Abstract
Pas.teu'ri.a. N.L. gen. n. Pasteuria of Pasteur, named after Louis Pasteur, French savant and scientist.
Firmicutes / “Bacilli” / Bacillales / Pasteuriaceae / Pasteuria
Gram-positive, endospore-forming bacteria. Propagation following germination within a nematode or cladoceran host proceeds through the formation of rounded to elliptical (termed “cauliflower-like” by Metchnikoff, 1888) vegetative microcolonies from which “daughter” microcolonies may be formed. The sporogenous cells at the periphery of the colonies are usually attached by narrow “sacrificial” intercalary hyphae that lyse, resulting in developing sporangia arranged in clumps of eight or more, but more often in quartets, triplets, or doublets and, finally, as single teardrop-shaped or cup-shaped or rhomboidal mature sporangia. The rounded end of the sporangium encloses a single refractile endospore (1.0–3.0 µm in major dimension), an oblate spheroid, ellipsoidal or almost spherical in shape, usually resistant to desiccation and elevated temperatures (one species has somewhat limited heat tolerance). Nonmotile. Sporangia and microcolonies are endoparasitic in the bodies of freshwater, plant, and soil invertebrates. Axenic cultivation has not been documented, but it can be grown in the laboratory with its invertebrate host. The pathogen is horizontally transmitted via soil or waterborne spores. Infected hosts fail to reproduce.
DNA G+C content (mol%): not known.
Type species: Pasteuria ramosa Metchnikoff 1888, 166AL.
Gram-positive, endospore-forming bacteria. Propagation following germination within a nematode or cladoceran host proceeds through the formation of rounded to elliptical (termed “cauliflower-like” by Metchnikoff, 1888) vegetative microcolonies from which “daughter” microcolonies may be formed. The sporogenous cells at the periphery of the colonies are usually attached by narrow “sacrificial” intercalary hyphae that lyse, resulting in developing sporangia arranged in clumps of eight or more, but more often in quartets, triplets, or doublets and, finally, as single teardrop-shaped or cup-shaped or rhomboidal mature sporangia. The rounded end of the sporangium encloses a single refractile endospore (1.0–3.0 µm in major dimension), an oblate spheroid, ellipsoidal or almost spherical in shape, usually resistant to desiccation and elevated temperatures (one species has somewhat limited heat tolerance). Nonmotile. Sporangia and microcolonies are endoparasitic in the bodies of freshwater, plant, and soil invertebrates. Axenic cultivation has not been documented, but it can be grown in the laboratory with its invertebrate host. The pathogen is horizontally transmitted via soil or waterborne spores. Infected hosts fail to reproduce.
DNA G+C content (mol%): not known.
Type species: Pasteuria ramosa Metchnikoff 1888, 166AL.
Number of validated species: 5
Further descriptive information
A complex of errors initially confused our understanding of the genus Pasteuria Metchnikoff (1888). Stated briefly, Metchnikoff (1888) described an endospore-forming bacterial parasite of cladocerans, which he named Pasteuria ramosa. Metchnikoff presented drawings and photomicrographs of the life stages of this parasite as they occurred in the hemolymph of the water fleas Daphnia pulex Leydig and Daphnia magna Straus. He was, however, unable to cultivate the organism in vitro. Subsequent workers (Henrici and Johnson, 1935; Hirsch, 1972; Staley, 1973), who were looking in cladocerans for Metchnikoff's unique bacterium, reported on a different bacterium with only superficial resemblance to certain life stages of Pasteuria ramosa. Their investigations led to the axenic cultivation of a budding bacterium that is occasionally found on the exterior surfaces of Daphnia species. Unlike the organism described by Metchnikoff, this bacterium divides by budding. It forms a major non-prosthecate appendage (a fascicle), it does not form endospores, it is not mycelial or branching, its staining reaction is Gram-negative, and it is not an endoparasite of cladocerans.
After searching for, but not finding, the bacterial endoparasite in water fleas as described by Metchnikoff, the erroneous conclusion was reached that this budding bacterium that occurs on the surfaces of Daphnia species was the organism Metchnikoff had described in 1888. As a result, a budding bacterium (strain ATCC 27377) was mistakenly designated (Staley, 1973) as the type culture for Pasteuria ramosa Metchnikoff (1888), the type (and, then, sole) species of the genus Pasteuria. This confusion between Metchnikoff's described cladoceran parasite and the quite different budding bacterium was resolved by Starr, Sayre and Schmidt (1983). The budding bacterium (with strain ATCC 27377 as its type culture) was named Planctomyces staleyi Starr, Sayre and Schmidt (1983), now named Pirellula staleyi (Butler, Wang, Webb and Fuerst, 2002). Conservation of the original descriptions of the genus Pasteuria and Pasteuria ramosa, as updated, was recommended (Starr, Sayre and Schmidt, 1983) and approved (Judicial-Commission, 1986). Unfortunately, vestiges of this nomenclatural disorder remained for some time. For example, certain evolutionary and taxonomic inferences (Woese, 1987) regarding the genus Pasteuria were based upon data concerning bacteria belonging to the Blastocaulis–Planctomyces group of budding and non-prosthecately appendaged bacteria rather than the mycelial and endospore-forming invertebrate parasites that actually comprise the genus Pasteuria. A Pasteuria ramosa-like strain was discovered infecting the cladoceran Moina rectirostris, a member of the Daphniidae (Sayre, Adams and Wergin, 1979), and this strain was used in the emendation of the species (Starr, Sayre and Schmidt, 1983). Ebert, Rainey, Embley and Scholz (1996), however, proposed that the Daphnia-parasitic Pasteuria ramosa that they had characterized from the same host as Metchnikoff (1888) be designated the neotype for Pasteuria ramosa Metchnikoff (1888) and that the Moina isolate be compared directly to the neotype in future studies.
Confusion of a different kind occurred in the nomenclature of the bacterial endoparasite of nematodes when Cobb (1906) erroneously reported the numerous highly refractive bodies infecting specimens of the nematode Dorylaimus bulbiferous as “perhaps monads” of a parasitic sporozoan. The incorrect placement in the protozoa of an organism now known to be a bacterial parasite of nematodes has persisted for nearly 70 years. Another incorrect placement was suggested by Micoletzky (1925), who found a nematode parasite similar in size and shape to those reported by Cobb. Micoletzky suggested that this organism belonged to the genus Duboscqia Perez (1908), another sporozoan group (Perez, 1908). Later, Thorne (1940) presented a detailed description of a new parasite from the nematode Pratylenchus pratensis (syn. Pratylenchus brachyurus, see Sayre, Starr, Golden, Wergin and Endo, 1988). Thorne assumed that this organism was similar to the nematode parasite described by Micoletzky, thereby assigning it to the microsporidian genus Duboscqia, as Duboscqia penetrans.
Thorne's description and nomenclature persisted until 1975 even though other investigators (Canning, 1973; Williams, 1960), who had examined this nematode parasite in some detail, questioned this placement. It was not until the nematode parasite was re-examined using electron microscopy that its true affinities to bacteria rather than to protozoa were recognized and the name Bacillus penetrans (Thorne, 1940) Mankau (1975) was applied to it (Imbriani and Mankau, 1977; Mankau, 1975). However, Bacillus penetrans was not included in the Approved Lists of Bacterial Names (Skerman, McGowan and Sneath, 1980), therefore it had no nomenclatural standing. Although this micro-organism forms endospores of the sort typical of the genus Bacillus Cohn (1872), its other traits (e.g., mycelial habit, endoparasitic associations with plant-parasitic nematodes) suggested that it did not belong in the genus Bacillus. Rather, it is closely related to Pasteuria ramosa (see Table 1 and Figure 1) and it has more properly been assigned (Sayre and Starr, 1985) to the genus Pasteuria Metchnikoff (1888) as Pasteuria penetrans.
| Characterisitic | Description |
|---|---|
| Morphological similarities as observed by light microscopy: | |
| Vegetative cells | Microcolonies consist of dichotomously branched mycelium. Diameter of mycelial filaments similar. Mycelial filaments are seen in host tissues only during early stages of infection. Daughter microcolonies seem to be formed by lysis of “sacrificial” intercalary cells. Nearly all vegetative mycelium eventually lyses, leaving only sporangia and endospores. |
| Endospores | Terminal hyphae or peripheral cells of the colony elongate and swell, giving rise to sporangia. A single endospore is produced within each sporangium. Endospores are in the same general size range. Refractivity of endospores, as observed on the light microscope, increases with maturity. |
| Staining reaction | Gram-positive |
| Ultrastructural similarities: | |
| Vegetative cells | Mycelial cell walls are typical of Gram-positive bacteria. Mycelial filaments divided by septa. Double-layered cell walls. Where they occur, mesosomes are similar in appearance and seem to be associated with division and septum formation. |
| Endospores | Typical endogenous spore formation. Identical sequences in endospore formation: (a) septa form within sporangia; (b) sporangium cytoplast condenses to form forespore; (c) endospore walls form; (d) final endospore matures; and (e) “light” areas adjacent to endospore give rise to extrasporal fibers. |
| Similar sequences of life stages | Microcolonies. Fragmentation of microcolonies. Quartets of sporangia. Doublets of sporangia. Single sporangia. Free endospores. |
| Host–bacterium relationships | All parasitize invertebrates. Colonies first observed in the host are sedentary and located in the host's musculature. Growth in muscle tissue eventually leads to fragmentation and entry of microcolonies into the coelom or pseudocoelom of the respective host. Microcolonies carried passively by body fluids. Colonization of hemolymph or pseudocoelomic fluid by the parasite is extensive. Host ranges are very narrow: Pasteuria ramosa occurs only in cladoceran water fleas, Pasteuria penetrans sensu stricto in the root-knot nematode Meloidogyne incognita, Pasteuria thornei in the lesion nematode Pratylenchus brachyurus, Pasteuria nishizawae in Heterodera and Globodera, and “Candidatus Pasteuria usgae” in Belonolaimus. Host is completely utilized by the bacteria; in the end, the host becomes little more than a bag of bacterial endospores. |
| Survival mechanisms | Survive in field soil and at bottom of ponds. Resist desiccation. Loss of infectivity after 5 min at 70°C may indicate moderate resistant to heat. |
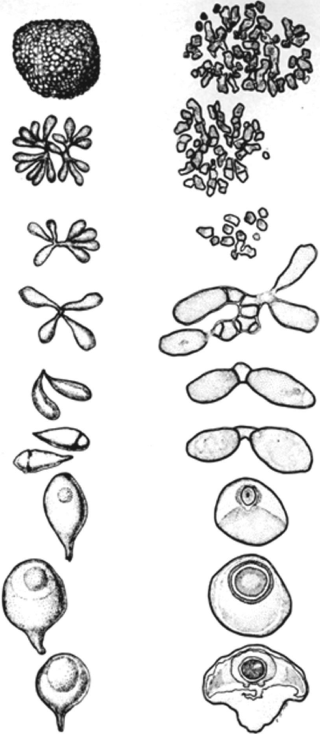
The developmental stages that occur before sporulation are similar for all Pasteuria spp. Except for Thermoactinomyces spp., the mycelial-like proliferation during development is not found in any other endospore-forming bacteria. The morphological changes that occur during sporulation appear to be generally similar for all of the endospore-forming bacteria (Atibalentja, Jakstys and Noel, 2004a, 2004b; Chen, Dickson, Freitas and Preston, 1997b; Ebert, Rainey, Embley and Scholz, 1996, 2004; Giblin-Davis, Williams, Brito, Dickson and Preston, 2003a, 2003b; Metchnikoff, 1888; Sayre and Starr, 1985).
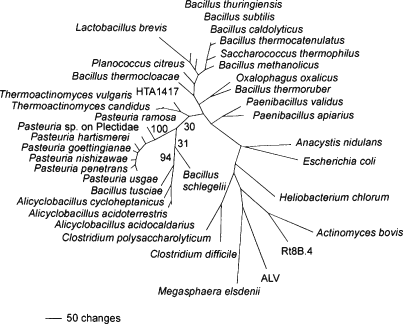
Recent molecular work with 16S rRNA gene sequences support Pasteuria as a monophyletic clade comprising well supported lineages (Anderson, Preston, Dickson, Hewlett, Williams and Maruniak, 1999; Atibalentja and Noel, 2008; Atibalentja, Noel and Domier, 2000; Bekal, Borneman, Springer, Giblin-Davis and Becker, 2001; Bishop, Gowen, Pembroke and Trotter, 2007; Ebert, Rainey, Embley and Scholz, 1996; Giblin-Davis, Williams, Bekal, Dickson, Brito, Becker and Preston, 2003b; Preston, Dickson, Maruniak, Nong, Brito, Schmidt and Giblin-Davis, 2003; Sturhan, Shutova, Akimov and Subbotin, 2005). Further genomic comparisons have been made through the sequences of sporulation and other genes in different isolates of Pasteuria penetrans and Pasteuria ramosa (Bird, Opperman and Davies, 2003; Charles, Carbone, Davies, Bird, Burke, Kerry and Opperman, 2005; Preston, Dickson, Maruniak, Nong, Brito, Schmidt and Giblin-Davis, 2003; Schmidt, Preston, Nong, Dickson and Aldrich, 2004; Trotter and Bishop, 2003). A phylogenetic tree (Atibalentja and Noel, 2008) comparing 16S rRNA sequences has been updated to depict the close relationship of Pasteuria spp. to other genera of the Firmicutes (Figure 2).
Comparison of spoIIAB gene sequences amplified by PCR from DNA from different isolates (biotypes) of Pasteuria penetrans identified single nucleotide polymorphisms or SNPs, indicating genetic heterogeneity within populations obtained from both individuals, as well as populations of Meloidogyne spp. (Nong, Chow, Schmidt, Dickson and Preston, 2007). DNA sequences from three different loci derived from a single-spore isolate of Pasteuria penetrans were identical, supporting the need for clonal populations for definitive studies on host preference (Trotter, Darban, Gowen, Bishop and Pembroke, 2004).
To clarify the characteristics of the genus Pasteuria, the meanings of the terms “endospore” and “sporangium” must be modified slightly from their usual definition in order to be applicable to this genus. Metchnikoff observed the several stages of endosporogenesis that occurred in Pasteuria ramosa. In his discussion, he noted within each sporangium a single refractile body that stained with difficulty; he called this structure, as we do today, an endospore. The Pasteuria endospore is not entirely typical of those found in Bacillus or Clostridium. For one thing, the Pasteuria endospore has a mass of fibrous outgrowths emanating from the central body or core. These microfibrillar strands (usually called parasporal fibers, peripheral fibers, or perisporium), which surround the central body of the endospore, are structures comprised of glycoproteins that are presumed to function as adhesins involved in attachment of the endospore to its invertebrate host (Figure 3) (Brito, Powers, Mullin, Inserra and Dickson, 2004; Davies, Redden and Pearson, 1994; Persidis, Lay, Manousis, Bishop and Ellar, 1991; Preston, Dickson, Maruniak, Nong, Brito, Schmidt and Giblin-Davis, 2003; Schmidt, Preston, Nong, Dickson and Aldrich, 2004). A monoclonal antibody that recognizes a glycan-containing epitope associated with adhesins on the surfaces of endospores of Pasteuria penetrans (Brito, Preston, Dickson, Giblin-Davis, Williams, Aldrich and Rice, 2003; Schmidt, Preston, Dickson, Rice and Hewlett, 2003) detects this epitope in extracts of endospores from other species of infected nematodes (Preston, Dickson, Maruniak, Nong, Brito, Schmidt and Giblin-Davis, 2003), as well as Pasteuria ramosa (Schmidt, Mouton, Nong, Ebert and Preston, 2008). The epitope was not detected in extracts of endospores obtained from a number of Bacillus spp. that were tested, but is common to endospores of all Pasteuria spp. that have been evaluated.
Albeit an integral part of the endospore, the parasporal fibers are arrayed differently in the different Pasteuria spp. discussed herein. It is difficult to include these somewhat amorphous fibrous masses in any precise measurements of endospores. For this reason, the measurements reported herein include only the major and minor axes of the central body of the endospore, the endospore proper, and explicitly exclude the parasporal fibers. Measurements and descriptions of parasporal fibers are presented separately.
The attachment of endospores to their invertebrate hosts is mediated by the adhesins in their parasporal fibers. The attached endospore is often overlaid by seemingly nonfunctional remnants of the old sporangium (Figure 3). The presence or absence of these sporangial remnants may be due in part to the length of time the sporangium has been subjected to degradative processes in the soil or the amount of abrasion received as the nematode host moves through its environs (Figure 4). When such sporangial material was significant, it was included in the measurements of the endospores reported herein. Even though sporangial material is sometimes seen, we have adopted the convention of calling the infectious unit on the nematode exterior cuticle surface an endospore. Pasteuria endospores, at least in the case of the nematode parasites, also differ from those of Bacillus in that the former produce a germ tube that penetrates the nematode cuticle and initiates bacterial colonization within the nematode body.
The type-descriptive material (Sayre and Starr, 1985) of Pasteuria penetrans (ex Thorne (1940) Sayre and Starr (1985) refers to the bacteria occurring on the root-knot nematode Meloidogyne incognita. Hence, the name Pasteuria penetrans must refer to that organism (Starr and Sayre, 1988a). This morphotype, however, has been isolated from other Meloidogyne spp. and comprises several genotypes that may defy or challenge easy species definition (Sturhan, Shutova, Akimov and Subbotin, 2005). Some other observed members of Pasteuria are demonstrably different from Pasteuria penetrans using a Linnaean species concept (typological) with molecular corroboration and should be assigned to other taxa. The first such assignment was made for the bacterium from the lesion nematode Pratylenchus brachyurus, to which the name Pasteuria thornei Starr and Sayre (1988a) was affixed (Starr and Sayre, 1988a). Because the obligate endoparasitic nature of Pasteuria currently prevents cultivation of axenic type strains, “Candidatus” status has been proposed for each new provisional species designation in this genus (Giblin-Davis, Williams, Bekal, Dickson, Brito, Becker and Preston, 2003b; Murray and Stackebrandt, 1995; Murray and Schleifer, 1994; Stackebrandt, Frederiksen, Garrity, Grimont, Kämpfer, Maiden, Nesme, Rosselló-Mora, Swings, Truper, Vauterin, Ward and Whitman, 2002). All of the previously named species in the genus Pasteuria Metchnikoff (1888) (Skerman, McGowan and Sneath, 1980) have nomenclatural standing and remain as species with validly published names.
The species that remain valid include Pasteuria ramosa Metchnikoff (1888) [Skerman, McGowan and Sneath (1980) as emended by Starr, Sayre and Schmidt (1983) serving as type; see Judicial Commission 1986, Wayne (1986)], Pasteuria penetrans (ex Thorne, 1940) [Sayre and Starr (1985) description and illustrations serving as type; Validation List no. 20; (Sayre and Starr, 1986)], Pasteuria thornei [Sayre, Starr, Golden, Wergin and Endo (1988) and Starr and Sayre (1988a) description and illustrations serving as type; Validation List no. 26; (Starr and Sayre, 1988b)], and Pasteuria nishizawae [Sayre, Wergin, Schmidt and Starr (1991) as emended by Noel, Atibalentja and Domier (2005) description and illustrations serving as type; Validation List no. 41; (Sayre, Wergin, Schmidt and Starr, 1992)]. We concur with the proposal of Ebert, Rainey, Embley and Scholz (1996) to accept the Daphnia parasite that they isolated and studied, and whose 16S rRNA gene has been sequenced, as the neotype for Pasteuria ramosa and the genus Pasteuria. Unfortunately, 16S rRNA gene sequence data are not available for Pasteuria thornei and this species of Pasteuria must be rediscovered before a more complete characterization can be made.
Enrichment and isolation procedures
Attempts to devise methods for in vitro cultivation of Pasteuria penetrans using defined media have proven difficult, but some success has been reported (Bishop and Ellar, 1991; Hewlett, Griswold and Smith, 2006). The cultivation of Pasteuria penetrans outside of its nematode host has been reported by an independent commercial enterprise, but complexities and undisclosed composition of the media used have precluded their application and confirmation in other laboratories. The distinctive morphology and unique relationship to invertebrate hosts shared by Pasteuria spp. (see Table 1) suggest that this commonality may be the harbinger of similarities in their physiological requirements for endospore germination, vegetative growth, sporulation, and their eventual axenic cultivation. Based on microscopic observations, the physiological and physical requirements for their growth in vivo would appear to be similar. Summarized briefly, vegetative growth of Pasteuria spp. seems linked through the environment provided in the coelom/pseudocoelom of their different invertebrate hosts. The hemolymph of the cladoceran or the pseudocoelomic fluid of the nematode allows for the exchange of nutrients and waste products. Also, the coelom/pseudocoelom provides space for mycelial colony development. These colonies fragment after they reach a critical size. Finally, it is reasoned that various factors, possibly the accumulation of bacterial biomass and metabolites, as well as the onset of senility in the invertebrate hosts, trigger sporulation of the bacteria. The similar physical conditions found in the separate host species suggests that the bacteria might have common nutritional requirements; hence, when the requirements for axenic cultivation of one Pasteuria sp. become known, they may, with slight modification, apply to other species. The unique host specificities of different Candidatus species (Giblin-Davis, Center, Williams, Schmidt, Brito, Dickson and Preston, 2004; Giblin-Davis, Williams, Brito, Dickson and Preston, 2003a; Sturhan, Shutova, Akimov and Subbotin, 2005), as well as biotypes of Pasteuria penetrans defined by a marked preference for different Meloidogyne spp. (Oostendorp et al., 1990; Stirling, 1985), supports a role for the host in conferring virulence and host specificity. The host may play an active role in the maturation of endospores and endow it with adhesins that serve as virulence factors through their recognition of receptors on the cuticle of the host and the attachment that is required for infection (Preston, Dickson, Maruniak, Nong, Brito, Schmidt and Giblin-Davis, 2003).
-
Cladocerans should be collected during the warmest part of the growing season. The parasite is most often found in Daphnia magna, but also in other Daphnia spp. (Ebert, 2005).
-
Because the frequency of occurrence of the parasite in a cladoceran population may be low (less than 10%), a large number of living specimens needs to be examined to increase the odds for detection. Prevalence, however, may reach up to 100% of all the adult hosts (Duncan and Little, 2007).
-
The internal parasites are most easily identified by using an inverted microscope at magnifications of × 100–250. Infected Daphnia are typically large and much less transparent than uninfected animals. The parasite fills the entire body cavity.
Although axenic cultivation of Pasteuria penetrans has been reported but not confirmed, investigations have been limited to studies on naturally or artificially infected nematode hosts (Bekal, Giblin-Davis and Becker, 1999; Chen, Dickson, Freitas and Preston, 1997b; Chen and Dickson, 1998; Imbriani and Mankau, 1977; Mankau, 1975; Mankau and Imbriani, 1975; Sayre, Gherna and Wergin, 1983, 1988; Sayre and Wergin, 1977; Starr and Sayre, 1988a) and exploration of the bacterium's potential as a biological control agent against plant-parasitic nematode populations (Chen and Dickson, 1998). Consequently, the studies have depended on finding, maintaining, and manipulating nematode populations infected with these bacteria. Because of this direct dependence on host–nematode populations, the procedures and methods used for maintaining members of the nematode-associated Pasteuria are by-and-large those used in maintaining the nematodes (Southey, 1986; Zuckerman, Mai and Harrison, 1984).
-
The group of nematodes that are parasitized by Pasteuria spp. is widespread and diverse. The bacterial parasite has been reported from about 116 nematode genera and 323 nematode species. Pasteuria spp. have been reported in a dozen states of the United States, as well as roughly 51 countries on five continents and on various islands in the Atlantic, Pacific, and Indian Oceans (Chen and Dickson, 1998; Ciancio, Bonsignore, Vovlas and Lamberti, 1994; Sayre and Starr, 1988; Sturhan, 1988).
-
Members of nematode-associated Pasteuria will most likely be found in soils where nematode populations have been consistently high and are causing crop damage (in the case of plant-parasitic nematodes only). However, numerous nematode suppressive soils have been identified where Pasteuria causes a precipitous drop in plant nematode numbers (Chen and Dickson, 1998). Planting of susceptible crops is necessary for maintenance of the nematode populations and multiplication of Pasteuria spp. Pasteuria spp. may also be associated with plant nematodes in greenhouse situations.
-
To find the bacterial endospores, nematodes are extracted from the suspected soil, e.g., by a centrifugal-flotation method (Jenkins, 1964). Increasing the sucrose concentration used for extraction of nematodes results in a higher percentage recovery of endospore-filled specimens (Oostendorp et al., 1991). Other separation methods, relying on the nematode's mobility (e.g., Baermann, 1917), may not yield those nematodes that are heavily encumbered with endospores or endospore-filled bodies since such nematodes are partially immobilized. Addition of healthy nematodes to soils, together with subsequent extraction and examination, is the most commonly used bioassay for determining the presence of members of the genus Pasteuria in soil. Attachment assays have been developed that allow estimations of the number of endospores per gram of soil and the extent to which soils are suppressive for plant nematodes (Chen and Dickson, 1998; Oostendorp et al., 1990). Also, methods have been developed for the quantification of Pasteuria endospore concentrations in tomato root material (Chen et al., 1996), the extraction and purification of endospores (Chen et al., 2000), and for determining suppressive soils caused by Pasteuria (Chen et al., 1997a; Dickson et al., 1994; Stirling, 1984; Walia, Hewlett and Dickson, 2004). PCR-based methods have been developed for amplifying and sequencing 16S rRNA genes from soil samples and single nematodes (Atibalentja, Noel and Ciancio, 2004b; Duan, Castro, Hewlett, White and Ogram, 2003). Immunoassays using polyclonal (Costa, Kerry, Bardgett and Davies, 2006; Fould, Dieng, Davies, Normand and Mateille, 2001) and monoclonal (Schmidt et al., 2003) antibodies directed against endospore surface proteins have been developed for the detection and quantification of bacterial endospores in soil and tissue samples. PCR-based assays for sporulation and other genes have been developed for the identification and quantification of the vegetative cells in plant tissues (Schmidt, Preston, Nong, Dickson and Aldrich, 2004).
-
Occurrence of endospores on the surfaces of nematodes is most easily observed by means of an inverted microscope at ×250–400 magnification. Several stains, e.g., crystal violet, cotton blue, Brilliant Blue G, etc., are useful for visualizing external spores. The endospores also may be readily identified with a fluorescent immunoassay and monoclonal antibodies that recognize adhesin-related epitopes (Davies, Redden and Pearson, 1994; Schmidt, Preston, Dickson, Rice and Hewlett, 2003). The application of immunoassays avoids potential misidentification of endospores of Paenibacillus spp. that may be associated with entomopathogenic rhabditid nematodes (El-Borai, Duncan and Preston, 2005; Enright, McInerney and Griffin, 2003).
-
Direct confirmation of the presence of members of the Pasteuria group inside the nematode can be made by microscopic examination of the pseudocoelom of nematodes, also at ×250–400 magnification. Both juveniles and adults should be examined for the characteristic mycelial colonies and endospores. Treating nematodes with methyl blue and lactophenol stain works well for visualizing the various developmental stages within the nematode pseudocoelom (Serracin, Schuerger, Dickson and Weingartner, 1997). In uninfected nematodes, the anterior region of the esophagus is clear, with internal structures visible; when filled with endospores, the region will not be clear and internal structures will be masked by the bacterium.
-
Detection of the bacterium in the sedentary endoparasitic nematodes depends on manual (Thorne, 1940) and/or enzymic (Dickson et al., 1970) removal of root-tissues from around the female nematodes. The freed females are placed on glass slides, crushed, and their body contents are examined microscopically for vegetative stages and endospores of the bacterium.
Maintenance procedures
Since the recently reported method of axenic cultivation is proprietary and has not been optimized or confirmed, Pasteuria spp. are maintained by co-cultivation with their respective invertebrate host.
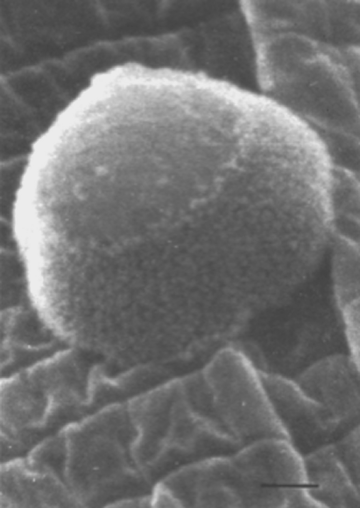
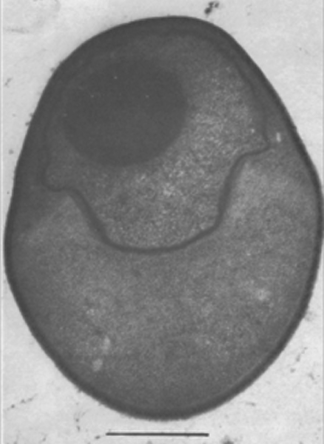
Pasteuria ramosa can be grown in the laboratory in clonal cultures of Daphnia magna. (Ebert et al., 2004). An endospore-filled body of Daphnia magna is shown in Figure 5. Daphnia are maintained on a diet of chemostat-grown unicellular green algae Scenedesmus sp. At 20°C, an infected Daphnia magna produces several million Pasteuria spores within about 40 d.

Endospores used to inoculate healthy Daphnia magna are obtained from two sources: (a) the crushed bodies of living or dead parasitized cladocerans in late-stage infections; and (b) sediments from the bottoms of aquaria (or ponds) in which dead and parasitized cladocerans have accumulated. Frozen cadavers can also be used. The infection rate is dose-dependent. High infection rates can be reached by adding 10,000 endospores to jars with 20 ml water and a single Daphnia magna in each jar (Regoes, Hottinger, Sygnarski and Ebert, 2003). Resistance of Daphnia magna clones to Pasteuria ramosa is widespread (Carius, Little and Ebert, 2001; Decaestecker, Vergote, Ebert and De Meester, 2003; Little and Ebert, 1999) and may be the most common reason for a failure to cultivate the bacterium. Furthermore, there are strong host clone–parasite isolate interactions (Carius, Little and Ebert, 2001).
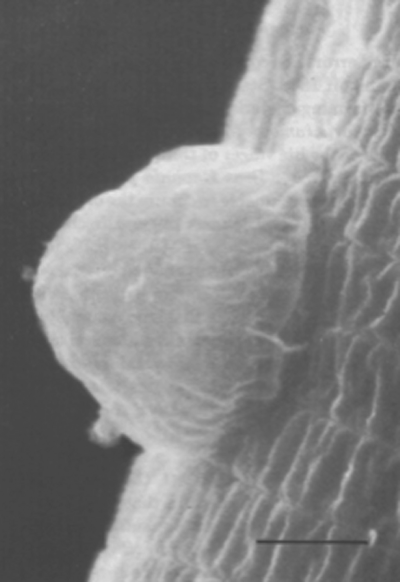
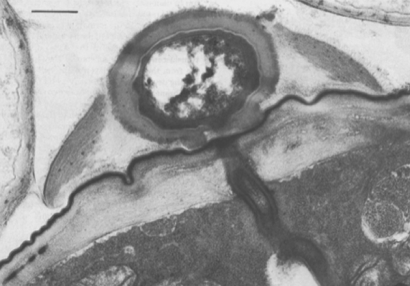
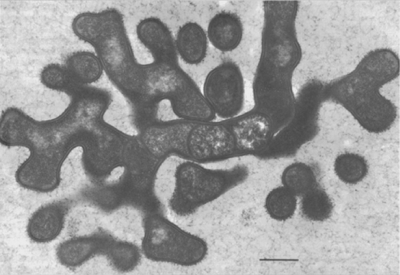
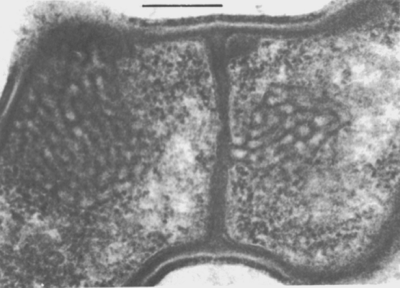
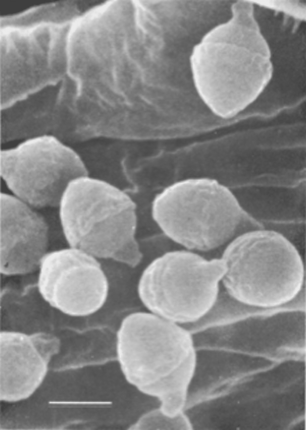
Nematode-associated Pasteuria can be maintained in a system consisting of the immediate nematode host and its host plant. A good example is the system consisting of Pasteuria penetrans–Meloidogyne incognita and tomato plants (Sayre and Wergin, 1977). To initiate and increase a bacterial population, dried bacterial endospore preparations (e.g., Stirling and Wachtel, 1980) are mixed into soils that are heavily infested with juveniles of Meloidogyne incognita. The juveniles become encumbered with the bacterial endospores as they move through the soil in a random fashion (Figure 6). The endospores adhering to the nematode cuticle are carried by the juvenile into tomato roots, where germination occurs after the nematode initiates feeding. An endospore penetrates the cuticle by means of a single germ tube (Figure 7), which extends into the pseudocoelomic cavity. The bacterium then enters into its vegetative endoparasitic developmental stages (Figure 8 and Figure 9). Finally, bacteria form in the mature and moribund host nematodes and in sporangia that contain endospores (Figure 10, Figure 11, and Figure 12). In summary, the developmental stages of the bacterium include recognition and attachment to a susceptible host nematode, infection of the host nematode via a germ tube, followed by vegetative growth, sporulation, and maturation within the host pseudocoelom.
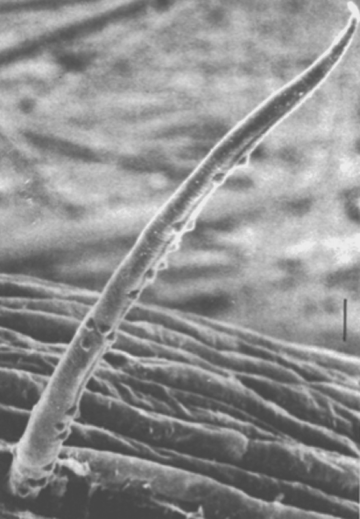
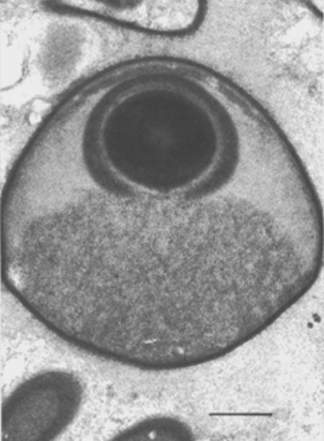
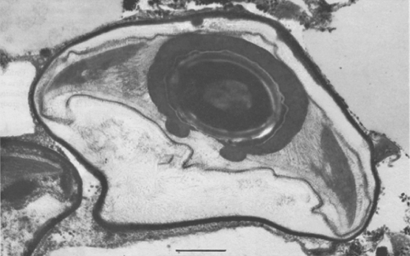
Endospores of Pasteuria penetrans growing inside Meloidogyne incognita can be harvested by two methods. The simplest procedure is to allow the nematode-infested plant roots to decay in situ in soil; during such decay, about 2 × 106 endospores are released from each female nematode. The soil containing the endospores is air-dried, mixed, and stored. Such preparations have yielded bacterial endospores that can attach to the juvenile of their respective host nematode even after several years in storage (Mankau, 1973). A second method for obtaining a more concentrated preparation of the bacterial endospores has been demonstrated (Stirling and Wachtel, 1980). Freshly hatched juveniles of Meloidogyne incognita may be encumbered with endospores by placing them in aqueous suspensions containing endospores. A centrifuge method can be used to help obtain consistent attachment of endospores to host nematodes (Hewlett and Dickson, 1993). These encumbered juveniles are then allowed to penetrate roots of tomato seedlings. After the life cycle of the nematode is completed in soil, the galled roots are harvested, washed, air-dried, ground into a fine powder, and stored. Such preparations have provided adequate sources of endospores for use in bioassays and other procedures. A more efficient and rapid method of obtaining endospore-filled female nematodes is by using an enzyme (cellulase and/or pectinase) preparation (Brito, Preston, Dickson, Giblin-Davis, Williams, Aldrich and Rice, 2003; Chen et al., 2000; Schmidt, Preston, Nong, Dickson and Aldrich, 2004). Purification can be achieved by selective filtration steps and centrifugation in sucrose or renografin (sodium diatrizoate) gradients (Chen et al., 2000).
Pasteuria thornei and “Candidatus Pasteuria usgae” can be maintained in similar systems, except that the bacteria must be maintained on the migratory endoparasitic host nematode Pratylenchus brachyurus or the ectoparasitic nematode Belonolaimus longicaudatus, respectively. Pratylenchus brachyurus may be collected from roots of infected hosts, whereas Belanolaimus longicaudatus may be collected from around roots of infected plants. Numerous bacterial endospores are liberated upon decay of the plant roots and the cadavers of infected nematodes. Healthy juveniles or adults of either nematode migrating through such soils can become encumbered with endospores of their respective bacterial parasite and repeat the developmental cycle, with not only maintenance of the bacterium, but also a net increase in the endospore content of the soil.
At present, the only method of producing endospores of Pasteuria nishizawae is to collect infected females and cysts. This is difficult due to the fragile nature of infected cysts and the difficulty in identifying them. At a certain stage, the infected cysts are usually a grayish-green color, but this coloration is often difficult to observe. Infected females cannot be readily identified without crushing them.
A number of investigators have cultivated members of Pasteuria in three-membered systems consisting of plant-tissue cultures, gnotobiotically reared nematodes, and the desired bacterium free of contaminating microbes (Bekal et al., 1999; Chen and Dickson, 1998). Once perfected, these cultural methods are useful for maintenance of these bacteria, at least until methods for their axenic cultivation become generally available.
Differentiation of the genus Pasteuria from other genera
Table 1 summarizes the characteristic features of species of the genus Pasteuria.
Taxonomic comments
De Toni and Trevisan (1889) provided the first generic diagnosis of Pasteuria; it followed quickly and closely the original description of Pasteuria ramosa by Metchnikoff (1888). However, some other early investigators, particularly those interested in taxonomic coherence, not having observed this enigmatic organism and relying solely on descriptions, rejected both the generic and specific concepts (Lehmann and Neumann, 1896; Migula, 1904). Laurent (1890) suggested that a bacteroid-forming species from nodules on leguminous plants, together with Pasteuria ramosa, comprised the new family he erected, Pasteuriaceae. Similarly, Vuillemin (1913) believed that a generic relationship existed between Nocardia and Pasteuria. De Toni and Revisan 1889 speculated that Pasteuria ramosa, because of its ability to form endospores, should be placed in the subtribe Pasteurieae of the tribe Bacilleae. The unusual morphology of Pasteuria ramosa became the basis for numerous suppositions about its affinities to other bacterial groups (Buchanan, 1925). This speculative process continued up until recently (Sayre et al., 1983; Sayre and Starr, 1985, 1988; Starr and Sayre, 1988a). The advent of 16S rRNA gene analyses indicated that Pasteuria is a deep lineage within the Bacillales (Atibalentja, Noel and Domier, 2000; Ebert, Rainey, Embley and Scholz, 1996). Although it has been classified within the “Alicyclobacillaceae” (Garrity, Bell and Lilburn, 2005), the low sequence similarity of 85% and its distinctive phenotype suggests that it would be more properly classified within its own family, the Pasteuriaceae.
A significant change made since the 9th edition of the Manual is the reduction in the confusion between Metchnikoff's Gram-positive, endospore-forming, mycelial cladoceran parasite with certain Gram-negative, budding, non-prosthecately appendaged aquatic bacteria. Conservation of Pasteuria ramosa sensu Metchnikoff (1888) on the basis of type-descriptive material, as well as rejection of ATCC 27377 as the type of Pasteuria ramosa Metchnikoff (1888) because it actually is a quite different organism (Planctomyces staleyi Starr, Sayre and Schmidt (1983), have been recommended (Starr et al., 1983) and approved (Judicial-Commission, 1986). The detailed drawings, photomicrographs, and lengthy description offered by Metchnikoff (1888) provided a sound basis for comparing his species with the current cladoceran parasites. As stated above, a Pasteuria ramosa-like strain was discovered infecting Moina rectirostris (Sayre, Adams and Wergin, 1979) that was used in the emendation of the species (Starr, Sayre and Schmidt, 1983). We support the proposal of Ebert, Rainey, Embley and Scholz (1996) that the Daphnia-parasitic Pasteuria ramosa characterized from the same host as Metchnikoff (1888) from Europe be designated the neotype for Pasteuria ramosa Metchnikoff (1888) and that the Moina isolate of Sayre et al. 1979 be compared directly to the neotype in future studies.
One reason investigators (Henrici and Johnson, 1935; Hirsch, 1972) have been interested in re-examining Pasteuria ramosa stems from the questions raised by Metchnikoff's assertion that the bacterium divides longitudinally. Metchnikoff suggested that cells of Pasteuria ramosa undergo a longitudinal fission, giving rise to a branched structure in which the two daughter cells remain attached at their tips. Based on a hypothesis about the evolution of division patterns in bacteria, he concluded that Pasteuria ramosa, a fairly primitive bacterium in his view, divided longitudinally.
Observations on the Pasteuria ramosa-like parasite of Moina (Sayre, Adams and Wergin, 1979) indicated that cleavage indeed occurs in three planes, as evidenced by the spherical mycelial colonies. However, no common plane of division was found, as illustrated in drawing 2 of the Metchnikoff (1888) paper, starting at the surface of the cauliflower-like growth and ending at its interior. The prominent bifurcations in the mycelium (Figure 13), which Metchnikoff also observed and which may have prompted his longitudinal fission theory, are not products of atypical fission but rather are probably the branched distal or terminal cells of the mycelium undergoing rapid enlargement during formation of endogenous spores. These terminal cells develop into sporangia.
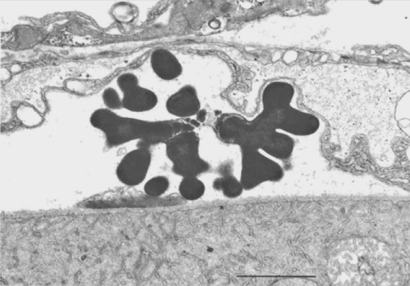
Of some historical interest are a few scattered reports in the literature about organisms similar in appearance to Pasteuria ramosa. In these reports, each author came to a different taxonomic decision about the organism, as follows: Torula or other yeast species (Ruehberg, 1933); two different genera of the microsporidia (Jirovec, 1939; Weiser, 1943), and a haplosporidium (Dermocystidium daphniae Jirovec (1939) and a possible intermediate stage in the life cycle of a Dermocystidium sp. (Sterba and Naumann, 1970). These results probably stem from the inability of these investigators to cultivate the particular organism they observed, taken together with their dependence solely on morphology and staining reactions as the basis for their descriptions and classifications. Surprisingly, Metchnikoff (1888), the first person to report on Pasteuria, recognized it correctly as a bacterium, whereas the later workers did not and, moreover, were apparently unaware of Metchnikoff's work.
Until rather recently, taxonomic studies of Pasteuria were carried out mainly by nematologists. Cobb (1906) erroneously designated such microbes as protozoan parasites of nematodes. At first glance, it would appear from the literature that subsequent workers (Steiner, 1938; Thorne, 1940) had independently come to the same conclusion. However, their conclusions were probably by consensus. Members of Pasteuria were then, and largely still are, essentially known only to the community of plant nematologists. When Thorne described Duboscqia penetrans as a protozoan, he could not have realized its bacterial nature because electron microscopes were not then available. Later, Williams (1960) studied a similar organism in a population of root-knot nematodes from sugarcane, presented an interpretation of its life stages, and indicated some reservations about Thorne's identification. Canning (1973) also doubted the identification as a protozoan and stressed the organism's fungal characteristics. Finally, Mankau (1975) and Imbriani and Mankau (1977) established the bacterial nature of the organism and brought its attendant taxonomic problems to the attention of bacteriologists. Some results of the ensuing interdisciplinary enterprise are summarized elsewhere (Sayre and Starr, 1988; Sayre, Starr, Golden, Wergin and Endo, 1988; Starr and Sayre, 1988a).
Differentiation and characteristics of the species and Candidatus species of the genus Pasteuria
The differential characteristics of nominal Pasteuria and Candidatus species are given in Table 2.
| Characteristic | 1. P. ramosa | 2. P. nishizawae | 3. P. penetrans | 4. P. thornei | 5. “Candidatus P. usgae” |
|---|---|---|---|---|---|
| Colony shape | Cauliflower-like floret initially, later fragments into cluster of elongated grape-like sporangia | Spherical to clusters of elongated grape-like sporangia | Small, elongate clusters | Cauliflower-like floret | |
| Sporangia | |||||
| Shape | Teardrop | Cup | Cup | Rhomboidal | Cup to rhomboidal |
| Diameter, µma | 2.12–2.77 | 4.1–4.7 | 3.0–3.9 | 2.22–2.7 | 4.7–7.1 |
| Height, µma | 3.40–4.35 | 2.8–3.4 | 2.26–2.60 | 1.96–2.34 | 2.73–4.97 |
| Fate of sporangial wall at maturity of endospore | Remains rigidly in place; external markings divide sporangium in three parts | Sporangial wall and mother cell matrix of endospore disintegrate, leaving exosporium as the outermost layer of the endospore; no clear external markings | Basal portion collapses inward in the developed endospore; no clear external markings | Remains essentially rigid, sometimes collapsing at bases; no clear external markings | Basal portion collapses inward in the developed endospore; no clear external markings |
| Exosporium | Present | Present, velutinous to hairy surface | Present | Present | Present |
| Stem cell | Remains attached to most sporangia | Seen occasionally | Seen occasionally | Neither stem cell nor second sporangium seen | Rarely seen |
| Central body | Oblate spheroid, an ellipsoid, narrowly elliptic in section | Oblate spheroid, ellipsoid to narrowly elliptical in section | Oblate spheroid, ellipsoid to broadly elliptical in section | Oblate spheroid; ellipsoid, sometimes almost spherical, narrowly elliptical in section | Oblate spheroid, ellipsoid to broadly elliptical in section |
| Orientation of major axis to sporangium base | Vertical | Horizontal | Horizontal | Horizontal | Horizontal |
| Cell dimensions, µma | 1.37–1.61 × 1.20–1.46 | 1.4–1.8 × 1.2–1.4 | 0.99–1.21 × 1.30–1.54 | 0.96–1.20 × 1.15–1.43 | 2.40–3.91 × 1.44–2.34 |
| Wall thickness, µma | 0.28–0.34 | 0.22–0.26 | 0.17–0.23 | 0.36–0.59 | |
| Protoplast | Contains pronounced stranded inclusions | Stranded inclusions sometimes seen | Stranded inclusions observed | Stranded inclusions observed | |
| Partial middle spore wall | Not observed | Surrounds endospore laterally, not in basal or polar areas | Surrounds endospore somewhat sublaterally | Surrounds endospores somewhat sublaterally | |
| Pore | |||||
| Occurrence | Absent | Present | Present | Present | Present |
| Characteristics | − | Thickness of basal wall constant and is the depth of pore | Basal annular opening formed from thickened outer wall | Basal cortical wall thins to expose inner endospore | Basal cortical wall thins to expose inner endospore |
| Diameter, µma | − | 0.3±0.1 | 0.28±0.11 | 0.13±0.01 | 0.29±0.1 |
| Parasporal structures, origin and orientation | Long primary fibers arise laterally from cortical wall, bending sharply downward to yield numerous secondary fibers arrayed internally toward the granular matrix | Same as P. penetrans but additional layer is formed on obverse surface of endospore | Fibers arise directly from cortical wall, gradually arching downward to form an attachment layer of numerous shorter fibers | Long fibers arise directly from cortical wall, bending sharply downward to form an attachment layer of numerous shorter fibers | Same as P. penetrans |
| Matrix, at maturity | Persists as fine granular material | Persists, numerous strands are formed and partial collapse may occur | Becomes coarsely granular; lysis occurs; sporangial wall collapses; base is vacuolate | Persists, but more granular; some strands are formed and partial collapse may occur | Same as P. penetrans |
| Host | Cladocerans (Daphnia, Moina) | Cyst nematodes (Heterodera glycines) | Nematodes Meloidogyne spp.) | Nematodes (Pratylenchus brachyurus) | Nematodes (Belonolaimus longicaudatus) |
| Completes life cycle in nematode juveniles | − | No, only in female and cyst | Mostly only in female, occasionally seen in 2nd stage juvenileb | Yes, in 2nd, 3rd, and 4th stage juveniles and adult | 3rd, 4th stage juveniles and adults |
| Location in host | Hemocoel and musculature; sometimes found attached to coelom walls | Pseudocoelom and musculature; no attachment to pseudocoelom walls seen | Pseudocoelom and musculature; no attachment to pseudocoelom walls seen | Pseudocoelom and musculature; no attachment to pseudocoelom walls seen | Pseudocoelom and musculature; no attachment to pseudocoelom walls seen |
| Attachment of spores on host | Spores not observed to attach or accumulate on surface of cladoceran | Spores accumulate on juveniles, rare on male | Spores accumulate in large numbers on cuticular surface | Spores accumulate in large numbers on cuticular surface | Spores accumulate in large numbers on cuticular surface |
| Mode of penetration of host | Not known; suspected to occur through gut wall | Direct penetration of nematode cuticle by germ tube | Direct penetration of nematode cuticle by germ tube | Direct penetration suspected but not seen | Direct penetration of nematode cuticle by germ tube |
| Source of host | Pond mud, freshwater | Soil, plants | Soil, plants | Soil, plants | Soil, plants |
- a Measurements are based on preparations examined by transmission electron microscopy. Somewhat different apparent sizes are obtained by phase-contrast light microscopy and scanning electron microscopy.
- b Rarely seen in males but, when observed, thought to be sex-reversed males (Hatz and Dickson, 1992).
List of species of the genus Pasteuria
Pasteuria ramosa
Metchnikoff 1888, 166AL [Nom. Cons. Opin. 61 Jud. Comm. 1986, 119. Not Pasteuria ramosa in the sense of Henrici and Johnson 1935, Hirsch 1972, and Staley 1973; see Starr et al. 1983 and Judicial-Commission (1986)]
ra.mo'sa. L. fem. adj. ramosa much-branched.
Gram-positive. Sporangia and microcolonies are parasitic in the hemocoel of cladocerans, water fleas of the genera Daphnia and Moina. Usually occur attached to one another at pointed ends of the teardrop-shaped sporangia, forming quartet, triplet, and doublet configurations. The rounded end of the sporangium encloses a single refractile endospore, having axes of 1.37–1.61 × 1.20–1.46 µm, narrowly elliptic in cross-section. Endospores are 4.2–5.4 × 4.9–6.0 µm. Nonmotile. Endospores are resistant to desiccation, but with only limited heat tolerance. They have been recovered from 30-year-old pond sediments (Decaestecker et al., 2004). Vegetative stages are cauliflower-like, septate, mycelial growths that branch dichotomously and fragment to form microcolonies. Has not been cultivated axenically, but can be grown in the laboratory with the invertebrate host. The type-descriptive material consists of descriptions and illustrations in Metchnikoff's original publication (Metchnikoff, 1888) and elsewhere (Ebert et al., 1996; Sayre et al., 1979, 1983; Starr and Sayre, 1988a; Starr et al., 1983).
DNA G+C content (mol%): not reported.
Type strain: descriptions and illustrations serving as type.
GenBank accession number (16S rRNA gene): AY762091 (Ebert et al., 1996).
Pasteuria nishizawae
Sayre, Wergin, Schmidt and Starr 1992, 327VP (Effective publication: Sayre, Wergin, Schmidt and Starr 1991, 562.) (emend. Noel, Atibalentja and Domier 2005, 1683.)
ni.shi.za'wae. N.L. gen. n. nishizawae of Nishizawa, named after Tsutomu Nishizawa, a Japanese nematologist who discovered and first investigated bacterial parasites of cyst-forming nematodes.
Gram-positive vegetative cells, forms endospores. Obligate endoparasitic bacterium of the pseudocoelom of Heterodera glycines (soybean cyst nematode). Microcolony shape is cauliflower-like initially and later fragments into clusters of elongated grape-like immature sporangia occurring in configurations of octets, quartets, and doublets. Sporangia are cup-shaped with diameters and heights (under the light microscope) of 5.3 and 4.3 µm, respectively, and (under the transmission electron microscope) of 4.4 and 3.1 µm, respectively. Sporangial wall and mother cell matrix disintegrate at maturity leaving the exosporium as the outermost layer of the endospore. The surface of the exosporium is velutinous to hairy. The stem cell is observed occasionally. The central body is oblate spheroid, ellipsoid to narrowly elliptical. Orientation of major axis to sporangium base is horizontal with diameter and height of 2.1 and 1.7 µm, respectively (when viewed under the light microscope) or 1.6 and 1.3 µm, respectively (when viewed by transmission electron microscopy). The epicortical layer entirely surrounds the cortex. A laminar inner spore coat with alternating layers of dense and light materials occurs between the outer spore membrane and the epicortical layer. The outer spore coat consists of several layers of electron-dense materials and is surrounded laterally and ventrally by microprojections. Including the microprojections, the outer spore coat is thickest at the top of the central body and then tapers gradually to 0.2 µm at the spore equator and to 0.1 µm or less around a 0.3 µm wide basal pore. Parasporal structures consist of long primary fibers arising laterally from the outer spore coat and bending downwards to yield numerous secondary fibers arrayed ventrally. Depending upon the extent of invagination of the basal adhesion layer, additional partial hirsute layers may be present on the obverse face of the central body. Spores attach to second-stage juveniles in the soil, but rarely to males in the soil. The cuticle and body wall are penetrated by the germ tube that develops after the infective second-stage juvenile penetrates the host plant root. The life cycle is completed only in the pseudocoelom of females, but may also be completed in the cyst (female cadaver). The only confirmed host is Heterodera glycines. Attachment of endospores to second-stage juveniles of Globodera rostochiensis (potato cyst nematode) with endospores obtained from Heterodera elachista (upland rice nematode), Heterodera lespedezae (lespedeza cyst nematode), Heterodera schachtii (sugarbeet cyst nematode), and Heterodera trifolii (clover cyst nematode) indicates that these nematode species may be hosts. Completion of the life cycle of Pasteuria nishizawae in these nematode species has not been confirmed.
DNA G+C content (mol%): not reported.
Type strain: descriptions and illustrations serving as type.
GenBank accession number (16S rRNA gene): AF134868 and AF516396.
Pasteuria penetrans
(ex Thorne 1940) Sayre and Starr 1986, 355VP (Effective publication: Sayre and Starr 1985, 163.) (emend. Sayre, Starr, Golden, Wergin and Endo 1988, 28; Duboscqia penetrans Thorne 1940, 51.)
pen'e.trans. L. part. adj. penetrans penetrating, entering.
Gram-positive vegetative cells. Mycelium is septate; hyphal strands, 0.2–0.5 µm in diameter, branch dichotomously. The sporangia, formed by expansion of hyphal tips, are cup shaped, approximately 2.26–2.60 µm in height with a diameter of 3.0–4.0 µm. Each sporangium is divided into two unequal sections. The smaller proximal body is not as refractile as the larger, rounded, cup-shaped portion, which encloses an ellipsoidal endospore broadly elliptic in section having axes of 0.99–1.21 × 1.30–1.54 µm. Endospores seem to be of the kind typical of the genus Bacillus; they are resistant to both heat and desiccation. Nonmotile. Sporangia and vegetative cells are found as parasites in the pseudocoelomic cavities of Meloidogyne spp. The epithet is now restricted to members of Pasteuria penetrans with cup-shaped sporangia and ellipsoidal endospores broadly elliptic in section occurring primarily as parasites of Meloidogyne spp. Has not been cultivated axenically; the type-descriptive material consists of the text and photographs in Sayre and Starr (1985) and Starr and Sayre (1988a). Pasteuria penetrans differs from other described members of Pasteuria in host specificity, in size and shape of sporangia and endospores, and in other morphological and developmental characteristics.
The influence of temperature on the development of Pasteuria penetrans in Meloidogyne spp. has been observed in growth chambers (Hatz and Dickson, 1992; Serracin et al., 1997; Stirling, 1981). The parasite's greatest endospore attachment rate to second-stage juveniles was at 30°C and the bacterium developed more quickly within its nematode host at 30 and 35°C than at 25°C. Development time quickly decreases as temperature decreases, e.g., at 35, 28, and 21°C, mature endospores were detected at 28, 35, and >90 d, respectively (Hatz and Dickson, 1992; Serracin et al., 1997).
DNA G+C content (mol%): not reported.
Type strain: descriptions and illustrations serving as type.
GenBank accession number (16S rRNA gene): AF077672 and AF375881 (Anderson et al., 1999).
Pasteuria thornei
Starr and Sayre 1988, 328VP (Effective publication: Starr and Sayre 1988a, 28.)
thor'ne.i. M.L. gen. n. thornei of Thorne, named after Gerald Thorne, a nematologist from the United States, who described and named this parasite of Pratylenchus as a protozoan parasite.
Gram-positive vegetative cells. Mycelium is septate; hyphal strands, 0.2–0.5 µm in diameter, branch dichotomously. Sporangia, formed by expansion of hyphal tips, are rhomboidal in shape, approximately 2.22–2.70 µm in diameter and 1.96–2.34 µm in height. Each sporangium is divided into two almost equal units. The smaller unit, proximal to the mycelium, is not refractile and contains a granular matrix interspersed with many fibrillar strands. The refractile apical unit is cone shaped; it encloses an ellipsoidal endospore, sometimes almost spherical, having axes of 0.96–1.20 × 1.15–1.43 µm, with cortical walls about 0.13 µm in thickness. A sublateral epicortical wall gives the endospore a somewhat triangular appearance in cross-section. The tapering outer cortical wall at the base of the endospore forms an opening approximately 0.13 µm in diameter. Sporangia and endospores are found as parasites of lesion nematodes (Pratylenchus spp.). Has not been cultivated axenically; the type-descriptive material consists of the text and photographs in Starr and Sayre (1988a) and Sayre et al. 1988. Pasteuria thornei differs from Pasteuria penetrans and other members of Pasteuria in host specificity, size and shape of sporangia and endospores, and other morphological and developmental traits.
DNA G+C content (mol%): not reported.
Type strain: descriptions and illustrations serving as type.
GenBank accession number (16S rRNA gene): not reported.
“Candidatus Pasteuria usgae”
Giblin-Davis, Williams, Bekal, Dickson, Brito, Becker and Preston 2003b, 197
us'gae. N.L. gen. n. usgae of U.S.G.A., the acronym for the United States Golf Association, in gratitude for their financial support to study this potential biological control agent against Belonolaimus longicaudatus in turfgrass ecosystems.
Organism is nonmotile with Gram-positive vegetative cells. Mycelium is septate; hyphal strands branch dichotomously with expansion of hyphal tip forming sporangium. With scanning electron microscopy, peripheral fibers of the mature endospore protrude around the exposed spherical outer coat of the spore creating a crenate border as opposed to other species of Pasteuria described from nematodes that have no scalloped border. The sporangium and central body diameters were on average at least 0.5 and 0.7 µm wider than these respective measurements for the other described species of Pasteuria. In lateral view with transmission electron microscopy, the shape of the central body is a rounded-rectangle to a rounded-trapezoid in transverse section that contrasts with the circular shape for Pasteuria ramosa, the horizontally oriented elliptical shapes for Pasteuria penetrans and Pasteuria nishizawae, and the rounded-square shape for Pasteuria thornei. The outer spore coat is thickest laterally, thinner on top and thinnest across the bottom of the spore, being 7–8 times thicker laterally than along the bottom. These measurements contrast with all other described species having outer spore coats with relatively uniform thickness. No basal ring exists around the pore opening as in Pasteuria penetrans. The outer coat wall thickness at its thickest point is >15% (both walls >30%) of the diameter of the central body compared with 3 to <13% (both walls 6 to <25%) for the other described species of Pasteuria. The epicortical wall remnant of the mature endospore occurs between the cortex and the inner spore coat in a sublateral band, similar to Pasteuria thornei but different from the other three described species. The epicortical wall in the other described species is as follows: completely concentric in Pasteuria ramosa and Pasteuria nishizawae, and lateral in Pasteuria penetrans.
Obligate endoparasitic bacterium of the pseudocoelom of Belonolaimus longicaudatus that cannot be cultivated on cell-free media. Cultivated only by attachment of endospores to Bacillus longicaudatus and co-cultivation on excised axenic root or green-house plant cultures. Transmission occurs horizontally. Host infection is via cuticular penetration by attached endospores that occurs on all stages of Belonolaimus longicaudatus except eggs. Sporogenesis, which leads to the death of the host, occurs in the pseudocoelom of J3 through adult stage nematodes. Sporogenesis is typical of other nematode-specific Pasteuria. Host range appears to be limited to Belonolaimus longicaudatus, although attachment of endospores has been observed on Bacillus euthychilus, but not other soil-inhabiting nematodes.
DNA G+C content (mol%): not reported.
Type strain: descriptions and illustrations serving as type.
GenBank accession number (16S rRNA gene): AF254387.
Further information
Pasteuria ramosa
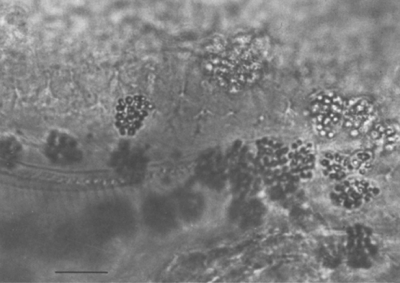
When using light microscopy, the earliest visible growth stages of Pasteuria ramosa in the water fleas Daphnia magna and Moina rectirostris Leydig 1860 are the cauliflower-like microcolonies usually found on the inner wall of the invertebrate's carapace Ebert, Rainey, Embley and Scholz (1996), Sayre, Adams and Wergin, 1979; Figure 14). The next detectable stage consists of quartets of sporangia carried in the hemolymph throughout the body of the cladoceran. Later, as the parasite develops, the hemolymph is noticeably clouded by the myriad immature sporangia of Pasteuria ramosa, mainly singles or doublets.
Electron micrographs of the “cauliflower” stage reveal circular patterns of septate hyphal strands of an actinomycete-like organism (Figure 13). The hyphae measure approximately 0.67 µm in width and their wall, which is fairly homogeneous in density, is 14.5–15 nm thick. The periplasmic region between the wall and the cell membrane is about 8.7–9.0 nm in width. The cell membrane measures 5.8 nm in thickness. Mesosomes (circular membrane complexes) are often found associated with the septa (Sayre et al., 1979; Figure 15).
The distal or terminal mycelial cells of the microcolonies enlarge to form teardrop-shaped sporangia that, when viewed by light microscopy, measure approximately 4.8–5.7 × 3.3–4.1 µm in diameter. Similar materials prepared for transmission electron microscopy and sectioned gave measurements of 3.40–4.35 µm in height and 2.12–2.77 µm in diameter. Early in this process, two septa form; these septa divide the sporangium into anterior, middle, and stem sections (Metchnikoff, 1888; Figure 16). The partitioning is reflected in the outer wall of the sporangia (Figure 17).
The anterior section, the upper two-thirds of the sporangium, gives rise to the forespore. Within the anterior section, granular material condenses to form an electron-dense endospore, slightly ellipsoidal to almost spherical in shape, having axes measuring 1.37–1.61 × 1.20–1.46 µm. It comprises a multilayered central cytoplasm containing numerous doubled fibrillar strands (Figure 18). Structural changes also occur within the median section, where electron-transparent areas appear to expand and attach laterally to the multilayered endospore wall to form fibrous appendages. The mode of penetration of spores into host cladocerans is not known.
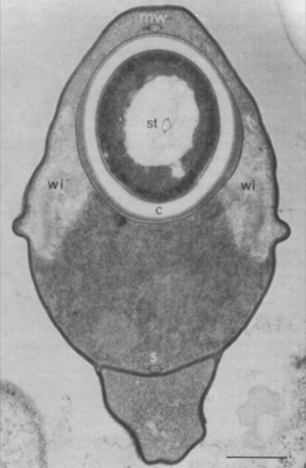
Parasitized water fleas (Moina rectirostris) taken from a pond in College Park, MD, USA, were found to yield about 2 × 105 Pasteuria ramosa sporangia per host individual. Generally, the parasite was found in mature females with no young in their egg pouches. Daphnia species, reported previously (Metchnikoff, 1888) as hosts of this organism, were not parasitized by this bacterial strain (Sayre, Adams and Wergin, 1979). Neither sporangia from crushed water fleas nor sediments from the rearing aquaria of parasitized Moina rectirostris (Sayre and Wergin, 1977) resulted in infection when added to healthy populations of Daphnia magna or Daphnia pulex. These two Daphnia species were listed by Metchnikoff (1888) as hosts for Pasteuria ramosa. However, because Moina rectirostris is in the same family (Daphniidae) as these two Daphnia spp., this result may suggest only a very marked host specificity in the particular strain of Pasteuria ramosa available to us, perhaps at the level of a forma specialis, the situation in which one form of a parasite reproduces only in one host species and not in others that are closely related taxonomically. Recent work on Pasteuria ramosa from populations of Daphnia magna demonstrated strong specificity (Carius, Little and Ebert, 2001). The type-descriptive material of this taxon (Metchnikoff, 1888) is attached to the form on Daphnia species. If later work should show differences warranting separation at the specific or subspecific level, the taxon on Moina would of course have to be given a different name from Pasteuria ramosa.
The influence of water temperature on the occurrence of Pasteuria ramosa in Moina rectirostris in nature was observed over a 3-year period. The parasite was not found until the surface water temperature in the pond reached 26°C or higher, usually about mid-July in the College Park (MD, USA) area. The apparent temperature requirement was confirmed in laboratory tests in which water in the aquaria was held at constant temperatures; the parasite was found in 6 and 3 d at 26° and 31°C, respectively, but not at all at 21°C. Pasteuria ramosa from Daphnia magna can be cultured from 15 to 25°C (Ebert et al., 1996).
Although endospores of Pasteuria ramosa appear to withstand desiccation, they have only limited resistance to heat. Air-dried sporangia, which were stored for 6 months, were capable of infecting healthy populations of cladocerans. However, when air-dried aquarium sediments were heated to 40, 60, or 80°C for 10 min and then added to cultures of healthy cladocerans, they failed to develop after treatment at 80°C (Sayre, Adams and Wergin, 1979). Endospores from Pasteuria ramosa from Daphnia populations can survive in pond sediments for decades (Decaestecker et al., 2004).
Pasteuria penetrans
Members of nematode-associated Pasteuria share several morphological, ultrastructural, and ecological features Sayre and Starr, 1985; Starr, Sayre and Schmidt, 1983; Starr and Sayre, 1988a; Table 1; Figure 1): all are Gram-positive; all form endospores; all form mycelia, septate and dichotomously branched, vegetative cells; and all parasitize invertebrates (Table 1). The members of the Pasteuria penetrans group differ in many respects from Pasteuria ramosa: colony shape, shape and size of sporangia (Figure 19) and endospores, and host relations (Table 2). Upon recognition (Sayre and Starr, 1985) of its relationship to the genus Pasteuria, the first of these nematode parasites to receive such taxonomic attention was renamed Pasteuria penetrans (ex Thorne) Sayre and Starr (1985). Subsequently, the name Pasteuria penetrans sensu stricto (i.e., in the strict sense) was limited in scope to the bacterium parasitic on Meloidogyne spp. and particularly Meloidogyne incognita (Starr and Sayre, 1988a). A second species, Pasteuria thornei, was erected for parasites of the lesion nematodes of the genus Pratylenchus and particularly Pasteuria brachyurus (Starr and Sayre, 1988a).
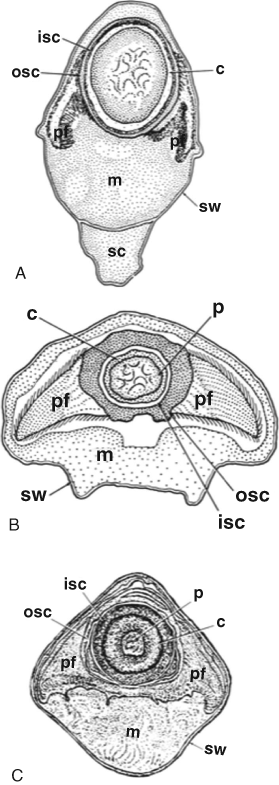
Much of the following information stems from studies of Pasteuria penetrans. Pasteuria thornei, which has received much less study than its relative, is similar in most respects examined (transmission electron micrographs of sporangia of Pasteuria thornei at various stages are shown in Figure 20, Figure 21, and Figure 22); where substantial differences have been observed, they are noted below. Cross-sections viewed by transmission electron microscopy (Imbriani and Mankau, 1977; Sayre and Starr, 1985; Sayre and Wergin, 1977; Starr and Sayre, 1988a) reveal that the endospore of Pasteuria penetrans sensu stricto consists of a central, highly electron-opaque core surrounded by an inner and an outer wall composed of several distinct layers (Figure 12). When observed with the transmission electron microscope, the peripheral matrix of the spore is fibrillar. Fine microfibrillar strands, about 1.5 nm thick, extend outward and downward from the sides of the endospore to the cuticle of the nematode, where they become more electron-dense.
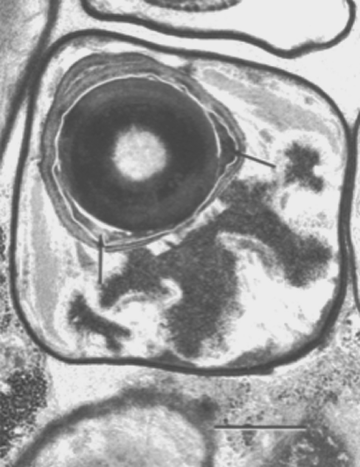
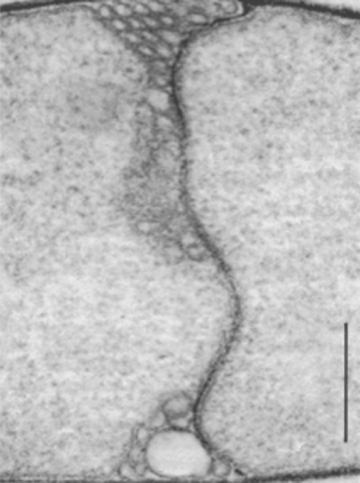
A mature endospore of Pasteuria penetrans sensu stricto attaches to the surface of a nematode so that a basal ring of wall material lies flatly against the cuticle. A median section through the endospore and perpendicular to the surface of the nematode bisects this basal ring. As a result, the ring appears as two protruding pegs, which are continuous with the outer layer of the spore wall and rest on the cuticular surface of the nematode (Figure 7).
The peripheral fibers of the endospore also are closely associated with the nematode's cuticle. These fibers, which encircle the endospore, lie along the surface of the nematode and follow the irregularities of the cuticular annuli. They seem not to penetrate the cuticle. The germ tube of the endospore of Pasteuria penetrans emerges through the central opening of the basal ring, penetrates the cuticle of the nematode, and enters the hypodermal tissue (Figure 7). Hyphae were initially encountered beneath the cuticle of the nematode near the site of germ-tube penetration. From this site, they apparently penetrate the hypodermal and muscle tissues and enter the pseudocoelom.
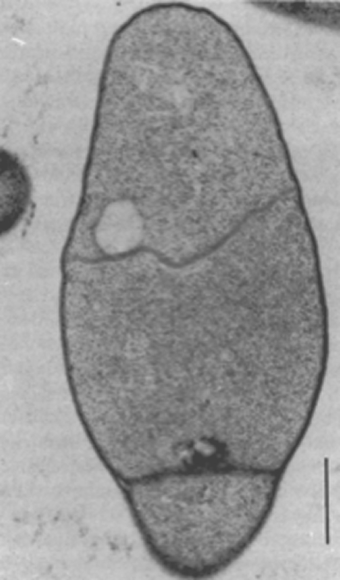
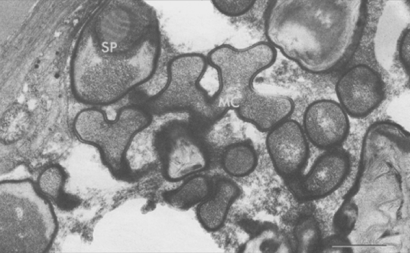
Mycelial colonies of Pasteuria penetrans up to 10 µm in diameter are formed in the pseudocoelom, where they are observed after the diseased juvenile penetrates plant roots (Figure 8). The hyphae comprising the colony are septate. A hyphal cell, which is 0.40–0.50 µm in cross-section, is bounded by a compound wall, 0.12 µm thick, composed of an outer and an inner membrane. The inner membrane of the wall forms the septation and delineates individual cells. In addition, this membrane is continuous with a membrane complex or mesosome that is frequently associated with the septum (Figure 9). Because of the sinuous and branching growth habit, cell length cannot be determined from thin section electron micrographs.
Sporulation of Pasteuria penetrans occurs in a developmental pattern that is similar to that observed in axenic cultures of Bacillus spp., e.g., Bacillus subtilis. During the controlled and semi-synchronous growth of Meloidogyne arenaria race 1 infected with a line (P20) of Pasteuria penetrans, endospore maturation was coincident with the formation of spore-associated adhesin, as determined by a specific monoclonal antibody (Brito et al., 2003).
Sporulation of Pasteuria penetrans is initiated in the female nematode. As the process begins, the terminal hyphal cells of the mycelium bifurcate and enlarge from typical hyphal cells to ovate cells measuring 2.0 × 4.0 µm. Structure and content of the cytoplast change from a granular matrix, which contains numerous ribosomes as found in the hyphal cells, to one lacking particulate organelles. During these changes, the developing sporangia separate from their parental hyphae, which cease to grow and eventually degenerate.
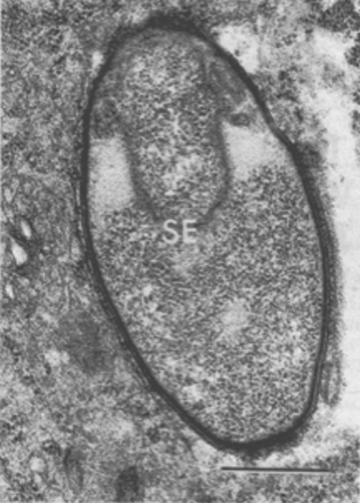
After these early structural alterations, a membrane forms within the sporangium and separates the upper third of the cell or forespore from its lower or parasporal portion (Figure 10). The granular matrix confined within the membrane then condenses into an electron-opaque body, 0.6 µm in diameter, which eventually becomes encircled by a multilayered wall. The resulting discrete structure is an endospore.
Coincident with the formation of an endospore in Pasteuria penetrans is the emergence of parasporal fibers. These fine fibers, which form around the base of the spore, differentiate from an electron-translucent, granular substance. They appear to connect with and radiate from the external layer of the wall of the endospore (Figure 11). During development of the parasporal fibers, the formation of another membrane, the exosporium, isolates the newly formed endospore within the sporangium. At this later stage of spore development, the granular content of the paraspore becomes less dense, degenerates, and disappears. As a result, the mature sporangium contains a fully developed endospore enclosed within the exosporium (Figure 12).
The cell wall of the sporangium of Pasteuria penetrans remains intact until the remnants of the infected nematode are disrupted, after which event the endospores are released. The exosporium apparently remains associated with the endospore until contact is made with a new nematode and the infection cycle restarts. The vermiform juvenile stages of the nematodes are encumbered by the parasite as they migrate through soils infested by the endospores of Pasteuria penetrans sensu stricto and the infectious cycle is repeated.
Much morphological, ultrastructural, developmental, and host diversity is evident in Pasteuria (Giblin-Davis, Wergin, Dickson, Hewlett, Bekal and Becker, 2001; Sayre and Starr, 1985; Sayre, Starr, Golden, Wergin and Endo, 1988). We believe this diversity speaks for the existence of many taxa within the group. Pasteuria thornei, Pasteuria nishizawae, and “Candidatus Pasteuria usgae” represent three cases where novel species have been proposed on substantial morphological, morphometric, host range, and molecular grounds (Table 2) (Giblin-Davis, Wergin, Dickson, Hewlett, Bekal and Becker, 2001; Giblin-Davis, Williams, Bekal, Dickson, Brito, Becker and Preston, 2003b; Sayre, Starr, Golden, Wergin and Endo, 1988; Starr and Sayre, 1988a). Other organisms–“Pasteuria hartismerei”. Pasteuria hartismerei Bishop, Gowen, Pembroke and Trotter (2007) was described from Meloidogyne ardenensis on the basis that the endospores lacked a basal ring on their ventral side and they had a unique clumping nature inside their host (Bishop, Gowen, Pembroke and Trotter, 2007). Also, a unique 16S rRNA sequence was obtained which differs from that of other described Pasteuria. This taxon will become a Candidatus species when validated according to the International Code of Nomenclature of Bacteria (1992) (Euzéby, 1998).
Acknowledgements
This chapter is dedicated to the memory of Dr Richard Sayre and his wife, Diane, who perished on 10 June 1998 in a boating accident in the Galapagos Islands.
We thank R. E. Davis for calling our attention to the genus Pasteuria and for his help in initiating its study. Thanks also go to W. P. Wergin, J. R. Adams, C. Pooley, R. Reise, and S. Ochs for sustained technical advice and assistance, particularly in the preparation of the figures involving transmission and scanning electron microscopy. Thanks are due R. L. Gherna for supplying strain ATCC 27377. We are grateful to J. M. Schmidt for her corroboration during the period when the taxonomic confusion between Pasteuria and Planctomyces was being corrected. Skillful bibliographic and redactional assistance was provided by P. B. Starr.



