. Colonies are smooth and glossy with a pink pigmentation on modified Ashdown's selective medium (see Enrichment and Isolation Procedures), while B. pseudomallei colonies are rough and wrinkled, with a dark purple pigmentation. The API 20NE and API 50CH biochemical profiles are similar to those of B. pseudomallei. Exceptions are the capacity to use l-arabinose, 5-ketogluconate, and adonitol, and the inability to utilize erythritol and dulcitol. Strains of the species are avirulent for Syrian golden hamsters. Growth occurs at temperatures between 25°C and 42°C. Production of siderophore, lipase, lecithinase, and protease are positive. The strains are resistant to aminoglycosides but sensitive to tetracycline, ceftazidime, and trimethoprim. Type strain was isolated from a rice field soil sample in central Thailand.
The mol% G + C of the DNA is: unknown.
Type strain: E264, ATCC 700388.
GenBank accession number (16S rRNA): U91838.
A comparison of sequences of 16S rRNA genes indicates a close relationship of B. thailandensis to B. pseudomallei. No DNA–DNA hybridization experiments between strains of the two species have been reported. In view of the low resolving power of 16S rRNA sequence similarity at the species level and the limited number of phenotypic differences, it is hoped that future studies may provide evidence supporting the taxonomic position of this group of organisms as an independent species of Burkholderia.
-
Burkholderia vandii Urakami, Ito-Yoshida, Araki, Kijima, Suzuki and Komagata 1994, 242VP
van' di.i. M.L. gen. n. vandii of Vanda, a genus of orchids.
Description taken from Urakami et al. (1994). The cells are 0.5–1.0 × 1.5–3.0 µm and have rounded ends. They occur singly, rarely in pairs, and are motile by one or several polar flagella. Abundant growth occurs in nutrient broth and peptone water. The colonies are white to light yellow. No diffusible fluorescent pigment is produced.
The methyl red and the Voges–Proskauer reactions are negative. Indole and hydrogen sulfide are not produced. Starch is not hydrolyzed. Denitrification and hydrolysis of gelatin are positive. Acids are weakly produced from inositol and glycerol oxidatively, but not from l-arabinose, d-xylose, d-glucose, d-mannose, d-fructose, d-galactose, maltose, sucrose, lactose, trehalose, d-sorbitol, d-mannitol, or soluble starch. No fermentation of sugars occurs. Nitrate is not used as a nitrogen source. Results of nutritional studies at the expense of many organic compounds, as well as extensive physiological information, are given in the original paper (Urakami et al., 1994) and in Gillis et al. (1995). The single known strain was isolated from orchids of the genus Vanda as an antibiotic-producing bacterium active against the plant pathogenic organisms Clavibacter michiganensis and Fusarium oxysporium.
According to Coenye et al. (1999b), B. vandii is a junior synonym of B. plantarii.
The mol% G + C of the DNA is: 68.5 (HPLC).
Type strain: ATCC 51545, DSM 9510, JCM 7957, LMG 16020, VA-1316.
GenBank accession number (16S rRNA): U96932.
Studies by Coenye et al. (1999b) based on SDS-PAGE of whole cell proteins, DNA–DNA hybridization, and extensive biochemical characterization indicate that B. vandii should be considered a junior synonym of B. plantarii.
-
Burkholderia vietnamiensis Gillis, Van, Bardin, Goor, Hebbar, Willems, Segers, Kersters, Heulin and Fernandez 1995, 287VP
vi.et' na.mi.en.sis. M.L. adj. vietnamiensis referring to Vietnam, where the rice strains were isolated.
Motile cells are 0.8–2 × 0.3–0.8 µm. No details on the number of flagella per cell are given. Colonies on nutrient agar are not pigmented and do not produce fluorescent pigment on King B medium, a property that is uniformly negative for species of Burkholderia. Growth occurs on nutrient agar between 20°C and 41°C. Strains are oxidase, catalase, β-galactosidase, and gelatinase positive. All strains fix N2 and produce ornibactin siderophores, but not pyochelin or cepabactin. Nutritional properties and characteristics useful for differentiation from other Burkholderia species have been summarized in Tables 2 and 3.
Further details are to be found in Gillis et al. (1995). In one of the tables in that article, denitrification is recorded as positive, although the property is not specifically mentioned in the text, where B. vietnamiensis is described as capable of reducing nitrate to nitrite. Further reduction to gases (N2O or N2) and the ability to grow under anaerobic conditions in the presence of nitrate are not mentioned. It may be inferred that strains of this species, as those of B. cepacia, are unable to denitrify. Differences between B. vietnamiensis and B. cepacia are in the utilization of l-arabitol, adonitol, butylamine, tryptamine, citraconate, and 5-ketogluconate by B. cepacia, and the ability of B. vietnamiensis to grow on itaconate. Strains of the latter do not synthesize the siderophores cepabactin and pyochelin (Gillis et al., 1995). B. vietnamiensis is capable of N2 fixation, a property recorded earlier for rhizosphere strains of B. cepacia (Bevivino et al., 1994). However, extracts from clinical strains also gave a single hybridization signal with a nifA probe from Klebsiella pneumoniae, although they did not fix nitrogen (Tabacchioni et al., 1995). Interestingly, the clinical strains produced pyochelin and its precursor, salicylic acid, a property absent from the rhizosphere strains (Bevivino et al., 1994). Therefore, it is very likely that the latter may have corresponded to the taxon to be described later as B. vietnamiensis (Gillis et al., 1995).
There is little doubt, however, that B. cepacia and B. vietnamiensis are closely related, as suggested by their relative position in the tree of Fig. 2. Aside from many phenotypic characteristics, their similarity extends to the production of the siderophore ornibactin (Meyer et al., 1995), and to the structure of the putative O-specific polymer isolated from the LPS of B. vietnamiensis, which resembles the O-antigen of B. cepacia serogroup J (Gaur and Wilkinson, 1996). The strains of B. vietnamiensis have been isolated from rice field soils and from clinical specimens. They are not pathogenic for onions.
The mol% G + C of the DNA is: 67.9 (Tm).
Type strain: DSM 11319, LMG 10929, TVV75.



