Abstract
Ba.cil'lus. N.L. masc. n. Bacillus a rodlet.
Firmicutes / “Bacilli” / Bacillales / Bacillaceae / Bacillus
Cells rod-shaped, straight or slightly curved, occurring singly and in pairs, some in chains, and occasionally as long filaments. Endospores are formed, no more than one to a cell; these spores are very resistant to many adverse conditions. Gram-positive, or Gram-positive only in early stages of growth, or Gram-negative. A meso-DAP direct murein cross-linkage type is commonest, but L-Lys-D-Glu, Orn-D-Glu and L-Orn-D-Asp have occasionally been reported. Motile by means of peritrichous or degenerately peritrichous flagella, or nonmotile. Aerobes or facultative anaerobes, but a few species are described as strictly anaerobic. The terminal electron acceptor is oxygen, replaceable by alternatives in some species. Most species will grow on routine media such as nutrient agar and blood agar. Colony morphology and size very variable between and within species. A wide diversity of physiological abilities is exhibited, ranging from psychrophilic to thermophilic, and acidophilic to alkaliphilic; some strains are salt tolerant and some are halophilic. Catalase is produced by most species. Oxidase-positive or -negative. Chemo-organotrophic; two species are facultative chemolithotrophs: prototrophs to auxotrophs requiring several growth factors. Mostly isolated from soil, or from environments that may have been contaminated directly or indirectly by soil, but also found in water, food and clinical specimens. The resistance of the spores to heat, radiation, disinfectants, and desiccation results in species being troublesome contaminants in operating rooms, on surgical dressings, in pharmaceutical products and in foods. Most species have little or no pathogenic potential and are rarely associated with disease in humans or other animals; an exception is Bacillus anthracis, the agent of anthrax; several other species may cause food poisoning and opportunistic infections, and strains of Bacillus thuringiensis are pathogenic to invertebrates.
DNA G + C content (mol%): 32–66 (Tm ).
Type species: Bacillus subtilis Cohn 1872, 174AL.
Cells rod-shaped, straight or slightly curved, occurring singly and in pairs, some in chains, and occasionally as long filaments. Endospores are formed, no more than one to a cell; these spores are very resistant to many adverse conditions. Gram-positive, or Gram-positive only in early stages of growth, or Gram-negative. A meso-DAP direct murein cross-linkage type is commonest, but L-Lys-D-Glu, Orn-D-Glu and L-Orn-D-Asp have occasionally been reported. Motile by means of peritrichous or degenerately peritrichous flagella, or nonmotile. Aerobes or facultative anaerobes, but a few species are described as strictly anaerobic. The terminal electron acceptor is oxygen, replaceable by alternatives in some species. Most species will grow on routine media such as nutrient agar and blood agar. Colony morphology and size very variable between and within species. A wide diversity of physiological abilities is exhibited, ranging from psychrophilic to thermophilic, and acidophilic to alkaliphilic; some strains are salt tolerant and some are halophilic. Catalase is produced by most species. Oxidase-positive or -negative. Chemo-organotrophic; two species are facultative chemolithotrophs: prototrophs to auxotrophs requiring several growth factors. Mostly isolated from soil, or from environments that may have been contaminated directly or indirectly by soil, but also found in water, food and clinical specimens. The resistance of the spores to heat, radiation, disinfectants, and desiccation results in species being troublesome contaminants in operating rooms, on surgical dressings, in pharmaceutical products and in foods. Most species have little or no pathogenic potential and are rarely associated with disease in humans or other animals; an exception is Bacillus anthracis, the agent of anthrax; several other species may cause food poisoning and opportunistic infections, and strains of Bacillus thuringiensis are pathogenic to invertebrates.
DNA G + C content (mol%): 32–66 (Tm ).
Type species: Bacillus subtilis Cohn 1872, 174AL.
Number of validated species: 95
Further descriptive information
Phylogeny. A phylogenetic tree, based on 16S rDNA sequences, is shown in Figure 1. The tree includes 142 named Bacillus species as listed in this chapter (but excludes Bacillus laevolacticus and Bacillus tequilensis). Bacillus tusciae and Bacillus schlegelii lie at the edge of the tree, and their respective closest neighbors, on the basis of 16S rDNA gene sequence comparisons, are an unknown Alicyclobacillus species and Aneurinibacillus.
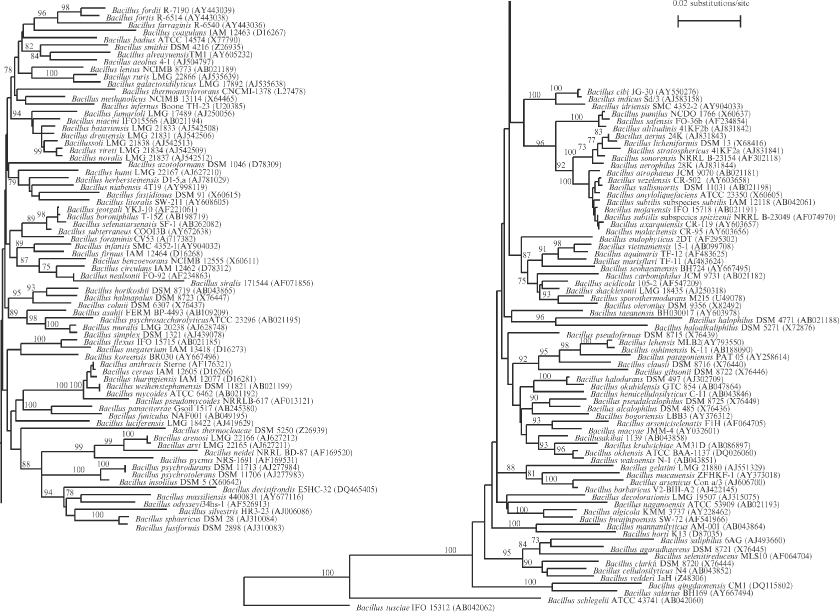
It is well known that 16S rDNA sequences do not always allow species to be discriminated, and that DNA–DNA hybridizations may be needed for this. However, sequences of other genes (the so-called core genes) may be more appropriate for discriminating these relatively recent branchings of the evolutionary tree that correspond to bacterial species. The ad hoc committee for the re-evaluation of the species definition in bacteriology (Stackebrandt et al., 2002) advised that genetic differences of the so-called core genes should be explored in order to come to a finer “bacterial species concept” in the future. The groupings (phylogenetic trees) that are obtained from comparisons either of sequences of individual core genes, or of concatenated gene sequences of several core genes, need to be validated against the phylogenetic species concept (Wayne et al., 1987). Recent data (Wang et al., 2007a) clearly show that in the Bacillus subtilis group, within which species delineation is very difficult, core genes such as gyrB allow differentiation on a genetic basis. A debate began recently concerning the impact of these new findings of genome analysis on bacterial taxonomy (Buckley and Roberts, 2007). Analysis of whole-genome sequences showed that about 80% of an individual genome may be shared by all pathogenic isolates of Streptococcus agalactiae (Tettelin et al., 2005), indicating that in closely related strains belonging to the same species, at least, a vast amount of the genetic information is shared. The interested reader is referred to the literature (e.g., Kunin et al., 2005, 2007; Dagan and Martin, 2006).
Cell morphology. Bacillus cells may occur singly and in pairs, in chains (which may be of great length), and as filaments. Trichome-forming “Arthromitus” strains from sow bug or wood louse (Porcellio scaber) guts, with endospore-forming filaments over 100 µm long and up to 180 cells per filament in animals cultivated in darkness, have been identified as Bacillus cereus (Jorgensen et al., 1997) and similar filamentous organisms have been isolated from moths, roaches and termites (Margulis et al., 1998; see Habitats, below). The rod-shaped cells of Bacillus species are usually round-ended, but the cells of members of the Bacillus cereus group have often been described as squared. Cell diameters range from 0.4 to 1.8 µm and lengths from 0.9 to 10.0 µm, but the cells of a particular strain are usually quite regular in size, and individual species normally have dimensions within fairly narrow limits. For example, cells of Bacillus pumilus are typically 0.6–0.7 by 2.0–3.0 µm, while those of Bacillus megaterium are usually 1.2–1.5 by 2.0–5.0 µm. Pleomorphism, showing as cells and filaments with swollen regions, and entirely swollen cells, may be observed in cultures grown in suboptimal conditions; this is seen, for example, in cultures of Bacillus fumarioli grown on relatively rich media (Logan et al., 2000), and such stressed cultures sporulate poorly. Bacillus cytoplasm may stain uniformly or be vacuolate; vacuolation (the presence of inclusions is visible by phase-contrast microscopy as areas less refractive than spores, and in Gram-stained preparations by unstained globules) is enhanced in some species (Bacillus cereus and Bacillus megaterium, for example) by cultivation on an agar medium containing a fermentable carbohydrate such as glucose, so that copious storage material is produced.
Sporangial morphologies are characteristic of species, and so often valuable in identification (see Life cycle, below), but an individual strain may show some variation and produce, for example, both oval and spherical spores. The commonest spore shape is ellipsoidal or oval, but shapes range from frankly cylindrical through ellipsoidal to spherical, and irregular forms such as kidney- or banana-shaped spores may be seen in some species. The position of the spore is also characteristic; the most frequently observed is a subterminal placement, and position can range from central through paracentral and subterminal to terminal. An individual strain may exhibit a range of spore positions. In small sporangia it is sometimes difficult to categorize spore positions with confidence. In just over half of the validly published Bacillus species the spores swell the sporangia slightly or appreciably, while in the remainder sporangial swelling has not been observed, but both swollen and unswollen sporangia may be observed within a single strain. The sporangia of Bacillus thuringiensis are characterized by their parasporal inclusions of crystalline protein known as δ-endotoxins, which are often toxic to insects and other invertebrates. Insecticidal strains of Bacillus sphaericus also produce crystalline parasporal inclusions; these are less prominent than those of Bacillus thuringiensis, but are generally visible with the aid of a good phase-contrast microscope (Priest, 2002).
L-form Bacillus cells have been reported from both humans, other animals and plants. Several authors have found L-forms in the blood of normal and arthritic persons in association with erythrocytes (Bisset and Bartlett, 1978; Pease, 1970, 1974), in other body fluids such as synovial fluids of arthritic patients (Pease, 1969), in association with neoplasms (Livingston and Alexander-Jackson, 1970), and in chickens and turkeys with infectious synovitis (Livingston and Alexander-Jackson, 1970; Roberts, 1964). As demonstrated by Bisset and Bartlett (1978), these organisms often revert to small, acid-fast diphtheroids, and on prolonged (up to 25 months) primary culture or subculture, and especially when grown in the presence of agents known to stimulate reversion of L-forms, some of them increase in size, lose their acid-fastness, and become Gram-positive endospore-forming rods. These organisms produce licheniform colonies on agar media, like the “Bacillus endoparasiticus” of Benedek (1955) from arthritic patients. The fully reverted isolates of Bisset and Bartlett (1978) were phenotypically similar to Bacillus licheniformis in other respects, and they named them “Bacillus licheniformis var. endoparasiticus.”
Symbiotic associations between L-form bacteria and plants have been observed (Paton and Innes, 1991), and this has encouraged the induction and characterization of a stable L-form of Bacillus subtilis (Allan, 1991; Allan et al., 1993). Artificially induced symbiosis of this stable L-form of Bacillus subtilis in strawberry plants has been demonstrated by ELISA (Ferguson et al., 2000), and a symbiosis of the same strain in Chinese cabbage seedlings has been shown to inhibit the germination of Botrytis cinerea conidia (Walker et al., 2002).
Cell-wall composition. Information on murein structure is known for only about half of the valid species of Bacillus (Table 1), but Bacher et al. (2001) have shown that matrix-assisted laser desorption/ionization time-of-flight mass spectrometry with nano-electrospray ionization quadrupole ion-trap mass spectrometry allows the ready determination of peptidoglycan structure in Bacillus subtilis vegetative cells and Bacillus megaterium spores. The vegetative cells of the majority of Bacillus species that have been studied have the most common type of cross-linkage in which a peptide bond is formed between the diamino acid in position 3 of one subunit and the D-Ala in position 4 of the neighboring peptide subunit, so that no interpeptide bridge is involved. The diamino acid in most Bacillus species is meso-diaminopimelic acid (meso-DAP), and this cross-linkage is now usually known as DAP-direct (A1γ in the classification of Schleifer and Kandler 1972). Where the structure is known, this cross-linkage is also typical of the examined representatives of several genera whose species were previously accommodated in Bacillus: Alkalibacillus, Brevibacillus, Geobacillus, Gracilibacillus, Paenibacillus, Salibacillus, and Virgibacillus (Table 1).
| Murein cross-linkagea | Reference | |
|---|---|---|
| Bacillus | ||
| B. subtilis | meso-DAP direct | Schleifer and Kandler (1972) |
| B. anthracis | (meso-DAP direct) | Schleifer and Kandler (1972) |
| B. aquimaris | meso-DAPb | Yoon et al. (2003a) |
| B. barbaricus | DAPb | Taubel et al. (2003) |
| B. badius | meso-DAP direct | Schleifer and Kandler (1972) |
| B. cereus | meso-DAP direct | Schleifer and Kandler (1972) |
| B. coagulans | meso-DAP direct | Schleifer and Kandler (1972) |
| B. fastidiosus | meso-DAP direct | Claus and Berkeley (1986) |
| B. firmus | (meso-DAP direct) | Schleifer and Kandler (1972) |
| B. funiculus | DAPb | Ajithkumar et al. (2002) |
| B. halophilus | meso-DAP direct | Ventosa et al. (1989) |
| B. hwajinpoensis | meso-DAPb | Yoon et al. (2004b) |
| B. horti | meso-DAPb | Yumoto et al. (1998) |
| B. indicus c | L-Orn-D-Asp | Suresh et al. (2004) |
| B. jeotgali | meso-DAP direct | Yoon et al. (2001a) |
| B. lentus | (meso-DAP direct) | Schleifer and Kandler (1972) |
| B. licheniformis | meso-DAP direct | Schleifer and Kandler (1972) |
| B. marisflavi | meso-DAPb | Yoon et al. (2003a) |
| B. megaterium | (meso-DAP direct) | Schleifer and Kandler (1972) |
| B. methanolicus | meso-DAP direct | Arfman et al. (1992) |
| B. mycoides | meso-DAP direct | Claus and Berkeley (1986) |
| B. oleronius | meso-DAP direct | Kuhnigk et al. (1995) |
| B. pumilus | meso-DAP direct | Schleifer and Kandler (1972) |
| B. schlegelii | meso-DAP direct | Krüger and Meyer (1984) |
| B. smithii | DAPb | Nakamura et al. (1988) |
| B. thermocloacae | meso-DAP direct | Demharter and Hensel (1989b) |
| B. thuringiensis | meso-DAP direct | Schleifer and Kandler (1972) |
| B. vietnamensis | meso-DAPb | Noguchi et al. (2004) |
| Alkaliphilic and alkalitolerant Bacillus species | ||
| B. cohnii | L-Orn-D-Asp | Spanka and Fritze (1993) |
| B. halmapalus | No DAP | Nielsen et al. (1994) |
| Alkaliphilic species in 6th 16S rRNA group of Nielsen et al. (1994) | ||
| B. horikoshii | No DAP | Nielsen et al. (1994) |
| Spherical-spored Bacillus species | ||
| B. fusiformis d | L-Lys-D-Asp | Ahmed et al. (2007c) |
| B. insolitus | Orn-D-Glu | Stackebrandt et al. (1987) |
| B. neidei | L-Lys-D-Glu | Nakamura et al. (2002) |
| B. psychrodurans | Orn-D-Glu | Abd El-Rahman et al. (2002) |
| B. psychrotolerans | Orn-D-Glu | Abd El-Rahman et al. (2002) |
| B. pycnus | L-Lys-D-Glu | Nakamura et al. (2002) |
| B. silvestris | L-Lys-D-Glu | Rheims et al. (1999) |
| B. sphaericus d | L-Lys-D-Asp | Schleifer and Kandler (1972) |
| Alkalibacillus | ||
| A. haloalkaliphilus | meso-DAP direct | Fritze (1996b) |
| Brevibacillus | ||
| Br. Brevis | meso-DAP direct | Schleifer and Kandler (1972) |
| Br. laterosporus | meso-DAP direct | Schleifer and Kandler (1972) |
| Geobacillus | ||
| G. stearothermophilus | (meso-DAP direct) | Schleifer and Kandler (1972) |
| G. thermoleovorans | DAPb | Zarilla and Perry (1987) |
| G. pallidus | meso-DAP direct | Scholz et al. (1987) |
| Gracilibacillus | ||
| Gr. dipsosauri | meso-DAP direct | Lawson et al. (1996) |
| Marinibacillus | ||
| M. marinus | L-Lys-direct | Yoon et al. (2001b) |
| Paenibacillus | ||
| P. polymyxa | (meso-DAP direct) | Schleifer and Kandler (1972) |
| P. alvei | meso-DAP direct | Schleifer and Kandler (1972) |
| P. amylolyticus e | (meso-DAP direct) | Schleifer and Kandler (1972) |
| P. lentimorbus | meso-DAP direct | Schleifer and Kandler (1972) |
| P. macerans | meso-DAP direct | Schleifer and Kandler (1972) |
| Sporolactobacillus | ||
| S. laevolacticus | meso-DAP direct | Andersch et al. (1994) |
| Sporosarcina | ||
| S. ureae | L-Lys-Gly-D-Glu | Stackebrandt et al. (1987) |
| S. globisporus | L-Lys-D-Glu | Stackebrandt et al. (1987) |
| S. psychrophilus | L-Lys-D-Glu | Stackebrandt et al. (1987) |
| S. pasteurii | L-Lys-D-Asp | Ranftl and Kandler (1973) |
| Ureibacillus | ||
| U. thermosphaericus | L-Lys-D-Asp | Andersson et al. (1995) |
| Virgibacillus | ||
| V. pantothenticus | meso-DAP direct | Schleifer and Kandler (1972) |
| V. halodenitrificans | meso-DAP direct | Denariaz et al. (1989) |
| V. marismortui | meso-DAPb | Arahal et al. (1999) |
| V. salexigens | meso-DAPb | Garabito et al. (1997) |
- a Data in parentheses were not obtained from the type strain of the species.
- b Configuration not determined.
- c This neutrophilic species is closely related to the alkaliphilic species Bacillus cohnii and Bacillus halmapalus.
- d Ahmed et al. (2007c) proposed the transfer of these species to the new genus Lysinibacillus.
- e The strain analyzed by Schleifer and Kandler (1972) as Bacillus circulans (ATCC 9966) has been reallocated to Paenibacillus amylolyticus.
A different type of cross-linkage is found in the spherical-spored members of the genus (informally known as the Bacillus sphaericus group) and in other genera containing spherical-spored organisms. Bacillus sphaericus and its close relatives typically have the cross-linkage type A4α (L-Lys-D-Asp or L-Lys-D-Glu), with L-Lys in position 3 of the peptide subunit with bridging to the D-Ala in position 4 of the neighboring peptide subunit by D-Asp or D-Glu. Bacillus sphaericus and Bacillus fusiformis have accordingly been transferred to the new genus Lysinibacillus (Ahmed et al., 2007c), but information on the peptidoglycan structure of other potential members of this genus is awaited). Three members of the Bacillus sphaericus group, however, Bacillus insolitus, Bacillus psychrodurans and Bacillus psychrotolerans, have L-Orn in position 3 of the peptide subunit with bridging by D-Glu to the D-Ala in position 4 of the neighboring peptide subunit (type A4β, or L-Orn-D-Glu) (Abd El-Rahman et al., 2002; Stackebrandt et al., 1987), and this structure is also found in the halophile Filobacillus milensis (Schlesner et al., 2001), which, although it bears spherical spores, is not related to any of the other spherical-spored groups but lies closest to Bacillus haloalkaliphilus (now reclassified into Alkalibacillus). Some spherical-spored species formerly classified in Bacillus have been transferred to other genera: Bacillus globisporus, Bacillus pasteurii and Bacillus psychrophilus have been transferred to Sporosarcina (Yoon et al., 2001b) and they share with the type species of that genus, Sporosarcina ureae, A4α cross-linking based on L-Lys in position 3 of the peptide subunit with interpeptide bridges of D-Asp, D-Glu, L-Ala-D-Asp, or Gly-D-Glu. Bacillus thermosphaericus, which has been transferred to the new genus Ureibacillus, also has a L-Lys-D-Asp structure (Fortina et al., 2001b). Two other, monospecific, genera of spherical-spored species have been proposed (Yoon et al., 2001c): Bacillus marinus has been transferred to Marinibacillus, and Jeotgalibacillus alimentarius accommodates a single isolate from a traditional food; both species have a direct L-Lys cross-linkage. meso-DAP has been found in the peptidoglycan of spores of Bacillus sphaericus and Bacillus pasteurii (Ranftl and Kandler, 1973).
Other than the absence of DAP from the walls of Bacillus horikoshii (Nielsen et al., 1994), no information is available about cross-linkage in members of the phylogenetically distinct group of alkaliphilic or alkalitolerant species which contains this species and Bacillus agaradhaerens, Bacillus alcalophilus, Bacillus clarkii, Bacillus clausii, Bacillus gibsonii, Bacillus pseudalcaliphilus, Bacillus pseudofirmus, and Bacillus vedderi. The two closely related species Bacillus cohnii (alkaliphilic) and Bacillus halmapalus (alkalitolerant) do not belong in this phylogenetic group, and lie nearer to Bacillus cereus: the cross-linkage of Bacillus cohnii is L-Orn-D-Asp (Spanka and Fritze, 1993), while Bacillus halmapalus has been shown to lack DAP (Nielsen et al., 1994).
Other cell-wall polymers have attracted less attention than murein, and the small amounts of reported data for a few strains do not allow the taxonomic values, if any, of these components to be recognized; the subject has been reviewed by Naumova and Shashkov (1997). Teichoic acids have been found in Bacillus coagulans, Bacillus licheniformis and Bacillus subtilis, and teichuronic acids have been found in Bacillus licheniformis, Bacillus megaterium and Bacillus subtilis. Aono and Ohtani (1990) and Aono et al. (1993) found the acidic polymers teichuronic acid and teichuronopeptide in the cell walls of alkaliphilic Bacillus strains and suggested that these components might be important in alkalophily as mutants deficient in them grew poorly at high pH. Fox et al. (1998) described the use of gas chromatography-mass spectrometry and liquid chromatography-mass spectrometry in the investigation of teichoic acids and teichuronic acids in Bacillus species.
Naumova and Shashkov (1997) also reviewed studies on sugar-phosphate polymers (found in Bacillus pumilus and Bacillus subtilis) and anionic polysaccharides (found in Bacillus cereus and Bacillus megaterium), but again the information is too sparse to reveal any taxonomic implications.
Capsules. Gram-positive bacteria may produce two kinds of capsule, composed of polyglutamic acid or polysaccharide, but their production by Bacillus species has not appeared to be of much taxonomic value. Although most Bacillus subtilis strains do not produce significant capsular material in the laboratory, the genome sequence of strain 168 indicates that this organism possesses the genes encoding both types of capsule (Foster and Popham, 2002). The production of poly-γ-glutamic acid by “Bacillus subtilis var. natto” during the stationary phase of growth is economically important in the manufacture of the fermented soybean product natto (Ueda, 1989).
The poly-γ-D-glutamic acid capsule of Bacillus anthracis is encoded by the three plasmid pXO2 genes capA, capB, and capC, and it is an important virulence factor for this organism as non-capsulate strains are avirulent (see Pathogenicity, below). The sequences of the enzymes encoded by the three genes suggest that they are membrane-associated (Mock and Fouet, 2001). The capsule is produced in vivo and when grown in appropriate conditions in the laboratory (see Procedures for testing special characters, below). Bacillus anthracis is a member of the Bacillus cereus group of closely related species, but none of the species besides Bacillus anthracis appears to produce this capsule. Although homologs of Bacillus anthracis virulence plasmid pXO1 genes were found in half of a set of 19 other members of the Bacillus cereus group in hybridization experiments, few pXO2 genes were found that hybridized with genomic DNA from the 19 Bacillus cereus group strains (Read et al., 2003). The capsule of Bacillus anthracis was reviewed by Mock and Fouet (2001). Other Bacillus species, outside the Bacillus cereus group, are known to produce poly-γ-glutamic acid. Synthesis by Bacillus licheniformis is carried out by a membrane-associated complex that catalyzes glutamic acid racemization, polymerization, and membrane translocation (Gardner and Troy, 1979); as with “Bacillus subtilis var. natto”, production of the capsular material is induced during the stationary phase (Foster and Popham, 2002). While D-glutamic acid is the predominant stereoisomer incorporated into the polymer, the ratio of D- and L-glutamic acids may vary according to the rate at which D-glutamic acid is being formed in the Bacillus subtilis cell (Aschiuchi et al., 1999), but in Bacillus licheniformis two glutamyl polypeptides are formed, one of each isomer, and the ratio is influenced by the concentrations of certain metal ions in the growth medium (Thorne, 1993). Bacillus megaterium is also known to produce poly-γ-glutamic acid, and can form a capsule comprising both polysaccharide and polypeptide, with the former at the cell poles and equators and the latter located laterally (Guex-Holzer and Tomcsik, 1956). Applications of bacterial poly-γ-glutamic acid are reviewed by Shih and Van (2001).
Carbohydrate polymers are formed by several Bacillus species, dextrans and levans being produced extracellularly by Bacillus licheniformis and Bacillus subtilis from sucrose (Claus and Berkeley, 1986), but true polysaccharide capsules have not been reported for Bacillus subtilis. The Bacillus subtilis genome contains two operons and some additional genes that show great similarity to capsule synthesis loci in Staphylococcus aureus and Streptococcus pneumoniae, but it is not known if they are truly genes for capsule synthesis (Foster and Popham, 2002). The extracellular polysaccharides of Bacillus licheniformis and Bacillus subtilis are of economic importance in the spoilage of bread and alcoholic beverages by “ropiness.” Analysis of the polysaccharide of a Bacillus licheniformis from ropy cider found that it was aheteropolymer containing over 80%mannose (Larpin et al., 2002). Aubert (1951) assumed that a heteropolysaccharide of D-glucose, D-galactose and D-ribose extractable from Bacillus megaterium KM with hot water was probably capsular material, but Cassity and Kolodziej (1984) concluded that a heteropolysaccharide of D-glucose, D-xylose, D-galactose, and L-arabinose produced by another strain of this species was intracellular and that it was used as a source of carbon and energy during sporulation. Several polysaccharides from Bacillus strains have been found to cross-react with antisera to capsules from other genera: Bacillus mycoides with Streptococcus pneumoniae type III, and Bacillus pumilus with Haemophilus influenzae type b and with Neisseria meningitidis group A (Myerowitz et al., 1973).
Flagella. Many species of Bacillus are motile by means of peritrichous flagella, which are not usually numerous and may be very few in number. Flagellation has not been considered a particularly useful taxonomic character for the genus, but the presence or absence of motility continues to be indicated in most species descriptions, and it is of some value in identification. For example, Bacillus anthracis and Bacillus mycoides are nonmotile, while most Bacillus cereus strains are motile. The flagella of Bacillus thuringiensis may bind to insect cells and be important in virulence (Zhang et al., 1995). The value of H-antigens in the typing of Bacillus cereus, Bacillus thuringiensis and Bacillus sphaericus, and other aspects of Bacillus flagellar antigens, are discussed in Antigens and vaccines, below. The flagella of Bacillus subtilis are well characterized, and reviews may be found in Sonenshein et al. (1993) and in Aizawa et al. (2002).
S-layers. Surface or S-layers are two-dimensional arrays composed of protein or glycoprotein molecules. The S-layer proteins assemble themselves into very stable structures which have oblique, square or hexagonal lattice symmetries, are 5–25 nm thick, and contain pores of 2–8 nm in diameter (Sleytr et al., 2001). The phylogenetic origins of the S-layers of some Bacillus cereus group strains was investigated by Mignot et al. (2001), and the possession of an S-layer was found to be largely restricted to a genetically clustered subgroup of clinical and insect isolates, suggesting a role in pathogenicity and the influence of ecological pressures to maintain the layer. It has been shown that the S-layer of Bacillus cereus is involved in the adhesion of the organism to host cell molecules, and polymorphonuclear leukocytes, as well as enhancing the organism's radiation resistance (Kotiranta et al., 2000). However, S-layers are apparently of no value as taxonomic markers, as in some species, including Bacillus cereus, their presence is strain-dependent (Kotiranta et al., 1998; Sleytr et al., 2001). The S-layer of Bacillus anthracis is reviewed by Mock and Fouet (2001).
Colony characteristics. Bacillus species show a very wide range of colonial morphologies, both within and between species, and of course medium composition and other incubation conditions have a strong influence. Despite this diversity, however, Bacillus colonies on routine media are not generally difficult to recognize. Some species have characteristic yet seemingly infinitely variable colonial morphologies: colonies of Bacillus cereus and relatives are very variable, but readily recognized (Figure 2a, b, h): they are characteristically large (2–7 mm in diameter) and vary in shape from circular to irregular, with entire to undulate, crenate or fimbriate edges; they have matt or granular textures, but smooth and moist colonies are not uncommon. Although colonies of Bacillus anthracis and Bacillus cereus can be similar in appearance, those of the former are generally smaller, non-hemolytic, may show more spiking or tailing along the lines of inoculation streaks, and are very tenacious as compared with the usually more butyrous consistency of Bacillus cereus and Bacillus thuringiensis colonies, so that they may be pulled into standing peaks with a loop. The colonies of Bacillus mycoides differ from those of other members of the Bacillus cereus group; they are characteristically rhizoid or hairy-looking and adherent, and they readily cover the whole agar surface (Figure 2d).
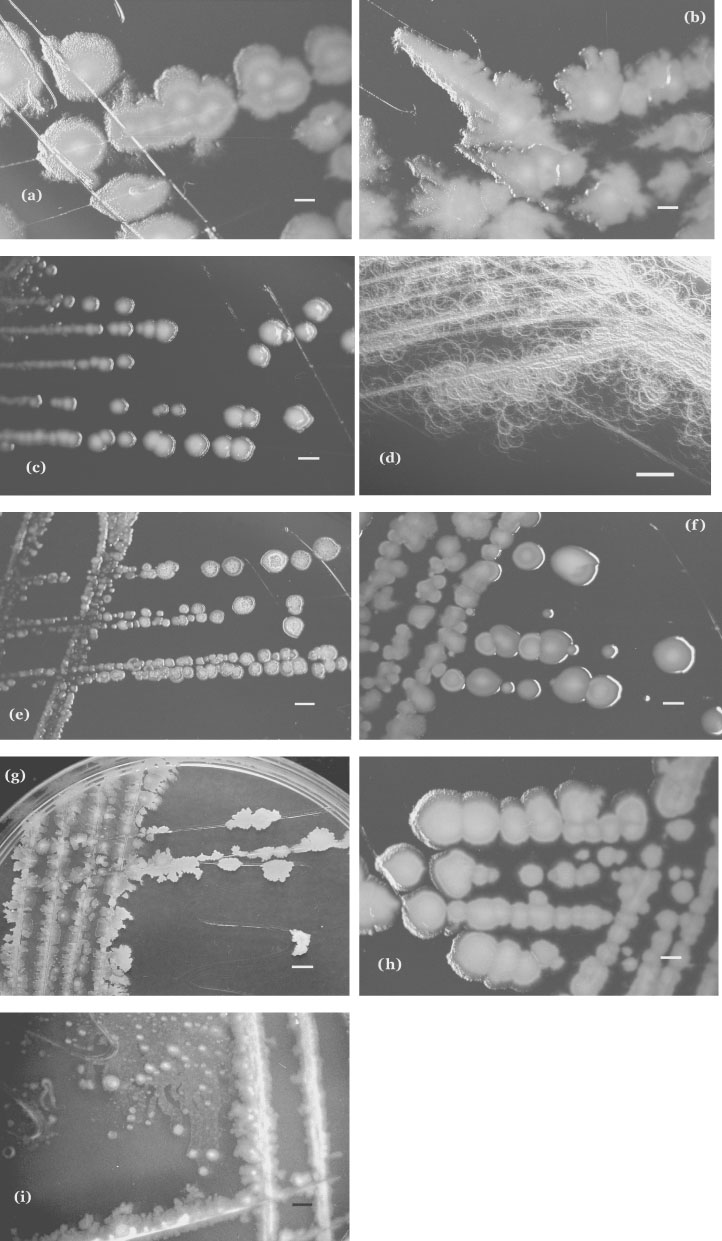
The colonies of other species vary from moist and glossy (Figure 2c, e, f) through granular to wrinkled (Figure 2h); shapes vary from round to irregular, sometimes spreading, with entire through undulate or crenate to fimbriate edges. After 24–48 h incubation, colonial sizes of mesophilic strains typically range from 1 to 5 mm; color commonly ranges from buff or creamy-gray to off-white, but occasional strains may produce black, brown, orange, pink or yellow pigments; such pigmentation tends to be characteristic of species or subspecies. Elevations range from effuse through raised to convex. Consistency is usually butyrous, but mucoid and dry, adherent colonies are not uncommon. Hemolysis may be absent, slight or marked, partial or complete. Bacillus subtilis (Figure 2g) and Bacillus licheniformis produce similar colonies which are exceptionally variable in appearance and often appear to be mixed cultures – the colonies are irregular in shape and of moderate (2–4 mm) diameter, and range in consistency from moist and butyrous or mucoid (with margins varying from undulate to fimbriate), through membranous with an underlying mucoid matrix (with or without mucoid beading at the surface), to a rough and crusty appearance as they dry. The “licheniform” colonies of Bacillus licheniformis tend to be quite adherent. Rotating and migrating microcolonies (Figure 2i), which may show spreading growth (the V morphotype, see below), were observed macroscopically in about 13% of strains received as Bacillus circulans (Logan et al., 1985) but this very heterogeneous species has undergone radical taxonomic revision, and organisms producing motile microcolonies are now allocated to Paenibacillus cookii, Paenibacillus glucanolyticus, Paenibacillus lautus, and some unidentified Paenibacillus species (Alexander and Priest, 1989; Logan et al., 2004a). Most of the colonial morphologies illustrated here are shown in color by Logan and Turnbull (2003).
Matsushita et al. (1998, 1999) have constructed a mathematical model to explain some of the morphological variation seen in colonies of Bacillus subtilis: it is a diffusion-reaction-type model, where colony patterns are influenced by substrate softness and nutrient concentration, and colonies comprise active and inactive cells. The active cells grow, divide and move, while inactive cells do not. Concentric ring-like colonies reflect alternate periods of advance and rest of the growing interface, which consists of the active cells. Active cells also form the tips of the growing branches of “dense branching morphology” colonies. Ben-Jacob et al. (1998) combined a detailed study of bacterial colony development with pattern-formation concepts derived from non-living systems to construct a model which suggested that cooperative cellular behavior, involving long-range chemorepulsion and short-range chemoattraction, occurs. They defined three colonial “morphotypes”: tip-splitting or branching (T), chiral (C), where the thin branches all have a same-handed twist, and vortex (V), where the tip of each branch bears a leading droplet containing many bacteria. The T morphotype is seen in Paenibacillus dendritiformis, the C morphotype particularly where a rapid growth transition from the T type occurs on softer agar (the reverse transition, C to T, occurring on harder agar), and the V morphotype is characteristic of some Paenibacillus strains formerly classfied as Bacillus circulans. Stecchini et al. (2001) found that the radial growth rate of Bacillus cereus colonies diminished as the agar content increased, and that colony density decreased during the incubation period, being lowest at the lower agar concentrations because the liquid film was thicker. Delprato et al. (2001) found that the bacteria in the central regions of Bacillus subtilis colonies migrated to the colony edge and formed a ring pattern following exposure of the whole colony to UV radiation, and that cells grew both inwards and outwards when the irradiation ceased; they proposed a diffusion-reaction model in which the radiation initiates a waste-limited chemotaxis.
Sporulation is strongly associated with the spatial development of the bacterial community; in Bacillus subtilis biofilms, sporulating aerial structures (primitive fruiting bodies) may be formed by motile cells that align themselves to form chains of attached cells (Branda et al., 2001). Bacillus subtilis uses an elaborate peptide quorum-sensing system to choose between the competent (i.e., for exogenous DNA uptake) state and the sporulation process, and sporulation occurs only poorly at low cell densities, even if the cells are starved (Miller and Bassler, 2001). To explain differences in the architectures of colonies grown from vegetative cells and those grown from spores, characterized by different glycocalyx wetting angles, Puzyr et al. (2002) suggested that germinating spores and vegetative cells of Bacillus subtilis adopt different strategies of substrate colonization.
Life cycle. Cohn 1876, Koch (1876) and Tyndall (1877) independently discovered that certain bacteria could spend part of their lives as the dormant cellular structures now known as endospores. The first two of these authors recognized the significance of these structures in the epidemiology of anthrax, and Koch's study of the life history of Bacillus anthracis proved the germ theory of disease and so marked the genesis of clinical bacteriology. Although Pasteur (1870) had previously figured endospores in a work on silkworm diseases, he did not clearly attribute the longevity of the pathogens to their spores. The ability to form endospores in aerobic conditions has been a defining character of the genus Bacillus since the 1920s, and has been applied in all editions of the Manual.
Spore formation is most important in identification to genus level. Before attempting to identify to species level it is important to establish that the isolate really is an aerobic endospore-former, and that other inclusions are not being mistaken for spores.
Endospores are so named because they are formed intracellularly, and they differ from their parent vegetative cells in many ways: they are optically refractile, and are highly resistant to chemical and physical stresses that are lethal to vegetative cells. These properties are conferred by the spores' special chemical composition and ultrastructure, and much effort has been expended over many years in order to elucidate the processes of spore formation and germination, and the molecular mechanisms that make endospores the hardiest form of life known on Earth. Although endospores are to be found in other genera, Clostridium for example, it is the spores of Bacillus subtilis that have been the most intensively studied, especially those of strain 168.
Under suitable nutritional, temperature, pH, gaseous and other conditions, Bacillus cells will grow and divide by binary fission, with the dividing septum traversing the middle of the cell. Depending on species, strain, and cultural conditions, daughter cells may separate so that the culture appears to be composed of single cells and pairs of dividing cells when viewed by phase-contrast microscopy. In other cases, daughter cells may remain attached to each other, so that chains of cells are seen. Filaments may also be observed, and these can often be symptomatic of a stressed culture. An organism that exists predominantly as regular rods in optimal growth conditions may produce swollen, pleomorphic, unhealthy-looking cells when stressed.
Endospores are formed at the end of the exponential growth phase, and at least two kinds of environmental factors have been implicated in the induction of sporulation. One trigger for sporulation is nutritional deprivation, for example when an actively growing culture is transferred from a rich to a poor growth medium. Many other factors are known to affect endospore formation, including growth temperature, environmental pH, aeration, presence of certain minerals, and carbon, nitrogen and phosphorus sources and their concentrations. Another influence is population density: as the mass of a culture increases, there is an extracellular accumulation of a secreted peptide (competence and sporulation factor, or CSF), which acts as an autoinducer for quorum sensing (Miller and Bassler, 2001). When this peptide reaches a concentration that relates to a particular cell density, high intracellular levels of CSF lead to an increase of the phosphorylated form of a response regulator (SpoOA), which leads to derepression of various stationary-phase genes, some of which are needed for sporulation (Sonenshein, 2000). Studies of Bacillus subtilis biofilms have shown that the cells do not behave as strictly unicellular organisms, but that sporulation is also tightly linked with the spatial development of the microbial community. Motile cells may form aligned chains of attached cells that produce aerial structures; these can be seen as primitive fruiting bodies, as they are the preferred sites of sporulation (Branda et al., 2001).
Sporulation is closely tied to the cell cycle, and a round of DNA replication must be initiated as a prerequisite for the sporulation pathway being activated (Michael, 2001). The cell division of vegetative growth is symmetrical, and yields two similar cells. During sporulation, however, cell division is asymmetrical and two quite different kinds of cells, the small forespore and the larger mother cell, are produced, each with its own copy of the chromosome. The two different kinds of division are believed to use essentially the same protein machinery (Errington, 2001). At the commencement of sporulation, the chromosomes form an elongated structure called the axial filament, with migration of a specific region of the chromosomes towards the poles, and polar septation bisects one end of this filament so that only part of the nucleoid lies within the forespore; the remainder of the chromosome is then transferred into the forespore from the mother cell (Errington, 2001; Levin and Grossman, 1998). The process of sporulation may be divided into seven morphologically recognizable stages following vegetative growth: I, preseptation, with the DNA forming the axial filament; II, asymmetric septation, the membrane of the developing spore surrounds the spore protoplast and becomes detached from the membrane of the mother cell; III, the forespore so formed becomes surrounded by the cytoplasm of the mother cell and so is contained within two membranes of opposing polarity; IV, spore cortex formation commences, with a primordial cell wall being laid down between the membranes, next to the forespore inner membrane; the cortex (a thicker layer of electron-transparent peptidoglycan, unique to bacterial endospores) is laid down on the outside of this primordial cell wall; an exosporium, a thin and delicate proteinaceous outermost covering, may be formed at this stage; V, proteinaceous spore coats are synthesized and begin to be deposited outside the cortex; VI, the spore matures, and acquires its refractility and heat resistance; VII, the sporangium lyses and releases the mature spore (Foster, 1994). In a laboratory culture of Bacillus subtilis, the whole process of sporulation may take about 8 h. The genetics of sporulation are reviewed by Piggot and Losick (2002).
Endospores are metabolically extremely dormant and do not contain ATP; this dormancy is the key to their resistance to many agents, including heat, radiation and chemicals, and their survival over long periods. Spore structure, resistance and germination are reviewed by Atrih and Foster (2001). The spore cortex is essential for spore dehydration (10–30% of the water content of the vegetative cell) and so for the maintenance of dormancy, and for the spore's heat resistance; the temperature of sporulation influences the mature spore's heat resistance (Nicholson et al., 2000). The mechanisms of spore resistance to chemical agents and radiation are not well understood, but saturation of the chromosome by protective small acid-soluble proteins (SASPs) is believed to play a part, while the spore coats are believed to prevent access of peptidoglycan-lytic enzymes to the spore cortex, and are also known to protect from hydrogen peroxide and UV radiation (Riesenman and Nicholson, 2000); coat assembly and composition are reviewed by Takamatsu and Watabe (2002). SASPs appear to be more important than the low core water content in protecting DNA from heat and oxidative damage (Setlow, 1995). Spore core and coat proteins are reviewed by Driks (2002). Pyridine-2,6-dicarboxylic acid (dipicolinic acid; DPA) is a unique and quantitatively important spore component (comprising 5–14% of the spore dry weight), and Ca2+ and other divalent cations are chelated by it, but precisely how it contributes to spore resistance is unclear (Slieman and Nicholson, 2001). DPA may be used as a marker for detecting the presence of spores by Curie-point pyrolysis mass spectrometry and by Fourier-transform infrared spectroscopy (Goodacre et al., 2000). Mechanisms of spore resistance have been reviewed by Nicholson et al. (2000) in the contexts of survival both in extreme terrestrial conditions and during travel through extraterrestrial environments. The function of the exosporium is not known; the exosporium of Bacillus cereus has been characterized by Charlton et al. (1999).
Conversion from the dormant spore to vegetative cell involves the three steps: activation, germination, and outgrowth. Dormancy may be broken by heat treatment at a time and sublethal temperature appropriate to the organism concerned, and by ageing at low temperatures, but endospores of many species do not require such activation. The heat treatment procedure used to assist the isolation of Bacillus species, by destruction of all kinds of vegetative cells, is often effective in activation (see Enrichment and isolation procedures, below). Following dormancy, with or without activation, the spore may encounter conditions that trigger germination; the cortex is rapidly hydrolyzed, SASPs are quickly degraded, and refractility is lost in a matter of minutes. The germinated spore protoplast then outgrows: it visibly swells owing to water uptake, biosynthesis recommences (taking advantage of the nutrients released by germination as well as those available in its new environment), and a new vegetative cell emerges from the broken spore coat; another period of vegetative reproduction ensues. It seems remarkable that a metabolically dormant spore can monitor its external environment in order to trigger germination within seconds in suitable conditions, and that this triggering mechanism can escape the constraints of dormancy while being resistant to damaging agents. Germination can be induced by exposure to nutrients such as amino acids and sugars, by mixtures of these, by non-nutrients such as dodecylamine, and by enzymes and high hydrostatic pressure; for many species, L-alanine is an important germinant, while D-alanine can bind at the same site as L-alanine and acts as a competitive inhibitor (Foster and Johnstone, 1990; Johnstone, 1994). The mechanism of germination has been most studied for Bacillus megaterium. Although spore structure is very similar between species, and cortex peptidoglycan structure is highly conserved (Atrih and Foster, 2001), there are many different germinant receptor specificities; nonetheless, the underlying mechanism of germination may be universal (Foster and Johnstone, 1990). Recent developments in the understanding of spore germination process and the spore components required for it are reviewed by Moir et al. (2002) and Paidhungat and Setlow (2002).
Various aspects of spores have been considered as taxonomic characters. Spore antigens are considered under Antigens and vaccines, below. The spore coat may open by splitting polarly, equatorially, transversely, or by expansion with the halves of the coat at each end of the outgrowing cell, or by the coat lysing. Small-celled organisms such as Bacillus subtilis tend to leave well-defined spore coat residues, while large-celled species such as Bacillus cereus and Bacillus megaterium may not. Lamana (1940a, 1954) studied modes of spore germination for nine species and found it to be of potential value for differentiation between the small-celled species and between this group and the large-celled species, but, with two exceptions (Burdon, 1956; Gould, 1962), little further attention has been paid to this character.
Bradley and Franklin (1958) showed that most of the 20 species they studied could be distinguished by electron microscopy of carbon replicas of spore surface patterns. Bulla et al. (1969) found that scanning electron microscopy gave inadequate resolution for such studies, but Murphy and Campbell (1969) achieved good resolution of Bacillus polymyxa spores by this method, and Gray and Hull (1971) considered this approach to be promising in the study of the Bacillus circulans complex. Later authors have sometimes described spore surface structure in proposals for new species, but too few such descriptions are available to judge the taxonomic value of spore surface characteristics across the genus.
Electron microscopy has revealed sword-shaped appendages radiating from one end of the exosporium of the spores of two phylloplane strains of Bacillus cereus (Mizuki et al., 1998). The proteinaceous spore appendages of 10 Bacillus cereus strains isolated from food-borne illness outbreaks and food industry sources showed some antigenic relationship, but when subjected to SDS-PAGE analysis none showed identical patterns (Stalheim and Granum, 2001). Smirnova et al. (1991) found that hemagglutination patterns of fimbriated Bacillus thuringiensis spores correlated with the subspecies of the strains rather than with their flagellar serovars. Song et al. (2000) reported that under strictly standardized growth conditions, spore fatty acid profiles, like those of vegetative cells, are stable and potentially of taxonomic value.
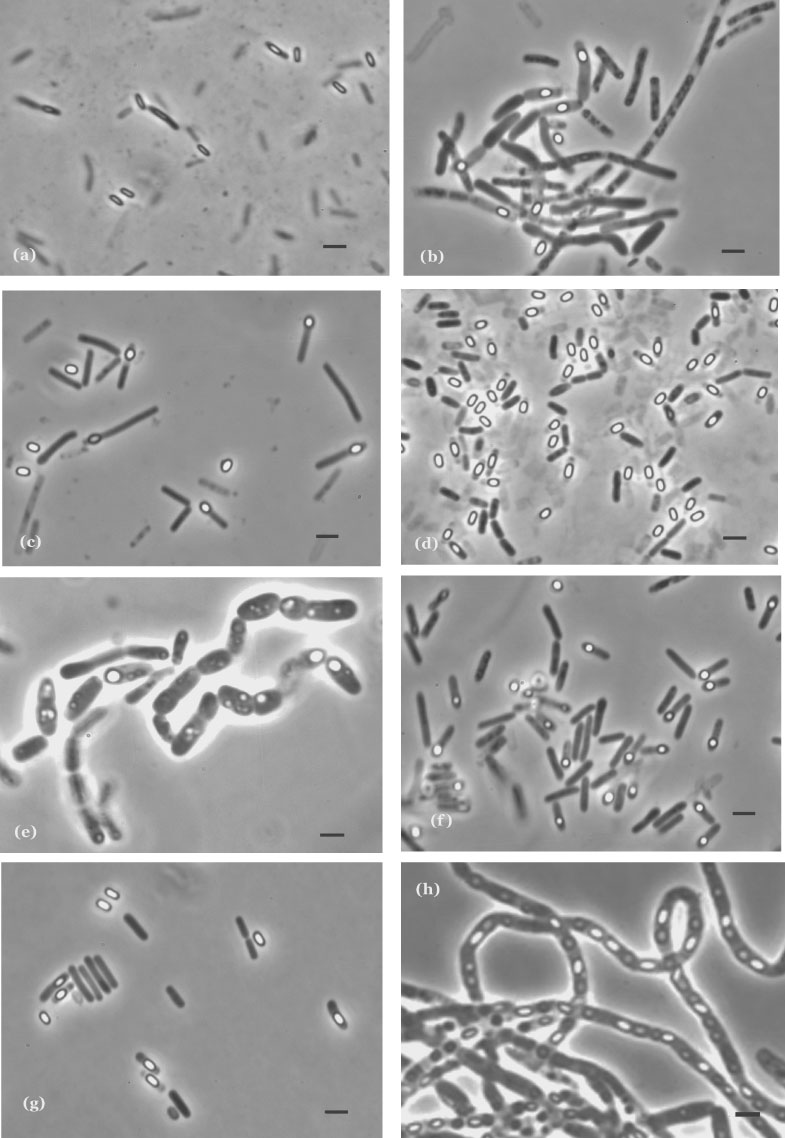
The microscopic morphologies of Bacillus species, especially of their sporangia, are well established as valuable characters. Smith et al. (1946, 1952) and Gordon et al. (1973) used cell size, appearance of cytoplasm and sporangial morphology as the basis of their division of the genus into three groups of species, and this arrangement still correlates quite well with the present classification of the aerobic endospore-formers. Sporangial morphology, and cell size, shape and cytoplasmic appearance, remain useful characters in polyphasic taxonomic studies, and sporangial characters are particularly valuable in identification. Spore shapes vary from cylindrical (Figure 3a) through ellipsoidal (Figure 3b–e, g) to spherical (Figure 3f); bean- or kidney-shaped, curved-cylindrical, and pear-shaped spores are also seen occasionally. Spores may be terminally (Figure 3f), subterminally (Figure 3a–g), paracentrally (Figure 3b, d, e, g) or centrally (Figure 3f) positioned within sporangia and may distend them (Figure 3c–f). Despite within-species and within-strain variation, sporangial morphologies tend to be characteristic of species, and for some species may allow tentative identification by the experienced worker. Routine recognition of Bacillus thuringiensis is largely dependent on observation of its cuboid or diamond-shaped parasporal crystals in sporangia (Figure 3h).
Nutrition and growth conditions. Despite the very wide diversity of the genus, most Bacillus species will grow well on routine media such as nutrient agar or trypticase soy agar, and most will grow on blood agar. However, some isolates, particularly those from nutritionally poor environments, may grow poorly if at all on these standard media and so require weaker formulations; for example, strains of Bacillus thuringiensis (Forsyth and Logan, 2000) isolated from Antarctic soils required Bacillus fumarioli agar or a half-strength formulation of this medium for reliable cultivation, and they would not grow consistently on trypticase soy agar.
In the First Edition of this Manual, Claus and Berkeley (1986) listed five of their 34 valid species that would not grow on nutrient agar. Three of these (Bacillus larvae, Bacillus lentimorbus and Bacillus popilliae) have been transferred to Paenibacillus, and one (Bacillus pasteurii) has been transferred to Sporosarcina, leaving Bacillus fastidiosus as the only exception. However, of the 68 Bacillus species newly described or validated in the two decades following the preparation of the First Edition of this Manual, some 24 grow poorly or not at all on nutrient agar because of its neutral pH, and/or insufficient salinity, or because it is nutritionally too weak or too rich. Bacillus benzoevorans does not grow on peptone or tryptone media, but may be cultivated on yeast extract media containing sodium acetate or benzoate (Pichinoty et al., 1984). Bacillus fastidiosus strains usually need allantoic acid, allantoin or uric acid as sole carbon, nitrogen and energy sources, but some strains will grow on certain peptones, especially at high concentrations. Bacillus laevolacticus requires glucose or other carbohydrate for growth (Andersch et al., 1994). Bacillus psychrodurans and Bacillus psychrotolerans do not grow, or grow only weakly, on nutrient agar or in nutrient broth, and require a rich medium such as casein-peptone soymeal-peptone agar (Abd El-Rahman et al., 2002). Bacillus sporothermodurans also grows weakly on nutrient agar but grows on Brain heart Infusion Agar or in nutrient agar supplemented with vitamin B12 (Pettersson et al., 1996). Both Bacillus fumarioli and Bacillus naganoensis are moderately acidophilic, and will not grow at pH 7.0 (Logan et al., 2000; Tomimura et al., 1990); also, Bacillus fumarioli sporulates poorly on trypticase soy agar even when adjusted to its optimum pH of 5.5, and requires a weaker medium such as Bacillus fumarioli agar or half-strength Bacillus fumarioli agar. Bacillus aeolius, Bacillus halodenitrificans, Bacillus halophilus, Bacillus horti (the type strain) and Bacillus jeotgali do not grow in routine media without added NaCl (Denariaz et al., 1989; Gugliandolo et al., 2003a; Ventosa et al., 1989; Yoon et al., 2001a; Yumoto et al., 1998). The majority of Bacillus species that do not grow on routine media, however, are alkaliphiles: Bacillus alcalophilus, Bacillus agaradhaerens, Bacillus clarkii, Bacillus cohnii, Bacillus krulwichiae, Bacillus pseudoalcalophilus, Bacillus pseudofirmus; Bacillus haloalkaliphilus (which also needs NaCl; now reclassified in Alkalibacillus), Bacillus thermocloacae and Bacillus vedderi will not grow at pH 7.0 (Agnew et al., 1995; Demharter and Hensel, 1989b; Fritze, 1996a; Nielsen et al., 1995a; Spanka and Fritze, 1993; Yumoto et al., 2003), while the alkalitolerant organisms Bacillus clausii, Bacillus gibsonii, Bacillus halmapalus, Bacillus horikoshii and Bacillus okuhidensis, will all grow at pH 7.0. The two arsenate- and selenate-reducing species Bacillus arseniciselenatis and Bacillus selenitireducens are both obligately alkaliphilic and halophilic (Switzer Blum et al., 1998). Table 2 shows differential characters of species with pH optima for growth of 8 or above.
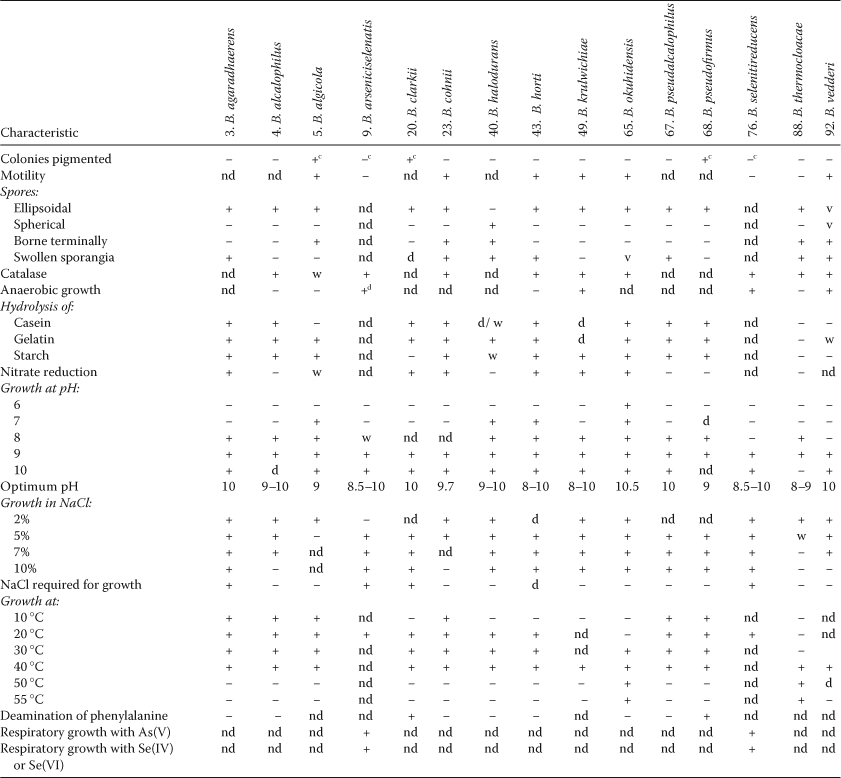
- a Symbols: +, >85% positive; d, different strains give different reactions (16–84% positive); −, 0–15% positive; v, variation within strains; w, weak reaction; d/w, d, different strains give different reactions, but positive reactions are weak; nd, no data are available.
- b Compiled from Claus and Berkeley (1986), Demharter and Hensel (1989b); Spanka and Fritze (1993); Agnew et al. (1995); Nielsen et al. (1995a); Fritze (1996a); Yumoto et al. (1998, 2003); Switzer Blum et al. (2001); Li et al. (2002); Ivanova et al. (2004a).
- c Bacillus algicola produces semitransparent, creamy, slightly yellowish colonies; Bacillus arseniciselenatis and Bacillus selenitireducens will produce red colonies, owing to elemental selenium precipitation, on selenium oxide media; Bacillus clarkii colonies may be cream-white to pale yellow in color, and one of the three strains described produces dark yellow colonies with age; Bacillus pseudofirmus colonies are yellow.
- d Bacillus arseniciselenatis does not grow aerobically; Bacillus selenitireducens grows weakly in microaerobic conditions.
Chemically defined media have been developed for several species, often with the optimization of industrial processes in mind. Minimal growth requirements have been established for rather few species, may be influenced by environmental conditions, and further emphasize the diversity of the genus.
Most species will use glucose and/or other fermentable carbohydrates as sole sources of carbon and energy. Patterns of acid production from, or assimilation of, carbon substrates are of great value in the characterization and identification of Bacillus species (Logan, 2002), but some species do not appear to utilize carbohydrates at all. Bacillus azotoformans uses a range of organic acids as carbon sources and does not attack carbohydrates; Bacillus badius and Bacillus benzoevorans assimilate certain amino acids and organic acids and do not produce acid from glucose and other carbohydrates. As indicated above, Bacillus fastidiosus usually uses allantoic acid, allantoin or uric acid as its sole carbon and energy source. The spherical-spored species Bacillus fusiformis, Bacillus neidei, Bacillus pycnus, Bacillus silvestris and Bacillus sphaericus do not produce acid or gas from D-glucose or other carbohydrates; Bacillus fusiformis utilizes acetate, citrate, formate, lactate and succinate. Bacillus carboniphilus, Bacillus insolitus, Bacillus siralis and Bacillus thermocloacae do not produce acid or gas from glucose or a range of other carbohydrates; the growth of Bacillus carboniphilus is promoted by activated carbon and graphite. Bacillus schlegelii and Bacillus tusciae will grow chemolithoautotrophically, using H2 as electron donor and CO2 as carbon source, and for the former species CO will satisfy both requirements. When growing chemoorgano-heterotrophically, Bacillus schlegelii utilizes acetate, butyrate, fumarate, propionate, succinate, phenol, 1-propanol and a small number of amino acids, and Bacillus tusciae uses a few alcohols, amino acids and organic acids, as their sole carbon sources, but neither species metabolizes carbohydrates. Bacillus methanolicus can grow on methanol, and some strains will also grow on ethanol.
Bacillus subtilis is attracted by many sugars (Ordal et al., 1979); following the genome sequencing of this species, its carbohydrate uptake and metabolism have been reviewed by Deutscher et al. (2002). In a theoretical analysis of metabolic fluxes, the capacity of Bacillus licheniformis for the production of certain industrial enzymes was found to be affected by the carbon sources used (Calik and Özdamar, 2001).
Bacillus species may use inorganic and organic sources of nitrogen. Many species will utilize an ammonium salt as their sole nitrogen source, amino acids are widely utilized, and strains of some species can use urea. The two facultative autotrophs Bacillus schlegelii and Bacillus tusciae can utilize ammonium ions, asparagine and urea as sole nitrogen sources. In the presence of molybdate, Bacillus niacini can use nicotinate as its sole source of carbon, nitrogen and energy. A soil isolate identified as Bacillus coagulans was found to use pyridine as sole carbon, nitrogen and energy source (Uma and Sandhya, 1997). Strains of Bacillus pumilus resistant to and able to utilize cyanide have been isolated (1983; Meyers et al., 1991; Skowronski and Strobel, 1969) and a cyanide-degrading enzyme purified and characterized (Meyers et al., 1991, 1993). In studies of the chemotaxis and motility of Bacillus subtilis, all 20 common amino acids have been found to attract the organism (Garrity and Ordal, 1995). Leucine, threonine and valine were found to be essential for growth and emetic toxin production by Bacillus cereus (Agata et al., 1999). Although Achouak et al. (1999) concluded that nitrogen fixation among aerobic endospore-formers is restricted to certain species of Paenibacillus, nitrogen fixation has been demonstrated in several Bacillus isolates from soil, including strains of Bacillus azotoformans, Bacillus cereus, Bacillus licheniformis, Bacillus megaterium (Rózycki et al., 1999) and Bacillus sphaericus. Some Bacillus species may stimulate the nitrogen-fixing activities of unrelated organisms, and so perhaps benefit from the nitrogen so fixed: a Bacillus firmus strain growing in association with a strain of Klebsiella terrigena was found to increase nitrogen fixation by the latter, probably owing to the protection of nitrogenase by the phenolic compounds it excreted (Zlotnikov et al., 2001); a Bacillus cereus strain was found to stimulate nodulation in legumes, so enhancing nitrogen fixation by bradyrhizobia (Vessey and Buss, 2002).
Little comprehensive information is available on the vitamin requirements of individual Bacillus species. Many do not require such growth factors, but yeast extract will often stimulate better growth. Adams and Stokes (1968) studied the requirements of the psychrophiles Bacillus insolitus and Bacillus psychrosaccharolyticus: the former required biotin and thiamine, while the latter needed niacin and thiamine, and biotin was essential or stimulator, depending upon the strain. Among spherical-spored species, Bacillus neidei and Bacillus sphaericus require both biotin and thiamin for growth, but Bacillus pycnus does not. In the presence of molybdate, Bacillus niacini can use nicotinate (niacin) as sole source of carbon, nitrogen and energy. Bacillus sporothermodurans and Bacillus subterraneus require biotin and thiamin for growth, but neither require cystine. For some species, such as Bacillus thermoamylovorans, vitamins and nucleic acid derivatives will stimulate growth, but are not essential.
Growth temperature ranges vary appreciably between the strains of species, and maxima and minima may be extended beyond the usual limits of a species for strains found in unusually hot or cold environments. Isolates of Bacillus licheniformis and Bacillus megaterium from an Antarctic geothermal lake, for example, were found to have maxima of 68°C and 63°C, 13°C and 18°C, respectively, higher than the previously published limits for these species (Llarch et al., 1997). The vast majority of established species are mesophiles, with optima between 25°C and 40°C and typically around 30°C, minima in the range 5–20°C, and maxima of 35–55°C. Several species, Bacillus coagulans, Bacillus fumarioli, Bacillus infernus, Bacillus methanolicus, Bacillus okuhidensis, Bacillus smithii, Bacillus thermoamylovorans and Bacillus tusciae, have higher growth temperature optima, ranging from 40°C to 55°C and above, with minima in the range 25–40°C and maxima of 55–65°C, and may be regarded as only moderately thermophilic. With minimum temperatures for growth of 37°C and above, optima in the range 55–70°C and maxima of 65–75°C, Bacillus schlegelii and Bacillus thermocloacae may be regarded as true thermophiles. Bacillus psychrodurans, Bacillus psychrosaccharolyticus, and Bacillus psychrotolerans grow and sporulate around 0°C and have maximum growth temperatures between 30°C and 35°C, while Bacillus insolitus, with a maximum growth temperature of 25°C, an optimum of 20°C and a minimum below 0°C, is a true psychophile. Growth temperature ranges and optima are given for most species in the List of species of the genus, below, and the differential characters of species with optimum temperatures of 50°C and above are shown in Table 3.
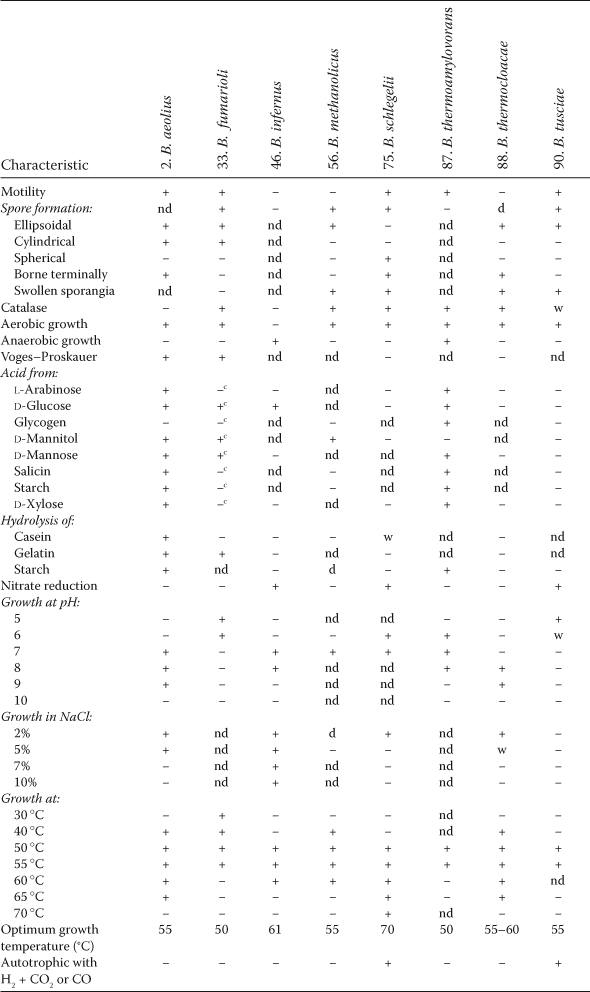
- a Symbols: +, >85% positive; d, different strains give different reactions (16–84% positive); −, 0–15% positive; w, weak reaction; nd, no data are available.
- b Compiled from Schenk and Aragno (1979); Bonjour and Aragno (1984); Demharter and Hensel (1989b); Arfman et al. (1992); Boone et al. (1995); Combet-Blanc et al. (1995); Logan et al. (2000); Gugliandolo et al. (2003a).
- c For Bacillus fumarioli, acid production from carbohydrates is tested at pH 6 – see Logan et al. (2000) and Testing for special characters.
Although aerobic growth has long been a defining character of members of the genus, some 20 species are facultatively anaerobic, and the definition was undermined by the discoveries of Bacillus infernus and Bacillus arseniciselenatis, which are strictly anaerobic (Boone et al., 1995; Switzer Blum et al., 1998). Nitrate respiration is a common property in the genus. Although Bacillus subtilis has long been regarded as a strict aerobe, which will like many Bacillus species, however, grow anaerobically using nitrate or nitrite as an electron acceptor, it has recently been shown to grow by fermentation in the absence of electron acceptors (Clements et al., 2002; Nakano and Zuber, 2002) (see Metabolism and metabolic pathways, below).
Survival. Spores are readily formed by strains of many species, but it is a mistake to assume that a primary culture or subculture in or on a routine growth medium will automatically yield spores if stored on the bench or in the incubator. Bacillus strains will not sporulate under all cultural conditions, and if conditions are not suitable for sporulation the culture may die (see Life cycle, above). Most strains will sporulate if grown for a few days on a routine, solid growth medium supplemented with 5 mg/l manganese sulfate; failure to sporulate on such a medium may be addressed by cultivating on a nutritionally weaker, manganese-supplemented, medium. Repeated subculture of a strain sometimes leads to the production of fewer spores or the complete loss of ability to sporulate; some strains, however, appear able to survive for long periods in refrigerated cultures, even though they have not sporulated.
It is best to grow the organism on nutrient agar containing manganese for a few days, and refrigerate when microscopy shows that most cells have sporulated. For most species sporulated cultures, sealed after incubation, can survive in a refrigerator for many years.
Metabolism and metabolic pathways. The majority of information on the metabolism and biochemistry of Bacillus species relates to Bacillus subtilis alone or to comparisons of this with other species, and further valuable information has been forthcoming from studies aimed at the optimization of various industrial processes employing several other aerobic endospore-forming species.
It is now established that Bacillus subtilis, which was long regarded as a strict aerobe, is capable of growing anaerobically, not only with nitrate as electron acceptor but also by fermentation in the absence of electron acceptors. This species and its close relatives apparently cannot use other electron acceptors such as dimethyl sulfoxide, fumarate and trimethylamine N-oxide, and have been considered to lie in an intermediate position between the true facultative anaerobes now allocated to Paenibacillus and the aerobes of the Bacillus sphaericus group (Priest, 1993), which are strictly oxidative. Bacillus cereus, Bacillus licheniformis and Bacillus thuringiensis can ferment carbohydrates in the absence of exogenous electron acceptors, and many Bacillus species can use nitrate as an electron acceptor in the absence of oxygen, but several species such as Bacillus megaterium and Bacillus pumilus are unable to do this. Bacillus subtilis uses pyruvate dehydrogenase for conversion of pyruvate to acetyl-coenzyme A in anaerobic as well as in aerobic conditions and fermentation is stimulated by pyruvate. The fermentation is of the mixed acid-butanediol type, and products include acetate, acetoin, 2,3 butanediol, ethanol and lactate (Nakano et al., 1997); Bacillus licheniformis also carries out a mixed acid fermentation (Shariati et al., 1995). During nitrate respiration, Bacillus subtilis reduces nitrate to nitrite and ammonium, and, unlike the denitrifier Bacillus licheniformis, it does not produce the gaseous products NO, N2O and N2 (Nakano and Zuber, 2002). A homolog of the Bacillus subtilis gene encoding membrane-bound respiratory nitrate reductase is found in Bacillus anthracis (Nakano and Zuber, 2002). Bacillus licheniformis shows poor anaerobic growth on fumarate, but it can grow in the presence of arginine using the arginine deiminase pathway (Maghnouj et al., 1998); Bacillus cereus also possesses genes for this pathway but Bacillus anthracis does not (Ivanova et al., 2003; Read et al., 2003). In the First Edition of this Manual (Claus and Berkeley, 1986), ability to grow and sporulate in air was implicit in the definition of Bacillus, but the proposals of the species Bacillus infernus and Bacillus arseniciselenatis, which are strictly anaerobic, undermine this long-held element of the genus definition. Bacillus arseniciselenatis and Bacillus selenitireducens are two alkaliphiles isolated from a lakewater containing unusually high levels of arsenic, and they will grow by respiratory (dissimilatory) reduction of As(V) to As(III) (arsenate to arsenite) and oxidation of lactate to acetate and CO2. The former will also grow by dissimulatory reduction of Se(VI) to Se(IV) (selenate to selenite) and the latter will reduce Se(IV) to Se(0), so that co-cultures will reduce selenate to elemental selenium (Switzer Blum et al., 1998). Such organisms or their enzymes are of interest for the bioremediation of environments contaminated with toxic oxyanions of arsenic and selenium. Lindblow-Kull et al. (1982) isolated a Bacillus strain from the seeds of the selenium-accumulating plant Astragalus crotalariae. It grew optimally in the presence of 3–100 mM selenite in nutrient broth, giving a strong red color owing to elemental selenium, and growth also occurred with selenate or tellurate. Bacillus infernus, a strict anaerobe, was isolated from a deep terrestrial subsurface environment and it can use Fe3+ and MnO2, as well as trimethylamine N-oxide and nitrate, as electron acceptors (Boone et al., 1995), while Bacillus subterraneus, which is a facultative anaerobe isolated from a deep subsurface thermal aquifer, also uses Fe3+ and MnO2, as well as fumarate, nitrate and nitrite as electron acceptors (Kanso et al., 2002).
The respiratory cytochromes and other heme proteins of Bacillus subtilis and relatives have been reviewed by von Wachenfeldt and Hederstedt (2002).
The natural habitat of Bacillus subtilis is soil, which contains a wide range of carbohydrates and polysaccharides from microorganisms, plants and animals, and so it can utilize a wide range of such substrates and possesses a large number of enzymes which degrade polysaccharides. Carbohydrates are taken into the cell by a range of means, including ATP-binding cassette (ABC) transporters and phosphotransferase systems (PTS); there are 77 putative ABC transporters and at least 16 PTS sugar transporters encoded in the genome (Kunst et al., 1997); 75 ABC transporter/ATP-binding proteins are encoded by the Bacillus halodurans genome (Takami et al., 2000). ABC transporters are important in Gram-positive organisms, given their single-membrane cell envelope, as they allow them to escape the toxic actions of many compounds. Bacillus anthracis has reduced numbers of PTS and other types of sugar transporters and lacks pathways for catabolism of several sugars compared with Bacillus subtilis (Read et al., 2003). Carbohydrate uptake and metabolism in Bacillus subtilis has been reviewed by Deutscher et al. (2002) and the regulation of carbon catabolism in Bacillus species was reviewed by Stulke and Hillen (2000).
Because many Bacillus species grow aerobically and produce acid from carbohydrates by oxidation rather than fermentation, they normally produce smaller amounts of acid from carbohydrates in comparison with most Paenibacillus species. Also, because the ammonia they produce from peptones may neutralize the small amount of acid produced, it is necessary to use a medium of low protein to carbohydrate ratio, and a sensitive indicator such as phenol red in order to detect acid production.
Most members of the Bacillus sphaericus group will not use carbohydrates as carbon or energy sources, and use certain organic acids and amino acids instead.
The genome of Bacillus subtilis encodes an Embden–Meyerhof–Parnas glycolytic pathway, coupled to a functional tricarboxylic acid (Krebs) cycle (Kunst et al., 1997), and the Bacillus halodurans genome is little different to that of Bacillus subtilis in this respect (Takami et al., 2000). Bacillus subtilis appears to have no glyoxylate shunt, but some Bacillus species, including Bacillus halodurans and Bacillus anthracis, produce glyoxylate shunt (or bypass) enzymes and/or have genes encoding components of this shunt, which allows acetate or fatty acids to be used as sole sources of carbon (Sonenshein, 2002). Inactivating mutations in the Krebs cycle genes of Bacillus subtilis cause defects in sporulation, and although most such defects are attributable to the conventional roles of the affected enzymes, other defects cannot be explained in this way and their mechanisms are unclear (Sonenshein, 2002). It appears that some Krebs cycle proteins may have regulatory as well as enzymic activities: the E2 subunit of the pyruvate dehydrogenase complex of Bacillus thuringiensis can bind to DNA, and in so doing has been implicated in regulation of the expression of a gene for toxin production (Walter and Aronson, 1999).
Bacillus subtilis can use ammonium, nitrate, amino acids, some purines, urea, uric acid, allantoin, and peptides as sole nitrogen sources. Glutamine, followed by arginine, is the best source for rapid growth. In order to utilize the nitrogen compounds that permit optimal growth rates, this organism, like other Gram-positive bacteria, regulates nitrogen metabolism genes by mechanisms very different to the pathway found in enteric bacteria. Bacillus subtilis controls gene expression in response to nitrogen availability with the three proteins GlnR, TnrA and CodY. Also, although σ54 factors were initially believed to be present only in Gram-negative bacteria, the Bacillus subtilis sigL regulon was found to contain a homolog of σ54, and is now known to contain genes involved in carbon and nitrogen source utilization (Fisher and Débarbouillé, 2002). Bacillus subtilis also possesses many genes involved in the degradation of opines and related molecules derived from plants (Kunst et al., 1997). Although Bacillus subtilis, Bacillus anthracis, Bacillus cereus and Bacillus halodurans have broad similarities in their metabolisms, Bacillus anthracis and Bacillus cereus have greater capacities for the utilization of amino acids and peptides. Bacillus anthracis and Bacillus cereus have wider ranges of coding sequences for secreted proteases, 48 and 51 respectively, compared with Bacillus subtilis, which has only 30, and wider ranges of peptidases too (Ivanova et al., 2003). The Bacillus anthracis genome also encodes 17 ABC-type peptide binding proteins, has nine homologs of the BrnQ branched chain amino-acid transporter, and has six LysE/Rht amino-acid efflux systems, whereas Bacillus subtilis has only four, two and two respectively. Bacillus anthracis and Bacillus cereus thus appear to be adapted to protein-rich environments such as animal matter (Ivanova et al., 2003; Read et al., 2003). Bacillus proteases are of considerable economic value, especially as detergent additives, are intensely studied with a view to enhancing their behaviors in industrial processes, and the search for new strains producing enzymes with novel properties continues (Outtrup and Jørgensen, 2002).
Although iron is an essential nutrient for most organisms, free iron availability is severely restricted in neutral, aerobic environments, including animal tissues, owing to its insolubility in such conditions. Bacteria secrete siderophores into their environments in order to chelate iron, and the ferri-siderophore complexes can then be assimilated. Bacillus subtilis regulates iron uptake by members of the ferric uptake regulator (Fur) family of proteins, and possesses three such homologs, Fur, PerR (peroxide stress response) and Zur (zinc uptake regulation). The Bacillus subtilis homolog BsuFur shows only 33% sequence similarity to Escherichia coli Fur (EcoFur) and, unlike EcoFur, it does not respond to Mn(II) in vivo (Herbig and Helmann, 2002). Bacillus anthracis has a wider range of iron-acquisition genes than does Bacillus subtilis; it possesses 15 ABC uptake systems for iron siderophores or chelates, and two clusters of genes for the biosynthesis of siderophores. There are in the sequence of Bacillus anthracis two genes involved in the synthesis of an aerobactin-like siderophore that are not found in the sequenced strains of Bacillus subtilis and Bacillus cereus (Read et al., 2003). Bacillus anthracis carries genes for two sphere-like proteins which have internal cavities and act as ferritins, and so are involved in iron uptake and regulation; the immunogenicities of these proteins make them of interest in the development of new anthrax vaccines (Papinutto et al., 2002). Bacillus cereus can use hemoglobin, heme and heme-albumin complex as its iron sources, but does not appear to use other iron-binding proteins such as lactoferrin and transferrin; it does not digest these two proteins, but it will digest heme-protein complexes to elicit release of heme which may then be captured as an iron source (Sato et al., 1999).
Halophilic species and some alkaliphilic Bacillus strains have obligate requirements for Na+. The strain of the alkaliphile Bacillus halodurans that was subjected to complete genome sequencing requires Na+ for growth in alkaline conditions, where the environmental sodium ions are essential for solute transport through the cytoplasmic membrane. ATP metabolism through the action of ATPases is considered to be important in generating a proton-motive force across the cytoplasmic membrane by extrusion of H+; Bacillus halodurans possesses genes for four types of ATPases which are well conserved between the genome of this species and Bacillus subtilis (Takami et al., 2000). The Bacillus halodurans genome was also found to carry protein coding sequences that are candidates for Na+/H+ antiporter genes, at least some of which are involved in halotolerance and alkalitolerance and allow the organism to maintain an intracellular pH lower than the environmental pH (Takami et al., 2000). The Bacillus subtilis 168 genome possesses a single ABC-type putative Na+ efflux system (Saier et al., 2002).
The abilities of some Bacillus strains to metabolize and transform complex organic compounds are of interest in both bioremediation and pharmaceutical production, and studies of isolates from special environments and searches for activities of potential value in biotechnological applications have revealed a number of unfamiliar substrates.
Reports of Bacillus strains or their enzymes capable of metabolizing environmental pollutants include: a Bacillus sp. capable of oxidizing H2S in chicken feces (Nakada and Ohta, 1998); a Bacillus sphaericus isolate from agricultural soil which oxidizes p-nitrophenol (Kadiyala et al., 1998); the naphthalene-degrading “Bacillus naphthovorans” from oil-contaminated tropical marine sediments (Zhuang et al., 2002; a Bacillus sp. that can utilize dimethylphthalate as sole carbon source (Niazi et al., 2001); a Bacillus sp. capable of using 4-chlorobiphenyl as sole carbon source, metabolizing it to 4-chlorobenzoic acid (Sàágua et al., 1998); and an engineered Bacillus megaterium cytochrome P450 that degrades polycyclic aromatic hydrocarbons (Carmichael and Wong, 2001).
The uricase of Bacillus fastidiosus catalyzes the oxidation of uric acid into the more soluble allantoin, and conjugates of this enzyme with soluble polymers to reduce antigenicity are of value in the therapy of gout, and of hyperuricemias associated with blood malignancies and chemotherapy (Schiavon et al., 2000). Some strains of Bacillus cereus, Bacillus megaterium and Bacillus sphaericus are capable of biotransformations of inexpensive natural steroidal substrates into high-value therapeutic compounds (Manosroi et al., 1999; Wadhwa and Smith, 2000). A Bacillus subtilis isolate from soil has been reported to produce the aroma compound vanillin by degradation of the phenylpropanoid isoeugenol, which it could use as sole carbon source (Shimoni et al., 2000). Decarboxylation of the abundant ferulic acid into the useful aromatic compound 4-vinylguaiacol has been described for Bacillus pumilus (Lee et al., 1998), while another strain of this species has been reported to be able to use phenols and cresols as sole carbon sources (Günther et al., 1995).
Genetics. Aerobic endospore-formers have been and continue to be important in many fields of basic research, and long-term studies of the sporulation process in Bacillus subtilis have led to its being probably the best understood developmental system. Although endospores are to be found in other genera, it is the spores of Bacillus subtilis that have been the most intensively studied, especially those of strain 168. Burkholder and Giles (1947) produced auxotrophic mutants of the Marburg (i.e., type) strain by exposure to UV light and X-rays in the 1940s, and their tryptophan auxotrophic strain 168 was chosen by Spizizen (1958) as the recipient in his demonstration of transformation of this species by bacterial DNA in the 1950s. Because studies on individual genes and gene products have been performed mainly on Bacillus subtilis, and to a lesser extent on pathogenic members of the Bacillus cereus group, such properties have as yet made little contribution to our understanding of the phylogeny of the genus Bacillus, but this will of course change as more species have their genomes sequenced (Stackebrandt and Swiderski, 2002).
The same laboratory strain, Bacillus subtilis 168, was also the first Bacillus, indeed the first Gram-positive bacterium, to have its genome sequenced (Kunst et al., 1997), and the implications of this knowledge in our understanding of the cellular architecture, chromosomal replication, cellular division, metabolism and metabolic regulation, macromolecular synthesis, adaption and differentiation of this organism have been reviewed by Sohenshein et al. 2002. As these authors observe, it has become clear from comparisons with the genome sequences of other organisms that the proteins of macromolecular synthesis, and the enzymes of biosynthesis and biodegradation, are widely conserved among prokaryotes, but that Gram-positive and Gram-negative organisms regulate gene expression and the activities of their gene products somewhat differently. The Bacillus subtilis genome is similar in size to that of Escherichia coli, and these two organisms have orthologous counterpart genes representing about one-quarter of their genomes (Kunst et al., 1997). The same authors found that of the 450 genes encoded by Mycoplasma genitalium, some 300 had products similar to proteins of Bacillus subtilis, and this is particular of interest given the belief that mycoplasmas are derived from Gram-positive bacteria.
Subsequent to this pioneering work on the Bacillus subtilis genome, those of three other Bacillus species, Bacillus halodurans, Bacillus anthracis and Bacillus cereus, have been sequenced (Ivanova et al., 2003; Read et al., 2003; Takami et al., 2000), so that comparisons can be made and both common and specific features of these organisms (a soil bacterium, an alkaliphile, a pathogen of humans and other animals, and an opportunistic pathogen, respectively) can be identified.
The genome of Bacillus subtilis has 4,214,810 bp comprising 4,100 protein-coding sequences (CDSs); although the mean mol% G + C is 43.5 for this organism, the ratio varies greatly throughout the chromosome. There are many gene duplications, including rRNA genes, and a particularly conspicuous duplication is a 190 bp element that is repeated 10 times, with five repeats lying each side of the origin of replication; similar sequences have been found in the closely related species Bacillus licheniformis (Kunst et al., 1997). The genome of Bacillus halodurans has 4,202,353 bp containing 4,066 predicted CDSs, its mean mol% G + C is 43.7, and 16S rDNA sequence analysis shows it to be a close relative of Bacillus subtilis. The principal apparent difference between the two organisms is the alkaliphily of Bacillus halodurans, and so it was naturally of interest to identify differences between the genomes and to try and correlate these with phenotype. Both genomes showed substantial conservation of a common region comprising, amongst others, the functions of cell division, DNA replication, RNA modification, nucleotide and nucleic acid metabolism, metabolism of enzymes and prosthetic groups, glycolytic pathways and the TCA cycle, protein secretion, motility and chemotaxis (Takami et al., 2000). Bacillus halodurans was found to carry 112 CDSs which showed similarity with transposases or recombinases from other prokaryotes, indicating their important evolutionary roles in horizontal gene transfer. Bacillus subtilis, on the other hand, has only ten transposons and transposon-related proteins (Takami et al., 2000). However, the genome of Bacillus subtilis was found to contain at least 10 prophages or remnants of prophages, which suggest that horizontal gene transfer by bacteriophages may have played an important part in the evolution of this organism (Kunst et al., 1997); Bacillus halodurans, on the other hand, has no intact prophage. The σ factors required for sporulation are well conserved between the two genomes, but of 11 σ factors belonging to the extracytoplasmic function (ECF) only one is found in Bacillus subtilis and 10 are unknown outside Bacillus halodurans; these unique σ factors may play parts in alkaliphily given the roles of ECF σ factors in the control of specific molecule or ion uptake or secretion, or of various extracellular stress signals (Takami et al., 2000). Other differences between the organisms concern genes affecting competence, the control of sporulation, and cell-wall components. The last of these includes teichuronopeptide (a compound known to contribute to alkaliphily) in Bacillus halodurans, and the genome of this organism also possesses five candidates for Na+/H+ antiporter genes that may relate to its haloduric and alkaliphilic phenotype.
The chromosomes of Bacillus anthracis and Bacillus subtilis encode similar sporulation machineries, and metabolic and transport genes, and both encode numbers of predicted drug efflux pumps common in soil bacteria. Particular differences include the extended capacity of Bacillus anthracis for amino acid and peptide utilization, including more peptide binding proteins, secreted proteases and peptidases, and amino-acid efflux systems, and Bacillus cereus is likewise well equipped with proteolytic enzymes, peptide and amino acid transporters and amino-acid degradation pathways (Ivanova et al., 2003). These may correlate with their being adapted to protein-rich environments, and they have lesser capacities than Bacillus subtilis for sugar utilization. Bacillus subtilis carries 41 genes for degradation of carbohydrate polymers, whereas Bacillus anthracis has 15 and Bacillus cereus only 14 (Ivanova et al., 2003). The Bacillus anthracis genome encodes several detoxification functions for which homologs are not apparent in Bacillus subtilis; one of these is cytoplasmic Cu-Zn superoxide dismutase (SodC) which counteracts nitric oxide-mediated killing in the macrophage and has an important role in several other intracellular bacteria.
Bacillus anthracis has 5,227,293 bp and 5,508 CDSs (5,503,799 bp and 5,838 CDSs when the virulence plasmids are included), while Bacillus cereus carries 5,426,909 bp and 5,366 CDSs. It is well established that the genes on its virulence plasmids are essential to the virulence of Bacillus anthracis, as strains cured of one or both plasmids are avirulent. Study of the genome sequence shows that this organism has chromosomally encoded proteins, including hemolysins, phospholipases and iron-acquisition proteins which might contribute to pathogenicity, and surface proteins which might have potentials as drug and vaccine targets. Nearly all of these potential virulence factors and surface proteins have homologs in Bacillus cereus, even the sequenced Bacillus cereus strain which is considered to be non-pathogenic (Ivanova et al., 2003), underlining yet again the well-established close relationship between these two organisms (Turnbull et al., 2002). The chromosome of Bacillus anthracis carries most of its housekeeping functions, and these mostly have homologs in the sequence of Bacillus cereus, while the two virulence plasmids pXO1 (toxic complex) and pXO2 (capsule) carry transposons, and genes of unknown function in addition to the key virulence determinants. Bacillus anthracis also chromosomally encodes proteins with homology to virulence factors of Listeria monocytogenes, and these may be significant for intracellular survival, multiplication and escape (Read et al., 2003). The genes for a complex of three non-hemolytic enterotoxins and two channel-forming hemolysins that have roles in the pathogenicities of Bacillus cereus and Bacillus thuringiensis have homologs in Bacillus anthracis. Bacillus anthracis also carries two (and Bacillus cereus three) homologs of Bacillus thuringiensis immune inhibitor A protease which has a role in virulence to insects, and both it and Bacillus cereus encode a homolog of the metalloprotease enhancin which boosts viral infectivity in insect guts. It has been suggested that these genes may be evidence that the Bacillus cereus group had an insect-infecting ancestor, and their possession of genes for chitinolytic enzymes is consistent with this idea (Ivanova et al., 2003; Read et al., 2003).
Comparative genome hybridization of 19 Bacillus cereus and Bacillus thuringiensis strains against a Bacillus anthracis microarray revealed 66–92% homology of chromosomal genes, and several major differences between Bacillus anthracis and Bacillus cereus reflect altered gene expression as opposed to gene gains or losses. In the Bacillus cereus and Bacillus thuringiensis strains were very few homologs of genes found on the virulence plasmid pXO2, but about half of the 19 strains carried homologs of genes (but not of the anthrax toxin genes) found in the virulence plasmid pXO1. There are many mobility genes on pXO1 (Okinaka et al., 1999), and plasmid transfer is known to occur within the Bacillus cereus group, but there is little localized variation in the G + C and dinucleotide contents of the Bacillus anthracis chromosome and virulence plasmids which suggests that most of the genes are native to this group of species (Read et al., 2003).
The great phylogenetic heterogeneity of Bacillus sensu lato was long evident from its wide mol% G + C range of 43–68 (Claus and Berkeley, 1986), and this heterogeneity has clearly been demonstrated by the 16S rRNA and rDNA sequence analyses that have followed. The impact of such analyses on the taxonomy of Bacillus sensu lato is discussed in Taxonomic comments (below) and in Stackebrandt and Swiderski (2002). Although 16S rDNA sequence comparisons are valuable in the determination of approximate phylogenetic relationships at the generic level and higher, they are not appropriate for the classification of strains at the species level (Stackebrandt and Goebel, 1994). Xu and Cote (2003) compared the sequences of the 16S–23S internal transcribed spacer region (ITS) of representatives of 27 Bacillus species and 19 strains representing five other endospore-forming genera. Although they found general agreement with polyphasic taxonomies incorporating 16S rDNA sequence comparisons, they also found support for the division of Bacillus into further new genera, and revealed unexpected groupings. For example, Bacillus coagulans was found to lie nearer to Geobacillus strains than to the other Bacillus species, Bacillus laevolacticus grouped with Virgibacillus pantothenticus, and Bacillus badius with Marinibacillus marinus, yet Bacillus circulans remained ungrouped (Xu and Cote, 2003). The ITS region is hypervariable in comparison with the more conserved 16S rRNA coding region, and ITS-PCR fingerprints have been used to investigate the relationships of members of the genus. Daffonchio et al. (1998a) were able to separate several species of Bacillus by this approach, but distinctions of very closely related species were not possible, and single-strand conformation polymorphism analysis was used to distinguish members of the “Bacillus subtilis group,” while Bacillus mycoides could be separated from Bacillus cereus/Bacillus thuringiensis by restriction analysis. When ITS-PCR, analysis of the regions between tRNA genes (tDNA-PCR), and RAPD were applied to Bacillus licheniformis the 10 strains studied fell into two clusters by all three fingerprinting methods. With Bacillus cereus, on the other hand, it was found that ITS-PCR and tDNA-PCR gave virtually identical profiles among the 21 strains, but that these strains showed great diversity in RAPD analysis and in plasmid profiles (Daffonchio et al., 1998b). Part of the ITS region has been used as a probe for the detection of Bacillus sporothermodurans (de Silva et al., 1998).
De Vos (2002) reviewed several other approaches to the analysis of nucleic acids that cover a wide range of taxonomic levels, and these are summarized here. As direct sequencing of 16S rDNA is still relatively expensive and not available to all microbiologists, indirect, fast and less expensive methods such as ARDRA, to characterize the 16S rDNA part of the ribosomal operon via restriction analysis, have offered a good alternative for sequencing aerobic endospore-forming bacteria (Heyndrickx et al., 1996c; Logan et al., 2000). This method also has the advantages that both computerized interpretation of the data and database construction are possible.
Because Bacillus sensu lato members contain 9–12 rRNA operons (e.g., Johansen et al., 1996; Okamoto et al., 1993), ribotyping of the aerobic spore-formers has been considered as a potentially useful approach to unravel their taxonomic structure. At present, the number of studies in which ribotyping has been used to characterize members of Bacillus is rather limited, and published reports deal mainly with intraspecific variation. In these studies the investigators try to find a correlation between the intraspecific distribution of ribopatterning in correlation with, for example, (i) food poisoning with Bacillus licheniformis (Salkinoja-Salonen et al., 1999), (ii) toxin production by members of the Bacillus cereus group, including strains of Bacillus cereus from food poisoning incidents (Pirttijärvi et al., 1999), (iii) tracing of certain Bacillus thuringiensis types (Akhurst et al., 1997) and (iv) differentiation between toxic and nontoxic Bacillus sphaericus strains (Aquino de Muro et al., 1992).
Although comparative data with other fine DNA fingerprinting methods are somewhat scarce for representatives of Bacillus, at least one study has revealed that randomly amplified polymorphic DNA (RAPD) analysis is only slightly more discriminative than the automated ribotyping (riboprinting) for Bacillus cereus isolates (Andersson et al., 1999a).
Denaturing-gradient gel electrophoresis (DGGE) and temperature-gradient gel electrophoresis (TGGE) are based on similar principles, and allow discrimination at the subspecies level and often at the strain level (De Vos, 2002). As patterns from both techniques can be obtained after amplification of target DNA taken from non-purified biological material such as soil or water samples, the methods allow visualization of the genetic biodiversity, including the non-cultivable bacterial components of biotopes. Comparison of the sequences of the dominant bands visualized by these approaches with databases such as EMBL may be indicative for the dominant bacterial component of the biotope under study. Using TGGE, for example, an unknown group of Bacillus members has been discovered as the main bacterial component in Drentse grassland in the Netherlands (Felske et al., 1998; Felske et al., 1999).
As identical 16S rDNA sequences do not guarantee species identity (Fox et al., 1992), DNA:DNA hybridizations are needed when 16S rDNA sequences between strains show 97% similarity or more with existing taxa. The study of DNA relatedness by different techniques has been widely applied to Bacillus, but only two methods are currently used for species delineation within the genus: the liquid renaturation method (De Ley et al., 1970, or a variant) and the later microplate method of Ezaki et al. (1989). Data obtained by both methods have been evaluated and compared (Goris et al., 1998). Neither of these methods allows the determination of Δthermostability (expressed as ΔT m) of the hybrid, but differences in ΔT m between the hybrid and the homologous duplex are important and can be decisive for taxonomic conclusions (Grimont et al., 1982).
Several typing methods are based on indirect comparative analysis of nucleic acid characteristics, and were originally developed for the discrimination of species, subspecies and even strains for epidemiological studies. Restriction Fragment Length Polymorphism (RFLP) analysis of whole bacterial genomes yielded very complex patterns of DNA fragments that are difficult to compare because of their smear-like appearance. The use of restriction enzymes that cut infrequently drastically reduces the number of the DNA fragments, the high molecular mass of which required the development of a specific technique known as pulsed field gel electrophoresis (PFGE) for the satisfactory separation of fragments on agarose gels. The method has been applied to differentiate between strains of Bacillus sphaericus (Zahner et al., 1998), and between very closely related species such as Bacillus anthracis, Bacillus cereus, Bacillus mycoides and Bacillus thuringiensis (Carlson et al., 1994; Harrell et al., 1995; Helgason et al., 2000; Liu et al., 1997). The last two of these studies dealt with infrequently reported clinical infections by Bacillus cereus.
Several other genomic typing techniques overcome the problem of interpreting complex banding patterns by visualizing only selected parts of bacterial genomes that have been amplified using the PCR. The banding patterns obtained using RAPD, in which oligonucleotides of about 10–20 bp are used as primers, are not always very reproducible, so that databases are of limited use and data exchanged between laboratories have to be interpreted with great care. Nonetheless, RAPD has been applied to the discrimination of Bacillus thuringiensis (Brousseau et al., 1993), Bacillus sphaericus (Woodburn et al., 1995) and thermophilic (now mostly assigned to Geobacillus) and mesophilic Bacillus members (Ronimus et al., 1997). A second group of PCR-based typing methods uses repetitive element primers and so is known as rep-PCR. It is based upon the observation that repetitive elements are dispersed throughout genomes of bacteria, and consensus motifs deduced from the sequence of these repetitive elements can be used as primers. The electrophoretic patterns revealed allow discrimination at the within-species level and sometimes at the strain level, and have been used to investigate the genetic diversity of novel species (Heyrman et al., 2003a; Heyrman et al., 2003b; Heyrman et al., 2004; Logan et al., 2002b), to unravel the genetic diversity of Bacillus sphaericus (da Silva et al., 1999; Miteva et al., 1999), and to demonstrate the presence of the thermoresistant organism Bacillus sporothermodurans in UHT treated milk (Klijn et al., 1997). Amplified Fragment Length Polymorphism (AFLP) is based upon a specific combination of PCR and restriction methodologies (Zabeau and Vos, 1993), and although much more complex than RAPD and Rep-PCR methods, it is also much more reproducible. It has been used in epidemiological studies of Bacillus cereus (Mantynen and Lindstrom, 1998; Ripabelli et al., 2000; Schraft et al., 1996) and for the genetic comparison of Bacillus anthracis and its closest relatives (Jackson et al., 1999; Keim et al., 1997; Turnbull et al., 2002). Further molecular characterization showed that variable number tandem repeats (VNTR), which are short, tandemly repeated sequences which undergo very rapid mutational change, were responsible for the variations seen by AFLP. Multiple-locus VNTR analysis (MVLA) thus offers greater discriminatory power than AFLP and should be useful for investigating the ecology and epidemiology of anthrax (Turnbull et al., 2002).
Hansen et al. (2001) developed a PCR assay for the detection of members of the Bacillus cereus group, using a 16S rRNA probe. Real-time PCR assays, which use primer and fluorescently labeled gene probe systems to allow the rapid and sensitive detection of genes specific for Bacillus anthracis, have been developed in several laboratories. Makino and Cheun (2003) described an assay that targeted genes for capsule and PA and allowed a single spore to be detected in 1001 of air in 1 h. Drego et al. (2002) outlined an assay targeting fragments of a chromosomal gene (rpo) for detecting the organism in clinical samples. Hoffmaster et al. (2002) evaluated and validated a three-target assay, with primers for capsule, PA and rpo, in order to test suspect isolates and to screen environmental samples during the outbreak that followed the 2001 bioterrorist attack in the USA, and a similar approach was evaluated by Ellerbrok et al. (2002).
Antigens and vaccines. Despite the promising findings of some early studies of somatic and spore antigens from a range of species (Doak and Lamanna, 1948; Lamana, 1940a, c, 1942), of flagellar, somatic and spore antigens of Bacillus (now Paenibacillus) polymyxa (Davies, 1951), and the potential taxonomic value of spore precipitinogens reported by Norris and Wolf (1961) following an extension of Davies' work to a wider range of species, serological studies have been taken little further for classification and identification of members of this genus. Paradoxically, however, although the Bacillus cereus group appeared to one of the least tractable in these early studies, where species-specific antigens were sought, the H-antigens of Bacillus cereus and Bacillus thuringiensis are now used with considerable success for typing purposes; Bacillus anthracis does not possess H-antigens as it is nonmotile. Smith et al. (1952) reviewed the earliest work, following their own disappointing results with antisera to vegetative cells of a range of species, and Berkeley et al. (1984) reviewed the application of serological methods to the identification of Bacillus species.
Somatic antigens have been little used for the identification of Bacillus strains. Investigations into the O-antigens of a Bacillus cereus and Bacillus licheniformis (Norris and Wolf, 1961) found them too strain-specific to be of taxonomic value. (Walker and Wolf, 1971) and Wolf and Sharp (1981) found the O-antigens of Bacillus (now Geobacillus) stearothermophilus to show some correlation with the three biochemical and physiological subgroups of this species that they recognized. Serotyping of Bacillus thuringiensis strains has been attempted on the basis of extracellular heat-stable somatic antigens (HSSAs; Ueda et al., 1989; Ohba et al., 1992), by observing the formation of immunoprecipitation haloes around colonies on antiserum-agar plates; they found a lack of correlation with H-antigen serogroups, while field isolates showed little HSSA variation within a single H-serovar. Concerns about the potential of Bacillus anthracis as a biological weapon have emphasized the need for a rapid method for the identification of Bacillus anthracis and diagnosis of anthrax. Polyclonal antibodies lack the desired specificity, because they react with other members of the Bacillus cereus group. Phillips and Ezzell (1999) were able to identify Bacillus anthracis by raising polyclonal antibodies against extracted vegetative cell antigens, absorbing with Bacillus cereus and Bacillus thuringiensis, and detecting reactions by immunofluorescence or immunoblotting. A monoclonal antibody specific to the Bacillus anthracis cell-wall polysaccharide antigen is effective in identification (Ezzell and Welkos, 1999), but this antigen may be masked in vivo by the organism's poly-γ-D-glutamic acid capsule. De et al. (2002) therefore developed a two-component direct fluorescent-antibody assay that allows rapid, sensitive and specific detection of the cell wall and capsule of Bacillus anthracis in clinical specimens.
Flagellar antigens have been more widely used in Bacillus typing than any other kind of antigen, as they provide the highest strain specificity, and valuable serotyping schemes have been developed for Bacillus thuringiensis and Bacillus cereus. High frequencies of H-antigen sharing between Bacillus cereus and Bacillus thuringiensis have been reported; in one study of Bacillus cereus strains from soils, phylloplanes and animal feces the seropositivity of the isolates with Bacillus thuringiensis H-antisera was 60–77% (Shisa et al., 2002). The common flagellar antigen of Bacillus cereus has been shown by SDS-PAGE and immunoblot assay to be due to a 61-kDa protein, and monoclonal antibody studies showed that the common antigenic epitope of the 61-kDa protein also exists in the flagella of Bacillus thuringiensis (Murakami et al., 1993).
Sixty-nine serotypes and 13 subantigenic groups of Bacillus thuringiensis have been recognized, giving 82 serovars (Lecadet et al., 1999). New strains of the species are screened by reference H-antisera and antisera are prepared against any strains that do not agglutinate. New antisera are then screened with all the known H-antigens, and a new serovar is recognized if cross-reactions do not occur or if new subfactors can be demonstrated by the antiserum-saturation technique (de Barjac, 1981). New serovars are registered at the International Entomopathogenic Bacillus Centre (IEBC) Collection at the Institute Pasteur, Paris, France; this laboratory was the international reference for Bacillus thuringiensis since 1965. Distinct serovars of Bacillus thuringiensis are given names and abbreviations, such as finitimus (FIN, H-antigen 2), sotto (SOT, H4a4b), tolworthi (TOL, H9) and pirenaica (PIR, H57). The first two of these two names were formerly used as the specific epithets of distinct species (Gordon et al., 1973). Although these serovar names have often been informally regarded as subspecific epithets, they do not represent validated subspecies of Bacillus thuringiensis and should instead be regarded as varieties. These varieties do not, unfortunately, show much correlation with toxicity to invertebrates; for example, serovar morrisoni (MOR, H8a8b) includes strains pathogenic to mosquitoes (Diptera), Coleoptera and Lepidoptera (Lecadet et al., 1999), while the invertebrate toxicity, if any, of many serovars (especially the recently recognized ones) is unknown.
A strain differentiation system for Bacillus cereus based on H-antigens is available at the Food Hygiene Laboratory, Central Public Health Laboratory, Colindale, London, UK, for investigations of food-poisoning outbreaks or other Bacillus cereus-associated clinical problems (Kramer and Gilbert, 1992). This system was developed by Taylor and Gilbert (1975) for the investigation of food poisoning outbreaks and recognized 18 serovars, but many strains from outbreaks were untypable (Gilbert and Parry, 1977). Terayama et al. (1978) extended the system and recognized further serovars in foods. Some of the serotypes show some correlation with pathogenicity and biotype; the distinction is not absolute, however, and it is probable that some organisms can produce both diarrheal and emetic toxins. Serovar 1, 3, 5 and 8 strains comprise a biotype distinguishable from other Bacillus cereus serovars and untypable strains, and these former four serovars have often been isolated in connection with cases of the emetic form of food poisoning (Logan and Berkeley, 1984; Logan et al., 1979), a form especially associated with cooked rice. Gilbert and Parry (1977) found that strains of serovar 1 are found more frequently in cooked rice than in uncooked rice, while strains of serovar 17 are commoner in uncooked rice than they are in cooked rice; they later showed that serovar 1 strains form more heat resistant spores than do serovar 17 strains (Parry and Gilbert, 1980).
Flagellar serotyping was developed for Bacillus sphaericus (de Barjac et al., 1985) independently of the recognition of DNA relatedness groups, so that the DNA relatedness group IIA (see Bacillus sphaericus in List of Species, below), the group in which the mosquitocidal strains of this species lie, is divided into 9 non-consecutively numbered serovars. The serotyping scheme for Bacillus sphaericus shows good agreement with a phage typing scheme (Yousten, 1984) for this species, but, as with Bacillus thuringiensis, the types do not always concur with pathogenicity (Priest, 2002).
The study of H-antigens of Bacillus subtilis (Simon et al., 1977) by agglutination tests was complicated by the tendency of the cells to clump spontaneously, and so double-diffusion in agar or complement fixation methods were applied. At least five distinct serovars were recognized, and although cross-reactions made the establishment of a practical serotyping scheme difficult, this approach was considered to be potentially useful in taxonomic studies.
As spores are so characteristic of the genus Bacillus, it is not surprising that their antigens have attracted much interest. Early workers (reviewed by Norris, 1962) recognized: (i) that any attempt to use living spores as antigens might be complicated by their germination in the host animal, giving rise to antisera against vegetative cells as well as spores; (ii) the need for a rapid method to overcome any problems of germination during the agglutination test itself; (iii) the tendency of spores to auto-agglutinate owing to their hydrophobic surfaces; and (iv) the need to remove vegetative cell debris from spore suspensions–by using media promoting complete sporulation, or by autolysis with lysozyme or thiomersalate, or by autoclaving. Once these problems are overcome, spore antigens can be useful for serological identification, and a revival of interest has been stimulated by the need to detect the spores of Bacillus anthracis in the contexts of biowarfare and bioterrorism (Iqbal et al., 2000). Several antigens common to endospores from different genera may be found on the exosporium, and an antibody to one of these antigens also reacted with vegetative cells of Bacillus cereus and Clostridium sporogenes (Quinlan and Foegeding, 1997).
Lamana (1940c) was able to differentiate between spores of several of the small-celled species by their antigens, but again was less successful with the large-celled species (Lamana, 1940b), and Lamana and Eisler (1960) were unable to separate Bacillus anthracis from Bacillus cereus using spore agglutinogens. Norris and Wolf (1961) reported a similar picture from their study of spore agglutinogens, but found that spore precipitinogens were of some taxonomic value, and the latter observation was confirmed for the Bacillus circulans complex by Wolf and Chowhury (1971a). Walker and Wolf (1971) found that spore agglutinogens supported the main biochemical subdivisions of their Bacillus (now Geobacillus) stearothermophilus strains, but could not detect spore precipitinogens. Smirnova et al. (1991) considered that hemagglutination patterns of fimbriated Bacillus thuringiensis spores might be of taxonomic value.
In order to circumvent cell-clumping problems, Kim and Goepfert (1972) developed a fluorescent antibody technique for confirming identifications of Bacillus cereus from food poisoning cases, but could not distinguish between spores of this species and Bacillus thuringiensis; it has subsequently been recognized that strains of the latter species may also cause food poisoning (Damgaard et al., 1997). Phillips and Martin (1983a, b) used radiolabeled polyclonal antibody to probe for Bacillus anthracis spores attached to solid supports. Spores would not attach reliably to microtiter plates, but although attachment to glass slides was better, the background signal in the immunoradiometric assay (IRMA) was higher; the best sensitivity was achieved with an indirect assay, but specificity was only moderate. Fluorescein-conjugated polyclonal antibodies to Bacillus anthracis spore surface antigens were found to cross-react with spores of several other Bacillus species, but these cross-reactions could be absorbed with strains of Bacillus cereus; however, spores of the Vollum strain (=type strain) did not react with antibodies to spores of most of the other strains of Bacillus anthracis tested (Phillips and Martin, 1988). A monoclonal antibody to viable and heat-killed spores of Bacillus anthracis was used in an immunofluorescence assay and achieved higher specificity but lower sensitivity than the IRMA approaches, but the epitope recognized by the antibody appeared to be unstable in spores stored for long periods (Phillips et al., 1988).
Anthrax vaccine The Sterne attenuated live spore vaccine, based on a toxigenic but non-capsulate strain, was introduced for animal vaccination in the late 1930s and spores of this strain remain in use as the basis of livestock vaccines in most parts of the world today. As this vaccine can show some slight virulence for certain animals, it is not considered suitable for human protection in the West, but live spore preparations are used in China and Russia. The former USSR vaccine was developed in the 1930s and 1940s, and licensed for administration by injection in 1959. It was based on two avirulent, non-capsulate Bacillus anthracis strains TI-I and 3, which were derived from virulent agents at the Sanitary-Technical Institute (STI), in Kirov (now Viatka). It was reported that in 30 years of use no adverse effects were associated with this vaccine, and so reconsideration of the suitability of live spore vaccines for human use has been suggested (Shiyakhov and Rubinstein, 1994). The UK vaccine is an alum-precipitated culture filtrate of the Sterne strain; it was first formulated in 1954, introduced for workers at risk in 1965, and licensed for human use in 1979. The current human vaccine in the USA is an aluminum hydroxide-adsorbed vaccine strain culture filtrate containing a relatively high proportion of protective antigen (PA) and relatively low amounts of lethal factor and edema factor; it was licensed in 1972 (Turnbull, 2000). Concerns about the lack of efficacy and safety data on the long-established UK and US vaccines, especially following the Sverdlovsk incident, and allegations that anthrax vaccination contributed to Gulf War syndrome in military personnel, have led to demands for new vaccines that would necessarily undergo stricter testing than was customary in the past. Favored active ingredients of these next-generation vaccines are whole-length recombinant PA or a mutant (non-toxic) portion of this molecule (Turnbull, 2000).
Antibiotic sensitivity. Most strains of Bacillus anthracis are susceptible to penicillin, there being few authenticated reports of resistant isolates (Lalitha and Thomas, 1997); consequently this antibiotic has been the mainstay of treatment and there have been few studies on the organism's sensitivity to other antibiotics. Mild and uncomplicated cutaneous infections may be treated with oral penicillin V, but the treatment usually recommended is intramuscular procaine penicillin or benzyl penicillin (penicillin G). In severe cases, and gastrointestinal and inhalational infections, the recommended therapy has been penicillin G by slow intravenous injection or infusion until the fever subsides, followed by intramuscular procaine penicillin; the organism is normally susceptible to streptomycin, which may act synergistically with penicillin (Turnbull et al., 1998). The use of an adequate dose of penicillin is important, as Lightfoot et al. (1990) found that strains grown in the presence of subinhibitory concentrations of flucloxacillin in vitro became resistant to penicillin and amoxycillin. The study of Lightfoot et al. (1990) on 70 strains, and that of Doganay and Aydin (1991) on 22 isolates, found that most strains were sensitive to penicillins, with minimal inhibitory concentrations of 0.03 mg/l or less; however, the former authors found that two resistant isolates from a fatal case of inhalational infection had MICs in excess of 0.25 mg/l. Bacillus anthracis is resistant to many cephalosporins. Coker et al. (2002) found that of 25 genetically diverse, mainly animal and human isolates from around the world, five strains were resistant to the “second generation” cephalosporin cefuroxime, and 19 strains showed intermediate susceptibility to this agent; all strains were sensitive to the “first generation” cephalosporin cephalexin, and to the “second generation” cefaclor, and three were resistant to penicillin but were negative for β-lactamase production. Mohammed et al. (2002) studied 50 historical isolates from humans and animals and 15 clinical isolates from the 2001 bioterrorist attack in the USA; the majority of their strains could be regarded as nonsusceptible to the “third generation” cephalosporin ceftriaxone, and three strains were resistant to penicillin. Genomic sequence data indicate that Bacillus anthracis possesses two β-lactamases: a potential penicillinase (class A) and a cephalosporinase (class B) which is expressed (Bell et al., 2002). Tetracyclines, chloramphenicol, gentamicin and erythromycin are suitable for the treatment of patients allergic to penicillin; tests in primates showed doxycycline to be effective, a finding confirmed by Coker et al. (2002), and indicated the suitability of ciprofloxacin (Turnbull et al., 1998). Mohammed et al. (2002) found that most of their strains showed only intermediate susceptibility to erythromycin. Esel et al. (2003) found that ciprofloxacin and the newer quinolone gatifloxacin had a good in vitro activity against 40 human isolates collected in Turkey, but that for another new quinolone, levofloxacin, it was observed that minimum inhibitory concentrations were high for 10 strains. Because human cases tend to be sporadic, clinical experience of alternative treatment strategies was sparse until the bioterrorist attack occurred in the US in late 2001. The potential and actual use of Bacillus anthracis as a bioweapon has also emphasized the need for post-exposure prophylaxis; recommendations include ciprofloxacin or doxycycline, with amoxycillin as an option for the treatment of children and pregnant or lactating women, given the potential toxicity of quinolones and tetracyclines; however, β-lactams do not penetrate macrophages well, and these are the sites of spore germination (Bell et al., 2002). Combination therapy, begun early, with a fluoroquinolone such as ciprofloxacin and at least one other antibiotic to which the organism is sensitive, appears to improve survival (Jernigan et al., 2001). Following the 2001 outbreak in the US, the recommendation for initial treatment of inhalational anthrax is ciprofloxacin or doxycycline along with one or more agents to which the organism is normally sensitive; given supportive sensitivity testing, a penicillin may be used to complete treatment. The same approach is recommended for cutaneous infections (Bell et al., 2002). Doxycycline does not penetrate the central nervous system well, and so is not appropriate for the treatment of meningitis.
Despite the well-established importance of Bacillus cereus as an opportunistic pathogen, there have been rather few studies of its antibiotic sensitivity, and most information has to be gleaned from the reports of individual cases or outbreaks. Bacillus cereus and Bacillus thuringiensis produce a broad spectrum β-lactamase and are thus resistant to penicillin, ampicillin, and cephalosporins; they are also resistant to trimethoprim. An in vitro study of 54 isolates from blood cultures by disk diffusion assay found that all strains were susceptible to imipenem and vancomycin and that most were sensitive to chloramphenicol, ciprofloxacin, erythromycin and gentamicin (with 2, 2, 6 and 7% strains, respectively, showing moderate or intermediate sensitivities), while 22 and 37% of strains showed only moderate or intermediate susceptibilities to clindamycin and tetraclycline, respectively (Weber et al., 1988); in the same study, microdilution tests showed susceptibility to imipenem, vancomycin, chloramphenicol, gentamicin and ciprofloxacin with MICs of 0.25–4, 0.25–2, 2.0–4.0, 0.25–2 and 0.25–1.0 mg/l, respectively. A plasmid carrying resistance to tetracycline in Bacillus cereus has been transferred to a strain of Bacillus subtilis and stably maintained (Bernhard et al., 1978).
Although strains are almost always susceptible to clindamycin, erythromycin, chloramphenicol, vancomycin, and the aminoglycosides and are usually sensitive to tetracycline and sulfonamides, there have been several reports of treatment failures with some of these drugs: a fulminant meningitis which did not respond to chloramphenicol (Marshman et al., 2000); a fulminant infection in a neonate which was refractory to treatment that included vancomycin, gentamicin, imipenem, clindamycin, and ciprofloxacin (Tuladhar et al., 2000); failure of vancomycin to eliminate the organism from cerebrospinal fluid in association with a fluid shunt infection (Berner et al., 1997); persistent bacteremias with strains showing resistance to vancomycin in two hemodialysis patients (A. von Gottberg and W. van Nierop, personal communication). Oral ciprofloxacin has been used successfully in the treatment of Bacillus cereus wound infections. Clindamycin with gentamicin, given early, appears to be the best treatment for ophthalmic infections caused by Bacillus cereus, and experiments with rabbits suggest that intravitreal corticosteroids and antibiotics may be effective in such cases (Liu et al., 2000).
Information is sparse on treatment of infections with other Bacillus species. Gentamicin was effective in treating a case of Bacillus licheniformis ophthalmitis and cephalosporin was effective against Bacillus licheniformis bacteremia/septicemia. Resistance to macrolides appears to occur naturally in Bacillus licheniformis (Docherty et al., 1981). Bacillus subtilis endocarditis in a drug abuser was successfully treated with cephalosporin, and gentamicin was successful against a Bacillus subtilis septicemia. Penicillin, or its derivatives, or cephalosporins probably form the best first choices for treatment of infections attributed to other Bacillus species. In the study by Weber et al. (1988), isolates of Bacillus megaterium (13 strains), Bacillus pumilus (4), Bacillus subtilis (4), Bacillus circulans (3), Bacillus amyloliquefaciens (2) and Bacillus licheniformis (1), along with five strains of Bacillus (now Paenibacillus) polymyxa and three unidentified strains from blood cultures, over 95% of isolates were susceptible to imipenem, ciprofloxacin and vancomycin; while between 75% and 90% were susceptible to penicillins, cephalosporins and chloramphenicol. Isolates of “Bacillus polymyxa” and Bacillus circulans were more likely to be resistant to the penicillins and cephalosporins than strains of the other species – it is possible that some or all of the strains identified as Bacillus circulans might now be accommodated in Paenibacillus, along with “Bacillus polymyxa.” An infection of a human bite wound with an organism identified as Bacillus circulans did not respond to treatment with amoxycillin and flucloxacillin, but was resolved with clindamycin (Goudswaard et al., 1995). A recurrent septicemia with Bacillus subtilis in an immunocompromised patient yielded two isolates, both of which could be recovered from the probiotic preparation that the patient had been taking; one isolate was resistant to penicillin, erythromycin, rifampin and novobiocin, while the other was sensitive to rifampin and novobiocin but resistant to chloramphenicol (Oggioni et al., 1998).
A strain of Bacillus circulans showing vancomycin resistance has been isolated from an Italian clinical specimen (Ligozzi et al., 1998). Vancomycin resistance was reported for a strain of Bacillus (now Paenibacillus) popilliae in 1965, and isolates of this species dating back to 1945 have been shown to carry a vanA- and vanB-like gene, that is to say a gene resembling those responsible for high-level vancomycin resistance in enterococci. Vancomycin-resistant enterococci (VRE) were first reported in 1986, and so it has been suggested that the resistance genes in Bacillus popilliae and VRE may share a common ancestor, or even that the gene in Bacillus popilliae itself may have been the precursor of those in VRE; Bacillus popilliae has been used for over 50 years as a biopesticide, and no other potential source of vanA and vanB has been identified (Rippere et al., 1998). Of two South African vancomycin-resistant clinical isolates, one was identified as Paenibacillus thiaminolyticus and the other was unidentified but considered to be related to Bacillus lentus (Forsyth and Logan, unpublished); the latter was isolated from a case of neonatal sepsis, and has been shown to have inducible resistance to vancomycin and teicoplanin; this is in contrast to the Bacillus circulans and Paenibacillus thiaminolyticus isolates mentioned above, in which expression of resistance was found to be constitutive (A. von Gottberg and W. van Nierop, personal communication).
Isolates of novel Bacillus species from pristine Antarctic environments showed sensitivity to: ampicillin, chloramphenicol, colistin sulfate, kanamycin, nalidixic acid (Bacillus fumarioli resistant), nitrofurantoin, streptomycin and tetracycline (Logan et al., 2000, 2002b, and unpublished information).
Pathogenicity. The majority of Bacillus species apparently have little or no pathogenic potential and are rarely associated with disease in humans or other animals. The principal exceptions to this are Bacillus anthracis (anthrax), Bacillus cereus (food poisoning and opportunistic infections), and Bacillus thuringiensis (pathogenic to invertebrates), but a number of other species, particularly Bacillus licheniformis, have been implicated in food poisoning and other human and animal infections. The resistance of the spores to heat, radiation, disinfectants, and desiccation also results in Bacillus species being troublesome contaminants in the operating room, on surgical dressings, in pharmaceutical products and in foods.
Bacillus anthracis. Anthrax is primarily a disease of herbivores, and before an effective veterinary vaccine became available in the late 1930s, it was one of the foremost causes worldwide of mortality in cattle, sheep, goats, and horses. In 1945 an outbreak in Iraq killed 1 m sheep. The development and application of veterinary and human vaccines together with improvements in factory hygiene and sterilization procedures for imported animal products, and the increased use of man-made alternatives to animal hides or hair, have resulted over the past half century in a marked decline in the incidence of the disease in both animals and humans. Nevertheless, the disease continues to be endemic in several countries of Africa, Asia, and central and southern Europe, particularly those that lack an efficient vaccination policy, and nonendemic regions must be constantly on the alert for the arrival of Bacillus anthracis in imported products of animal origin. Sites where these materials were formerly handled, such as disused tanneries, may be sources of infection when they are disturbed during redevelopment. Likewise, anthrax carcasses can remain infectious for many years, even when buried with quicklime. The cycle of infection is as follows: the spores are ingested by a grazing animal and may gain access to the lymphatics, and so to the spleen, though abrasions in the alimentary canal; following several days of the organism multiplying and producing toxin in the spleen, the animal suffers a sudden and fatal septicemia and collapses; hemorrhagic exudates escape from the mouth, nose and anus and contaminate the soil, where the vegetative cells sporulate in the air. The spores remain viable in soil for many years and their persistence does not depend on animal reservoirs, so that Bacillus anthracis is exceedingly difficult to eradicate from an endemic area. Bacillus anthracis continues to be generally regarded as an obligate pathogen. Its continued existence in the ecosystem appears to depend on a periodic multiplication phase within an animal host, with its environmental presence reflecting contamination from an animal source at some time (Lindeque and Turnbull, 1994); however, some authorities believe that self-maintenance may occur within certain soil environments (Cherkasskiy, 1999). Direct animal-to-animal transmission within a species (that is to say, excluding carnivorous scavenging of meat from anthrax carcasses) is very rare.
Bacillus anthracis has long been considered a potential agent for biological warfare or bioterrorism. It is believed its first use was against livestock during World War I (Barnaby, 2002; Christopher et al., 1997). It has been included in various development and offensive programmes in several countries since (Alibek, 1999; Barnaby, 2002; Mangold and Goldberg, 1999; Mikkola et al., 2000; Zilinskas, 1997) and it has also been used in terrorist attacks (Christopher et al., 1997; Lane and Fauci, 2001; Takahashi et al., 2004). In public consciousness, Bacillus anthracis is associated more with warfare and terrorism than with a disease of herbivores, and it is feared accordingly. Apart from artificial attacks, humans almost invariably contract anthrax directly or indirectly from animals. It is a point-source type of disease, and direct human-to-human transmission is exceedingly rare. Circumstantial evidence shows that humans are moderately resistant to anthrax as compared with obligate herbivores; infectious doses in the human inhalational and intestinal forms are generally very high (LD50 2,500 to 55,000 spores). Naturally acquired human anthrax may result from close contact with infected animals or their carcasses after death from the disease, or be acquired by those employed in processing wool, hair, hides, bones, or other animal products. Most cases (about 99%) are cutaneous infections, but Bacillus anthracis meningitis and gastrointestinal anthrax are occasionally reported. In industrial settings, inhalation of spore-laden dust may also occur; anthrax weapons are normally intended to cause the inhalational form, but are likely to cause cutaneous cases as well. There have been a few reports of laboratory-acquired infections, none of them recent (Collins, 1988), but a major outbreak of anthrax occurred in April 1979 in the city of Sverdlovsk (now Yekaterinburg) in the Urals as a result of the accidental release of spores from a military production facility; 77 cases were recorded and 66 patients died (Meselson et al., 1994).
Cutaneous infection occurs through a break in the skin, and as Bacillus anthracis is not invasive the lesions generally occur on exposed regions of the body; this includes the eyelids. Before the availability of antibiotics and vaccines, 10–20% of untreated cases of cutaneous anthrax were fatal, and the rare fatalities seen today are due to obstruction of the airways by the edema that accompanies lesions on the face or neck, and sequelae of secondary cellulitis or meningitis. Inhalational anthrax cases are more often fatal, because they go unrecognized until too late for effective therapy, but undiagnosed, low-grade infections with recovery may occur. The number of recorded cases of inhalational anthrax is lower than might be expected from the high profile given to this condition. In the 20th century there were just 18 reported cases (two of them laboratory-acquired) in the USA, 16 (88.9%) fatal (Brachman and Kaufmann, 1998); figures in the UK showed a similar picture. In 11 confirmed cases of inhalational anthrax that followed a bioterrorist attack, in which spores were delivered in mailed letters and packages, early recognition and treatment helped a survival level of 55% to be achieved (Bell et al., 2002; Jernigan et al., 2001). Oropharyngeal and gastrointestinal anthrax are not uncommon in regions of the world where animal anthrax is endemic and socio-economic conditions are poor, and people eat the meat of animals that have died suddenly; such cases are greatly underreported (Anonymous, 1994; Dietvorst, 1996). Gastrointestinal infections are mainly characterized by gastroenteritis, and asymptomatic infections and symptomatic infections with recovery are not uncommon (CDC, 2000). The symptoms of oropharyngeal infections are fever, toxemia, inflammatory lesions in the oral cavity and oropharynx, cervical lymph node enlargement, and edema, and there is a high case-fatality rate (Sirisanthana and Brown, 2002). Meningitis can develop from any of the forms of anthrax. The emergence of clinical signs is rapidly followed by unconsciousness, and the prognosis is poor. Outbreaks of primary anthrax meningoencephalitis have been reported from India and elsewhere (George et al., 1994; Kwong et al., 1997).
Infection occurs when endospores enter the body from the environment, and the spore is the primary infectious form of the organism (Hanna and Ireland, 1999). Spores are rapidly phagocytosed by macrophages, some of which undergo lysis, and in cases of inhalational anthrax the surviving macrophages are carried towards the mediastinal lymph nodes by the lymphatics. Phagocytosed spores may not germinate for up to 60 d, and so incubation of the inhalational form of the disease may take between 2 d and 6–8 weeks; this latency does not appear to occur in the cutaneous form of the disease. By analogy with other Bacillus species, germination is presumed to be triggered by a specific chemical germinant, but this remains unidentified. In several Bacillus species L-alanine is a germinant, and its binding to a receptor causes loss of spore refractility and resistance, the cortex swells, and metabolic activity commences; triggering of the germinant receptor is believed to activate endogenous proteolytic activity which converts the proenzyme of a germination-specific cortex-lytic enzyme to its active form which allows hydrolysis of the cortex, uptake of water, and all the other events associated with germination. The elevated CO2 level and body temperature of the host cause the organism to transcriptionally activate the capsule and toxin genes. These genes are carried on two plasmids: plasmid pXO1 encodes the toxin genes, and plasmid pXO2 encodes the capsule genes; loss of either plasmid effectively renders the organism avirulent. Spores germinating in the presence of serum and elevated levels of CO2 release blebs of capsular material through openings in the spore surface; the capsule of poly-γ-D-glutamic acid is purported to resist phagocytosis by virtue of its negative charge (Ezzell and Welkos, 1999). The anthrax toxin complex comprises three components: edema factor (EF), protective antigen (PA), and lethal factor (LF), none of which is toxic alone; EF and LF are active in binary combinations with PA and have different activities. PA molecules bind to molecules of a particular host cell membrane protein (anthrax toxin receptor or ATR; Bradley et al., 2001) and form ring-shaped prepores of heptameric oligomers (aggregates of seven); PA is then cleaved and so activated by a furin-like protease on the surface of the cell under attack. An active PA heptamer can then bind one or more molecules of EF, LF or both. The complex passes into the cell by receptor-mediated endocytosis and into an acidified endosome; following conformational change of the heptamer in the low-pH environment, the complex escapes directly to the cytosol by insertion of the heptamer into the endosomal membrane. The PA-EF binary toxin interacts with the abundant host protein calmodulin (CaM; the major intracellular calcium receptor) and becomes an active adenylyl cyclase in most cell types; this elevates levels of the secretogogue cAMP and leads to hypovolaemic shock. The crystal structure of EF in complex with CaM has been elucidated (Drum et al., 2002). The PA-LF binary toxin is a zinc metalloprotease which cleaves members of the mitogen-activated protein kinase kinase family, so affecting certain signaling pathways, and levels of shock-inducing cytokines. This toxin primarily affects macrophages, and removal of macrophages from mice using silica renders them insensitive to the toxin (Hanna, 1999); however, the process which leads to macrophage lysis is unclear (Pannifer et al., 2001). The importance of any interaction between EF and LF awaits clarification. The molecular pathogenesis of infection with Bacillus anthracis was reviewed by Little and Ivins (1999).
Bacillus cereus. Bacillus cereus is next in importance to Bacillus anthracis as a pathogen of humans (and other animals), causing food-borne illness and opportunistic infections, and its ubiquity ensures that cases are not uncommon. In relation to food-borne illness, Bacillus cereus is the etiological agent of two distinct food poisoning syndromes (Kramer and Gilbert, 1992): (i) the diarrheal-type, characterized by abdominal pain with diarrhea 8–16 h after ingestion of the contaminated food and associated with a diversity of foods from meats and vegetable dishes to pastas, desserts, cakes, sauces, and milk, and (ii) the emetic-type characterized by nausea and vomiting 1–5 h after eating the offending food, predominantly oriental rice dishes, although occasionally other foods such as pasteurized cream, milk pudding, pastas, and reconstituted formulas have been implicated. One outbreak followed the mere handling of contaminated rice in a children's craft activity (Briley et al., 2001), and fulminant liver failure associated with the emetic toxin has been reported (Mahler et al., 1997). Both syndromes arise as a direct result of the fact that Bacillus cereus spores can survive normal cooking procedures. Under improper storage conditions after cooking, the spores germinate and the vegetative cells multiply. In diarrheal illness, the toxin(s) responsible are produced by organisms in the small intestine (infective doses 104–109 cells per gram of food), while the emetic toxin is preformed and ingested in food (about 105–108 cells per gram in order to produce sufficient toxin). Variations in infective dose of the diarrheal illness will reflect the proportion of ingested cells that are sporulated, and so can survive the acid barrier of the stomach. The capacity of the strain concerned to produce toxin(s) will, of course, influence the infective or intoxicating dose in both types of illness. It is likely that cases showing both diarrheal and emetic symptoms are caused by organisms producing both diarrheal and emetic toxins. Strains of Bacillus thuringiensis, which are close relatives of Bacillus cereus, may also produce the diarrheal toxin, and Bacillus thuringiensis has indeed been implicated in cases of gastroenteritis (Damgaard et al., 1997); strains of this species commonly carry genes for Bacillus cereus enterotoxins (Rivera et al., 2000) and Fletcher and Logan (1999) found that strains of both Bacillus mycoides and Bacillus thuringiensis were positive in commercial tests for enterotoxin and in a cytotoxicity assay. Cases of illness caused by Bacillus thuringiensis may have been diagnosed as caused by Bacillus cereus, as the former may not produce its characteristic insecticidal toxin crystals when incubated at 37°C, owing to the loss of the plasmids carrying the toxin genes (Granum, 2002). The safety of using Bacillus thuringiensis as a biopesticide on crop plants has been reviewed by Bishop (2002); Bishop et al. (1999) found that the main pesticide strains that they assayed produced low titers of enterotoxin.
The toxigenic basis of Bacillus cereus food poisoning and other Bacillus cereus infections has begun to be elucidated, and a complex picture is emerging (Beecher, 2001; Granum, 2002). Bacillus cereus is known to produce six toxins, four of which are enterotoxins, and the emetic toxin. The enterotoxins are (i) Hemolysin BL (Hbl), a 3-component proteinaceous toxin which also has dermonecrotic and vascular permeability activities, and causes fluid accumulation in ligated rabbit ileal loops; Hbl is produced by about 60% of strains tested (Granum, 2002), and it has been suggested that is a primary virulence factor in Bacillus cereus diarrhea, but the mechanism of its enterotoxic activity is unclear (Granum, 2002); (ii) Non-hemolytic enterotoxin (Nhe) is another 3-component proteinaceous toxin which is produced by most strains tested (Granum, 2002), and whose components show some similarities to Hbl; (iii) and (iv) Enterotoxin T (BceT) and Enterotoxin FM (EntFM) are single-component proteinaceous toxins whose roles and characteristics are not known; also, Cytotoxin K (CytK) is similar to the β-toxin of Clostridium perfringens and was associated with a French outbreak of necrotic enteritis in which three people died (Lund et al., 2000). The genetics of toxin production are summarized by Granum (2002).
The emetic toxin, cereulide, is a dodecadepsipeptide comprising a ring of four amino- and/or oxy-acids: [D-O-Leu-D-Ala-L-O-Val-L-Val] thrice repeated; chemically speaking, it is closely related to the potassium ionophore valinomycin (Agata et al., 1994). It is resistant to heat, pH and proteolysis, but it is not antigenic (Kramer and Gilbert, 1989). Cereulide is probably an enzymically synthesized peptide rather than a direct genetic product; it is produced in larger amounts at lower incubation temperatures, its production does not appear to be connected with sporulation (Finlay et al., 2000), and it is produced in aerobic, and microaerobic, but not in anaerobic conditions (Finlay et al., 2002). Its mechanism of action is unknown, but it has been shown to stimulate the vagus afferent through binding to the 5-HT3 receptor (Agata et al., 1995). The earliest detection system for emetic toxin involved monkey-feeding tests (Logan et al., 1979), but a semi-automated metabolic staining assay has now been developed (Finlay et al., 1999).
Bacillus cereus is also a destructive ocular pathogen. Endophthalmitis may follow penetrating trauma of the eye, intraocular surgery, or hematogenous spread, and it may evolve very rapidly. Loss both of vision and the eye is likely if appropriate treatment is instituted too late (Das et al., 2001; Davey and Tauber, 1987). Bacillus cereus keratitis associated with contact lens wear has also been reported (Pinna et al., 2001) Other Bacillus cereus infections occur mainly, though not exclusively, in persons predisposed by neoplastic disease, immunosuppression, alcoholism and other drug abuse, or some other underlying condition, and fatalities occasionally result. Reported conditions include bacteremia, septicemia, fulminant sepsis with hemolysis, meningitis, brain hemorrhage, ventricular shunt infections, infections associated with central venous catheters, endocarditis, pseudomembranous tracheobronchitis, pneumonia, empyema, pleurisy, lung abscess, brain abscess, osteomyelitis, salpingitis, urinary tract infection, dermatolymphangioadenitis associated with filarial lymphedema, and primary cutaneous infections. Wound infections, mostly in otherwise healthy persons, have been reported following surgery, road traffic and other accidents, scalds, burns, plaster fixation, drug injection (including a case associated with contaminated heroin; (Dancer et al., 2002) and close-range gunshot and nail bomb injuries; some became necrotic and gangrenous. A fatal inflammation was caused by a blank firearm injury; blank cartridge propellants are commonly contaminated with the organism (Rothschild and Leisenfeld, 1996). Neonates also appear to be particularly susceptible to Bacillus cereus, especially with umbilical stump infections; respiratory tract infections associated with contaminated ventilation systems have also occurred (Van Der Zwet et al., 2000). Other infections reported in neonates include intestinal perforation, meningoencephalitis, and bacteremia refractory to therapy. There have been reports of wound, burn, and ocular infections with Bacillus thuringiensis (Damgaard et al., 1997), but there is as yet no evidence of infections associated with the use of this organism as an insecticide.
Bacillus cereus also causes infections in domestic animals. It is a well-recognized agent of mastitis and abortion in cattle, and can cause these conditions in other livestock (Blowey and Edmondson, 1995).
Other species. Reports of infections with non-Bacillus cereus group species are comparatively rare, but very diverse (Berkeley and Logan, 1997; Logan, 1988), and there have been several hospital pseudoepidemics associated with contaminated blood culture systems. Bacillus licheniformis has been reported from ventriculitis following the removal of a meningioma, cerebral abscess after penetrating orbital injury, septicemia following arteriography, bacteremia associated with indwelling central venous catheters (Blue et al., 1995), bacteremia during pregnancy with eclampsia and acute fibrinolysis, peritonitis in a CAPD patient and in a patient with volvulus and small-bowel perforation, ophthalmitis, and corneal ulcer after trauma. There have also been reports of L-form organisms, phenotypically similar to Bacillus licheniformis, occurring in blood and other body fluids of patients with arthritis, patients with neoplasms, clinically normal persons, and in association with infectious synovitis in birds (see Cell morphology, above). Although some authors have claimed a relationship between these organisms and diseases with postulated immunological elements, and higher isolations from the synovial fluids and membranes of arthritic patients have been reported, Bartlett and Bisset (1981) were unable to confirm the latter association. Bacillus licheniformis can cause food-borne diarrheal illness, and has been associated with an infant fatality (Mikkola et al., 2000). A toxin possibly associated with Bacillus licheniformis food poisoning has been identified (Mikkola et al., 2000), and toxigenic strains of Bacillus pumilus have been isolated in association with food-borne illness and from clinical and environmental specimens (Suominen et al., 2001). Bacillus licheniformis is frequently associated with bovine abortion and has been reproduced by experimental infection of cows, which demonstrated the tropism of the organism for the bovine placenta (Agerholm et al., 1997); this species has also been associated with abortion in water buffalo (Galiero and De Carlo, 1998), and is occasionally associated with bovine mastitis (Blowey and Edmondson, 1995). Many of these types of Bacillus licheniformis and Bacillus cereus infections are associated with wet and dirty conditions during winter housing, particularly when the animals lie in spilled silage (Blowey and Edmondson, 1995); in one outbreak, a water tank contaminated with Bacillus licheniformis was implicated (Parvanta, 2000).
The name Bacillus subtilis was often used to mean any aerobic, endospore-forming organism, but since 1970 there have been reports of infection in which identification of this species appears to have been made accurately. They include bacteremias associated with immunosuppression, surgical intervention, neoplastic disease, and trauma (de Boer and Diderichsen, 1991); other cases associated with neoplastic disease include: fatal pneumonia and bacteremia, a septicemia and an infection of a necrotic axillary tumour in breast cancer patients; breast prosthesis and ventriculo-atrial shunt infections; endocarditis in a drug abuser; meningitis following a head injury; cholangitis associated with kidney and liver disease; and isolations from dermatolymphangioadenitis associated with filarial lymphedema (Olszewski et al., 1999), and from surgical wound-drainage sites. Bacillus subtilis has also been associated with cases of bovine mastitis and of ovine abortion (Logan, 1988).
Bacillus subtilis has been implicated in food-borne illness: vomiting has been the commonest symptom, but with accompanying diarrhea frequently reported, the onset periods have been short (ranging from 10 min to 14 h; median 2.5 h), the bacterial loads of the organism were high (105–109 c.f.u./g), and the implicated foods were often prepared dishes in which meat or fish were served with cereal-based components such as bread, pastry, rice or stuffing (Kramer and Gilbert, 1989).
A probiotic preparation labeled as containing strains Bacillus subtilis led to a fatal septicemia in an immunocompromised patient (Oggioni et al., 1998); subsequently, the organisms concerned were identified as Bacillus clausii (Spinosa et al., 2000). These authors reported another Bacillus clausii infection, cholangitis in polycystic kidney disease in a 15-year-old French boy who had undergone renal transplant. The original authors (Wallet et al., 1996) had identified the organism as Bacillus subtilis; their patient had not been taking a probiotic preparation and the source of the infecting Bacillus clausii was unclear (Spinosa et al., 2000).
Organisms identified as Bacillus circulans have been isolated from cases of meningitis, a cerebrospinal fluid shunt infection, endocarditis, a wound infection in a cancer patient, a bite wound, and endophthalmitis (Tandon et al., 2001). Roy et al. (1997) reported epidemic endophthalmitis associated with isolates identified as Bacillus circulans that contaminated a product used during cataract surgery. It must be noted, however, that many isolates previously identified as Bacillus circulans might have been misallocated (see comments on Bacillus circulans in the List of species, below). Bacillus coagulans has been isolated from corneal infection, bacteremia and bovine abortion. Bacillus pumilus has been found in cases of pustule and rectal fistula infection, and in association with bovine mastitis. Bacillus sphaericus has been implicated in a fatal lung pseudotumour, and meningitis. Among 18 cancer patients with 24 bacteremic episodes, Banerjee et al. (1988) isolated Bacillus cereus (eight cases), Bacillus circulans (3), Bacillus subtilis (2), Bacillus coagulans (1), Bacillus licheniformis (1), Bacillus pumilus (1), Bacillus sphaericus (1) and six unidentified aerobic endospore-formers.
Fish. There have been several reports of Bacillus infections among farmed fish. Bacillus mycoides was isolated from necrotic muscular lesions in channel catfish (Ictalurus punctatus) during an epizootic in a commercial pond in Alabama, USA, and similar lesions could be reproduced by subcutaneous injection of the isolate (Goodwin et al., 1994). An unidentified aerobic endospore-former was associated with a septicemic condition affecting a variety of widely cultivated freshwater fish in Nigeria, and its etiological role was confirmed by reinfection trials (Oladosu et al., 1994). Ferguson et al. (2001) isolated an unidentified Bacillus from a severe multi-focal, necrotizing and granulomatous infection of the intensively reared catfish Pangasius hypophthalmus in the Mekong delta; the condition was reproduced experimentally, but subsequently the pathogenicity of this organism has not been demonstrable (M. Crumlish, personal communication).
Insect pathogens. Bacillus thuringiensis strains produce crystalline, proteinaceous, parasporal bodies within the sporangia (Figure 3h), and the insecticidal activities of many of these δ-endotoxins have made the organism (often referred to as Bt) one of the most widely produced and studied bacteria in biotechnology. The δ-endotoxins are produced as the organism begins to sporulate (in most cases; Sekar, 1988), and may represent up to 30% of sporangial dry weight by the completion of sporulation (Baum and Malvar, 1995). The δ-endotoxin genes are designated with the “cry” prefix to indicate that their product proteins are crystalline; they are nearly always located on large conjugative plasmids (Aronson, 1993), and transposons and insertion elements have been found associated with them. During infection, plasmids may be transferred between strains by conjugation (Thomas et al., 2000). There are over 80 different classes and subclasses of Cry proteins, representing at least four distinct protein families, and they have their own nomenclatural system (Crickmore et al., 1998). Related proteins are produced by Clostridium bifermentans (Barloy et al., 1996) and Paenibacillus popilliae (Zhang et al., 1997). Some strains of Bt which are active against Diptera produce structurally unrelated crystalline proteins known as Cyt toxins (Ellar, 1997). The full list of Cry and Cyt toxins is maintained at: http://www.biols.susx.ac.uk/Home/Neil_Crickmore/Bt/. An individual sporangium may carry a single type of insecticidal crystal protein, or several different types, comprising one or more parasporal bodies. Despite the name, the targets of insecticidal crystal proteins are not restricted to insects such as Lepidoptera (butterflies and moths), Coleoptera (beetles) and Diptera (flies); some mites, nematodes, flatworms and protozoa are also susceptible (Feitelson et al., 1992). The protein structure and mode of action of the δ-endotoxins remain subjects of intensive study, which has been reviewed by Ellar (1997), Schnepf et al. (1998) and Aronson and Shai (2001). The δ-endotoxins have three-domain structures (Groschulski et al., 1995; Li et al., 1991), with molecular masses usually of around 70 kDa or 130–140 kDa. Their phylogenetic relationships have been investigated by Bravo (1997). Domain I forms pores in susceptible gut epithelia, while Domain II has receptor-binding properties which influence the toxin's spectrum of activity (reviewed by Dean et al., 1996). Several roles have been suggested for Domain III; it is believed to play a part in receptor binding – perhaps an initial and reversible binding which is followed by an irreversible interaction mediated by Domain II (Lee et al., 1999b). An aminopeptidase N (Knight et al., 1994) and a cadherin-like glycoprotein (Vadlamudi et al., 1995; Vladmudi et al., 1995) have been identified as δ–endotoxin-binding proteins in insect larval gut epithelia.
The ingested protoxin dissolves in the alkaline midgut of a susceptible insect larva and undergoes proteolytic activation. Binding to the specific receptor on the brush-border cell is followed by insertion into the membrane to form a pore (Knowles, 1994) through which small molecules and ions can pass, so that water is taken up by osmosis and the cells swell and lyse (Ellar, 1997). It has been suggested that the concomitant fall in midgut pH is followed by spore germination and a fatal septicemia, while the micro-organism benefits from the nutrients so liberated (Ellar, 1990). With neutral or acidic guts, proteolytic nicking may allow protoxin solubilization (Carroll et al., 1997), and pore characteristics may vary with pH (Schwarz et al., 1993). The applications and development of Bt pesticides, and their safety, have been reviewed by Bishop (2002), while Van Rie 2002 has reviewed the development of transgenic crop plants.
Bacillus sphaericus has been divided into six taxa on the basis of DNA relatedness, supported by other molecular studies, and some strains belonging to group IIA (Rippere et al., 1997) are pathogenic to mosquitoes and have been exploited for biological control purposes. The parasporal crystal proteins synthesized by pathogenic strains of Bacillus sphaericus share no homology with those produced by Bacillus thuringiensis (Baumann et al., 1991; Charles et al., 1996; Porter et al., 1993). Early isolates, recovered in California, had mosquitocidal activities too low to be of practical use (Kellen et al., 1965), but further isolates, strain numbers 1,593 from Indonesia, 2,362 from Nigeria and 2,297 from Sri Lanka, showed toxicities high enough to be of value in vector control programmes (reviewed by Baumann et al., 1991; Charles et al., 1996). These later, highly toxic, strains all produced parasporal toxin crystals which were absent from the earlier, poorly toxic, isolates. The crystals, although smaller than those seen in Bacillus thuringiensis, are visible by phase-contrast microscopy (Priest, 2002). Screening programmes around the world have yielded over 560 further mosquitocidal isolates, and these are held by the International Entomopathogenic Bacillus Centre at the Institute Pasteur, Paris. Toxicity for different species of mosquito vary: there is no activity against Aedes aegypti; other Aedes species are moderately susceptible; intermediate activity against Anopheles species has attracted interest for malaria control; high activity against Culex quinquefasciatus is used to combat Japanese encephalitis and filariasis (Priest, 2002). Insects other than mosquitoes, and mammals, are unaffected by the toxins.
In all cases, it is the mosquito larvae that are attacked. As they feed, they ingest the spore/crystal complex, and symptoms follow rapidly; the spores then germinate so that the organism can take advantage of the rich nutrient supply. The parasporal crystal produced by high-toxicity strains of Bacillus sphaericus lies within the exosporium and is a binary toxin (Bin) reminiscent of cholera and diphtheria toxins. Its bin genes are highly conserved, only four variants being known (Priest, 2002). When ingested by a mosquito larva the component polypeptides, BinA and BinB, dissolve in the alkaline conditions of the midgut; BinA is slowly reduced from 42 kDa to an active form of about 39 kDa by host proteases, while BinB is more rapidly reduced from 51 kDa to an active form of about 43 kDa (Aly et al., 1989; Broadwell and Baumann, 1987). The exact mechanism of toxicity is not clear, but it is known that BinB is responsible for specific binding while BinA effects channel formation (Priest, 2002). Following mitochondrial swelling, large vacuoles appear in cells of the gastric caecum and posterior midgut (Davidson, 1981), peristalsis stops, and the larvae may die within 6 h. The Mtx1 protein, which is formed within the vegetative cells, is responsible for the toxicity of some strains that lack parasporal crystals. This protein of about 100 kDa is broken into subunits of 27 kDa and 70 kDa which respectively resemble an ADP-ribosylating toxin and a glycoprotein-binding protein (Hazes and Read, 1995; Thanabalu et al., 1993). Although highly toxic to a wide range of mosquitoes, it is not highly expressed and is subject to proteolytic attack within the bacterium (Ahmed et al., 1995; Wati et al., 1997). Other mosquitocidal toxin genes include mtx2 (Liu et al., 1996) and mtx3 (Thanabalu and Porter, 1996), whose related products act by pore formation. As some isolates that lack all the toxins described above show weak pathogenicity, it is likely that further toxins await discovery (Priest, 2002).
Ecology. Most Bacillus species are saprophytes widely distributed in the natural environment, but some species are opportunistic or obligate pathogens of animals, including humans, other mammals, and insects. Bacillus anthracis is, to all intents and purposes, an obligate pathogen of animals and humans. The habitats of most species are soils of all kinds, ranging from acid through neutral to alkaline, hot to cold, and fertile to desert, and the water columns and bottom deposits of fresh and marine waters. Their endospores readily survive distribution in soils, dusts and aerosols from these natural environments to a wide variety of other habitats, and Nicholson et al. (2000) has considered the roles of Bacillus spores in the natural environment. Some species appear to be ubiquitous contaminants of man, other animals, their foodstuffs, water and environments, natural, domestic, industrial and hospital. Their wide distribution is in part owing to the extraordinary longevity of their endospores, which show much greater resistance to physical and chemical agents, such as heat, cold, desiccation, radiation, disinfectants, antibiotics and other toxic agents, than their counterpart vegetative cells. Endospores are typically more resistant to heat than vegetative cells by a factor of 105 or more, while resistance to UV and ionizing radiation may be 100-fold or more. If protected from radiation, spores may survive for very long periods. Striking claims include the isolation of a viable strain of Bacillus sphaericus from an extinct bee preserved in 25- to 40-million-year-old amber (Cano and Borucki, 1995), and the recovery of an aerobic endospore-former related to Salibacillus and Virgibacillus from a water droplet trapped in a Permian salt crystal for an estimated 250 million years (Vreeland et al., 2000); the latter report has been contested on account of the apparent modernity of its DNA (Graur and Pupko, 2001; Nickle et al., 2002), and both isolations need to be confirmed by independent laboratories. Bacillus infernus was isolated from Triassic shales, lying at depths of around 2.7 km below the land surface, that may have been hydrologically isolated for 140 million years, and although they have not been demonstrated for this organism, endospores are likely to have contributed to its survival (Boone et al., 1995). Another organism from a deep subsurface environment is Bacillus subterraneus, which was isolated from the Great Artesian Basin of Australia, a thermal aquifer up to 2 km deep (Kanso et al., 2002); spores have not been demonstrated for this species either.
The commonly isolated species, such as Bacillus subtilis and Bacillus cereus are very widely distributed worldwide; Bacillus thuringiensis has been isolated from all continents, including Antarctica (Forsyth and Logan, 2000); as well as being a common organism in the natural environment and foods, Bacillus cereus frequently contaminates domestic kitchen environments (Beumer and Kusumaningrum, 2003). In studies of indoor and outdoor air and dusts, Bacillus species commonly dominate the cultivable flora or form a large part of it (Aldagal and Fung, 1993; Andersson et al., 1999b; Dutkiewicz et al., 2001; Marafie and Ashkanani, 1991; Schaffer and Lighthart, 1997; Venkateswaran et al., 2001), and honeybees have been found to scavenge airborne spores electrostatically (Lighthart et al., 2000). The presence of Bacillus fumarioli strains showing similar phenotypic behavior and substantial genotypic similarity from Candlemas Island in the South Sandwich archipelago and from volcanoes some 5600 km distant on continental Antarctica, is most convincingly explained by their carriage in the air as free spores or spores attached to plant propagules, as no birds are known to visit the latter sites (Logan et al., 2000). Strains indistinguishable from these Antarctic isolates have been cultivated from gelatin production plants in Belgium, France and the USA (De Clerck et al., 2004a).
Bacillus species are often isolated following heat treatment of specimens in order to select for spores, and presence of spores in a particular environment does not necessarily indicate that the organism is metabolically active there; however, it is reasonable to assume that large numbers of endospores in a given environment reflects former or current activity of vegetative Bacillus cells there. Indeed, the ease with which strains of a close relative of Bacillus pumilus and strains of Bacillus thuringiensis have been isolated from pristine environments along the Victoria Land coast of Antarctica (Forsyth and Logan, 2000; and unpublished observations) cannot easily be explained as widespread and chance contamination with endospores from another source; the organisms almost certainly undergo some multiplication in those environments. Bacillus fumarioli was found as both spores and vegetative cells at geothermal sites in Antarctica where soil temperatures ranged from 3.4°C to 62.5°C; the proportions of sporulated cells tended to be higher at the temperature extremes and lower at temperatures approaching the optimum growth temperature (50°C) of the organism (Logan et al., 2000). Isolation of organisms showing special adaptions to the environments in which they are found, such as acidophily, alkaliphily, halophily, psychrophily, and thermophily, suggests that these organisms must be metabolically active in these niches, but it tells us little about the importances of their roles in the ecosystems, and nothing about their interactions with other members of the flora. Bacillus thermantarcticus, which warrants transfer to Geobacillus (see Species Incertae Sedis, below), was also found in the geothermal soil of Cryptogam Ridge, Mount Melbourne, Antarctica (Nicolaus et al., 1996), a site from which Bacillus fumarioli was isolated (Logan et al., 2000).
Many Bacillus species will degrade biopolymers, with versatilities varying according to species, and it is therefore assumed that they have important roles in the biological cycling of carbon and nitrogen; it is further assumed that their activities in food spoilage and biodegradation reflect the contamination of these materials by endospores derived from dusts and other vehicles. Valid though these assumptions may be, the ever-increasing diversity of known Bacillus species and their apparent primary habitats implies that such generalizations may deserve reconsideration in some cases, and that certain species may have quite specialized activities.
Habitats. Although isolates of many of the established species have been derived from soil, or from environments that may have been contaminated directly or indirectly by soil, the range of isolation sources is very wide, and includes, in addition to temperate, acidic, neutral and alkaline soils, fresh and marine waters, foods and clinical specimens: air (Bacillus carboniphilus), arsenic-rich sediments (Bacillus arseniciselenatis, Bacillus selenitireducens) and arsenic-contaminated mud and water (Bacillus indicus, Bacillus macyae), bauxite-processing waste (Bacillus vedderi), brine (Bacillus haloalkaliphilus), compost (Bacillus circulans, Bacillus coagulans, Bacillus licheniformis, Bacillus sphaericus, Bacillus subtilis), emperor moth caterpillars (“phane”; Bacillus cereus, Bacillus circulans, Bacillus licheniformis, Bacillus megaterium, Bacillus mycoides, Bacillus pumilus, Bacillus subtilis), feathers (Bacillus cereus, Bacillus licheniformis, Bacillus pumilus, Bacillus subtilis), feces (Bacillus alkalophilus, Bacillus badius, Bacillus cohnii, Bacillus flexus, Bacillus halodurans, Bacillus megaterium, Bacillus pseudofirmus), geothermally heated soils (Bacillus fumarioli, Bacillus luciferensis, Bacillus schlegelii), honey bee and greater wax moth frass (Bacillus cereus, Bacillus megaterium, Bacillus sphaericus), inner tissues of plants (Bacillus amyloliquefaciens, Bacillus cereus, Bacillus endophyticus, Bacillus insolitus, Bacillus licheniformis, Bacillus megaterium, Bacillus pumilus, Bacillus subtilis), invertebrates (Bacillus oleronius, Bacillus sphaericus, Bacillus thuringiensis), leather (Bacillus cereus, Bacillus firmus, Bacillus licheniformis, Bacillus megaterium, Bacillus pumilus, Bacillus sphaericus, Bacillus subtilis), milk (Bacillus cereus, Bacillus coagulans, Bacillus licheniformis, Bacillus smithii, Bacillus sporothermodurans, Bacillus weihenstephanensis), naturally heated waters (Bacillus methanolicus, Bacillus okuhidensis), poultry litter and manure (Bacillus cereus, Bacillus fastidiosus, Bacillus halodurans, Bacillus pumilus, Bacillus subtilis), paper and paperboard (Bacillus amyloliquefaciens, Bacillus cereus, Bacillus circulans, Bacillus coagulans, Bacillus firmus, Bacillus flexus, Bacillus halodurans, Bacillus licheniformis, Bacillus megaterium, Bacillus mycoides, Bacillus pumilus, Bacillus sphaericus, Bacillus subtilis, Bacillus thuringiensis), recycled paper pulp (Bacillus pumilus), seaweed (Bacillus algicola), saline and hypersaline environments (Bacillus alcalophilus, Bacillus firmus, Bacillus halodenitrificans, Bacillus halophilus, Bacillus megaterium), sewage and wastewater treatment processes (Bacillus funiculus, Bacillus thermocloacae), sheep fleece (Bacillus cereus, Bacillus thuringiensis), silage (Bacillus coagulans, Bacillus siralis), soda lakes (Bacillus agaradhaerens, Bacillus cohnii, Bacillus pseudofirmus, Bacillus vedderi), solfatara (Bacillus tusciae), gemstones (Bacillus badius, Bacillus cereus, Bacillus circulans, Bacillus coagulans, Bacillus firmus, Bacillus lentus, Bacillus licheniformis, Bacillus mycoides, Bacillus subtilis; Khan et al., 2001), stone surfaces of ancient monuments (Bacillus licheniformis, Bacillus megaterium, Bacillus mycoides, Bacillus subtilis; Turtura et al., 2000), subterranean soil and water (Bacillus infernus, Bacillus subterraneus), and wall paintings (Bacillus decolorationis, Heyrman et al., 2003a; Bacillus barbaricus, Taubel et al., 2003).
Most species of Bacillus are heterotrophic organisms that have been isolated on complex organic media. Relatively few attempts have been made to isolate aerobic endospore-formers which can utilize inorganic sources of carbon and energy, or to demonstrate that established heterotrophic species are capable of facultative autotrophy. The two thermophiles Bacillus schlegelii and Bacillus tusciae remain the only species in the genus shown to be facultatively chemolithoautotrophic. Nitrogen fixation is well established for certain species in Paenibacillus (Paenibacillus azotofixans, Paenibacillus macerans, Paenibacillus polymyxa), but less is known about Bacillus species utilizing atmospheric nitrogen; although Bacillus edaphicus and Bacillus mucilaginosus were isolated on nitrogen-free media, these species are actually members of Paenibacillus (see Species Incertae Sedis, below). However, several studies have demonstrated nitrogen fixation by strains of Bacillus cereus, Bacillus licheniformis, Bacillus megaterium, Bacillus sphaericus, and unidentified strains (some of which may have been Paenibacillus species) isolated from rhizospheres and phylloplanes, and from endophytic sites and mycorrhizae (Rózycki et al., 1999). A comparative phylogenetic study, however, concluded that nitrogen fixation among aerobic endospore-formers is restricted to certain species of Paenibacillus (Achouak et al., 1999). Nitrogen-fixing Bacillus and Paenibacillus growing in the rhizosphere may help to promote plant growth; other ways in which aerobic endospore-formers may promote the growth of plants include (Chanway, 2002): the production of phytohormones, increasing nutrient availability (to the plant or to other, nitrogen-fixing, bacteria; Zlotnikov et al., 2001), the suppression of ethylene production by the plant in its rhizosphere, interactions with symbiotic bacteria and fungi (Medina et al., 2003), enhancement of root nodulation, and biological control of plant pathogens by various mechanisms including the production of antibiotics. In one study, Bacillus subtilis and Bacillus mycoides were found to dominate the rhizosphere of tea bushes (Pandey and Palni, 1997), a strain of the latter species having antifungal activity. Epiphytic Bacillus strains can have protective roles in the phyllosphere (Collins and Jacobsen, 2003; Jock et al., 2002). Representatives of several species, including Bacillus amyloliquefaciens, Bacillus cereus, Bacillus endophyticus, Bacillus insolitus, Bacillus licheniformis, Bacillus megaterium, Bacillus pumilus and Bacillus subtilis, have been isolated from the inner tissues of healthy plants, including cotton, grape, pea, spruce and sweet corn, and some strains appear to have important roles in growth promotion and plant protection (Reva et al., 2002). These endophytes and epiphytes can have potential as agents for the biocontrol of plant diseases, and their spores offer advantages in the formulation of such preparations (Emmert and Handelsman, 1999). Hosford (1982) and Leary et al. (1986), on the other hand, reported Bacillus species showing pathogenicity for plants.
Bacillus species are known to have roles in the postharvest processing and flavor development of cocoa (Schwan et al., 1995), coffee (Silva et al., 2000), tobacco (English et al., 1967) and vanilla (Röling et al., 2001), in the production of natural fibers and other vegetable products, in several traditional fermented foods based on leaves and seeds (and poultry eggs) (often dominated by Bacillus subtilis; Wang and Fung, 1996; Beaumont, 2002; Sarkar et al., 2002), and in composting (Blanc et al., 1999; Strom, 1985).
Bacillus species may cause deterioration of hides intended for leather production (Birbir and Ilgaz, 1996). Keratinolytic strains of Bacillus cereus, Bacillus licheniformis, Bacillus pumilus, Bacillus subtilis and of unidentified Bacillus species have roles in the degradation of feathers in poultry waste and may be found in the plumage of many bird species (Burtt and Ichida, 1999; Kim et al., 2001). Bacillus species also play a part in the degradation of chitin. This activity has been demonstrated for strains of Bacillus circulans, Bacillus coagulans, Bacillus lentus, Bacillus licheniformis, Bacillus megaterium, Bacillus pumilus and Bacillus thuringiensis (Clements et al., 2002), and strains of Bacillus amyloliquefaciens, Bacillus cereus, Bacillus megaterium, Bacillus sphaericus and Bacillus subtilis which utilize the chitin in crustacean wastes have been isolated (Sabry, 1992; Wang and Hwang, 2001). The value of chitinolysis to insect-pathogenic strains of Bacillus thuringiensis is evident, and an exochitinase from a strain of “B thuringiensis subsp. pakistani” was found to be toxic to Aedes aegypti larvae (Thanthiankul et al., 2002). Chitinases from a soil isolate of Bacillus amyloliquefaciens have been found to have antifungal properties (Wang et al., 2002). (The two species Bacillus chitinolyticus and Bacillus ehimensis, which were isolated using a chitin medium, are members of Paenibacillus).
Strains of several Bacillus species have been found to accumulate metal ions non-enzymically by adsorption to their cell surfaces and this can be of importance in waste treatment and natural environments: Bacillus licheniformis cells can accumulate cerium, cobalt and copper ions from aqueous and simulated waste solutions (Hafez et al., 2002), Bacillus subtilis may accumulate aluminum, cadmium, iron and zinc, and aluminosilicates (Urrutia and Beveridge, 1995), and an unidentified Bacillus strain bound chromium, copper and lead ions (Nourbakhsh et al., 2002). Bacillus megaterium biomass was found to bioreduce ions of the precious metals gold, palladium, platinum, rhodium and silver (Lin et al., 2001). Bacillus arseniciselenatis and Bacillus selenitreducens can use oxyanions of the two highly toxic elements arsenic and selenium as terminal electron acceptors in anaerobic respiration, and the environmental impact of such activity is becoming appreciated (Stolz and Oremland, 1999).
The trichome-forming bacteria “Anisomitus”, “Arthromitus”, “Entomitus”, “Coleomitus”, “Metabacterium”, and “Sporospirillum”, which occur in the alimentary tracts of animals, and which have been reported to form endospores, were listed as Genera Incertae Sedis in the First Edition of this Manual (Claus and Berkeley, 1986). Since that time, molecular methods have allowed considerable progress to be made in the taxonomy of some of these organisms. A cultivable “Arthromitus” strain from sow bug or wood louse (Porcellio scaber) has been identified as Bacillus cereus, and similar organisms have been isolated from moths, roaches and termites (Jorgensen et al., 1997; Margulis et al., 1998). Bacillus oleronius was first isolated from the hindgut of the termite Reticulitermes santonensis, and cellulolytic strains of the Bacillus cereus group and Bacillus megaterium have been found in the gut of another termite, Zootermopsis angusticollis (Wenzel et al., 2002). An “Arthromitus”-like endospore has been reported from a Miocene termite preserved in amber (Wier et al., 2002). On the other hand, several nonculturable, segmented, filamentous bacteria from chickens, mice, rats and trout have been shown to represent a distinct subline of the Clostridium subphylum, which has been proposed as “Candidatus Arthromitus” (Snel et al., 1995; Urdaci et al., 2001). Strains of “Metabacterium polyspora” from guinea pig cecum are closely related to the extremely large, viviparous, intestinal symbionts of the surgeonfish, Epulopiscium species, and also belong to the Clostridium subphylum (Angert et al., 1996).
Cellular fatty acids. This approach was recently reviewed by Kämpfer (2002). On the basis of the fatty acid compositions of 19 Bacillus sensu lato species Kaneda (1977) recognized six groups (A-F). All except group D were found to contain major amounts of branched chain acids, while within groups A–C only insignificant amounts (<3%) of unsaturated fatty acids were found; these latter three groups could be separated on the basis of their predominant fatty acids. Group A contained species now allocated to Bacillus (Bacillus circulans, Bacillus licheniformis, Bacillus megaterium, Bacillus pumilus and Bacillus subtilis), Brevibacillus and Paenibacillus and contained C14:0 anteiso (26–60%) and C15:0 iso (13–30%) acids, with chain lengths of 14–17. Among group B strains, now all allocated to Paenibacillus, C15:0 anteiso acid predominated (39–62%), with chain lengths between 14 and 17. In group C species, now accommodated in Geobacillus, C15:0 iso acid was predominant. In group D, now Alicyclobacillus, a unique fatty acid pattern with up to 70% cyclohexane fatty acids of chain length 17–19 was found. Group E comprised members of the Bacillus cereus group of species which, unlike the other groups, always had small proportions (7–12%) of unsaturated fatty acids present; the predominant fatty acids (19–21%) were of the C15:0 iso type. The psychrophiles Bacillus (now Sporosarcina) globisporus and Bacillus insolitus formed group F and contained large proportions (17–28%) of unsaturated fatty acids, and the predominant branched-chain fatty acids in these two species were C15:0 anteiso acids. The work of Kaneda was largely confirmed, and was supplemented, by the comprehensive study of Kämpfer (1994). For many species of Bacillus sensu stricto (but excepting Bacillus badius, the Bacillus cereus group, Bacillus circulans, Bacillus coagulans, Bacillus simplex and Bacillus smithii) profiles (with ranges as percentage of total given in parentheses) were C15:0 anteiso (25–66%), C15:0 iso (22–47%), and C17:0 anteiso (2–12%). For the Bacillus cereus group (Bacillus anthracis, Bacillus cereus, Bacillus mycoides, Bacillus pseudomycoides, B thuringiensis), levels of C15:0 anteiso were lower (3–7%), and amounts of unsaturated fatty acids were generally higher (>10%). Bacillus smithii and Bacillus coagulans showed higher amounts of C17:0 anteiso (17–42%) and lower amounts of C15:0 anteiso and C15:0 iso, and they also form a separate lineage within the Bacillus rRNA group. Other findings were low amounts of C15:0 anteiso (<10%) and relatively high amounts of unsaturated acids in Bacillus badius, and very low amounts of C15:0 iso in Bacillus circulans. These results indicated that the Bacillus rRNA group is still heterogeneous, and it was predicted that further taxonomic rearrangements would follow (Kämpfer, 2002).
With the division of Bacillus sensu lato into several phylogenetically distinct genera, the re-evaluation of the ability of fatty acid data to differentiate taxa has become possible. Numerical analyses of fatty acid data have shown that, in terms of level of taxonomic resolution, profiles vary largely within the species (Kämpfer, 1994). Although Bacillus species often cannot be differentiated by fatty acid analysis, especially in cases when large numbers of strains are examined, distinction of individual species, or even subspecies in certain cases, can be possible if small numbers of strains are studied (because possible intraspecific variation is not detected and hence cannot influence the interpretation of the results), or if genomically well-characterized groups of strains such as Bacillus cereus and its relatives are being investigated. Thus, given highly standardized growth conditions to achieve reproducible results, and a reliable database containing information on genomically homogeneous strains, fatty acid patterns can be used for Bacillus species identification. Also, whole-cell fatty acid analysis is valuable as a rapid and fairly inexpensive screening and identification method as part of polyphasic taxonomic studies (Kämpfer, 2002).
A GLC system developed for the identification of bacteria and yeasts by fatty acid methyl ester (FAME) analysis is marketed as the Microbial Identification System (MIDI, Newark, DE, USA). It requires highly standaridized cultivation and extraction procedures and provides a species-specific fatty acid database. Fatty acid peaks can be named by comparing retention times with those of a known mixture, but definitive identification can be made only by mass spectrometry. Species identification within Bacillus is not always possible by this approach, and additional phenotypic and/or genotypic characterization is often necessary (Kämpfer, 2002).
Sodium-Dodecylsulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) of whole-cell proteins. Whole-cell protein patterning (SDS-PAGE) is a rapid and cost-effective method for the comparison of large groups of bacteria, and is a valuable initial step in polyphasic characterization. It requires highly standardized conditions of growth, combined with a rigorously standardized procedure for analysis, and normalization of the data for computer-assisted comparison of the results. Normalization between different electrophoretic runs can be achieved by the inclusion of a carefully chosen bacterial pattern in which a protein extract of a standard organism is loaded into the outer lanes and the central lane of the gel. If a molecular mass marker is loaded as well, the molecular mass of the protein bands can be estimated easily. This method was reviewed by De Vos 2002. It has made important contibutions to polyphasic taxonomic studies within Bacillus (De Clerck and De Vos, 2002) and to the recognition of novel Bacillus species such as Bacillus fumarioli, Bacillus luciferensis and Bacillus shackletonii (Logan et al., 2000, 2002b, 2004b).
Whole-cell spectrometric analysis. A variety of instrument-based physico-chemical techniques for whole-cell analysis, including pyrolysis mass spectrometry (PyMS), matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF-MS) and Fourier transform infrared spectrometry (FT-IR), have been applied to Bacillus. These “fingerprinting” methods require expensive instruments and complex data handling, but offer simple sample preparation, speed of analysis, high throughput, and low processing costs, and they yield data that can discriminate at genus, species and strain level. They can be valuable in polyphasic taxonomic studies, but better long-term reproducibility of spectra is required for the building of databases for routine identification applications. These approaches are reviewed by Magee and Goodacre (2002).
Bacteriophage. The potential for phage typing of Bacillus species has been little investigated. The γ phage has long been used as a specific test for identifying Bacillus anthracis, but preparations of this phage are not widely available, and the specific molecular tests for this species now exist. Ackermann et al. (1995) developed phage typing schemes for Bacillus subtilis and Bacillus thuringiensis. They examined 200 Bacillus subtilis strains and were able to distinguish 29 phagovars using 10 phages; with 500 strains of Bacillus thuringiensis, 10 phages allowed the recognition of 25 phagovars, but the phagovars showed no correlation with H-serovars. Pantasicocaldas et al. (1992) investigated the population dynamics of Bacillus subtilis and its phages in soil.
Enrichment and isolation procedures
Detection of Bacillus strains by cultivation and isolation relies mainly upon resistance of their endospores to heat (and to other conditions) which are lethal to vegetative cells of most kinds of bacteria. Only endospores and extremely themophilic, non-spore-forming bacteria are able to survive the heat treatment, so that subsequent aerobic culture at lower temperatures yields only isolates germinating from the surviving spores. Such an approach may also heat-activate the spores and so enhance their germination. However, an obvious disadvantage is that vegetative cells of Bacillus will be lost along with the other, nonspore-forming bacteria; as a result, it will not be known whether the isolation of a Bacillus strain from a particular habitat reflects its multiplication in and colonization of that niche, or mere survival of a dormant, contaminant spore. Furthermore, spores formed in natural environments may have heat sensitivities that are different to those formed in laboratory cultures, spores may be present in very small numbers or even absent, and heat treatment may be mutagenic. It is therefore valuable to reserve part of the specimen or sample for cultivation from an unheated control preparation for study in parallel with the heat-treated sample. With many environments this will allow isolates arising from vegetative cells to be obtained – problems of heavy bacterial load can, of course, be addressed by dilution of the sample. Repeated and consistent isolations of similar strains from heat-treated samples taken from pristine or sparsely populated environments would suggest that the organisms must be colonizing the habitat rather than just be reflecting heavy contamination, and it seems likely that populations of Bacillus strains in many habitats will exist as both vegetative and sporulated cells (see the comments on Bacillus fumarioli in the Ecology section of Further descriptive information, above). Notwithstanding this, it is clear that spores may be carried for long distances by water or the air, and locally large numbers of spores may be regularly deposited in sites that are apparently unsuitable for their germination and continued development; it is well known, for example, that thermophilic endospore-formers may easily be isolated from cold environments (Marchant et al., 2002).
Heat treatment may vary from 60°C to 80°C for 10 min or longer; 80°C for 10 min is widely used. The given time allows for a period of heat penetration of the sample followed by a sufficient holding period at temperature; this assumes that the specimen is in an aqueous suspension in a water bath, and is adequately immersed. Solid samples may be emulsified in sterile, deionized water, 1:2 (w/v) prior to heating; the unheated control is prepared in the same way, but is unheated, or else the suspension intended for heating may be sampled for cultivation prior to heating. Direct plate cultures are made on appropriate solid media by spreading up to 250 µl volumes from undiluted, and 10-, 100- and 1000-fold dilutions of the treated sample.
Enrichment culture of samples from habitats expected to be sparsely populated with bacteria can be done by heat-treating suspensions in a suitable broth medium (perhaps 1–5 g of soil in 10 ml broth, for example), then incubating them and streakdiluting onto solid media as soon as they show signs of turbidity, with further subcultures from the reincubated broths being made at intervals thereafter. This approach, using different incubation temperatures and a range of pH, and with paired cultures from samples that had not been subjected to heat treatment, yielded a variety of isolates from pristine soils (Logan et al., 2000). Broths for particular kinds of organisms (nitrogen-free broths – in flasks so as to give a large broth surface/air interface – to isolate nitrogen fixers, for example) can be made selective or elective as desired.
Using a disinfectant to inactivate vegetative cells is for most such agents constrained by the difficulty of removing or neutralizing the agent prior to cultivation. Ethanol, however, may easily be removed by evaporation, and it does not have the potentially mutagenic effect of heat treatment. Filter-sterilized 95–100% ethanol is added to a final concentration of 1:1 (v/v) and held for 30–60 min at room temperature. The ethanol must first be filter-sterilized, because some commercial batches may be contaminated with spores.
For soil and certain other kinds of samples, desiccation by air drying will destroy many vegetative cells, but inactivation of cystforming bacteria such as Azotobacter may require an extended period of drying.
Although useful for the detection and isolation of well-represented species, the approaches outlined above are rather crude for the isolation of organisms present only in relatively small numbers, and for the slow-growing, less vigorous types. Methods that have been described for the isolation of individual species are outlined below. For isolation of species from clinical cases, most strains will survive carriage in freshly collected specimens or in a standard transport medium (for safety concerning Bacillus anthracis, see below); heat treatment is not appropriate for fresh clinical specimens, where spores are usually sparse or absent. All the clinically significant isolates reported to date are of species that grow, and often sporulate, on routine laboratory media at 37°C. It seems unlikely that many clinically important, but more fastidious, strains are being missed for the want of special collection, media or growth conditions.
Safety. With the exception of Bacillus anthracis, species of aerobic endospore-forming bacteria that may be isolated from clinical specimens can be handled safely on the open bench, and no special precautions are required for specimen collection. Isolation and presumptive identification of Bacillus anthracis can be performed safely in the routine clinical microbiology laboratory, provided that normal good laboratory practice is observed; vaccination is not required for minimal handling of the organism. If aerosols are likely to be generated, the work should be performed in a safety cabinet. Anthrax is not highly contagious; cutaneous anthrax is readily treated and is only life-threatening in exceptional cases, and the infectious doses in the human inhalation and intestinal forms (also treatable if recognized) are generally very high (LD50 > 10,000 spores). Precautions, therefore, need to be sensible, not extreme. When collecting specimens related to suspected anthrax, disposable gloves, disposable apron or overalls, and boots which can be disinfected after use should be worn; for dusty samples that might contain many spores, the use of head-gear and dust masks should be considered. Discard disposable items into suitable containers for autoclaving followed by incineration. Non-autoclavable items should be immersed overnight in 10% formalin (5% formaldehyde solution). Glutaraldehyde (5%) is also effective. Items that cannot be immersed should be bagged and sent for formaldehyde fumigation. Ethylene oxide and hydrogen peroxide vapor are also effective fumigants, but the latter is inappropriate if organic matter is being treated. The best disinfectant for specimen spillages is again formalin; where this is considered impractical, 10% hypochlorite solution can be used, although its limitations should be appreciated; it is rapidly neutralized by organic matter, and it corrodes metals. Other strong oxidizing agents, such as hydrogen peroxide (5%) and peracetic acid (1%) are also effective but with the same organic matter limitations. Specimen collection and handling from humans and animals is described by Logan and Turnbull (2003), Logan et al. (2007) and Turnbull et al. (1998); non-clinical materials associated with attempts at deliberate release of the organism as a weapon may be very hazardous and no attempt to sample or process them should be made without the appropriate instructions from the correct authorities.
Bacillus aeolius was isolated from a 45°C water sample collected from a shallow (15 m depth) marine hot spring, by aerobic enrichment in marine broth at 65°C for 3 d, followed by plating on marine agar (Gugliandolo et al., 2003a).
Bacillus agaradhaerens, Bacillus alcalophilus and other alcaliphiles. These may be isolated by direct plating of heat-treated samples onto alkaline nutrient media, or by enrichment for 1 or more days in alkaline liquid nutrient media, with shaking, at the chosen incubation temperature, followed by plating. Media may be adjusted to a pH between 8.6 and 11.0, by using sodium hydrogen carbonate, sodium carbonate, sodium sesquicarbonate, trisodium phosphate or sodium perborate. A modification (Grant and Tindall, 1980) of the widely used medium of Horikoshi (1971) is: glucose, 10.0 g; peptone, 5.0 g; yeast extract, 5.0 g; KH2PO4, 1.0 g; MgSO4·7H2O, 0.2 g; distilled water 900 ml. Agar is added at the recommended concentration if a solid medium is required. After autoclaving, 100 ml of 20% (w/v) Na2CO3·10H2O, which has been sterilized by autoclaving separately, is added (at 60°C if the medium contains agar); the final pH of the medium is 10.5, and the concentration of the Na2CO3 solution can be reduced to give lower pH values. Other carbon sources, such as starch, may be substituted for the glucose. Most alkaliphilic Bacillus species require Na+ ions for growth, germination and sporulation (Horikoshi, 1998). Optimal growth of Bacillus agaradhaerens is at pH 10.0 or above, no growth occurs at pH 7.0, and up to 16% NaCl is tolerated; optimal growth of Bacillus alcalophilus is about pH 10.0, no growth occurs at pH 7.0, and 5–8% NaCl is tolerated (Nielsen et al., 1995a).
Bacillus algicola was isolated from 5 g seaweed allowed to decay in 200 ml sterilized natural sea water for 2 months at 22°C, supplemented with and aqueous ethanol extract of a protein inhibitor of endo(1→3)-β-D-glucanases (Yermakova et al., 2002). 0.1 ml of suspension was then plated onto plates of Marine Agar 2216 (Difco) and on plates of medium B (peptone, 2.0 g; casein hydrolysate, 2.0 g; yeast extract, 1.0 g; glucose, 1.0 g; KH2PO4, 0.02 g; MgSO4·7H2O, 0.05 g; agar, 15 g; natural sea water, 500 ml; distilled water at pH 7.5–7.8, 500 ml), which was also used as the maintenance medium.
Bacillus amyloliquefaciens. This organism has been isolated from a wide range of environments, including industrial amylase fermentations, foods and soil. It is phenotypically similar to Bacillus subtilis. Welker and Campbell (1967) isolated strains of this organism from commercial α-amylase concentrates as follows: 1 ml or 0.5 g of α-amylase concentrate is placed in a tube containing 5 ml of sterile distilled water and heated at 80°C for 10 min.; the heated suspension is then streaked onto plates of tryptose blood agar base containing 1% starch; after incubation at 37°C for 18–20 h, colonies showing wide haloes of starch hydrolysis are purified by restreaking on fresh plates of the same medium. The screening method of Effio et al. (2000), which uses dye-linked starch to reveal colonies of amylolytic bacteria, may also be of value.
Bacillus anthracis. For isolation of this organism from old carcasses, animal products or environmental specimens, the organisms will mostly be present as spores. The heat treatment recommended for isolating this species is 62.5°C for 15 min; this will both heat-shock the spores to enhance germination, and effectively destroy nonspore-forming contaminants [solid samples should first be emulsified in sterile, deionized water, 1:2 (w/v)]. Direct plate cultures are made on blood, nutrient, or, selective agars, as appropriate, by spreading up to 250 µl volumes from undiluted, and 10- and 100-fold dilutions of the treated sample. There is no effective enrichment method for Bacillus anthracis in old animal specimens or environmental samples; isolation from these is best done with polymyxin-lysozyme EDTA-thallous acetate (PLET) agar (Knisely, 1966). Aliquots (250 µl) of the undiluted and 1:10 and 1:100 dilutions of heat-treated suspension of the specimen are spread across PLET plates which are read after incubation for 36–40 h at 37°C. Roughly circular, creamy-white colonies, 1–3 mm diameter, with a ground-glass texture must be distinguished from those of other members of the Bacillus cereus group; they are subcultured on (i), blood agar plates to test for γ phage and penicillin susceptibility, and for hemolysis, and (ii), directly or subsequently in blood to look for capsule production using M'Fadyean's stain. PCR-based methods are being used increasingly for confirming the identity of isolates (Turnbull et al., 1998).
Bacillus aquimaris and Bacillus marisflavi were isolated from sea water of a tidal flat by dilution plating onto marine agar (Yoon et al., 2003a).
Bacillus arseniciselenatis. The only reported isolate of this organism was isolated from the arsenic-rich sediment of Mono Lake, California. Lake sediment (15 ml) was added to 45 ml supplemented, autoclaved lake water and sealed under N2 in a 160 ml serum bottle. Supplements were 10 mM sodium lactate, 10 mM Na2SeO4, 0.5 g yeast extract, in 1 l lake water, with 2.5% cysteine-sulfide reducing agent. The slurry was incubated statically in the dark at 20°C for 18 d, by which time red deposits of elemental selenium were evident. A 1.0 ml aliquot was transferred to a smaller serum bottle containing 20 ml of the supplemented lake water and incubated in the same way for 1 week. A stable enrichment culture was achieved by weekly transfers into 10 ml medium in anaerobic, crimp-sealed test tubes for over 1 year. Manipulation was done in an anaerobic glove box using standard anaerobic methods. The enrichment was streaked onto solidified supplemented lake water medium and the plates incubated under N2 for 1 week, after which red colonies were seen. Colonies were inoculated into further sealed test tubes of supplemented lake water, and non-spore-forming organisms were destroyed by heat treatment at 70°C for 20 min (Switzer Blum et al., 1998).
Bacillus asahii was cultivated from soil using PY-1 medium (peptone, 8 g; yeast extract, 3 g; agar, if required, 15 g, distilled water, 1 l).
Bacillus atrophaeus is essentially a variant of Bacillus subtilis which forms a soluble black pigment on media containing utilizable carbohydrates. Most strains have been isolated from soil. No method for its specific enrichment or selective isolation are known to the authors, but its pigment formation is evidently of value in its recognition on plate cultures; it forms bluish-black colonies on glycerol-glutamate agar (Arai and Mikami, 1972).
Bacillus azotoformans. For selective isolation, soil samples are heat-treated (80°C, 10 min) in peptone broth, and incubated under an atmosphere of pure N2O at 32°C; gassy and foaming cultures are subcultured several times in fresh medium under the same conditions. Pure cultures are obtained by streak plating from the enrichment cultures onto the same medium solidified with agar, and incubating at 32°C in air (Pichinoty et al., 1976). The enrichment medium contains: peptone, 10 g; Na2HPO4·12H2O, 3.6 g; KH2PO4, 1.0 g; NH4Cl, 0.5 g; MgSO4·7H2O, 0.03 g; trace elements solution (Pichinoty et al., 1977), 0.2 ml; distilled water, 1 l; adjust pH to 7.0 and autoclave at 121°C for 15 min.
Bacillus badius. No methods for the specific enrichment or selective isolation of this species are known to the authors.
Bacillus barbaricus was isolated from samples of an experimental wall painting by suspending in sterile saline, shaking for 1 h, then plating dilutions onto PYES agar and incubating at room temperature. PYES agar contained: peptone from casein, 3 g; yeast extract, 3 g; disodium succinate, 2.3 g; agar to solidify; distilled water, 1 l; pH 7.2 (Taubel et al., 2003).
Bacillus bataviensis was isolated from soil by plating two dilution series, one unheated and the other heated at 80°C for 15 min, onto 35 different agar media, most of which were mineral media with different complex components as sole carbon sources (Felske et al., 1999; Heyrman et al., 2004).
Bacillus benzoevorans was originally isolated by aerobic enrichment culture of heat-treated soil samples at 32°C for a week in a minimal medium containing benzoate, p-hydroxybenzoate or cyclohexane carboxylate as carbon and energy sources (Pichinoty, 1983). The enrichment medium comprises: Na2HPO4·12H2O, 3.575 g; KH2PO4, 0.98 g; MgSO4·7H2O, 0.03 g; NH4Cl, 0.5 g; trace element solution (Pichinoty et al., 1977), 0.2 ml; benzoate, p-hydroxybenzoate or cyclohexane carboxylate, 2 g; yeast extract (except in case of p-hydroxybenzoate), 0.1 g; distilled water to 1 l; pH adjusted to 7.0 with NaOH.
Bacillus carboniphilus was isolated from the air using a otherwise nonpermissive medium which had been spotted with sterilized graphite suspension (Fujita et al., 1996). The basal medium was Penassay Broth (Bacto Antibiotic Medium 3; Difco), the potassium and sodium levels of which were stressful to the organism: beef extract, 1.5 g; yeast extract, 1.5 g; peptone, 5.0 g; glucose, 1.0 g; NaCl, 3.5 g; K2HPO4, 3.68 g; KH2PO4, 1.32 g; agar 15 g; distilled water, 1 l. 200 µl of a thick suspension of graphite in 1% Triton X-100, sterilized by heating for 1 min at about 700°C on a gas burner, was spotted on the surface of each agar plate, and plates were incubated at 44°C for 48–96 h. Colonies appearing around the graphite spots were purified by streaking on trypticase soy agar plates.
Bacillus cereus. With food specimens submitted for the investigation of food-poisoning incidents, heating part of the specimen at 62.5°C for 15 min will both heat-shock the spores and effectively destroy nonspore-forming contaminants [solid samples should first be emulsified in sterile, deionized water, 1:2 (w/v)]. The other part of the specimen is cultivated without heat treatment in case spores are very heat-sensitive, or absent. Direct plate cultures are made on blood, nutrient, or, selective agars, as appropriate, by spreading up to 250 µl volumes from undiluted, and 10-, 100- and 1000-fold dilutions of the treated sample. Heat treatment is not suitable for human specimens where spores are usually sparse or absent. Enrichment procedures are generally inappropriate for isolations from clinical specimens, but when searching for Bacillus cereus in stools ≥3 d after a food-poisoning episode, nutrient or tryptic soy broth with polymyxin (100,000 U/l) may be added to the heat-treated specimen. Several media have been designed for isolation, identification and enumeration of Bacillus cereus. They exploit the organism's egg-yolk reaction positivity and acid-from-mannitol negativity; pyruvate and polymyxin may be included for selectivity. Three satisfactory formulations are MEYP (Mannitol, Egg Yolk, Polymyxin B agar), PEMBA (Polymyxin B, Egg yolk, Mannitol, Bromthymol blue Agar) and BCM (Bacillus cereus Medium) (van Netten and Kramer, 1992).
Bacillus circulans. Although several methods for isolating this organism have been described (Claus and Berkeley, 1986), recent developments in the taxonomy of this species (which had long been known as a complex of species) indicate that very few of the strains that have been allocated to this taxon on the basis of phenotypic properties are actually closely related to the type strain. It is not possible, therefore, to recommend a method for its specific isolation.
Bacillus clarkii. Optimal growth of this alkaliphilic organism is above pH 10.0, no growth occurs at pH 7.0, and up to 16% NaCl is tolerated (Nielsen et al., 1995a); see Bacillus agaradhaerens, Bacillus alcalophilus and other alcaliphiles.
Bacillus clausii. Optimal growth of this alkalitolerant organism is above pH 8.0, good growth occurs at pH 7.0, and 8–10% NaCl is tolerated (Nielsen et al., 1995a); see Bacillus agaradhaerens, Bacillus alcalophilus and other alcaliphiles.
Bacillus coagulans. Methods for the specific isolation of this species are based upon its thermotolerance and acid tolerance; it may be isolated from soil and other sources by enriching and then subculturing at pH 5.5–6.0 with incubation at around 50°C. Allen (1953) used an enrichment medium of glucose, 10 g; yeast extract, 5.0 g; distilled water to 1 l, adjusted with lactic acid to pH 5.5 and autoclaved at 121°C for 15 min.; flasks containing 200 ml are inoculated with 1 ml of heat-treated suspension of soil or other material and incubated for 24 h at 50°C; 0.1 ml aliquots are then transferred to fresh medium and incubated for 24 h at 50°C; loopfuls are streaked onto nutrient agar and incubated at 45°C for 48 h. Small. round, whitish. opaque or opalescent colonies are likely to be Bacillus coagulans. A similar approach was used by Emberger (1970): heat-treated soil samples were incubated anaerobically at 54°C in nutrient broth which had been supplemented with 1% (w/v) glucose and adjusted to pH 6.0. Loopfuls were then streaked onto plates of the same medium solidified with agar and incubated aerobically at 45°C; this yielded colonies of Bacillus coagulans and Bacillus licheniformis.
Bacillus cohnii. Samples of alkaline or possibly alkaline soil from horse meadows, and from horse feces that have been lying for some time, or other potential sources, are heat treated at 80°C for 10 min and 1–2 drop inocula are added to nutrient broth which has been adjusted to pH 9.7 by adding sodium sesquicarbonate (Na2CO3) to a final concentration of 0.1 mol/l (Spanka and Fritze, 1993). Cultures are incubated aerobically at 45°C for 1–2 d, and streaked onto plates of the same medium solidified with agar, with incubation at 45°C or 28–30°C for 2–7 d. Small, cream-white, seldom-occurring colonies are picked for purification.
Bacillus decolorationis was isolated from scrapings of biofilms on ancient mural paintings by homogenizing the samples in physiological water, preparing a dilution series, and then plating on five different media: (i) trypticase soy broth (BBL) solified with Bacto agar (Difco); (ii) the aforementioned trypticase soy agar supplemented with 10% NaCl; (iii) R2A agar (Difco); (iv) R2A agar supplemented with 10% NaCl; (v) starch-casein medium, which contained starch, 10 g; casein, 0.3 g; KNO3, 2 g; K2HPO4, 2 g; NaCl, 2 g; MgSO4·7H2O, 0.05 g; CaCO3, 0.02 g; FeSO4·7H2O, 0.01 g; agar, 20 g; water, 1 l. All media were supplemented with 0.03% cycloheximide to inhibit fungal growth. Plates were incubated aerobically at 28°C for 3 weeks (Heyrman et al., 1999).
Bacillus drentensis – see Bacillus bataviensis.
Bacillus endophyticus. Strains of this species were isolated from the inner tissues of healthy cotton plants (Reva et al., 2002). Pieces of stem about 1 cm in diameter were flamed with ethanol and the outer layers removed with a sterile scalpel; slices of inner stem were placed on nutrient agar plates and incubated for 48 h at 30°C. Bacterial growth associated with the stems was then purified by repeated streak cultivation on plates of the same medium.
Bacillus farraginis, Bacillus fordii and Bacillus fortis were isolated from samples of raw milk, feed concentrate and green fodder, and samples talen from milking apparatus, by heat treating at 100°C for 30 min followed by plating on brain heart infusion (Oxoid) supplemented with bacteriological agar no. 1 (Oxoid) and filter-sterilized vitamin B12 (1 mg/l) (Sigma).
Bacillus fastidiosus uses uric acid, allantoin and allantoic acid as sole carbon, nitrogen and energy sources, and this property may be used in its enrichment and isolation. The method of Fahmy (personal communication to Claus and Berkeley, 1986) uses the following enrichment medium: K2HPO4, 0.8 g; KH2PO4, 0.2 g; MgSO4·7H2O, 0.5 g; CaCl2·2H2O, 0.05 g; FeSO4·7H2O, 0.015 g; MnSO4·H2O, 0.01 g; uric acid, 10 g; distilled water to 1 l. The pH is not adjusted. 300 ml Erlemneyer flasks containing 30 ml of this medium are inoculated with 5 ml of a soil suspension in water that has been heat treated for 5 min at 80°C, and the inoculated flasks are shaken at 30°C for 24 h. One milliliter quantities are transferred to fresh flasks of medium for further incubation under the same conditions. Loopfuls, or 0.1 ml quantities, of serial dilutions of the enrichment culture are streaked or spread on plates of the following medium: a layer of the medium described above, from which the uric acid has been omitted but which has been solidified with agar, is overlaid with uric acid agar; the latter is prepared by sterilizing a 3% (w/v) agar solution and a 2% (w/v) uric acid suspension separately and then combining equal parts and pouring to a depth of about 3 mm. Colonies of uric acid-degrading organisms produce clear haloes in the milky layer of uric acid agar, owing to utilization of the acid and its solubilization in the rising pH caused by splitting of the urea that is formed from the uric acid. Bacillus fastidiosus colonies usually show rhizoid outgrowths on this medium. Suspect colonies are suspended in nutrient broth and streaked on uric acid agar and nutrient agar; isolates developing only on the former medium are likely to be this species, and they may be purified on plates of the mineral agar to which 2% (w/v) allantoin has been added before autoclaving.
Bacillus firmus. Methods for isolating members of this species complex, particularly pigmented organisms from salt marshes and marine environments, have been described (Claus and Berkeley, 1986), but none of them are specific, and recent developments in the taxonomy of the group have revealed that few authentic strains of Bacillus firmus originate from salty environments or are strongly pigmented; most strains have been isolated from soil, as laboratory contaminants, and as contaminants of food and pharmaceutical production environments. Until the species is more tightly defined, it is not possible to recommend a method for its specific isolation.
Bacillus flexus was revived by Priest et al. (1988) to accommodate two strains that showed low DNA homology to Bacillus megaterium but which showed phenotypic similarity to that species. No method has been described for its specific isolation.
Bacillus fordii and Bacillus fortis – see Bacillus farraginis.
Bacillus fumarioli. Strains of this species were isolated from soil samples that were collected from geothermal sites in the Antarctic and transported and stored both chilled and frozen. 1 g quantities of soil are added to 9 ml Bacillus fumarioli broth (BFB) in duplicate at pH 5.5, and one of each pair is heat treated at 80°C for 10 min to kill vegetative cells. Broths are incubated at 50°C in air (bottles loose-capped), and subcultured by streaking onto Bacillus fumarioli agar (BFA, which is BFB containing 5 mg/l MnSO4·4H2O, to enhance sporulation, and 18 g/l agar) and incubated at 50°C (with the plates in loosely closed polythene bags to avoid drying-out) for 1–2 d (Logan et al., 2000). BFB is an adaption of medium B of Nicolaus et al. (1998) and contains yeast extract, 4 g; (NH4)2SO4, 2 g; KH2PO4, 3 g and 4 ml each of solutions A and B in distilled water, 1 l; adjust to pH 5.5. Solution A: (NH4)2SO4, 12.5 g; MgSO4·7H2O, 5.0 g; distilled water, 100 ml; Solution B, CaCl2·2H2O, 6.25 g; distilled water, 100 ml. Growth and sporulation may be enhanced by enriching and subculturing on 1/2 BFB and 1/2 BFA, in both of which all components excepting the water, and the agar in the latter, are reduced by half. Using the same medium and conditions, further isolates have been obtained from gelatin production plants in France, Belgium and the USA (De Clerck et al., 2004a).
Bacillus funiculus was described on the basis of a single isolate from a domestic wastewater treatment tank (Ajithkumar et al., 2001). Water from a sludge-circulating tank was diluted to 104 in 0.5% NaCl solution and plated onto the following medium: nutrient broth (Oxoid CM-1), 4 g; potato starch, 5 g; glucose, 8 g; NaCl, 5 g; yeast extract, 0.5 g; agar, 15 g; distilled water, 1 l. Plates were incubated at 32°C for 24 h, and then at 20°C for 1 week, and colonies of this species were white, opaque, round and umbonate.
Bacillus fusiformis was revived for four strains, three of which were urease-positive, that had been assigned to Bacillus sphaericus. No method for its specific enrichment or isolation have been described, but urease positivity may be of value when screening colonies from Bacillus sphaericus isolations (see below); however, other spherical-spored organisms, including mosquito pathogenic strains of Bacillus sphaericus (Krych et al., 1980) and Sporosarcina psychrophila (formerly Bacillus psychrophilus), are also urease-positive.
Bacillus galactosidilyticus. Two strains of this species were isolated from raw milk after a heat treatment of 80°C for 10 min in one case, and 100°C for 30 min in the other, in order to pasteurize the samples and activate any endospores present; samples were then plated on brain heart infusion (BHI) (Oxoid) solidified with Bacteriological Agar no. 1 (15 g/l) (Oxoid) and supplemented with filter-sterilized vitamin B12 (1 mg/l), incubating at 37°C for 48 h (Heyndrickx et al., 2004).
Bacillus gelatini. Strains were isolated from samples of gelatin batches from a gelatin production plant by enrichment of 30 g of sample in 70 ml Trypticase Soy Broth (Oxoid) at 45°C and 55°C for 24 h, and plating on Trypticase Soy Agar (Oxoid) and Brain heart Infusion Agar (BBL), supplemented with 1 mg vitamin B12/l and Nutrient Agar supplemented with 1.2% gelatin at 45°C and 55°C (De Clerck et al., 2004c).
Bacillus gibsonii. Optimal growth of this alkalitolerant organism is about pH 8.0, growth occurs at pH 7.0, and up to 9% NaCl is tolerated (Nielsen et al., 1995a); see Bacillus agaradhaerens, Bacillus alcalophilus and other alcaliphiles.
Bacillus halmapalus. Optimal growth of this alkalitolerant organism is about pH 8.0, and good growth occurs at pH 7.0, but 5% NaCl is not tolerated (Nielsen et al., 1995a); see Bacillus agaradhaerens, Bacillus alcalophilus and other alcaliphiles.
Bacillus halodurans. Optimal growth of this alkaliphilic organism is pH 9.0–10.0, most strains grow at pH 7.0, and good growth is obtained at up to 12% NaCl (Nielsen et al., 1995a); see Bacillus agaradhaerens, Bacillus alcalophilus and other alkaliphiles.
Bacillus halophilus. This species is based upon one strain isolated from rotting wood on a seashore (Ventosa et al., 1989) by enrichment in Sehgal and Gibbons complex medium (Onishi et al., 1980): NaCl, 234 g; vitamin-free Casamino acids (Difco), 7.5 g; yeast extract, 10 g; sodium citrate, 3 g; KCl, 2 g; MgSO4·7H2O, 2 g; FeCl3·nH2O, 2.3 mg; distilled water 1 l; pH 6.6), followed by inoculation onto 15% (w/v) salt MH medium: yeast extract, 10 g; proteose peptone, 5 g; glucose, 1 g; agar, 20 g; 15% (w/v) NaCl solution 1 l.
Bacillus horikoshii. Optimal growth of this alkalitolerant organism is about pH 8.0, growth occurs at pH 7.0, and 8–9% NaCl is tolerated (Nielsen et al., 1995a); see Bacillus agaradhaerens, Bacillus alcalophilus and other alcaliphiles.
Bacillus horti. This species is based upon two strains isolated from garden soil in Japan using an alkaline peptone-yeast extract agar (Yumoto et al., 1998); details of sample preparation and inoculation were not reported. The isolation medium comprised: peptone, 8 g; yeast extract, 3 g; K2HPO4, 1 g; FeSO4·7H2O, 10 mg; EDTA, 3.5 mg; ZnSO4·7H2O, 3 mg; Co(NO3)2·6H2O, 2 mg; MnSO4·nH2O, 2 mg; CuSO4·5H2O, 1 mg; H3BO3, 1 mg; agar, 15 g; NaHCO3/Na2CO3 buffer (100 mM; pH 10) in deionized water, 1 l.
Bacillus hwajinpoensis was isolated from sea water of the East Sea in Korea by dilution plating onto marine agar (Yoon et al., 2004b).
Bacillus indicus was isolated from a sand sample from an arsenic-contaminated aquifer by cultivation on nutrient agar containing 5% sodium arsenate. Nutrient agar without sodium arsenate was used for growth and maintenance.
Bacillus infernus. This anaerobic species was isolated from shale taken from a depth of about 2.7 km below the land surface, where conditions were estimated to be anoxic, thermic (60°C), and brackish (1.2% NaCl). Core sample material was placed in an inert atmosphere, pared to remove potential surface contamination, and transferred to the laboratory on ice. Enrichment was done by adding 50 mg pieces of rock to MSA medium (Boone et al., 1995) and incubating for 40 d at 50°C in pure nitrogen at pH 8.2. The medium comprised: yeast extract, 0.5 g; peptone, 0.5 g; NaCl, 10 g; NaOH, 4 g; NH4Cl, 1 g; MgCl2·6H2O, 1 g; K2HPO4, 0.4 g; CaCl2·2H2O, 0.4 g; Na2S·9H2O, 250 mg; disodium EDTA·2H2O, 5 mg; CoCl2·6H2O, 1.5 mg; resazurin, 1 mg, MnCl2·4H2O, 1 mg; FeSO4·7H2O, 1 mg; ZnCl2, 1 mg; AlCl3·6H2O, 0.4 mg; Na2WO4·2H2O, 0.3 mg; CuCl2·2H2O, 0.2 mg, NiSO4·6H2O, 0.2 mg; H2SeO3, 0.1 mg; H3BO3, 0.1 mg; Na2MoO4·2H2O, 0.1 mg; 20 mM formate, 20 mM acetate, 20 mM MnO2 (MnO2 prepared by mixing a 9.5% KMnO4 solution with an equal volume of a 17.8% MnCl2·4H2O solution) in deionized water, 1 l. Ingredients excepting sulfide were added together and solution equilibrated under pure N2; the medium was dispensed into bottles sealed to exclude air. Sulfide was added from O2-free stock solution 1 h before use. The enrichment culture was serially diluted and inoculated onto MSA medium lacking the NaCl, but solidified with 18 g agar per liter, in roll tubes. Pinpoint colonies became visible in the zones of clearing of the MnO2 in 3–4 weeks, and they were picked, suspended in MSA medium, serially diluted and reinoculated into the roll tube medium to purify.
Bacillus insolitus. This psychrophilic organism, along with other organisms, was isolated from marshy and normal soil by enrichment in trypticase soy broth at 0°C for 2 weeks; cultures showing turbidity were streaked on trypticase soy agar and incubated at 0°C for 2 weeks, and isolates were purified on nutrient agar (Larkin and Stokes, 1966). Cells of this species appear coccoid when grown on nutrient agar, but rod-shaped on richer media such as trypticase soy agar (Larkin and Stokes, 1967).
Bacillus jeotgali. Strains of this species were isolated by dilution plating of jeotgal, a Korean traditional fermented seafood, on trypticase soy agar supplemented with artificial sea water (per liter: NaCl, 24 g; MgSO4·7H2O, 7 g; MgCl2·6H2O, 5.3 g; KCl, 0.7 g; CaCl2, 0.1 g) at pH 7.5 and incubated at 30°C. Colonies were cream-yellow or light orange-yellow, smooth, flat and irregular (Yoon et al., 2001a).
Bacillus krulwichiae was isolated from garden soil contaminated with aromatic compounds. Soil samples were added to alkaline mineral basal salt medium (AMBS) and incubated aerobically for 48 h at 30°C; 0.5 ml amounts were transferred to fresh medium and incubated for 24 h, then plated onto ABMS agar plates, purified five times, and maintained on peptone-yeast extract-alkaline (PYA) agar at 27°C. AMBS contained: yeast extract, 0.2 g; hydroxybenzoate, 3 g; NH4NO3, 2.5 g; K2HPO4, 1.5 g; Na2HPO4, 1.5 g; MgSO4·7H2O, 0.5 g; CaCl2·2H2O, 20 mg; FeSO4·7H2O, 10 mg; MnSO4·nH2O, 1 mg; ZnSO4·7H2O, 0.5 mg; Na2CO3, 10 g; distilled water, 1 l; pH 10. PYA agar contained: peptone, 8 g; yeast extract, 3 g; K2HPO4, 1 g; FeSO4·7H2O, 10 mg; EDTA, 3.5 mg; ZnSO4,·7H2O, 3 mg; Co(NO3)2·6H2O, 2 mg; MnSO4·nH2O, 2 mg; CuSO4·5H2O, 1 mg; H3BO3, 1 mg; NaHCO3/Na2CO3 buffer (100 mM in deionized water; pH 10), 1 l (Yumoto et al., 2003).
Bacillus lentus. Gibson originally isolated this species from soil by plating dilutions of soil suspensions on nutrient agar supplemented with 10% urea (peptone, 10 g; meat extract, 10 g; agar, 15 g; distilled water to 1 l; adjusted to pH 7.0–7.5 and autoclaved at 121°C for 15 min; 100 g crystalline urea is added to molten agar immediately before use and steamed for 10 min prior to cooling and pouring) and incubating at 25°C. However, recent work on the taxonomy of the species (Logan and De Vos, unpublished observations) has revealed that few authentic strains of Bacillus lentus are available for study and that many unrelated strains have been assigned to the species. Until the species is more tightly defined, it is not possible to recommend a method for its specific isolation.
Bacillus licheniformis. Strains of this species may be obtained from soil by anaerobic enrichment in peptone-meat extract-KNO3 medium (Claus, 1965): soil suspensions are heat treated at 80°C for 10 min, and 2 ml quantities are added to glass-stoppered bottles of a medium containing peptone, 5 g; meat extract, 3 g; KNO3, 80 g, distilled water 1 l; pH 7.0. The bottles are filled completely, without trapping bubbles of air, and incubated at 40–45°C for 48 h, whereupon most will show turbidity and gas production. Loopfuls are streaked onto plates of glucose mineral base agar: glucose, 10 g; (NH4)2SO4, 1 g; K2HPO4, 0.8 g; KH2PO4, 0.2 g; MgSO4·7H2O, 0.5 g; CaSO4·2H2O, 0.05 g; FeSO4·7H2O, 0.01 g; agar, 12 g; distilled water to 1 l; adjust to pH 6.8. Colonies are often reddish and have lobes and mounds of slime.
Bacillus luciferensis. Strains of this species were isolated from soil samples that were collected from a geothermal site on Candlemas Island, South Sandwich archipelago, and transported and stored both chilled and frozen. 1 g quantities of soil were added to 9 ml trypticase soy broths in duplicate at pH 6.5 and one of each pair was heat treated at 80°C for 10 min to kill vegetative cells. Spread plates were inoculated with 0.1 ml soil suspension on trypticase soy agar at pH 6.5 (containing 5 mg/l MnSO4 to enhance sporulation) and incubated at 30°C. The suspensions remaining were incubated at the same temperature in a waterbath, and streaked onto the solid medium as soon as they became turbid (Logan et al., 2000).
Bacillus macyae was isolated from arsenic-contaminated mud from a gold mine, as described by Santini et al. (2002). The medium for Bacillus macyae is NaCl, 1.2 g; KCl, 0.3 g; NH4Cl, 0.3 g; KH2PO4, 0.2 g; Na2SO4, 0.3 g; MgCl2·6H2O, 0.4 g; CaCl2.2H2O, 0.15 g; NaHCO3, 0.6 g; trace element solution with 5.2 g/l Na2-EDTA and pH 6.5, 1.00 ml; resazurin, 0.5 mg; yeast extract, 0.8 g; Na-lactate, 1.1 g; KNO3, 0.5 g; distilled water 1000.00 ml. Prepare the medium anaerobically under 100% N2. Add sodium lactate (10 mM; electron donor) and sodium nitrate (or sodium arsenate; electron acceptors) to 5 mM from sterile, anaerobic stock solutions. Adjust final medium pH to 7.4–7.8. The trace element solution contains: HCl (25%; 7.7 M); FeCl2·4H2O, 1.5 g; CoCl2·6H2O, 190 mg; MnCl2·4H2O, 100 mg; ZnCl2, 70 mg; H3Bo3, 6 mg; Na2MoO4·2H2O, 36 mg; CuCl2·2H2O, 2 mg; NiCl2·6H2O, 24 mg; distilled water, 990 ml; dissolve the FeCl2 in the HCl, then dilute in water, add and dissolve other salts, and make up to 1 l. Incubate at 28°C.
Bacillus marisflavi – see Bacillus aquimaris.
Bacillus megaterium. The method of Claus (1965) may be used to isolate strains of this species from soil. Plate 0.1 ml volumes of dilutions of heat-treated soil suspensions on glucose mineral base agar: glucose, 10 g; (NH4)2SO4 or KNO3, 1 g; K2HPO4, 0.8 g; KH2PO4, 0.2 g; MgSO4·7H2O, 0.5 g; CaSO4·2H2O, 0.05 g; FeSO4·7H2O, 0.01 g; agar, 12 g; distilled water to 1 l; adjust to pH 7.0. Plates are incubated at 30°C. On the nitrate medium, white, round, smooth and shiny colonies 1–3 mm in diameter may develop in 36–48 h. On the ammonium medium (necessary, because not all strains can use nitrate), a variety of colonies may develop in 24–36 h, but colonies of Bacillus megaterium can be detected by their appearance. Suspect colonies from either medium should be observed microscopically for the typically large cells of this species, then purified on nutrient agar or trypticase soy agar.
Bacillus methanolicus. Dijkhuizen et al. (1988) isolated their thermotolerant, methanol-utilizing strains using the following enrichment medium: filter-sterilized methanol to 50 mMol; yeast extract, 0.5 g; Casamino acids, 0.5 g; peptone, 0.5 g; (NH4)2SO4, 1.5 g; K2HPO4, 4.65 g; NaH2PO4 H2O, 1.5 g; MgSO4·7H2O, 0.2 g; trace element solution, 0.2 ml; vitamin solution, 1 ml; distilled water, 1 l; the medium was adjusted to pH 7.0. The trace element solution contained: EDTA, 50 g; ZnSO4·7H2O, 22 g; CaCl2, 5.54 g; MnCl2·4H2O, 5.06 g; FeSO4·7H2O, 4.99 g; (NH4)6Mo7O24·4H2O, 1.10 g; CuSO4·5H2O, 1.57 g; CoCl2·6H2O, 1.61 g; distilled water, 1 l; pH adjusted to 6.0 with KOH. The vitamin solution contained biotin, 100 mg; thiamin, HCl, 100 mg; riboflavin, 100 mg; pyridoxalphosphate, 100 mg; pantothenate, 100 mg; nicotinic acid amide, 100 mg; p-aminobenzoic acid, 20 mg; folic acid, 10 mg; vitamin B12, 10 mg; lipoic acid, 10 mg; distilled water, 1 l. To conical flasks containing 25 ml of the medium, 1–5 ml of liquid samples or 1–5 g of soil samples (both previously heat treated at 80°C for 10 min, the soil samples suspended in 5 ml mineral medium) were added, and incubated at 50–55°C. Dense growth usually appeared within 72 h, and this was subcultured onto methanol agar plates (the enrichment medium solidified with 1.5% agar), which were incubated at 50°C. Colonies were transferred to the liquid medium after 24 h incubation. Cells apparently lysed rapidly on plates, and so repeated transfers (about 10) to liquid culture, followed by plating, were used to select variants less susceptible to lysing on solid medium. Cultures were then serially diluted in mineral medium and plated on methanol agar.
Bacillus mojavensis. This species is phenotypically virtually indistinguishable from Bacillus subtilis. Original strains were isolated from desert soil using a non-specific method for Bacillus species (see Bacillus subtilis, below), and the emerging colonies screened for the Bacillus subtilis phenotype (Roberts et al., 1994). See also Bacillus subtilis.
Bacillus mycoides. Strains of this species are readily isolated from a wide variety of sources, including soil, and are easily recognized by the characteristic rhizoid morphologies of their colonies (Figure 2d). Small drops of heat-treated or untreated soil suspensions (or soil crumbs, or appropriate preparations of other source material) are placed in the centers of nutrient or trypticase soy agar plates and incubated at 28°C. A Bacillus mycoides colony will develop as rhizoid growth which may spread to cover the agar surface within 2–3 d of incubation. Purification is best attempted by subculturing from the outer edge of the colony, and is aided by suspending growth (which may be quite adherent) in a nutrient broth, shaking vigorously, and then serially diluting prior to plating.
Bacillus naganoensis. The single original strain of this species was isolated on a medium containing colored starch and pullulan, designed to screen for organisms producing α-amylases and pullulanases (Tomimura et al., 1990). The initial screening medium contained: tryptone, 2 g; (NH4)2SO4, 1 g; KH2PO4, 0.3 g; MgSO4·7H2O, 0.2 g; CaCl2·2H2O, 0.2 g; FeSO4·7H2O, 0.01 g; MnCl2·4H2O, 0.001 g; agar, 20 g; soluble starch, 10 g; blue-colored soluble starch (Rinderknecht et al., 1967), 3 g; red-colored pullulan, 7.5 g; distilled water to 1 l; adjust to pH 4.0 using 0.2 N sulfuric acid. The colored pullulan was prepared by dissolving 100 g pullulan in 2 l distilled water, heating to 50°C and adding 10 g Brilliant Red and 100 ml of 10% Na3PO4; after 75 min incubation the product was precipitated with 1600 ml 99.5% ethanol, collected by decantation, washed twice with 60% ethanol, washed once with 99.5% ethanol, and air-dried (Tomimura et al., 1990). A 2 g portion of soil sample was suspended in 10 ml water and a 0.1 ml volume spread onto the screening medium and incubated at 30°C. Discrete colonies surrounded by blue zones (indicating pullulan, but not starch, hydrolysis), were subcultured onto a variant of the screening medium in which amylopectin (10 g/1 l) replaced the starches and pullulan; following incubation, pullulanase producing colonies were revealed by the dark blue zones surrounding them, against a light-purple background, when exposed to iodine vapor.
Bacillus nealsonii was isolated from a spacecraft-assembly plant by exposing 2.5 × 5 cm (0.05–0.08 cm thick) stainless steel witness plates, which had been cleaned by ultrasonication and solvent treatment, then sterilized by heating at 175°C for 2 h, on 2-m-high stands for 9 months. Each plate was placed in 30 ml sterile phosphate-buffered rinse solution (pH 7.2) and sonicated (25 kHz, 0.35 W/cm2) for 2 min. Each rinse was divided into two equal parts and one was heat treated at 80°C for 15 min, the other not. Total aerobic counts were determined in tryptic soy agar pour plates, incubated at 32°C for 3–7 d (Venkateswaran et al., 2003).
Bacillus neidei. This species contains soil isolates previously allocated to Bacillus sphaericus, and is distinguished from that species by a small number of phenotypic characters. Unlike Bacillus sphaericus, it has a requirement for cystine (Nakamura et al., 2002). No method for its specific enrichment or isolation has been described. See Bacillus sphaericus.
Bacillus niacini. Strains of this and other nicotinate-utilizing species were recognized by their production of blue or brown haloes on nicotinate medium. About 1 g of sample, which may have been heat treated (60°C or 80°C for 10 min), was suspended in 20 ml nicotinate medium (Nagel and Andreesen, 1989) and in 20 ml of the same medium supplemented with 0.05% yeast extract. This medium comprised: nicotinic acid or 6-hydroxynicotinic acid, 40 mM; CaCl2, 0.18 mM; MnSO4, 0.14 mM; MgSO4, 2.0 mM; NH4Cl, 5,6 mM; NaCl, 0.85 mM; FeSO4, 0.036 mM; potassium phosphate buffer (0.6 M, pH 7.5), 5 ml; Tris/HCl (1.0 M, pH 7.5), 100 ml; trace element solution, 1 ml; filter-sterilized vitamin solution, 5 ml; distilled water to 1 l; pH adjusted to 7.5. After 3–5 d incubation at 30°C on a rotary shaker, aliquots are plated onto nicotinate-yeast extract medium solidified with agar. The trace element solution contained: HCl (25% = 7.7 M), 12.5 ml; FeSO4·7H2O, 2.1 g; H3BO3, 0.03 g; MnCl2·4H2O, 0.1 g; CoCl2·6H2O, 0.19 g; NiCl2·6H2O, 0.024 g; CuCl2·2H2O, 0.002 g; ZnSO4·7H2O, 0.144 g; Na2MoO4·2H2O, 0.036 g; distilled water, 987 ml; autoclaved in sealed bottles with about 1/3 head space of air. The vitamin solution comprised: lipoic acid, 60 mg; p-aminobezoic acid, 50 mg; calcium-D-pantothenate, 50 mg; cyanocobalamin, 50 mg; nicotinic acid, 50 mg; riboflavin, 50 mg; thiamine hydrochloride, 50 mg; biotin, 20 mg; folic acid, 20 mg; pyridoxal hydrochloride, 10 mg; distilled water,1 l. Colonies with blue or brown haloes were streaked on the same medium to purify, then single colonies were suspended in saline and plated onto tryptone-yeast extract-succinate medium (tryptone, 10 g; yeast extract, 5 g; disodium succinate, 2 g; agar, 20 g; distilled water, 1 l; pH 7.5) or other complex medium, allowed to sporulate, and subjected to heat treatment then further cultivation on the same medium (Nagel and Andreesen, 1991).
Bacillus novalis – see Bacillus bataviensis.
Bacillus odysseyi. The description of this species (La Duc et al., 2004) indicates that the two isolates were isolated from two spa waters in Japan; one at 59°C and pH 6.4 and the other at 51°C and pH 8.1; both isolates showed optimal growth between 45°C and 50°C at pH 10.5 in heart infusion broth.
Bacillus okuhidensis. The description of this species (Li et al., 2002) indicates that the two isolates were isolated from two spa waters in Japan; one at 59°C and pH 6.4 and the other at 51°C and pH 8.1; both isolates showed optimal growth between 45°C and 50°C at pH 10.5 in heart infusion broth.
Bacillus oleronius. The original isolate of this species was cultivated from the hindgut contents of a termite (Reticulitermes santonensis) using the following enrichment medium: yeast extract, 1 g; vitamin and trace element solution (Balch et al., 1979), 10 ml; distilled water,1 l; NaCl, 24.1 mM; KCl, 21.5 mM; K2HPO4, 10.8 mM; KH2PO4, 6.9 mM; MgSO4·5.3 mM; CaCl2, 0.53 mM; adjusted to pH 7.2; after sterilization 1 mM of each of the following aromatic substrates were added: benzoic acid, coumaric acid, ferulic acid, 4-hydroxybenzoixc acid and vanillic acid. After enrichment, the organism was maintained on trypiticase soy agar, with monthly transfers. Further strains of this species have been isolated from animal feed concentrate, raw milk and dairy plant using a method devised for the isolation of Bacillus sporothermodurans (Scheldeman et al., 2002): samples were heat treated for 30 min at 100°C, followed by plating on brain heart infusion broth solidified with bacteriological agar and supplemented with vitamin B12 (1 mg/l, filter-sterilized); plates were incubated for 48 h at 37 and 55°C, but 37°C is the optimal temperature for growth and isolation of this species.
Bacillus pseudalcalophilus. Optimal growth of this alkaliphilic organism is about pH 10.0, no growth occurs at pH 7.0, and up to 10% NaCl is tolerated (Nielsen et al., 1995a); see Bacillus agaradhaerens, Bacillus alcalophilus and other alcaliphiles.
Bacillus pseudofirmus. Optimal growth of this alkaliphilic organism is about pH 9.0, most strains do not grow at pH 7.0, and 16–17% NaCl is tolerated (Nielsen et al., 1995a); see Bacillus agaradhaerens, Bacillus alcalophilus and other alcaliphiles.
Bacillus pseudomycoides is indistinguishable from Bacillus mycoides by conventional characters, and will probably be isolated along with strains of that species. It was proposed on the basis of differences in fatty acid composition and DNA relatedness between strains of Bacillus mycoides (Nakamura, 1998). See Bacillus mycoides.
Bacillus psychrosaccharolyticus. This psychrophilic organism was isolated by the same methods as were used for Bacillus insolitus. Cells of this species appear granular when grown on nutrient agar and lightly stained, and are larger and vacuolate when grown on glucose agar (Larkin and Stokes, 1967).
Bacillus pumilus. There is no specific method of enrichment or isolation of this species that is known to the authors; however, Knight and Proom (1950) found that when their suspensions of soil in distilled water were incubated at 37°C for 3 d, then plated on nutrient agar and incubated at 37°C, the mixed collection of colonies arising included strains of this species.
Bacillus pycnus. Like Bacillus neidei, this species contains soil isolates previously allocated to Bacillus sphaericus, and is distinguished from that species by a small number of phenotypic characters. Unlike Bacillus sphaericus, it does not have a requirement for biotin and thiamin (Nakamura et al., 2002). No method for its specific enrichment or isolation has been described. See Bacillus sphaericus.
Bacillus schlegelii. Aragno (1978) isolated this thermophilic, hydrogen-oxidizing organism by adding superficial samples (0.5 g) of sediment from a eutrophic lake in Switzerland to 100 ml bottles containing 20 ml of a basal mineral medium and Bonjour et al. (1988) made further isolations from the same lake, from air and from geothermal sites in Iceland and Italy using this three-part medium: Solution 1: Na2HPO4·2H2O, 4.5 g; KH2PO4, 1.5 g; NH4Cl, 1 g; MgSO4·7H2O, 0.2 g; trace elements solution, 1 ml; distilled water, 1 l; Solution II: CaCl2·2H2O, 100 mg; ferric ammonium citrate, 50 mg; distilled water, 100 ml; Solution III: NaHCO3, 5 g; distilled water, 100 ml; the three solutions were autoclaved separately and mixed in the proportions I, 1 l; II, 10 ml; III, 10 ml after cooling; pH 7.0. The trace elements solution contained: H3BO3, 300 mg; CoCl2·6H2O, 200 mg; ZnSO4·7H2O, 100 mg; MnCl2·4H2O, 30 mg; Na2MoO4·2H2O, 30 mg; NiCl2·6H2O, 20 mg; CuCl2·2H2O, 10 mg; distilled water, 1 l. Cultures were incubated in desiccators at 65°C under an atmosphere of 0.05 atm O2 + 0.1 atm CO2 + 0.45 atm H2 (partial pressure measured at room temperature). Dense growth was apparent after 4 d, and it was twice subcultured in fresh medium using loopful inocula. Pure cultures were obtained by streaking on plates of the same medium solidified with agar and incubating in the same conditions as before.
Further strains have been obtained by Krüger and Meyer (1984) from sludge samples of a sugar factory settling pond, by, and by Hudson et al. (1988) from geothermally heated Antarctic soil. Krüger and Meyer used carbon monoxide as the sole carbon and energy source for enrichment, and cultivated at 65°C in the mineral medium of Meyer and Schlegel (1983) under an atmosphere of (v/v) 5% CO2, 35% CO and 60% air. After 1 week of incubation, positive enrichments were serially subcultured in fresh medium using a 10% inoculum. Hudson et al. (1988) isolated their strain from a soil sample taken from Mount Erebus, Antarctica. The temperature of the sample site was 37°C, and the sample was kept unfrozen; 1 g was added to 100 ml of a thiosulfate medium: Na2S2O3, 10 g; NaHCO3, 2 g; NaNO3, 0.689 g; NH4Cl, 0.4 g; Na2HPO4, 0.111 g; KNO3, 0.103 g; MgSO4·7H20, 0.1 g; CaSO4·2H2O, 0.06 g; NaCl, 0.008 g; nitrilotriacetic acid, 0.1 g; (also, for later work, yeast extract, 0.1 g); phenol red, 0.024 g; distilled water to 1 l; pH 7.0. Trace elements and ferric chloride, and vitamins were also added. Incubation was at 60°C, and growth was evident from a fall in pH shown by the phenol red indicator. pure cultures were obtained by streaking on the same medium (without yeast extract) solidified with agar.
Bacillus selenitireducens. The single reported strain of this medium was isolated using the same method employed for Bacillus arseniciselenatis (see above), but it was found not to use selenate as the electron acceptor and could use arsenate in the lake water medium or selenite instead (Switzer Blum et al., 1998).
Bacillus shackletonii – see Bacillus luciferensis.
Bacillus silvestris. The single strain upon which this species is based was isolated from a 10 g sample of beech forest soil by storing it in humid conditions and incubating at 40°C for 15 min followed by suspension in 100 ml of a germination solution comprising: malt extract, 1 g; glucose, 0.4 g; yeast extract, 0.4 g; CaCO3, 0.2 g; L-asparagine, 1 g; distilled water, 1 l; filtersterilized cycloheximide solution (0.1 g in 10 ml water) was added after cooling to room temperature (Rheims et al., 1999). Dilutions in the germination medium were prepared to 10−3 and this final dilution was stirred at room temperature for 15 min and then aliquots of 100 µl were streak diluted on various media and incubated at different temperatures. The strain was isolated on a plate of tryptic soy broth supplemented with 0.3% yeast extract and solidified with agar; incubation was at 25°C for 1 d.
Bacillus simplex. This species is based on two soil isolates received as “Bacillus simplex” and “Bacillus teres” (Priest et al., 1988), and no method for isolating strains of the species is known to the authors.
Bacillus siralis. The original strain of this species was isolated from silage by heat treating the sample at 100°C for 60 min and plating onto brain heart infusion (BHI) agar (de Silva et al., 1998). Pettersson et al. (2000) made further isolations by suspending 1 g of lyophilized silage in 1 ml sterile water, heating at 100°C for 90 min, then diluting to 10−2 in sterile water; 50 ml aliquots were spread on plates of BHI agar and on plates of the following medium: yeast extract, 0.5 g; salts solution, 1 ml; nutrient agar, 100 ml. The salts solution comprised: MgCl2, 1 × 10−3 M; CaCl2, 7 × 10−4 M; MnCl2, 5 × 10−5 M. Plates were incubated at 24–48 h at 37°C, and colonies resembling those of the original isolate were purified on plates of the supplemented nutrient agar.
Bacillus smithii. This species contains isolates from evaporated milk, canned foods, cheese, and sugar beet juice extraction plant which were previously assigned to Bacillus coagulans; no methods for its specific enrichment or isolation are known to the authors – see Bacillus coagulans.
Bacillus soli – see Bacillus bataviensis.
Bacillus sonorensis. This species is phenotypically similar to Bacillus licheniformis, although yellow/cream pigmentation has been given as a distinguishing character, and colonies are bright yellow on a medium containing glucose 40 g; neopeptone, 10 g; agar 15 g, distilled water, 1 l; pH 5.6; whereas those of Bacillus licheniformis are cream (Palmisano et al., 2001). Original strains were isolated from desert soil using a general method for Bacillus species (see Bacillus subtilis below).
Bacillus sphaericus. Members of this species and closely related, spherical-spored organisms may be enriched using a method described by Beijerinck and Minkman (1910): 10–20 g of casein or its sodium salt is added to 1 l tap water, and the medium is dispensed in 30 ml quantities in 300 ml Erlenmeyer flasks; about 1 g of soil is added to each and the suspensions are heated to boiling, cooled and then incubated at 37°C for 3 d. Sphericalspored organisms will usually predominate in the resulting mixture of sporulating organisms. The flasks are heated to 80°C for 5 min and loopfuls are streaked on plates of nutrient agar at pH 7.0 and incubated at 30°C; colonies yielding sporangia swollen with spherical spores are further purified. Massie et al. (1985) described a medium containing sodium acetate as the only major source of carbon, for isolating Bacillus sphaericus from soil: Na2HPO4·12H2O, 11.2 g; KH2PO4, 2.4 g: (NH4)2SO4, 2.0 g; MgSO4·7H2O, 50 mg; MnCl2·4H2O, 4 mg; FeSO4·7H2O, 2.8 mg; sodium acetate·3H2O, 5.0 g; trisodium citrate ·2H2O, 20 mg; distilled water to 1 l; pH 7.2. The basal medium, without salts of Mg2+, Mn2+ and Fe2+ and the carbon source, was autoclaved for 10 min at 121°C. The salts were dissolved together at ×50 strength in 0.005 M-H2SO4 and autoclaved separately. The carbon source was autoclaved separately as a 25% w/v aqueous solution. The medium was solidified with agar. The medium was used unsupplemented or else it was supplemented with (per liter): glutamate, 1 g; thiamine, 10 mg; biotin 0.001 mg; in the combinations: biotin plus thiamine, thiamine plus glutamate, glutamate alone. The medium was also prepared with the acetate concentration doubled, and this version also used supplemented or unsupplemented. About 1 g moist soil was suspended in 5 ml sterile water and heated at 60°C for 30 min; 50 ml medium in a 250 ml flask was inoculated with 1 g soil suspension and incubated at 30°C with shaking; the next day, a loopful of culture was streaked onto solid medium, of the same composition as that in the original flask, and incubated at 30°C.
Strains of the species pathogenic to mosquito larvae may be naturally resistant to chloramphenicol and streptomycin (Burke and McDonald, 1983) and a selective, differential medium containing streptomycin has been described by Hertlein et al. (1979). The BATS medium of Yousten et al. (1985) uses arginine as the sole carbon and nitrogen source and uses streptomycin as the selective agent; it was found to inhibit the growth of 68% the nonpathogenic Bacillus sphaericus strains tested: Na2HPO4, 5.57 g; KH2PO4, 2.4 g: MgSO4·7H2O, 50 mg; MnCl2·4H2O, 4 mg; FeSO4·7H2O, 2.8 mg; CaCl2·2H2O, 1.5 mg; L-arginine, 5 g; thiamine, 20 mg; biotin, 2 µg; streptomycin sulfate, 100 mg; agar, 20 g; distilled water, 1 l. The arginine, thiamine, biotin and streptomycin are prepared as a filter-sterilized stock solution. The Mg2+, Mn2+, Fe2+ and Ca2+ salts are prepared as an acidified [0.03% (v/v) concentration of H2SO4], autoclaved stock solution. Stock solutions are added to the autoclaved phosphate salts-agar medium when it has cooled to 50°C after autoclaving.
Bacillus sporothermodurans. Strains of this species are characterized by their highly heat-resistant spores (HHRS), and they were first detected in ultrahigh-temperature (UHT) treated milk which is heated to 135–142°C for a few seconds; further strains were isolated from silage. The organism has been sought in milk, feed and silage by heating samples to 100°C for 60 min, plating onto brain heart infusion (BHI) agar, and incubating at 37°C for 2 d. Colonies resembling Bacillus sporothermodurans are selected and purified by streaking on plates of BHI agar or nutrient agar which have been supplemented with 1 mg per liter of vitamin B12 (Pettersson et al., 1996). The approach described is not specific for this species, and a range of other HHRS is likely to be isolated. Further strains of this species have been isolated from raw, UHT and sterilized milks, animal feed concentrate, and soy meal using the following method (Scheldeman et al., 2002): samples were heat treated for 30 min at 100°C, followed by plating on brain heart infusion broth solidified with bacteriological agar and supplemented with vitamin B12 (1 mg/l, filtersterilized); plates were incubated for 48 h at 20, 37 and 55°C, but 37°C is the optimal temperature for growth and isolation of this species.
Bacillus subterraneus. The single isolate of this species was cultivated from deep subterranean thermal water (71°C, pH 7.8) collected in a sterile glass container from a bore well tapping the Great Artesian Basin of Australia; the containers were completely filled and sealed with air-proof enclosures. For enrichment, 1 ml of water was injected into 10 ml of metal reduction (MR) medium, supplemented with 0.016 g iron oxide, in Hungate tubes and incubated at 40°C for 1 week (Kanso et al., 2002). MR medium was prepared as follows: yeast extract, 2 g; NH4Cl, 1 g; K2HPO4·3H2O, 0.08 g; MgCl2·6H2O, 4.5 g; CaCl2·2H2O, 0.375 g; NaCl, 20 g; vitamin solution (Patel et al., 1985), 10 ml; trace element solution, 1 ml; distilled water to 1 l. The trace element solution contained: nitrilotriacetic acid neutralized to pH 6.5 with KOH, 12.5 g; FeCl3·4H2O, 0.2 g; MnCl2·4H2O, 0.01 g; CoCl2·6H2O, 0.017 g; CaCl2·2H2O, 0.1 g; ZnCl2, 0.1 g; CuCl2, 0.02 g; H3BO3, 0.01 g; Na2MoO4·2H2O, 0.01 g; NaCl, 1.0 g; Na2SeO3, 0.02 g; distilled water, 1 l. The medium was boiled and then cooled under a stream of oxygen-free N2 gas to about 50°C, 3.6 g of NaHCO3 were added, and the pH adjusted to 7.1; 10 ml aliquots were dispensed under N2 into Hungate tubes (Hungate, 1969), and this gas phase was subsequently replaced with N2/CO2 (80:20) and the medium autoclaved at 121°C for 15 min. The enrichment culture that developed could reduce Fe(III), and nitrate (20 mM) was used as an alternative electron acceptor; serially diluted enrichments in MR medium with nitrate were purified on plates of the same medium solidified with 2% agar and incubated at 40°C for 1 week.
Bacillus subtilis. This species is readily isolated from dried grass, and has long been known as the “hay bacillus.” Zopf (1885) recommended the following method: hay is soaked in water for 4 h at 36°C, using as small a volume of water as possible, and the fluid is decanted and diluted to a specific gravity of 1.004; the pH is adjusted to 7.0 and 500 ml of the suspension is transferred to a sterile 1-l Erlenmeyer flask, which is plugged with cotton wool and then boiled for 1 h. The flask is incubated at 36°C for about 28 h, and the pellicle that usually forms will often be found to yield only Bacillus subtilis. Knight and Proom (1950) added air-dried soil to 10 ml of nutrient broth to a give total volume of 15 ml, shook the vessel well, plated loopfuls of the suspension on nutrient agar, and then incubated at 37°C for 2 d; the resulting flora was predominantly Bacillus subtilis. Roberts and Cohan (1995) and their colleagues isolated the Bacillus subtilis strains which they subsequently allocated to Bacillus mojavensis, Bacillus vallismortis and Bacillus subtilis subsp. spizizenii (and close relatives of Bacillus licheniformis allocated to Bacillus sonorensis) by suspending 1 g quantities of desert soils in 5 ml amounts of sterile water, heat-treating suspensions at 80°C for 10 min, vortex mixing for 1 min and allowing to settle for a further minute, then plating 50–100 µl onto tryptone blood agar base plates and incubating at 37°C for 48 h (F.M. Cohan, personal communication).
Bacillus thermoamylovorans. The single original strain of this species was isolated from palm wine (Combet-Blanc et al., 1995) using Hungate's anaerobic methods (Hungate, 1969; Macy et al., 1972; Miller and Wolin, 1974). Palm wine, 10 ml; 10% NaHCO3, 1 ml; 2% Na2S·9H2O, 0.2 ml were added to a 60 ml serum bottle containing 20 ml of basal medium supplemented with 0.3% glucose. Bottles were incubated at 50°C for 24 h, and then a 1-ml sample was transferred to a fresh serum bottle of basal medium. This step was twice repeated. A pure culture was obtained by the roll-tube method of Hungate, using Hungate tubes containing 4.5 ml of basal medium supplemented with 0.3% glucose. Basal medium contained: yeast extract, 5 g; Biotrypcase (bioMérieux), 5 g; KH2PO4, 1 g; NH4Cl, 1 g; MgCl2·6H2O, 0.4 g; FeSO4·7H2O, 5 mg; mineral solution, 25 ml; trace element solution, 1 ml; Tween 80, 1 ml; distilled water, 1 l; pH adjusted to 7.5 with 10 M KOH. The mineral solution contained: KH2PO4, 6 g; (NH4)2SO4, 6 g; NaCl, 12 g; MgSO4·7H2O, 2.6 g; CaCl2·2H2O, 0.16 g; distilled water, 1 l. The trace element solution contained: nitrilotriacetic acid (neutralized to pH 6.5 with KOH), 1.5 g; MgSO4·7H2O, 3 g; MnSO4·2H2O, 0.5 g; NaCl, 1 g; FeSO4·7H2O, 0.1 g; CoCl2.or CoSO4, 0.1 g; CaCl2·2H2O, 0.1 g; ZnSO4, 0.1 g; CuSO4·5H2O, 0.01 g; AlK(SO4)2, 0.1 g; H3BO3, 0.01 g; Na2MoO4·2H2O, 0.01 g; distilled water, 1 l; pH adjusted to 7.0 with KOH. The medium was boiled, and cooled under a stream of O2-free N2 at room temperature, distributed into serum bottles and Hungate tubes, and autoclaved at 110°C for 45 min. Energy sources were injected into the bottles and tubes from separately sterilized stock solutions.
Bacillus thermocloacae. Strains of this species were isolated from an aerobic culture at 60°C of municipal sewage sludge in a laboratory fermenter (Demharter and Hensel, 1989b). Samples were taken from the fermenter, homogenized, diluted and plated on Ottow's 1974 medium (glucose, 1.0 g; peptone, 7.5 g; meat extract, 5.0 g; yeast extract, 2.5 g; Casamino acids, 2.5 g; NaCl, 5.0 g; agar, 13 g; tap water, 1 l; pH 7.2–7.4.) supplemented with 100 ml per liter of aqueous sludge extract, adjusted to a final pH of 8.5, and incubated for 3–4 d at 60°C.
Bacillus thuringiensis. The isolation of Bacillus thuringiensis from environmental samples can be very laborious. After heat treatment and plate cultivation, it requires the microscopic examination of material from all colonies with Bacillus cereus-type morphologies for the parasporal crystals characteristic of Bacillus thuringiensis. Therefore, several methods have been devised in order to increase the proportion of such colonies which are Bacillus thuringiensis. Travers et al. (1987) described a method which uses acetate to inhibit germination of Bacillus thuringiensis spores, followed by heat to kill germinated cells of other spore-formers and cells of non-spore-forming bacteria: L broth (tryptone, 10 g; yeast extract. 5 g; NaCl, 5 g; distilled water, 1 l) is buffered with 0.25 M sodium acetate. 0.5 g of sample is added to 10 ml of this broth in a 125 ml triple-baffled flask, and the flask is shaken at 250 r.p.m. at 30°C for 4 h. A sample of this suspension is then heat treated for 3 min at 80°C in a flow-through heat treater, and used to inoculate plates of L agar (as L broth, but solidified with agar; sodium acetate is not included). Carozzi et al. (1991) used a similar approach, but with heat treatment at 65°C for 10 min. Johnson and Bishop (1996) enriched Bacillus thuringiensis in penicillin broth, followed by plating on penicillin agar, and found their method to be superior to those of Travers et al. (1987) and Carozzi et al. (1991): 0.25 g of sample is placed in a tube containing 2 ml of nutrient broth which has been supplemented with 1 ml/l CCY salts and 20 IU/ml penicillin G. The CCY salts solution (to aid sporulation) contains: acid casein hydrolysate, 1 g/l; enzymic casein hydrolysate, 1 g; glycerol, 0.6 g; enzymic yeast extract, 0.4 g; glutamine, 20 mg; distilled water, 1 l; and the following salts: K2HPO4, 26 mM; KH2PO4, 13 mM; MgCl2·6H2O, 0.5 mM; CaCl2·6H2O, 0.2 mM; FeCl3·6H2O, 0.05 mM; ZnCl2, 0.05 mM; MnCl2,·4H2O, 0.01 mM. The suspension is heat-shocked at 70°C for 10 min and then added to 50 ml of the same medium in a 250 ml flask. The flask is shaken at 200 r.p.m. at 30°C until sporulation is complete. The suspension is centrifuged at 3,600 r.p.m. for 1 h, and the pellet resuspended in 2 ml of fresh medium and the heat treatment and shaking procedure repeated; serial dilutions of the suspension are then inoculated onto plates of the supplemented nutrient broth which has been solidified with agar, and incubated at 30°C until sporulation is complete (as judged by microscopy), and observed for the characteristic sporangial morphology of Bacillus thuringiensis. Forsyth and Logan (2000) found that a penicillin-based method was unsuitable for the isolation of Bacillus thuringiensis from Antarctic soils, as their isolates from these environments showed some penicillin sensitivity. Heat treatment and enrichment in BFB (see Bacillus fumarioli, above), followed by plating from turbid enrichments onto BFA and observation for parasporal crystals gave best results; over all the samples studied, however, acetate selection methods gave yields no better than the BFB method (Logan and Grieg, unpublished observations).
Bacillus tusciae. Strains of this hydrogen-oxidizing thermoacidophile were isolated from ponds in a geothermally heated area in Italy (Bonjour and Aragno, 1984). The enrichment medium was the mineral medium used by Bonjour et al. (1988) (see Bacillus schlegelii, above): 0.5 g per liter of NaHCO3 were added for autotrophic growth, and the medium was acidified to pH 3.5 using 5 M HCl. About 1 g amounts of pond sediment mixed with overlying water were added to 20 ml of medium in 100 ml Pyrex screw-capped bottles, and cultures were incubated in desiccators under a gas mixture of 0.05 atm O2 + 0.1 atm CO2 + 0.45 H2 (partial pressures measured at room temperature; the reduced total pressure of 0.6 atm allowed incubation at high temperatures without overpressure) for 5 d at 55°C. For isolation, cultures were subcultured by streaking onto plates of the same medium solidified with agar and incubated under the same conditions as for enrichment.
Bacillus vallismortis. This species is phenotypically virtually indistinguishable from Bacillus subtilis, and original strains were isolated from desert soil using a non-specific method for Bacillus species (see Bacillus subtilis, above).
Bacillus vedderi. Samples of mud from a bauxite-processing red mud tailing pond were inoculated into alkaline oxalate medium and incubated at room temperature or 45°C for 2 weeks (Agnew et al., 1995). Bottles showing turbidity were subcultured into bottles of fresh medium which, once turbid, were subcultured by streaking on plates of the same medium solidified with 2% (w/v) agar. Isolates were routinely grown at 37°C. The alkaline oxalate medium comprised: Na2CO3, 2.65 g; (NH4)2SO4, 1 g; K2HPO4, 0.17 g; MgSO4, 0.15 g; distilled water, 1 l; pH adjusted to 10.5 with 5 M NaOH before autoclaving. After cooling, the following additions were made from sterile stock solutions to the final concentrations shown: sodium oxalate, 0.67% (w/v); yeast extract, 0.1% (w/v); mineral solution, 0.2% (v/v). The mineral solution contained: nitrilotriacetic acid, 1.5 g; MgSO4·7H2O, 3 g; MnSO4·2H2O, 0.5 g; NaCl, 1 g; FeSO4·7H2O, 0.1 g; CaCl2·2H2O, 0.1 g; CoCl2, 0.1 g; ZnSO4, 0.1 g; CuSO4·5H2O, 0.01 g; AlK(SO4)2, 0.01 g; H3BO3, 0.01 g; Na2MoO4·2H2O, 0.01 g; distilled water, 1 l.
Bacillus vietnamensis was isolated from nuoc mam (Vietnamese fish sauce); trypticase soy agar was used as basal and maintenance medium.
Bacillus vireti – see Bacillus bataviensis.
Bacillus weihenstephanensis. This species is phenotypically similar to Bacillus cereus and only distinguishable from it by ability to grow at 7°C, inability to grow at 43°C, and by certain 16S rDNA signature sequences (Lechner et al., 1998), but not all psychrotolerant organisms resembling Bacillus cereus are Bacillus weihenstephanensis. Strains were isolated from German dairies by plating pasteurized milk samples on plate count agar and incubating at 7 ± 0.5°C for 10–16 d. Psychrotolerance was confirmed by inoculating purified colonies into liquid culture of plate count medium and incubating at 7 ± 0.5°C with agitation until growth was visible. Plate count medium contained: peptone, 5 g; yeast extract, 2.5 g; glucose, 1 g; distilled water, 1 l; pH, 7.0.
Maintenance procedures
Bacillus strains may be preserved on slopes of a suitable growth medium that encourages sporulation, such as nutrient agar or trypticase soy agar containing 5 mg/l of MnSO4·7H2O. Slopes should be checked microscopically for spores before sealing, to prevent drying out, and storage in a refrigerator; on such sealed slopes the spores should remain viable for many years. For longer-term preservation, lyophilization and liquid nitrogen may be used, as long as cryoprotectants are added.
Procedures for testing special characters
Introduction. The ubiquity and huge diversity of Bacillus species presents a huge diagnostic challenge. In the First Edition of this Manual, the genus Bacillus was essentially defined as: “aerobic, endospore-forming, Gram-positive rod-shaped bacteria,” and the methods of Gordon et al. (1973) could confidently be recommended for identification of the majority of the 34 valid species (five further species were added in proof) listed by Claus and Berkeley (1986). The scheme of Smith et al. (1952) split the species into three groups according to their sporangial morphologies, and then further divided them by biochemical and physiological tests, and this system culminated in the monograph of Gordon et al. (1973). This approach was effective for some years, but Bacillus identification was still generally perceived as complicated, the chief difficulties being the need for special media, and between-strain variation. Much of the latter was a reflection of unsatisfactory taxonomy, but, as the studies of Logan and Berkeley (1981) revealed, test inconsistency exacerbated the problems. Logan and Berkeley (1984) addressed these problems with a large database for 38 clearly defined taxa (species) using miniaturized tests in the API 20E and 50CHB Systems (bioMérieux, Marcy l'Etoile, France), and this scheme, with updates, remains in common use. Since the development of the Gordon et al. and Logan and Berkeley schemes, the genus has been radically changed, and the task of identification made more complicated, by: (i) the proposal of many new species (frequently from exotic habitats, and often primarily on the basis of molecular analyses), (ii) the allocation of strict anaerobes (Bacillus arseniciselenatis, Bacillus infernus) and organisms in which spores have not been observed (Bacillus infernus, Bacillus thermoamylovorans) to the genus, and (iii) the transfer of many species to the new genera, Alicyclobacillus, Aneurinibacillus, Brevibacillus, Geobacillus, Gracilibacillus, Marinibacillus, Paenibacillus, Salibacillus (now merged with Virgibacillus), Ureibacillus and Virgibacillus, and to the long-established genus Sporosarcina.
Unfortunately, taxonomic progress has not revealed readily determinable features characteristic of each genus. Also, many recently described species represent genomic groups disclosed by DNA–DNA pairing experiments, and routine phenotypic characters for distinguishing some of them are very few and of unproven value. Furthermore, several recently described species were proposed on the basis of very few strains so that the within-species diversities of such taxa, and so their true boundaries, remain unknown. It is clear, therefore, that the identification of Bacillus species has become increasingly difficult since the publication of the schemes of Gordon et al. and Logan and Berkeley, a period during which demands to identify such organisms have greatly increased, especially in the medical and biotechnological fields.
The identification scheme of Gordon et al. (1973) embraced 18 species, only half of which now remain in Bacillus. The genus now contains 90 species, and encompasses acidophiles, alkaliphiles, neutrophiles, halophiles, mesophiles, psychrophiles and thermophiles, in addition to the largely neutrophilic and mesophilic species studied by Gordon et al. (1973), so that their methods can no longer be expected, even with substantial modifications and an expanded database, to allow recognition of all the species now allocated to the genus. Identification of Bacillus species with routine phenotypic tests must therefore call upon a variety of characterization methods, and a unified approach is no longer possible. Despite this, the traditional characterization tests used by Gordon et al. retain their place in Bacillus identification, because the most commonly encountered species are still distinguishable by these methods.
Fritze (2002) recommended a stepwise approach to identification of the aerobic endospore-formers: (i) differential cultivation at a range of temperature (say 5, 30 and 55°C) and pH conditions (say pH 4.5, 7–7.2 and 9) [to which we may add salt concentrations appropriate to halotolerant and halophilic organisms], (ii) selection of spores by heat treatment or alcohol treatment (the latter being preferred, because not all spores are sufficiently heat-resistant to survive the former procedure), and (iii) characterization using the appropriate media and incubation temperatures.
Reva et al. (2001) described a phenotypic identification scheme for aerobic endospore-formers based on 115 characters and a key. Their database included just 69 strains of validly published Bacillus species, with numbers of representatives of each species ranging from one for Bacillus badius, Bacillus mycoides, Bacillus lentus and Bacillus thuringiensis to 27 for Bacillus subtilis. Four of the 13 Bacillus species they included were not represented by their type strains. They also included strains of two Brevibacillus species, four Paenibacillus species and one Virgibacillus species. Identification schemes are naturally limited by their databases, and schemes based on keys are prone to failure with atypical isolates. The strengths and weaknesses of the Reva et al. (2001) scheme can only be revealed by usage; the characters they employed were investigated by the traditional procedures described by Gordon et al. (1973) and Claus and Berkeley (1986), and the discriminative efficiencies they calculated for these characters might be valuable in the construction of “home-grown” identification systems.
Reference strains. In the following accounts of media and methods, the above-mentioned constraints must be borne in mind, and it is recommended that the original and emended descriptions of the more recently described species are consulted wherever possible, and that cultures of those organisms are obtained for comparison. It should also be remembered that 16S rDNA sequencing is not always reliable as a standalone tool for identification, and that a polyphasic taxonomic approach is advisable for the identification of some of the more rarely encountered species and the confident recognition of new taxa. It must be appreciated that the species descriptions given in the text and tables below are largely lifted from authors' descriptions of their proposed species, and that a number of such descriptions were based upon few strains, so that within-species diversities in such cases are unknown. Characterization methods and their interpretation vary, and typographic errors in the compilation of descriptions are bound to occur (several being encountered in the preparation of this review), so original descriptions should never be relied upon entirely. Nomenclatural types exist for a good reason and are usually easily available; there is no substitute for direct laboratory comparisons with authentic reference strains. However, collections of Bacillus species in laboratories around the world harbor many misnamed strains. This is not necessarily a reflection on the competence of those assembling the collections; it is more a symptom of the unsatisfactory state of the classification of the organisms at the time the cultures were acquired, and this underlies the difficulty that many bacteriologists frequently encounter with Bacillus identification.
Unfortunately, several of the groups whose taxonomies are the most complex are the ones whose members are frequently submitted to reference laboratories. Such organisms are frequently included in the databases of commercial kits, but it can be difficult to obtain sufficient authentic strains of some these species to allow a satisfactory database entry to be made. Ideally, an entry in the database should reflect at least ten representative strains of the species, but for some taxa, and particularly for the new species which have been based upon just one strain or very few strains, this can be impossible. It can also be a problem for some of the older-established species, such as Bacillus circulans and Bacillus lentus, as the representative strains found in culture collections around the world are sometimes the widely dispersed subcultures of a few original isolates. These problems emphasize the importance of basing proposals for new taxa on adequate numbers of strains to reflect the diversities of the taxa.
A further problem has emerged with the splitting of well-established (but not necessarily homogeneous) species or groups into large numbers of new taxa over a short period. Bacillus circulans was long referred to as a complex rather than a species, but the revision of the taxonomy of this group, and consequent proposals for several new species to be derived from it, have led to difficulties in identification. Although the proposals were mostly based upon polyphasic taxonomic studies, initial recognition of the new taxa depended largely upon DNA relatedness data. A DNA:DNA reassociation study of Bacillus circulans strains yielded Bacillus circulans sensu stricto, Bacillus amylolyticus, Bacillus lautus, Bacillus pabuli and Bacillus validus and evidence for the existence of five other species (Nakamura, 1984a; Nakamura and Swezey, 1983). Bacillus amylolyticus, Bacillus lautus, Bacillus pabuli and Bacillus validus are now accommodated in Paenibacillus, and these species and Bacillus circulans are difficult to distinguish using routine phenotypic tests.
Such radical taxonomic revisions have left many culture collections worldwide with few representatives of Bacillus circulans sensu stricto, but with numerous misnamed strains of this species, which may or may not belong to one of the newly proposed taxa. The curators will normally not be able to know which are which without considerable expenditure in scholarship and experimental work, and in many cases a collection will hold only one authentic strain, the type strain, of a species - be it an old or new species.
When attempting to construct an identification scheme for Bacillus the implication of such rapid taxonomic progress is huge. Accessing authentic strains of many species, even well-established ones, may require much time and effort, and for several of them the strains available may be too few to allow the diversities of the taxa to be adequately reflected in the identification scheme. Smith et al. (1952) and Gordon et al. (1973) showed commendable restraint in their concept of a bacterial species, saying in the latter monograph: “When only a few strains of a group are available, as often happens, their species descriptions must remain tentative until verified by the study of more strains”. Just as taxonomists can only be as good as their culture collections, so identification systems can only be as good as their databases.
Although many new characterization methods have been developed over the last 30 years, the principle of identification remains the same; identifications cannot be achieved, strictly speaking; the best that can be done is to seek the taxon to which the unknown strain probably belongs. The outcome is expressed as a probability and, as with the classification upon which the scheme is based, the answer cannot be final (Logan, 2002). It should not be assumed that, because traditional approaches for identifying Bacillus are perceived as being difficult and unreliable, any newer approach is likely to be superior regardless of the size and quality of its database; whatever characterization method is used, considerable amounts of time, money and expertise need to be invested in the construction of reliable and detailed databases, which must be founded upon wide diversities of authentic reference strains.
Standardization. Whatever methods are used to generate the characters upon which identifications are based, standardization of methodologies and inclusion of reference strains is crucial. The methods used to generate the characters included in species descriptions have not usually been standardized between laboratories, and the test results shown in differentiation tables often include information lifted from the literature, so that data are often not comparable. As the number of valid Bacillus species increases, the task of studying related and reference organisms in parallel becomes more demanding for classification and identification work, and authors may be tempted to lean ever more heavily on data presented in the literature. In addition, standardization of methodologies for many phenotypic tests is inherently impossible between those organisms whose temperatures and pH ranges for growth do not overlap. Miniaturized versions of traditional biochemical tests (API kits, VITEK cards, and Biolog plates) offer standardized methods for a range of biochemical characters; the first named offers some versatility in temperature and pH, while the last named can be incubated at a range of temperatures.
Media. The most widely used solid media for cultivating neutrophilic Bacillus species are nutrient agar and trypticase soy agar; these media may be adjusted to higher or lower pH for cultivating alcaliphiles and acidiphiles, and NaCl may be added for the cultivation of halophiles. Sporangial morphology remains an important character, and although many strains will sporulate on these media within a few days of incubation, the addition of 5 mg/l of MnSO4·xH2O is recommended for encouraging sporulation. Gordon et al. (1973) recommended soil extract agar to encourage sporulation for the purpose of strain maintenance, but this approach is unnecessarily laborious as the addition of manganese ions appears to serve just as well. Rich media such as blood agar may not yield sporulated cells and the culture might die without sporulating. Many of the more recently described Bacillus species have been found in unusual environments and/or have been isolated and studied using special media; these media are described in the descriptions of these species (see below). Most of the media (or revisions described by Claus and Berkeley, 1986) used by Gordon et al. (1973) for separation of the nine species that they listed, and which remain in the genus, are given below. Other media employed by Gordon et al., such as litmus milk, are not listed because they gave very poorly reproducible results (Logan and Berkeley, 1981) and/or are now rarely used for some other reason.
Anaerobic agar. Trypticase (trypsin hydrolysate of casein), 20 g; glucose, 10 g; NaCl, 5 g; sodium thioglycolate (mercaptoacetic acid, sodium salt), 2 g; sodium formaldehydesulfoxylate (hydroxymethanesulfinic acid, monosodium salt dihydrate), 1 g; agar 15 g; distilled water, 1 l; pH 7.2. Distribute into 15 mm diameter glass tubes to 75 mm depth and autoclave at 121°C for 20 min. Several commercial preparations are available. (Note: Gordon et al. omitted the indicator and glucose from the usual formulation of this medium).
Citrate and propionate utilization media. Trisodium citrate ·2H2O, 1 g (or sodium propionate, 2 g); MgSO4·7H2O, 1.2 g; (NH4)2HPO4, 0.5 g; KCl, 1 g; trace element solution, 40 ml; phenol red (0.04% w/v solution), 20 ml; agar, 15 g; distilled water, 920 ml; pH 6.8; distribute in tubes, autoclave at 121°C for 20 min and set as slopes. Trace element solution: ethylenediaminetetraacetate, 500 mg; FeSO4·7H2O, 200 mg; H3BO3, 30 mg; CoCl2·6H2O, 20 mg; ZnSO4,·7H2O, 10 mg; MnCl2·4H2O, 3 mg; Na2MoO4·2H2O, 3 mg; NiCl2·6H2O, 2 mg; CuCl2·2H2O, 1 mg; distilled water, 1 l.
Egg-yolk reaction medium. Tryptone (trypsin hydrolysate of casein), 10 g; Na2HPO4, 5 g; KH2PO4, 1 g; NaCl, 2 g; MgSO4·7H2O, 0.1 g; glucose, 2 g; distilled water, 1 l; pH 7.6; autoclave at 121°C for 20 min. Add sterile, commercially prepared egg-yolk emulsion (at the concentration recommended by the manufacturer), or 1.5 ml egg yolk (aseptically aspirated from a hen's egg) to 100 ml of basal medium and allow to stand overnight in a refrigerator. Dispense the supernatant in 2.5 ml amounts; basal medium without yolk is similarly dispensed. Modern practice replaces this medium with egg-yolk agar, without a noticeable difference in sensitivity: add 10 ml commercially prepared egg-yolk emulsion to 90 ml molten nutrient agar held at 45–50°C; mix and pour as plates.
Glucose agar. D-Glucose, anhydrous, 10 g; nutrient agar, 1 l; pH 6.8; mix thoroughly and autoclave at 115°C for 20 min.
Medium for acid production from carbohydrates. Basal medium: (NH4)2HPO4, 1 g; KCl, 0.2 g; MgSO4·7H2O, 0.2 g; yeast extract, 0.2 g; agar 15 g; distilled water, 1 l; adjust to pH 7.0; bromcresol purple (0.04% w/v solution), 15 ml; autoclave at 121°C for 20 min. Aqueous solutions (10% w/v) of carbohydrates are filter-sterilized or may be autoclaved at 121°C for 20 min. Gordon et al. (1973) used L-arabinose, D-glucose, D-mannitol and D-xylose, but on the basis of API 50CHB tests (see Miniaturized biochemical test systems, below) the following other carbohydrates may be valuable for differentiation of certain species: N-acetylglucosamine, D-mannose, D-tagatose, galactose, gluconate, glycerol, glycogen, inulin, melezitose, methyl α-D-mannoside, β-methylxyloside, salicin and starch. Aseptically add carbohydrate solution to molten base to a final concentration of 0.5% w/v, and set the medium as slopes. Media for testing acidophilic and alkaliphilic strains are described in Methods, pH below.
Milk agar. Skim milk powder, 5 g in 50 ml of distilled water; agar, 1 g in 50 ml of distilled water. Autoclave separately at 121°C for 20 min, cool to 45°C, mix, and pour into Petri dishes. Dry the surfaces of the plates before use.
Nitrate broth. Peptone (trypsin hydrolysate of meat), 5 g; beef extract, 3 g; KNO3, 1 g; distilled water, 1 l; pH 7.0. Distribute into test tubes containing inverted Durham's tubes, and autoclave at 121°C for 20 min.
Gelatin medium. Gelatin, 120 g; distilled water, 1 l; pH 7.0; autoclave at 121°C for 20 min; distribute in test tubes. Alternatively, tubes of commercially prepared nutrient gelatin may be used, or plates of nutrient agar supplemented with 0.4% gelatin.
Phenylalanine agar. NaCl, 5 g; yeast extract, 3 g; DL-phenylalanine, 2 g; Na2HPO4, 1 g; agar, 12 g; distilled water, 1 l; pH 7.3; distribute in tubes, autoclave at 121°C for 20 min and set as slopes. Claus and Berkeley (1986) favored the commercially prepared product available from BBL (www.voightglobal.com).
Resistance to lysozyme medium. Prepare a solution of lysozyme containing 10,000 enzyme units/ml in distilled water and sterilize it by filtration. Add 1 ml of this medium to 99 ml sterile nutrient broth and distribute the mixture in 2.5 ml amounts.
Sabouraud dextrose broth. Neopeptone (enzyme digest of casein and meat), 10 g; D-glucose, 40 g; agar, 15 g; distilled water, 1 l; pH 5.7; dispense into test tubes, sterilize by autoclaving at 121°C for 20 min, and set as slopes. The broth is prepared by reducing the glucose content to 20 g, omitting the agar, and distributing in tubes.
Sodium chloride media. Tubes of nutrient broth are prepared with 0, 5, 7 and 10% (w/v) NaCl.
Starch agar. Suspend 1 g of potato starch in 10 ml cold distilled water and mix with 100 ml nutrient agar. Autoclave at 121°C for 20 min, cool to 45°C, mix thoroughly, and pour into Petri dishes.
Tyrosine agar. L-tyrosine, 0.5 g; distilled water, 10 ml; autoclave at 121°C for 20 min. Mix aseptically with 100 ml sterile, molten nutrient agar, cool to about 50°C and pour into Petri dishes, taking care to achieve a uniform distribution of the tyrosine crystals. Dry plates before use.
Voges – Proskauer broth. Proteose peptone (enzyme digest of meat), 7 g; glucose, 5 g; NaCl, 5 g; distilled water, 1 l; pH 6.5; dispense 5 ml amounts into 20 mm test tubes and sterilize by autoclaving at 115°C for 20 min.
Methods. Current schemes for identifying Bacillus species may be roughly divided into four categories according to the kinds of characters they use: (i) traditional biochemical, morphological and physiological characters, (ii) miniaturized versions of traditional biochemical tests (API kits, VITEK cards, and Biolog plates), (iii) chemotaxonomic characters [such as fatty acid methyl ester (FAME) profiles, and pyrolysis mass spectrometry], and (iv) genomic characters (ribotyping, and nucleic acid probes). However, as early as the work of Smith et al. (1952) it was becoming clear that no one phenotypic technique would be suitable for identifying all Bacillus species. The problems have mounted up as further species from extreme environments have subsequently been proposed, and the potentials of chemotaxonomic analyses and studies of nucleic acids have therefore been investigated. The sections that follow outline these approaches and summarize their current contributions to identification for Bacillus and its relatives. However, it is impossible to devise standard conditions to accommodate the growth of strains of all species for chemotaxonomic work, and it remains unknown to the taxonomist if differences between taxa are consequences of genetic or environmental factors. The need to substantiate each characterization method by other techniques (be they phenotypic or genotypic) has become increasingly important as new techniques emerge. This need is satisfied by the polyphasic approach now usual for the better classification studies, and the same approach may sometimes be necessary in order to identify strains from some of the less familiar species.
Sporulation and microscopic appearance. Before attempting to identify to species level it is important to establish that the isolate really is an aerobic endospore-former. Isolates of large, aerobic Gram-positive rods have often been submitted to reference laboratories as Bacillus species, even though sporulation had not been observed, or because PHB granules or other storage inclusions had been mistaken for spores. It should also be borne in mind that Bacillus species do not always stain Gram-positive. See Media (above) for comments on suitable sporulation media; cultures grown on rich media may lyse and die rather than sporulate. Sporulation has not been observed in several recently described species (Bacillus infernus, Bacillus thermoamylovorans), but the potential to form endospores may be detected using a PCR method based upon certain genes for sporulation (Brill and Wiegel, 1997).
A Gram-stained smear showing cells with unstained areas suggestive of spores can be stripped of oil with acetone/alcohol, washed, and then stained for spores. Spores are stained in heat-fixed smears by flooding with 10% aqueous malachite green for up to 45 min. (without heating), followed by washing and counterstaining with 0.5% aqueous safranin for 30 s; spores are green within pink/red cells at 1000 × magnification. Phase-contrast (at 1000 × magnification) should be used if available, as it is superior to spore-staining and more convenient. Spores are larger, more phase-bright, and more regular in shape, size and position than other kinds of inclusion such as polyhydroxybutyrate (PHB) granules (Figure 3e), and sporangial appearance is valuable in identification (Figure 3). Members of the Bacillus cereus group and Bacillus megaterium will produce large amounts of storage material when grown on carbohydrate media such as glucose agar, but on routine media this vacuolate or foamy appearance is rarely sufficiently pronounced to cause confusion (Figure 3e).
General morphology should be studied in relatively young (18–24 h at 30°C) cultures grown in nutrient broths aerated by shaking. Morphologies of cells raised on nutrient agar plates or slopes may be heterogeneous owing to varying conditions of oxygen supply within colonies. Wet preparations may be viewed by phase-contrast microscopy at 1000 × magnification, and observed for cell size (diameter), shape, shapes of ends of cells (rounded, squared, tapered), chains, filaments, and motility; for cells grown on glucose agar observe for storage inclusions (use the type strains of Bacillus cereus and Bacillus subtilis as positive and negative controls, respectively). Study cultures grown for 24 h and up to 7 d on medium supplemented with 5 mg/l MnSO4 for spores: observe for spore shape (spherical, cylindrical, ellipsoidal), position (central or paracentral, subterminal, terminal), presence of parasporal bodies (use the type strain of Bacillus thuringiensis as positive control), and for swelling of the sporangium. Cells in wet preparations may be immobilized by coating clean slides with a thin (0.5 mm) layer of sterile 2% water agar; a drop of turbid suspension of the organism is placed on the solidified agar, overlaid with a coverslip, and viewed by phase-contrast microscopy in the normal way.
Capsule formation by Bacillus anthracis. The capsule of virulent Bacillus anthracis can be demonstrated on nutrient agar containing 0.7% sodium bicarbonate incubated overnight under 5–7% CO2 (candle jars perform well). Colonies of the capsulated Bacillus anthracis appear mucoid, and the capsule can be visualized by staining smears with M'Fadyean's polychrome methylene blue or India ink, or by indirect fluorescent antibody staining (Logan and Turnbull, 2003). More simply, 2.5 ml of blood (defibrinated horse blood seems best; horse or fetal calf serum are quite good) can be inoculated with a pinhead quantity of growth from the suspect colony, incubated statically for 6–18 h at 37°C, and M'Fadyean stained. The M'Fadyean stain is preferable to other capsule staining methods, as it is more specific for Bacillus anthracis capsules. As Bacillus anthracis is suspected, safety precautions must be taken throughout capsule staining procedures; all materials coming into contact with the specimen, including spent reagents and rinsings, must either be discarded into a disinfectant effective against endospores or autoclaved. For the M'Fadyean stain, make a thin smear from the specimen, and also from a positive control, on a clean slide and allow to dry. Fix by immersion in 95% or absolute alcohol for 30–60 s. Put a large drop (approx. 50 µl) of polychrome methylene blue (M'Fadyean stain) on the smear and ensure all the smear is covered by spreading the stain with an inoculating loop (“flooding” the slide is wasteful, unnecessary and ecologically undesirable). Leave for one minute and wash the stain off with water (into 10% hypochlorite solution). Blot the slide and allow to dry. At 100–400× magnification, the organisms can be seen as fine short threads; at 1,000 × magnification (oil immersion), if virulent Bacillus anthracis is present, the capsule should be seen as a clearly demarcated zone around the blue-black, often square-ended rods which lie in short chains of two to a few cells in number. The positive control can be prepared by culturing a virulent strain in defibrinated horse blood as described above. For India ink negative-staining, place a large loopful of undiluted India ink on a cleaned slide, and mix in a small portion of the bacterial colony or a small loopful of the deposit from a centrifuged liquid culture. Drop a cleaned cover glass on, avoiding air bubbles, and press firmly between two sheets of blotting paper. When examined at 1000× under oil, the capsules appear as haloes around the highly refractive outlines of the bacterial cells. When capsules are absent, the ink particles directly abut the cell wall, and the cells are not easily seen. Phase-contrast is superior to bright-field microscopy, as the bacterial cells can be seen clearly in all cases.
Gamma phage sensitivity of Bacillus anthracis. Schuch et al. 2002 found that the PlyG lysin of γ phage may be used to detect Bacillus anthracis by luminescence, and that the same lysin could kill vegetative cells and germinating spores. blood agar plates to test for γ phage and penicillin susceptibility. Enquiries about gamma phage and indirect fluorescent antibody capsule staining should be addressed to the Diagnostics Systems Division, USAMRIID, Fort Detrick, Frederick, MD 21702-5011, USA.
Incubation temperature and time. Select an incubation temperature that matches the optimum growth temperature of the organism(s) as closely as is practical. Convenient temperatures are 20°C for psychrophiles, 30°C for mesophiles, and 45, 50 or 55°C for strains growing up to 55–65°C. Claus and Berkeley (1986) suggested that the incubation temperature should lie 10–15°C below the maximum growth temperature, and this remains sound advice; however, it should be noted that some new isolates of well-established species and strains of certain more recently described species may have unusually narrow or wide growth temperature ranges, so that this rule of thumb can be difficult to apply. Although Gordon et al. (1973) stipulated incubation periods of up to 14 d for many tests, most strains will have realized their potentials on these test media within 7 d.
pH. The media and the methods used by Gordon et al. (1973), and presented here in updated form, were mainly developed for mesophilic, neutrophilic species. They will not be applicable to acidophilic and alkaliphilic organisms. Certain media and methods may be adapted by adjusting the acidity as far as pH 6 for moderate acidophiles; for tests based upon acid production from carbohydrates, an indicator with a lower end point such as bromcresol purple will need to be selected (see Logan et al., 2000, and Miniaturized biochemical test systems, below).
For alkaliphilic organisms, the methods described by Fritze et al. (1990) may be recommended: the alkalinity of the casein, gelatin, nitrate and phenylalanine media, and media for determining growth temperatures and salt tolerance may be raised as far as pH 9.5–10 by adding 100 ml/l 1 M sodium sesquicarbonate after autoclaving; the phenylalanine medium is prepared with only 50 ml/l 1 M sodium sesquicarbonate, and it and the nitrate reduction medium should be acidified at the time of reading the results. Acid production from carbohydrates may be detected by using thymol blue in the following basal medium: K2HPO4, 7 g; NaCl, 5 g; KH2PO4, 2 g; (NH4)2SO4, 1 g; MgSO4·7H2O, 0.1 g; vitamin solution, 1 ml; distilled water, 1 l; adjust to pH 8.9–9.1 with NaOH. The vitamin solution contained: pyridoxine HCl, 100 mg; p-aminobenzoic acid, 50 mg; calcium D-pantothenate, 50 mg; nicotinic acid, 50 mg; riboflavin, 50 mg; thiamin HCl, 50 mg; D-biotin, 20 mg; folic acid, 20 mg; vitamin B12, 1 mg; distilled water, 1 l. The same basal medium, containing glucose as the carbon source and with pH adjusted with sodium sesquicarbonate, is used to test for ability to grow in neutral and alkaline conditions. Other tests useful for characterizing alkaliphiles are: diaminopimelic acid in cell walls, glucuronidase, pullulanase, Tween hydrolysis, and urease. The presence of diaminopimelic acid in cell walls is tested as follows: hydrolyze 1 mg dried cells with 1 ml 6 N HCl in a sealed, hard-glass tube held at 100°C for 18 h; cool; filter the sample through paper and wash with 1 ml H2O; remove HCl by drying 2–3 consecutive times under reduced pressure at 40°C on a rotary evaporator; take up residue with 0.3 ml H2O and spot 5 ml onto a cellulose-coated (microcrystalline) thin layer chromatography plate; separate the amino acids using the solvent mixture methanol-water-10 N HCl-pyridine (80:17.5:2.5:10, by volume), and detect them using acetonic ninhydrin spray (0.1% w/v) followed by heating at 100°C for 2 min. DAP spots are olive green fading to yellow, while other amino acids give purple spots. β-glucuronidase is detected by 4-methyl-umbel-liferone glucuronide (MUG) agar: tryptose, 20 g; NaCl, 5 g; cysteine ·HCl, 1 g; agar, 12 g; distilled water, 1 l; pH 9.7; autoclave and hold molten at 45–50°C; dissolve MUG in warm water and filter through a 0.22 µm membrane or autoclave the solution; add this solution to the agar base at 100 µg/ml and distribute in microtiter plate wells; when solidified, stab inoculate the wells and seal the microtiter plate with plate tape; observe after overnight incubation for fluorescence at about 366 nm. For pullulanase, add 0.3% (w/v) pullulan to a minimal medium at pH 9.7, and supplement with sterile solutions of peptone (0.5%), yeast extract (0.05%), and vitamin supplements; inoculate, incubate and reveal the reaction as for starch hydrolysis (below). For hydrolysis of Tween 20, 40 and 60, use the following medium: peptone, 10 g; NaCl, 5 g; CaCl2·H2O, 0.1 g; agar, 1 l water; pH 9.7; autoclave at 121°C for 20 min and cool to 45–50°C; add Tween to final concentration of 1% and pour plates; inoculate as single streaks, incubate up to 7 d, and observe for opaque halo. For urease detection, strains are cultivated on slopes of nutrient agar and growth is washed off at 3 and 7 d with 2 ml distilled water into a test tube; a drop of phenol red indicator is added and the reaction brought to pH 7 with dilute HCl; the suspension is equally divided and one tube has 0.1 g crystalline urea added (the other tube is a negative control) and allowed to stand; an alkaline reaction demonstrates the presence of urease. Nielsen et al. (1995a) determined carbohydrate utilization profiles of alkaliphiles using the API 50CH gallery (see Miniaturized biochemical test systems, below).
Salinity. Halotolerant and halophilic organisms may be tested in the Gordon et al. (1973) media, but supplemented with up to 10% NaCl; reactions of these salt-loving organisms in routine tests may often be very weak. Alternatively, media prepared in a marine broth or marine salts base may be used. Halophiles may often also be akaliphilic.
Traditional characterization tests. Bacillus cereus, Bacillus circulans, Bacillus coagulans, Bacillus firmus, Bacillus licheniformis, Bacillus megaterium, Bacillus pumilus, Bacillus sphaericus and Bacillus subtilis are the only species listed and distinguished by Gordon et al. (1973) that remain in Bacillus after later taxonomic revisions, and the tests outlined below are still valuable for distinguishing between these commonly encountered species.
Inocula. Use 1 drop inocula of overnight (for mesophiles at 30°C) nutrient broth cultures delivered with Pasteur pipettes for liquid and sloped media. For plate media, use the same culture, but apply the inoculum with a moderate sized (2–3 mm outside diameter) loop.
Maximum and minimum growth temperatures. Use slopes of nutrient agar or some other suitable growth medium for the organism, immerse bottles to their necks in a waterbath at the chosen temperature and allow to equilibrate prior to inoculation. Intervals of 5°C are recommended. Take care to maintain the water levels in the waterbaths throughout the test. Observe for growth after 3 d for temperatures of 55°C or higher, after 5 d at 30–50°C, after 14 d at 20 and 25°C, and after 21 d for temperatures below 20°C.
Acid production from carbohydrates. Inoculate, incubate, and observe for growth and acid production (shown by the indicator passing from mauve through gray to yellow) for up to 14 d. Use the type strain of Bacillus subtilis as positive control and the type strain of Bacillus sphaericus as negative control for L-arabinose, D-glucose, D-mannitol and D-xylose.
Anaerobic growth. Inoculate a tube of anaerobic agar with a small loopful of broth culture by stabbing to the bottom of the tube, or use a Pasteur pipette to seed molten medium held at about 40°C and then allow the agar to solidify. Incubate mesophiles for 3–7 d. Use the type strains of Bacillus cereus and Bacillus megaterium as positive and negative controls, respectively.
Casein decomposition. Inoculate plates of milk agar with one-streak inocula, incubate, and observe for zones of clearing around the growth over 7 d and up to 14 d. At the termination of the test, scrape growth aside with a loop and observe for weak reactions which may have occurred beneath the colony. Use the type strains of Bacillus megaterium and Paenibacillus macerans as positive and negative controls, respectively.
Citrate and propionate utilization. Inoculate slants of the citrate and propionate utilization media and incubate for up to 7 d. Observe for a red (alkaline) reaction which indicates utilization of the substrate as sole carbon source. Use the type strains of Bacillus subtilis and Bacillus badius as positive and negative controls for citrate, and the type strains of Bacillus licheniformis and Bacillus subtilis as positive and negative controls for propionate, respectively.
Egg-yolk reaction medium. Inoculate into an egg-yolk broth and a control broth lacking egg yolk, incubate for up to 7 d, observing the egg-yolk broth at 1 or 2-d intervals for a heavy white precipitate in or on the surface of the medium. If using plates of egg-yolk agar, apply one-streak inocula, incubate as for tubes, and observe for a zone of whitish opacity in the medium around the growth. Use the type strains of Bacillus cereus and Bacillus megaterium as positive and negative controls, respectively.
Gelatin hydrolysis. Inoculate tubes of nutrient gelatin and incubate at 28°C; at 2- to 3-d intervals, for up to 4 weeks, hold the tubes at 20°C for 4 h and observe for liquefaction. If using plate medium, inoculate with a single streak and incubate for 3–5 d, scrape some growth aside with a loop to reveal weak reactions which may have occurred beneath the colony, then flood the plate with 10 ml of 1 N H2SO4 saturated with Na2SO4; unchanged gelatin forms an opaque precipitate within 1 h, and a clear zone indicates hydrolysis. Use the type strains of Bacillus cereus and Bacillus coagulans as positive and negative controls, respectively.
Growth at pH 5.7 in Sabouraud media. Inoculate a slope of Sabouraud dextrose agar and a tube of Sabouraud dextrose broth, and a tube of nutrient broth as a control, incubate, and observe for growth in either or both Sabouraud media for up to 14 d. Use the type strains of Bacillus cereus and Bacillus badius as positive and negative controls, respectively.
Nitrate reduction. Inoculate nitrate broths and incubate. After 3 and 7 d, observe for gas in the Durham tube, indicating reduction of nitrate through nitrite to nitrogen gas), and touch a loopful of culture onto a strip of potassium iodide/starch paper which has been moistened with a few drops of 1 N hydrochloric acid and observe for a purple color which indicates the presence of nitrite. Strains negative at 7 d are tested at 14 d by mixing 1 ml culture with 3 drops of each of: (i) sulfanilic acid, 0.8 g; 5 N acetic acid (glacial acetic acid and water 1:2.5), 100 ml; (ii) dimethyl-α-naphthylamine, 0.6 ml; acetic acid, 100 ml. A red or yellow (=high concentration) color indicates the presence of nitrite. If still negative, add 4–5 mg zinc dust to the tube; if a red color develops (owing to reduction of nitrate to nitrite by the zinc) it indicates the absence of nitrate reduction by the organism, and confirms that rapid reduction of nitrate to nitrogen gas (not trapped by the Durham tube) has not occurred within the first 3 d of incubation. Use the type strains of Bacillus cereus and Bacillus megaterium as positive and negative controls, respectively.
Phenylalanine deamination. Inoculate duplicate slopes of phenylalanine agar and incubate for 7 d. Pipette 4–5 drops of 10% (w/v) ferric chloride solution over the slope and observe for a green color beneath the growth; this indicates the formation of phenylpyruvic acid from the phenylalanine. If negative, the second tube is tested after 14 d further incubation. Use the type strains of Bacillus megaterium and Bacillus cereus as positive and negative controls, respectively.
Resistance to lysozyme. Lightly inoculate a tube of resistance-to-lysozyme medium and a control tube of 2.5 ml nutrient broth and incubate. Observe for growth or its absence in the lysozyme medium after 7–14 d. Use the type strains of Bacillus cereus and Bacillus megaterium as positive and negative controls, respectively.
Sodium chloride tolerance. Lightly inoculate NaCl broths, incubate at a slant in order to enhance aeration, and observe for growth after 7 and 14 d.
Starch hydrolysis. Inoculate duplicate plates of starch agar and incubate. At 3 and 5 d scrape some growth aside with a loop to reveal weak reactions which may have occurred beneath the colony and flood the plates with 95% ethanol in order to make the unchanged starch turn white and opaque; observe for a clear zone around and under the growth which indicates starch hydrolysis. Use the type strains of Bacillus cereus and Bacillus sphaericus as positive and negative controls, respectively.
Tyrosine decomposition. Use one-streak inocula on plates of tyrosine agar and incubate; protect from drying during incubation. Observe for clearing of the tyrosine crystals around and below the growth after 7 and 14 d. Use the type strains of Bacillus cereus and Bacillus sphaericus as positive and negative controls, respectively.
Voges–Proskauer reaction. Inoculate Voges–Proskauer broths in triplicate and test for acetyl methyl carbinol production after incubation for 3, 5 and 7 d by adding 3 ml of 40% (w/v) NaOH to the culture and adding 0.5–1 mg creatine. Vortex mix to aerate and observe for the production of a red color after 30–60 min at room temperature. Use the type strains of Bacillus cereus and Bacillus megaterium as positive and negative controls, respectively.
Chemotaxonomic characters. Chemotaxonomic fingerprinting techniques applied to aerobic endospore-formers include FAME profiling (Kämpfer, 2002), polyacrylamide gel electrophoresis (PAGE) analysis (De Vos, 2002), pyrolysis mass spectrometry, and Fourier-transform infra-red spectroscopy (Magee and Goodacre, 2002). Only one of these approaches, FAME analysis, is supported by a commercially available database for routine identification. Fatty acid analysis can play a very useful part in polyphasic taxonomic studies of Bacillus. However, fatty acid profiles across the aerobic endospore-forming genera do not, given frequent and considerable within-species heterogeneity, form the basis of a reliable, stand-alone identification scheme (Kämpfer, 1994, 2002). A further difficulty is the need for a standardized media and incubation temperature for preparing isolates for FAME analysis, making databases for acidophiles, alkaliphiles, neutrophiles, mesophiles, psychrophiles and thermophiles incompatible. The commercially available Microbial Identification System software (MIDI, Newark, Delaware, USA) includes a FAME database for the identification of aerobic endospore-formers. Although it cannot be expected to give an accurate or reliable identification with every isolate, it is certainly a valuable screening tool when used with caution.
Serology. See Antigenic structure in Further descriptive information, above.
Genotypic methods. As with other groups of bacteria, studies of 16S rDNA and of DNA have very valuable applications in the classification of aerobic endospore-formers (De Vos, 2002). Nucleic acid fingerprinting techniques are also of great potential for typing work, of course. A good example is the ability to differentiate Bacillus anthracis strains by amplified fragment length polymorphism (AFLP) analysis (Keim et al., 1997) on account of variable number tandem repeats (VNTR; Keim et al., 2000; Turnbull et al., 2002), as the distinction of isolates of this species for epidemiological or strategic purposes has long been a challenge. AFLP also shows promise for the epidemiological typing of Bacillus cereus (Ripabelli et al., 2000).
At present, however, nucleic acid analyses are not entirely suitable for the routine identification of aerobic endospore-formers; their value in classification does not necessarily make them suitable as routine diagnostic tools at the species level. Amplified rDNA restriction analysis (ARDRA), for example, has been and continues to be exceptionally effective in the classification of Bacillus (Logan et al., 2000). It is a very powerful technique for recognizing new taxa and can be used to screen large numbers of strains much faster than is reasonably possible with 16S rDNA sequencing, but it is not always capable of distinguishing closely related species (Logan et al., 2002b). Sequencing of 16S rDNA is not always capable of resolving species either; not only are the sequences within and adjacent to this gene almost identical among Bacillus anthracis, Bacillus cereus and Bacillus thuringiensis, but the variable sites within these sequences can differ among multiple rRNA cistrons within a single strain (Turnbull et al., 2002).
Other fingerprinting methods such as ribotyping, which is commercially available, are presently limited by the appropriateness of the restriction enzymes they use, and by the sizes of the databases available to those developing them – both in terms of the numbers of species included and of the numbers of authentic strains representing those species.
The use of gene probes in conjunction with the PCR, to allow rapid and sensitive detection, is covered in Genetics, above.
Miniaturized biochemical test systems. Over 50 years after Smith et al. (1946) published their first identification scheme, the most widely used commercially available methods for identifying members of the genus Bacillus and its relatives are still based upon miniaturized developments of traditional, routine biochemical tests: the API 20E and 50CHB Systems (bioMérieux, Marcy l'Etoile, France), the VITEK System (bioMérieux, Hazelwood, Missouri, USA) and Biolog (Biolog Inc., Hayward, California, USA) (Logan et al., 2000).
The API 20E/50CHB kits contain miniaturized and standardized versions of conventional biochemical tests, the API 50CHB comprising 48 tests for acid production from carbohydrates, esculin hydrolysis, and a negative control. They can be used for distinguishing between a number of well-established Bacillus species, can also recognize biotypes within the Bacillus cereus group (Logan et al., 1979) and may be used for the presumptive distinction of Bacillus anthracis from other members of the Bacillus cereus group within 48 h. The overall findings of an international reproducibility trial employing code-numbered Bacillus sensu lato strains showed that better test reproducibility could be achieved with API tests than with the conventional tests of Gordon et al. (1973), even when the latter had been carefully standardized by the Subcommittee on the Taxonomy of the Genus Bacillus of the International Committee on Systematic Bacteriology (Logan and Berkeley, 1981). API tests have proved valuable in the characterization of several novel species of aerobic endospore-formers (Heyndrickx et al., 1998, 1999; Heyrman et al., 2003a, b, 2004; Logan et al., 2000, 2002b, 2004a). The API 50CH gallery offers some flexibility in incubation temperature and in pH and salinity of the suspension medium. Deinhard et al. (1987a) used an ammonium salts-yeast extract medium at pH 4, with bromphenol blue as indicator, to investigate acid production from carbohydrates in the API 50CH gallery by strains of Bacillus (now Alicyclobacillus) acidoterrestris, and Logan et al. (2000) used a similar medium at pH 6 with 0.033% bromcresol purple as indicator to characterize strains of Bacillus fumarioli: KH2PO4, 3 g; MgSO4·7H2O, 0.5 g; CaCl2·2H2O, 0.25 g; (NH4)2SO4, 0.2; yeast extract, 0.5 g; trace element solution, 1 ml; bromcresol purple, 0.33 g; distilled water, 1 l; pH 6.0. Trace element solution contained: FeSO4·7H2O, 0.05 g; ZnSO4·7H2O, 0.05 g; MnSO4·3H2O, 0.05 g; distilled water, 100 ml; 0.1 M H2SO4, 1 ml. Nielsen et al. (1995a) used the 50CH gallery for testing carbohydrate utilization by alkaliphilic Bacillus species. Heyrman et al. (2003b) added up to 10% NaCl to the API 50CHB suspension medium in order to detect acid production by strains of halotolerant and halophilic Bacillus and Virgibacillus strains.
The Vitek system arose from the Auto Microbial System (AMS) which was used for the direct identification of microbes from urine samples (Aldridge et al., 1977) and which led from the Microbial Load Monitor (MLM), which was an instrumental system developed for NASA in the 1960s for the detection of specific micro-organisms in a space-craft environment. bio-Mérieux offers a Bacillus card for the VITEK automated identification system; identifications are automatically attempted hourly between 6 h of incubation and the final reading at 15 h.
As many new species have been proposed since the API and Vitek schemes were established, updated databases are being prepared following the study of a large number of strains which have been carefully authenticated by polyphasic taxonomic study. Biolog also offers a database for the aerobic endospore-forming bacteria. The Biolog system is based on carbon source utilization patterns, indicated by the reduction of a tetrazolium dye, using a 96-well MicroPlate that is inoculated with a standardized suspension of a pure culture and incubated as appropriate. Unlike the API 50CH, the system is based on the process of metabolism itself rather than the release of metabolic by-products such as acid. For Bacillus identification the first release of the Biolog Gram-positive panel was dogged by problems of false positive results (Baillie et al., 1995), and this problem has been addressed by using a more viscous suspension medium to reduce flocculation and pellicle formation. The MicroPlates can be read after 4–6 h incubation, then reincubated for a further 12–18 h if an acceptable similarity threshold has not been reached.
The effectiveness of such kits can vary with the genera and species of aerobic endospore formers concerned, but they are improving with continuing development and expanding data-bases (Logan, 2002), and many of the characters they test are valuable in polyphasic taxonomic studies. It is stressed that their use for identification should always be preceded by the basic characterization tests, especially endospore formation, described above.
Differentiation from closely related taxa
The 142 species of Bacillus present such a wide diversity of routine phenotypic features that there are no characters that reliably allow distinction of Bacillus species from members of the other 18 genera in the family Bacillaceae, or from some genera of aerobic endospore-forming bacteria in other families. Table 4 summarizes some routine phenotypic characters that may be of value for differentiating between members of the family.
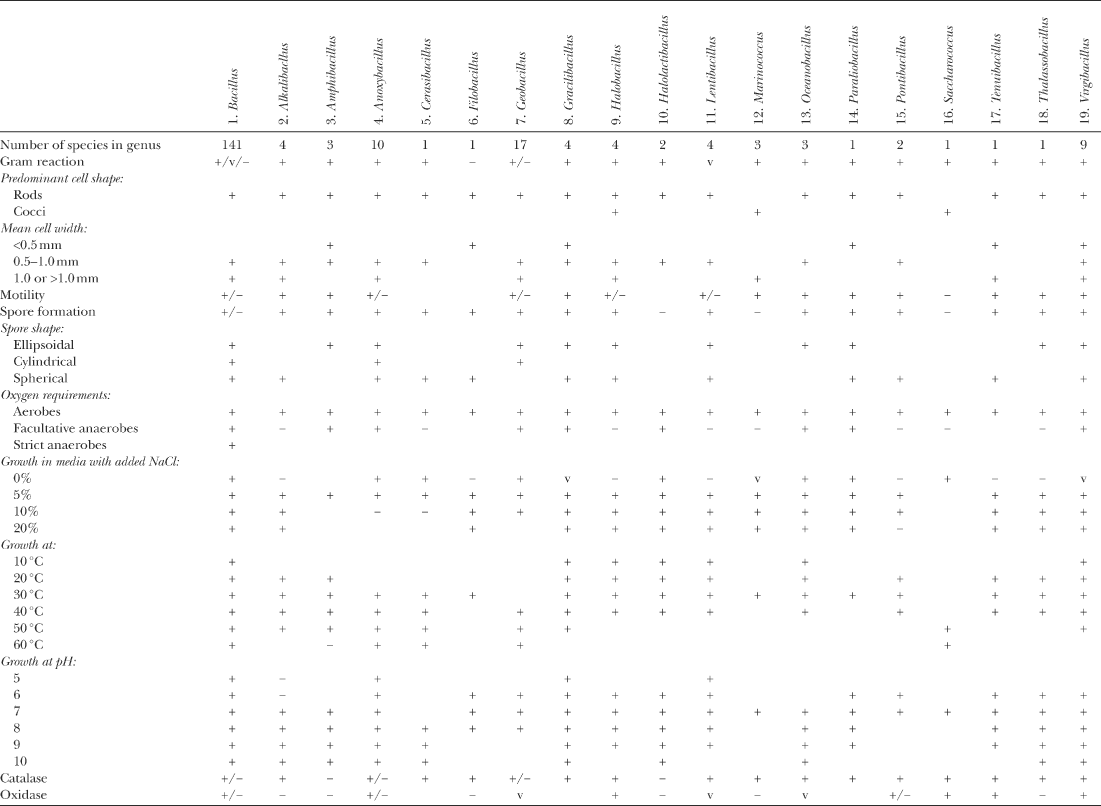
- a Symbols: +, at least one species within the genus gives a positive reaction; +/−, some species are positive, some are negative; v, varies within the genus; −, negative; w, weak.
Taxonomic comments
Ferdinand Cohn established the genus Bacillus in 1872 to include the three species of rod-shaped bacteria, Bacillus subtilis (type species), Bacillus anthracis and Bacillus ulna, without taking motility or sporulation into account. Ehrenberg had described Vibrio subtilis in 1835, and “subtilis” is one of the earliest bacterial species epithets still in use. Davaine (1868) had proposed the genus Bacteridium to accommodate the nonmotile organism that causes anthrax. Cohn illustrated spores, and in later publications he discussed the resistance of spores and their significance in anthrax epidemiology, but Winter (1880) was the first to include “propagation through spores” in the description of the genus, and Prazmowski (1880) was the first to use sporulation as a differential (i.e., taxonomic) characteristic; he proposed the genus name Clostridium for organisms that differed from Bacillus in having spindle-shaped sporangia. At this time some workers still believed that all bacteria existed in several morphological and physiological forms, and that classification of the “fission fungi” was of no scientific value – Buchner (1882) claimed that shaking cultures of Bacillus subtilis at different temperatures could yield Bacillus anthracis!
The definition of the genus Bacillus as rod-shaped, aerobic or facultatively anaerobic organisms forming resistant endospores has been used for many years, and it remains of practical value despite the proposal of the strictly anaerobic species Bacillus infernus (Boone et al., 1995), Bacillus arsenicoselenatis and Bacillus selenitireducens (Switzer Blum et al., 1998). However, it was not until the 1880s that the name Bacillus and sporulation were brought together, and not until 1920 that aerobic growth became a defining character. From the 1880s to the 1900s Bacillus was variously used to contain rod-shaped organisms, all the rods except those in Clostridium, all the spore-formers, only those spore-formers producing unswollen sporangia, all motile rods, peritrichously flagellate rods (a classification that became the best-established in the American literature up to 1920), and nonmotile spore-formers with unswollen sporangia. The term Bacillus has thus been used in two senses: as a genus name, and, as “bacillus,” as a general reference to shape; unfortunately the latter remains the most widely accepted definition of the term, especially by medical bacteriologists. As early as 1913, Vuillemin (1913) considered the name Bacillus so vulgarized by its various applications that it should lose nomenclatural status.
Although some took physiological characters as well as morphological ones into account in their classifications, it was not until the Committee of the Society of American Bacteriologists on Characterization and Classification of Bacterial Types reported in the early volumes of the Journal of Bacteriology (Winslow et al., 1917, 1920), that satisfactory and largely uncontested definitions of the bacterial groups emerged. The family Bacillaceae was defined as “Rods producing endospores, usually Gram-positive. Flagella when present peritrichic. Often decompose protein media actively through the agency of enzymes” and Bacillus was described as “Aerobic forms. Mostly saprophytes. Liquefy gelatin. Often occur in long threads and form rhizoid colonies. Form of rod usually not greatly changed at sporulation”. The Committee also used the requirement of oxygen and sporangial shape for differentiation between Bacillus and Clostridium, the other genus in the family Bacillaceae, and this description was applied in the first and second editions of Bergey's Manual of Determinative Bacteriology (Bergey et al., 1923, 1925). So, as noted by Gordon (1981), the definition of the genus Bacillus as aerobic endospore-forming rods had become widely established by the 1920s.
Identification remained difficult, however, and the early editions of Bergey's Manual were not practical bench books for many taxa. Although the commonest spore-forming bacteria were accurately described in papers published early in the 20th century, Ford and his coworkers found that identification of their fresh isolates from milk remained difficult. This stimulated an extensive investigation of many strains from various environments in order to test the classification devised for the milk strains (Laubach et al., 1916; Lawrence and Ford, 1916), and 26 species were recognized – four of them new.
In 1937 it was agreed that “the genus Bacillus should be so defined as to exclude bacterial species which do not produce endospores” (Nomenclature Committee of the International Society for Microbiology, 1937; St. John-Brooks and Breed, 1937), over 100 years after Ehrenberg first described what is now the type species of the genus.
Some late-19th century classification schemes excluded motile organisms from Bacillus, and so abandoned Bacillus subtilis as the type species of the genus. Although other schemes retained Bacillus subtilis, in the late 1890s and early 1900s some confusion was emerging about the identity of the type. When describing Bacillus cereus, Frankland and Frankland (1887) noted its similarity both to Bacillus anthracis and a culture of Bacillus subtilis received from Koch. That could not have been Bacillus subtilis as recognized nowadays, and two very different type strains seemed to exist: one bore small spores and germinated equatorially, and the other formed much larger spores with germination occurring at the pole. The former was a strain from the University of Marburg and the latter was the Michigan type originating from the laboratory of Koch in 1888, and then maintained at the University of Michigan.
Following studies of strains from various culture collections, and after finding that the small-spored type tended to overgrow the large-spored type in cultures that mimicked the methods used by Cohn and other earlier workers, Conn (1930) suggested that the Marburg type should be called Bacillus subtilis Cohn. The Nomenclature Committee of the International Society for Microbiology sought the opinions of its members (Breed and St. John-Brooks, 1935), and in 1936, at the Second International Congress for Microbiology in London, the Marburg strain of Bacillus subtilis was officially adopted as the generic type (Nomenclature Committee of the International Society for Microbiology, 1937; St. John-Brooks and Breed, 1937).
With the resolution of the type strain controversy, the discovery that many pathogenic “Bacillus subtilis” strains were in fact Bacillus cereus stimulated a large taxonomic study of the genus (Clark, 1937) followed by a grouping of the mesophilic species in the form of a diagnostic key (Smith and Clark, 1937). In another study with a key, Gibson and Topping (1938) considered the Bacillus circulans and Bacillus fusiformis groups to be species complexes exhibiting several variations, and the problems so presented remain incompletely resolved to this day. Smith's team meticulously characterized their cultures and then emphasized the similarities rather than the differences between their strains, so “lumping” their taxa rather than splitting them. In a report published a decade after the study began (Smith et al., 1946), they recognized three groups of species: Group One comprised those with oval to cylindrical spores without definite swelling of the sporangia, and included the Bacillus cereus and Bacillus subtilis groups, Bacillus pumilus, Bacillus lentus, Bacillus megaterium and Bacillus firmus. Group Two contained those with oval spores and swollen sporangia, and included Bacillus alvei, Bacillus brevis, Bacillus circulans, Bacillus laterosporus, Bacillus macerans and Bacillus polymyxa. Group Three consisted of Bacillus pasteurii and Bacillus sphaericus, both of which produced round spores with distinct swelling of the sporangia. The first truly workable diagnostic key designed for Bacillus identification emerged as a result of this work and appeared in the 6th edition of Bergey's Manual (Breed et al., 1948). The effectiveness of the scheme was demonstrated by Knight and Proom (1950); in their study of 296 strains all but 51 could be allocated to the species or groups previously described.
Smith et al. (1952) published a revision of their 1946 report, another classic example of painstaking and objective work. It was based on the study of 1134 strains, and such were the problems of synonymy in the genus that although 491 of these had 158 species names on receipt, all but 20 could be assigned to only 19 species. The classification outlined was used in the 7th edition of Bergey's Manual (Breed et al., 1957) and was adopted by most bacteriologists working with Bacillus. The designation of Bacillus anthracis as a variety of Bacillus cereus resulted in much controversy, but Smith et al. (1946, 1952) cited several reports on the loss of pathogenicity by Bacillus anthracis and considered such strains to be indistinguishable from Bacillus cereus. These studies from Smith's laboratory shaped the future of Bacillus taxonomy, and various research groups began applying existing techniques and new methods to the taxonomy of the genus Bacillus.
Numerical taxonomic methods were first applied to Bacillus by Sneath, 1962) using the data of Smith et al. (1952), and a phenogram was constructed which “largely agreed” with the 1952 classification. Although current classifications were criticized by Bonde (1975) on the grounds that their systems depended more on laboratory culture collection strains than on fresh isolates, his own classification generally agreed with that of Gordon et al. (1973).
During the 1970s several new approaches to characterization such as serology, enzyme and other molecular studies, and pyrolysis gas-liquid chromatography (Oxborrow et al., 1977) began to emerge as potentially useful taxonomic tools in bacterial taxonomy as a whole but despite this, the taxonomy of Bacillus remained relatively untouched, with few new species or subspecies being described and names validated. With Bacillus the emphasis during this time was on facilitating the identification of members of the genus.
One of the most influential and significant studies of the genus Bacillus was published in by Gordon et al. (1973), 1,134 strains were included in a classification which formed the basis for the Bacillus section in the Eighth Edition of Bergey's Manual (Gibson and Gordon, 1974) but numerical methods were not used in the analysis. The classification was very similar to that proposed by Smith et al. (1946, 1952), the main difference being that subgrouping was not attempted and the species were arranged as a spectrum of morphological characteristics. Given the period at which the work was done, it is surprising that numerical analysis was not attempted, and so the success of the arrangement that was made is all the more impressive. The characterization tests applied by Gordon et al. (1973) were used by the International Committee on Systematic Bacteriology Subcommittee on the Taxonomy of the Genus Bacillus as standard methods, but international reproducibility trials found that, even in the hands of Bacillus experts, the miniaturized, highly standardized tests in the API System gave more rapid and consistent results (Logan and Berkeley, 1981). The increasing incidence of Bacillus isolations from clinical environments emphasized the need for a rapid identification scheme, which could only follow an improved taxonomy of the genus. Consequently, Logan and Berkeley (1984) developed a Bacillus identification scheme based upon API tests.
It was also the classification of Gordon et al. (1973) with support from the work of Logan and Berkeley (1981) that formed the basis of the list of Bacillus included in the Approved Lists of Bacterial Names published by the International Committee of Systematic Bacteriology (Skerman et al., 1980). These lists marked a new starting date for bacterial nomenclature, the previous date being that of Linnaeus' monumental classification work, Species Plantarum, which was published in 1753. Since that time, many synonyms had been inadvertently been proposed, and the number of Bacillus species described fluctuated greatly through the eight editions of Bergey's Manual, ranging from a peak of 146 species in 1939 to the smallest number of 22 in 1974 (a further 26 appeared as species incertae sedis, and many of these were represented by very few strains).
The need for subdividing Bacillus had long been recognized from its DNA base composition range of 32–69 mol% G + C and the arrangements that had emerged from numerical taxonomies of phenotypic data (Logan, 1994). Early phylogenetic studies based on 16S rRNA cataloging (e.g., Fox et al., 1977, 1981; Stackebrandt et al., 1987) confirmed the evolutionary heterogeneity of Bacillus, and were found to be entirely consistent with the distribution of murein types among the species (Stackebrandt et al., 1987), but it was considered too early to split Bacillus to provide a more “natural” classification, as very few species had yet been analyzed by 16S rRNA cataloguing or full sequence comparative studies. The genus was thus still kept as one taxonomic entity in Bergey's Manual of Systematic Bacteriology (Claus and Berkeley, 1986).
Ash et al. (1991) recognized five phylogenetically distinct clusters among 51 type strains of Bacillus species on the basis of 16S rRNA sequence similarities. Rössler et al. (1991) published the results of a similar study in the same year, finding four major clusters which showed high correlation with those described by Ash et al. A weakness of both studies was their reliance upon single (type) strains of each species, so that within-species diversities were not indicated and the authenticities of the strains were not controlled. Indeed, in the Ash et al. study, Bacillus acidoterrestris and Bacillus lautus were misplaced owing, presumably, to contaminants (Heyndrickx et al., 1996b; Wisotzkey et al., 1992). A sixth rRNA group containing Bacillus alcalophilus was recognized by Nielsen et al. (1994) but insufficient phenotypic and genotypic data were available to allow the proposal of a new genus.
With the accumulation of further 16S rRNA (rDNA) sequence data, Bacillus has been divided into more manageable and better-defined groups. So far, nine new genera have been established. However, this taxonomic progress has not revealed readily determinable features characteristic of each genus, and they show wide ranges of sporangial morphologies and phenotypic test patterns.
The proposal of Alicyclobacillus (Wisotzkey et al., 1992) initiated the splitting of the genus Bacillus and extensive reclassification ensued. Alicyclobacillus contains 19 species of thermoacidophiles, including organisms formerly called Bacillus acidocaldarius (Darland and Brock, 1971), Bacillus acidoterrestris (Deinhard et al., 1987a) and Bacillus cycloheptanicus (Deinhard et al., 1987b). These organisms exhibit ω-alicyclic fatty acids as the major natural membranous lipid components, a phenotypic trait not found in other Bacillus species.
Ash et al. (1993) proposed the genus Paenibacillus to encompass their previously described Group 3 (Ash et al., 1991) as they considered these organisms to be phylogenetically “so removed” from the cluster which contained Bacillus subtilis as to warrant such an action. Data from their 1991 publication were used along with previously published phenotypic characters, and a gene probe based on 16S rRNA. Paenibacillus contains 95 species and includes organisms formerly called Bacillus alginolyticus (Nakamura, 1987), Bacillus alvei (Cheshire and Cheyne, 1885), Bacillus amylolyticus (Nakamura, 1984a), Bacillus chondroitinus (Nakamura, 1987), Bacillus curdlanolyticus (Kanzawa et al., 1995), Bacillus glucanolyticus (Alexander and Priest, 1989), Bacillus gordonae (Pichinoty et al., 1986), a synonym of Bacillus validus, now Paenibacillus validus (Heyndrickx et al., 1995), Bacillus kobensis (Kanzawa et al., 1995), Bacillus larvae (White, 1906) and Bacillus pulvifaciens (Nakamura, 1984c) (now both subspecies of Paenibacillus larvae (Heyndrickx et al., 1996c), Bacillus lautus (Nakamura, 1984a), Bacillus lentimorbus (Dutky, 1940), Bacillus macerans (Schardinger, 1905), Bacillus macquariensis (Marshall and Ohye, 1966), Bacillus pabuli (Nakamura, 1984a), Bacillus peoriae (Montefusco et al., 1993) Bacillus polymyxa (Prazmowski, 1880), Bacillus popilliae (Dutky, 1940), Bacillus thiaminolyticus (Nakamura, 1990) and Bacillus validus (Nakamura, 1984a). It was perhaps surprising to find that Bacillus circulans sensu stricto was recovered within the cluster containing Bacillus subtilis, as it is phenotypically similar to Paenibacillus polymyxa and, Paenibacillus macerans; however, strains originally named Bacillus amylolyticus, Bacillus pabuli and Bacillus validus had been members of the Bacillus circulans “complex” (Gibson and Topping, 1938) until these three species were revived by Nakamura (1984c). Another member of this complex, Bacillus lautus, was also revived by Nakamura (1984c) and later transferred to Paenibacillus (Heyndrickx et al., 1996c). Unfortunately, there is no phenotypic character which might allow the differentiation of the genus Paenibacillus from other genera of aerobic endospore-forming genera, and so the taxonomic gain in phylogenetic accuracy was not accompanied by easier routine identification.
Following 16S rRNA gene sequence analyses of type strains of species closely related to Bacillus brevis and Bacillus aneurinolyticus Shida et al. (1996) recognized two distinct clusters and proposed the Bacillus brevis cluster as a new genus Brevibacillus and the Bacillus aneurinolyticus cluster as Aneurinibacillus. Brevibacillus (Shida et al., 1996), contains the 10 species formerly known as Bacillus agri (Nakamura, 1993), Bacillus borstelensis (Shida et al., 1995), Bacillus brevis (Migula, 1900), Bacillus centrosporus (Nakamura, 1993), Bacillus choshinensis (Takagi et al., 1993), Bacillus formosus (Shida et al., 1995), Bacillus laterosporus (Laubach et al., 1916), Bacillus parabrevis (Takagi et al., 1993), Bacillus reuszeri (Shida et al., 1996) and Bacillus thermoruber (Manachini et al., 1985). Bacillus galactophilus (Takagi et al., 1993) is a synonym of Bacillus agri (Shida et al., 1994). Aneurinibacillus (Shida et al., 1996) emend. Heyndrickx et al. (1997) accommodates the three species Bacillus aneurinilyticus (Shida et al., 1994), Bacillus migulanus (Takagi et al., 1993), and Bacillus thermoaerophilus (MeierStauffer et al., 1996).
Subsequent developments included proposals for three genera of halotolerant and halophilic species: Virgibacillus (Heyndrickx et al., 1998) containing Bacillus pantothenticus (Proom and Knight, 1950); Gracilibacillus (Wainø et al., 1999) containing Bacillus dipsosauri (Lawson et al., 1996); Salibacillus (Wainø et al., 1999) containing Bacillus marismortui (Arahal et al., 1999, 2000) and Bacillus salexigens (Garabito et al., 1997).
It is possible that the boundaries between some of the new genera, in terms of 16S rDNA sequence differences, may become obscured as and when further species are discovered; indeed, Heyrman et al. (2003b) transferred the species of Salibacillus to Virgibacillus.
Several of the familiar Bacillus thermophiles comprise a distinct evolutionary line, and so the genus Geobacillus (Nazina et al., 2001) accommodates species formerly called Bacillus kaustophilus (Priest et al., 1988), Bacillus stearothermophilus (Donk, 1920), Bacillus thermocatenulatus (Golovacheva et al., 1975), Bacillus thermodenitrificans (Manachini et al., 2000), Bacillus thermoglucosidasius (Suzuki et al., 1983) and Bacillus thermoleovorans (Zarilla and Perry, 1987). However, the longer-established species in this genus await polyphasic taxonomic study and circumscription, as many misnamed strains are known to exist in collections.
The thermophilic, round-spored, organism Bacillus thermosphaericus (Andersson et al., 1996) has been accommodated within the new genus Ureibacillus (Fortina et al., 2001b). Several other round-spored species, Bacillus globisporus (Larkin and Stokes, 1967), Bacillus pasteurii (Chester, 1898) and Bacillus psychrophilus (Nakamura, 1984b), have been transferred (Yoon et al., 2001b) to Sporosarcina (Kluyver and van Neil, 1936) which was established to accommodate the motile, spore-forming coccus Sporosarcina ureae (Beijerinck, 1901). As this proposal placed spore-forming rods and cocci in the same genus, the definition of Sporosarcina had to be emended. Two further new genera of round-spored organisms were subsequently proposed to accommodate single species, but they were only represented by single strains in the study concerned: Jeotgalibacillus alimentarius (Yoon et al., 2001c), and Marinibacillus to which Bacillus marinus was transferred (Yoon et al., 2001c).
Bacillus continues to accommodate the best-known species such as Bacillus subtilis (the type species), Bacillus anthracis, Bacillus cereus, Bacillus licheniformis, Bacillus megaterium, Bacillus pumilus, Bacillus sphaericus and Bacillus thuringiensis. It still remains a large genus, with 90 species (May 2004), since losses of species to other genera have been balanced by proposals for new Bacillus species. Seventy-one of the present members of Bacillus were proposed after the treatment of the genus for the first edition of the Systematics was compiled, and of the 34 species described in that edition, only 18 remain in Bacillus.
Members of the Bacillus cereus group, Bacillus anthracis Bacillus cereus Bacillus thuringiensis, are really pathovars of a single species (Turnbull et al., 2002), and yet the phylogenetic and phenetic distinction of this group probably support generic status. The internal division of the different 16S rRNA groups within the genus Bacillus sensu lato is presently far from clear. Many of its species fall into several apparently distinct rRNA sequence groups such as the “Bacillus subtilis group”, the “Bacillus cereus group”, and the “Bacillus sphaericus group”, but although such divisions may also be phenotypically distinguishable, intermediate organisms may make satisfactory subdivision difficult.
Numerous Bacillus species mentioned in the literature over the years are not now recognized because of synonymy, incomplete characterization, or the lack of type strains, or even the complete loss of original isolates. Some invalid names persist, however, and can cause much confusion. Good examples are “Bacillus subtili var. niger” and “Bacillus globigii”. Strains bearing these names are used for sterilization control and other purposes, but the names themselves are invalid. Most of these currently used strains may be regarded as Bacillus subtilis, and are listed as such in the catalogues of several culture collections; all three names may appear on the packaging of the commercially available biological indicator products that use them. Bacillus subtilis var. niger is a name that was applied to strains that produce a distinctive black pigment, hence their popularity as biological indicators. However, N. R. Smith's strain 1221A of Bacillus subtilis var. niger, which is a standard strain for sterility testing, is noted for its red pigment, and is also known as Bacillus globigii. Gordon et al. (1973) found their strains labeled as Bacillus globigii were Bacillus circulans (sensu lato), Bacillus licheniformis, Bacillus pumilus or Bacillus subtilis var. niger, and they commented: “strains of Bacillus subtilis, Bacillus pumilus and Bacillus circulans labeled Bacillus globigii are extant, and some have been widely distributed. As a result, the name Bacillus globigii is meaningless”.
New species and subspecies of aerobic endospore formers are regularly described: 182 new species were proposed between the publication of the First Edition of this Manual in 1986 and the time of preparing the present edition (May 2004); of these, 121 were initially assigned to Bacillus, 43 were then transferred to new genera, and three others await transfer (these are Bacillus edaphicus and Bacillus mucilaginosus, which belong in Paenibacillus, and Bacillus thermantarcticus, which belongs in Geobacillus). The remaining new species were distributed among the 11 new genera mentioned in the preceding sentence and ten further new genera; 15 of the new genera contain a single species. During that period only six proposals for merging species (Heyndrickx et al., 1996a; Heyndrickx et al., 1995; Rosado et al., 1997; Shida et al., 1994; Sunna et al., 1997b), and one proposal for merging genera (Heyrman et al., 2003b) had been made. At the time of writing there are 440 valid species of aerobic endospore-formers among 47 genera. Unfortunately, new taxa have often been proposed on the basis of very few strains (48 of the present Bacillus species are based upon the study of a single strain, and nine on the basis of only two strains), so that their within-species variations are unknown. It is of small comfort that the proportion of new Bacillus species proposed on the basis of a single isolate is, at 33%, less than the proportion of single-isolate taxa described for prokaryotes overall between 1990 and 2000 (Christensen et al., 2001).
Another regrettable circumstance is that many recently described species of Bacillus, and species of the genera recently derived from it, represent genomic groups disclosed by DNA–DNA pairing experiments, and routine phenotypic characters for distinguishing some of them are very few and of unproven value. An extreme example of this kind of problem is the splitting of strains of Bacillus subtilis into two subspecies and three new species: Bacillus atrophaeus (Nakamura, 1989), Bacillus mojavensis (Roberts et al., 1994), Bacillus vallismortis (Roberts et al., 1996), Bacillus subtilis subsp. spizizenii (Nakamura et al., 1999) and Bacillus subtilis subsp. subtilis. These proposals were based principally upon DNA relatedness studies (the 70% relatedness threshold for species being rigorously applied), with distinctions between these “cryptic” individual taxa being supported by a miscellany of approaches which included small differences in fatty acid compositions, multilocus enzyme electrophoresis, restriction digest analysis of selected genes, and transformation resistance. The only distinctive phenotypic character cited among these proposals was the production of brown pigment by Bacillus atrophaeus on media containing tyrosine, and so the recognition of the four new taxa appears to be of little practical value.
It is known (Fox et al., 1992; Stackebrandt and Goebel, 1994) that rDNA sequence analysis alone does not allow unequivocal differentiation at the species or finer taxonomic levels, and may even lead to completely mistaken conclusions on the exact phylogenetic positions of certain strains (Clayton et al., 1995), especially when taxa are proposed on the basis of lone strains. Christensen et al. (2001) proposed an addition to Recommendation 30b of the Bacteriological Code (1990 Revision), which advises that proposals for new taxa be based upon at least five strains from different sources, and that descriptions be based upon comparative studies including reference strains. Adoption of this suggestion, and its application by reviewers of proposals for new taxa, would do much to improve the practical usefulness of future developments in Bacillus taxonomy.
An important proposal, published at the time that this account was going to press, was for the transfer of Bacillus sphaericus and Bacillus fusiformis to the new genus Lysinibacillus (Ahmed et al., 2007c). Other transfers of species listed below include a proposal by Jeon et al. (2005b) for Bacillus haloalkaliphilus to be reclassified in a new genus Alkalibacillus, and a proposal by Hatayama et al. (2006) for the transfer of Bacillus laevolacticus to Sporolactobacillus, and the reclassification of Bacillus halophilus as Salimicrobium halophilum (Yoon et al., 2007b) and Bacillus arvi, Bacillus arenosi and Bacillus neidei into a new genus, Viridibacillus (Albert et al., 2007).
It is clear that the taxonomic reshuffling of the genus has not yet come to an end. Furthermore, none of the other new genera of aerobic endospore formers, Sulfobacillus, Amphibacillus, Halobacillus, Ammoniphilus, Thermobacillus, Filobacillus, Oceanobacillus, Lentibacillus, Paraliobacillus, Cerasibacillus, Halolactibacillus, Pontibacillus, Tenuibacillus, Salinibacillus, Pullulanibacillus, Tuberibacillus, Caldalkalibacillus, Ornithinibacillus, Paucisalibacillus, Vulcanibacillus, Pelagibacillus, and Piscibacillus (Golovacheva and Karavaiko, 1978; Hatayama et al., 2006; Ishikawa et al., 2002; Ishikawa et al., 2005; Kim et al., 2007b; L'Haridon et al., 2006; Lim et al., 2005b; Lu et al., 2001; Mayr et al., 2006; Nakamura et al., 2004b; Niimura et al., 1990; Nunes et al., 2006; Ren and Zhou, 2005a, b; Schlesner et al., 2001; Spring et al., 1996; Tanasupawat et al., 2007; Touzel et al., 2000; Xue et al., 2006; Yoon et al., 2002; Zaitsev et al., 1998) contain species formerly assigned to Bacillus. “Bacillus flavothermus” is accommodated in Anoxybacillus as Anoxybacillus flavithermus (Pikuta et al., 2000a).
Differentiation of the species of the genus Bacillus
Differential characteristics of the species of the genus Bacillus are shown in Table 5, and additional data are shown in Table 6.
- a Symbols: +, >85% positive; d, different strains give different reactions (16–84% positive); −, 0–15% positive; v, variation within strains; w, weak reaction; −/w, negative or weak reaction; d/w, different strains give different reactions and reactions are weak when positive; ng, no growth in the test medium; no entry indicates that no data are available.
- b Compiled from Larkin and Stokes (1967); Nakayama and Yanoshi (1967); Gordon et al. (1973), Pichinoty et al. (1976, 1983, 1984); Aragno (1978); Schenk and Aragno (1979); Pichinoty (1983); Bonjour and Aragno (1984); Logan and Berkeley (1984), Claus and Berkeley (1986), Priest et al. (1987, 1988); Nakamura et al. (1988, 1999, 2002); Demharter and Hensel (1989b); Denariaz et al. (1989); Nakamura (1989, 1998); Ventosa et al. (1989); Tomimura et al. (1990); Nagel and Andreesen (1991); Arfman et al. (1992); Spanka and Fritze (1993); Andersch et al. (1994); Roberts et al. (1994, 1996); Agnew et al. (1995); Boone et al. (1995); Combet-Blanc et al. (1995); Nielsen et al. (1995a); Fritze (1996a); Fujita et al. (1996); Kuhnigk et al. (1995); Kuroshima et al. (1996); Pettersson et al. (2000, 1996); Shelobolina et al. (1997); Lechner et al. (1998); Switzer Blum et al. (1998); Yumoto et al. (2004c, 2003, 1998); Rheims et al. (1999); Logan et al. (2002a, 2002, 2000, 2004b); Palmisano et al. (2001); Yoon et al. (2001a, 2003a); Abd El-Rahman et al. (2002); Ajithkumar et al. (2002); Kanso et al. (2002); Li et al. (2002); Reva et al. (2002); Venkateswaran et al. (2003); Gugliandolo et al. (2003a); Heyrman et al. (2003a, 2005a, 2004); Taubel et al. (2003); De Clerck et al. (2004b, 2004c); Heyndrickx et al. (2004); La Duc et al. (2004); Ivanova et al. (2004a); Noguchi et al. (2004); Santini et al. (2004); Scheldeman et al. (2004); Suresh et al. (2004).
- c See Table 7 for a comparison of the subspecies of Bacillus subtilis and the closely related species Bacillus subtilis, Bacillus atrophaeus, Bacillus mojavensis and Bacillus vallismortis, and the species Bacillus amyloliquefaciens, Bacillus licheniformis, Bacillus pumilus, and Bacillus sonorensis.
- d See Table 2 for a comparison of alkaliphilic species: Bacillus agaradhaerens, Bacillus alcalophilus, Bacillus algicola, Bacillus arseniciselenatis, Bacillus clarkii, Bacillus cohnii, Bacillus halodurans, Bacillus horti, Bacillus krulwichiae, Bacillus okuhidensis, Bacillus pseudoalcaliphilus, Bacillus pseudofirmus, Bacillus selenitireducens, Bacillus thermocloacae, Bacillus vedderi.
- e See Table 8 for comparison of the closely related species Bacillus anthracis, Bacillus cereus, Bacillus mycoides, Bacillus pseudomycoides, Bacillus thuringiensis, and Bacillus weihenstephanensis.
- f See Table 3 for comparison of the thermophilic species (optimum growth at 50 °C or above): Bacillus aeolius, Bacillus fumarioli, Bacillus infernus, Bacillus methanolicus, Bacillus schlegelii, Bacillus thermoamylovorans, Bacillus thermocloacae, and Bacillus tusciae.
- g See Table 9 for comparison of the neutrophilic, non-thermophilic species that form spherical spores: Bacillus fusiformis, Bacillus insolitus, Bacillus neidei, Bacillus psychrodurans, Bacillus psychrotolerans, Bacillus pycnus, and Bacillus sphaericus.
- h Pigmentation: Bacillus subtilis may form pigments, varying from cream through yellow, orange, pink and red, to brown or black, on potato or agar media containing glucose, and strains forming brown or black pigment were often formerly called “Bacillus subtilis var. aterrimus”; Bacillus algicola produces semitransparent, creamy, slightly yellowish colonies; Bacillus aquimaris colonies are pale orange-yellow; Bacillus arseniciselenatis and Bacillus selenitireducens will produce red colonies, owing to elemental selenium precipitation, on selenium oxide media; Bacillus atrophaeus forms a dark brownish-black soluble pigment in 2–6 d on media containing tyrosine or other organic nitrogen source; Bacillus carboniphilus produces grayish yellow pigment on nutrient agar and brownish red pigment on trypto-soya agar; some strains of Bacillus cereus may produce a yellowish-green fluorescent pigment on various media, some strains may produce a pinkish brown diffusible pigment on nutrient agar, and on starch-containing media containing sufficient iron some strains produce the red pigment pulcherrimin; Bacillus clarkii colonies may be cream-white to pale yellow in color, and one of the three strains described produces dark yellow colonies with age; Bacillus endophyticus colonies may be white or pink-red, even on the same plate, and media containing ampicillin and lysozyme commonly yield red colonies; colonies of Bacillus fastidiosus on uric acid medium may become yellowish; Bacillus firmus colonies are creamy-yellow to pale orangey-brown after 3 d on TSA at 30 °C; Bacillus gibsonii colonies are yellow; Bacillus indicus colonies are yellowish-orange; Bacillus hwaijinpoensis colonies are light yellow; Bacillus jeotgali colonies are cream-yellow to light orange-yellow; many strains of Bacillus licheniformis can produce red pigment (assumed to be pulcherrimin) on carbohydrate media containing sufficient iron, and colonies on glycerol/glutamate medium are reddish-brown; Bacillus marisflavi colonies are pale yellow; Bacillus megaterium colonies may become yellow and then brown or black on long incubation; Bacillus pseudofirmus colonies are yellow; Bacillus sonorensis colonies are yellowish-cream on routine media, and bright yellow on pH 5.6 agar; Bacillus subterraneus colonies are dark yellow to orange on tryptic soy agar.
- i Citrate test results may vary according to the test method used; Gordon et al. (1973) found citrate utilization to be a variable property among 23 strains of Bacillus anthracis, while Logan and Berkeley (1984) and Logan et al. (1985) obtained negative results for 37 strains using the API 20E test method. For Bacillus badius, Gordon et al. (1973) obtained negative results with two strains, while Logan and Berkeley (1984) obtained positive results for two strains using the API 20E test method.
- j Strains of Bacillus cereus of serovars 1, 3, 5, and 8, which are particularly associated with outbreaks of emetic-type food poisoning, do not produce acid from salicin and starch, whereas strains of Bacillus cereus of other serotypes are usually positive for these reactions. See Table 8.
- k For Bacillus fumarioli, acid production from carbohydrates is tested at pH 6 – see Logan et al. (2000) and Testing for special characters.
- l Gordon et al. (1973) found the Voges–Proskauer reaction to be negative for 60 strains of Bacillus megaterium, while Logan and Berkeley (1984) obtained positive results for all but one of 33 strains using the API 20E test method.
- m Acid production from carbohydrates by Bacillus naganoensis is slow, and shows only after extended (>14 d) incubation.
- n The published description of Bacillus pseudomycoides (Nakamura, 1998) records that 7% salt is tolerated, but the differentiation table in that publication indicates the opposite result.
- o Spores of Bacillus psychrodurans and Bacillus psychrotolerans are rarely formed; on casein-peptone soymeal-peptone agar spores are predominantly spherical, but on marine agar they are predominantly ellipsoidal.
- p Mosquitocidal strains of Bacillus sphaericus produce parasporal toxin crystals which are smaller than those produced by Bacillus thuringiensis, but which are nonetheless visible by phase-contrast microscopy.
- q Growth occurs within the range pH 7.2–9.5 in media adjusted with NaOH and HCl, but not in media where the pH is adjusted using buffered systems as described by Nielsen et al. (1995a).
- r Negative when incubated at 30 °C, but may become positive slowly when incubated at 40 °C.
- s Growth is poor in the absence of NaCl.

- a Symbols: +, >85% positive; d, variable (16–84% positive); −, 0–15% positive; w, weak reaction; d/w, variable and weak when positive; no entry indicates that no data are available.
- b Compiled from Larkin and Stokes (1967); Nakayama and Yanoshi (1967); Gordon et al. (1973), Pichinoty et al. (1984, 1976, 1983); Aragno (1978); Schenk and Aragno (1979); Pichinoty (1983); Bonjour and Aragno (1984); Logan and Berkeley (1984); Claus and Berkeley (1986); Priest et al. (1987, 1988); Nakamura et al. (1988, 1999, 2002) Demharter and Hensel (1989b)<qu ref=81>; Denariaz et al. (1989); Nakamura (1989, 1998); Ventosa et al. (1989); Tomimura et al. (1990); Nagel and Andreesen (1991); Arfman et al. (1992); Spanka and Fritze (1993); Andersch et al. (1994); Roberts et al. (1994, 1996); Agnew et al. (1995); Boone et al. (1995); Combet-Blanc et al. (1995); Nielsen et al. (1995a); Fritze (1996a); Fujita et al. (1996); Kuhnigk et al. (1995); Kuroshima et al. (1996); Pettersson et al. (2000, 1996); Shelobolina et al. (1997); Lechner et al. (1998); Switzer Blum et al. (1998); Yumoto et al. (1998, 2003, 2004c); Rheims et al. (1999); Logan et al. (2002a, b, 2000, 2004b); Palmisano et al. (2001); Yoon et al. (2001a); Abd El-Rahman et al. (2002); Ajithkumar et al. (2002); Kanso et al. (2002); Li et al. (2002); Reva et al. (2002); Venkateswaran et al. (2003); Gugliandolo et al. (2003a); Heyrman et al. (2003a, 2005a, 2004); Taubel et al. (2003); Yoon et al. (2003a); De Clerck et al. (2004b, 2004c); Heyndrickx et al. (2004); La Duc et al. (2004); Ivanova et al. (2004a); Noguchi et al. (2004); Santini et al. (2004); Scheldeman et al. (2004); Suresh et al. (2004).
- c Reactions differ between strains of the emetic biotype of Bacillus cereus for these substrates.
- d Results obtained when inocula are supplemented with 7% NaCl.
- e Results obtained when grown at pH 10.
- f Assimilation data for Bacillus simplex are for the type strain only.
-
List of species of the genus Bacillus
1
-
Bacillus subtilis (Ehrenberg 1835) Cohn 1872, 174AL Nom. cons. Nomencl. Comm. Intern. Soc. Microbiol. 1937, 28; Opin. A. Jud. Comm. 1955, 39 (Vibrio subtilis Ehrenberg 1835, 279.)
sub'ti.lis. L. adj. subtilis slender.
Aerobic, Gram-positive, motile rods, forming ellipsoidal to cylindrical spores which lie centrally, paracentrally and subterminally in unswollen sporangia (Figure 3g). Cells grown on glucose agar stain evenly. Cells 0.7–0.8 by 2.0–3.0 µm, occurring singly and in pairs, seldom in chains. Colonial morphology is exceptionally variable, within and between strains, and may give the appearance of a mixed culture. Colonies are round to irregular in shape and of moderate (2–4 mm) diameter, with margins varying from undulate to fimbriate; they become opaque, with surfaces that are dull and which may become wrinkled; color is whitish, and may become creamy or brown; textures range from moist and butyrous or mucoid, through membranous with an underlying mucoid matrix, with or without mucoid beading at the surface, to rough and crusty as they dry (Figure 2g). Pigments, varying from cream through yellow, orange, pink and red, to brown or black, may be formed on potato or agar media containing glucose; strains forming brown or black pigment were often formerly called “Bacillus subtilis var. aterrimus.” Strains forming brownish-black pigment on tyrosine (and so often evident on the crude media available to earlier workers), and often formerly called “Bacillus subtilis var. niger,” have been split from Bacillus subtilis as Bacillus atrophaeus.
Optimum growth temperature 28–30°C, with minimum of 5–20°C and maximum of 45–55°C. Growth occurs between pH 5.5 and 8.5, but limits have not been recorded. Some restricted anaerobic growth may occur in complex media with glucose or (less effectively) nitrate. Growth occurs on minimal medium with glucose and an ammonium salt as sole sources of carbon and nitrogen. Grows in presence of up to 7% NaCl, some strains will tolerate 10% NaCl. Catalase-positive, oxidase variable. Casein, esculin, gelatin and starch are hydrolyzed, phenylalanine and urea are not hydrolyzed. Pectin and polysaccharides of plant tissues are decomposed. Dextran and levan are formed extracellularly from sucrose. Citrate is utilized as sole carbon source by most strains; propionate is not utilized. Nitrate is reduced to nitrite. Voges-Proskauer-positive. Acid without gas is produced from glucose and from a wide range of other carbohydrates.
The practical values of the distinction of the subspecies of Bacillus subtilis and of the species Bacillus mojavensis and Bacillus vallismortis are questionable. The distinction of Bacillus atrophaeus from the Bacillus subtilis subspecies and from Bacillus mojavensis and Bacillus vallismortis is dependent upon brownish-black pigment production on tyrosine agar by strains of Bacillus atrophaeus. See Table 7.
Table 7. Differentiation of Bacillus subtilis from closely related Bacillus speciesa, b 
- a Symbols: +, >85% positive; d, different strains give different reactions (16–84% positive); −, 0–15% positive; w, weak reaction; nd, no data are available.
- b Compiled from Claus and Berkeley (1986), Priest et al. (1987), Nakamura (1989), Roberts et al. (1994, 1996), Nakamura et al. (1999), and Palmisano et al. (2001).
- c The subspecies of Bacillus subtilis are not distinguishable by routine phenotypic tests.
- d Bacillus subtilis may form pigments, varying from cream through yellow, orange, pink and red, to brown or black, on potato or agar media containing glucose; strains forming brown or black pigment were often formerly called “Bacillus subtilis var. aterrimus.”
- e Colonies are yellowish-cream on routine media, and bright yellow on pH 5.6 agar.
- f This species accommodates strains forming brownish-black pigment on tyrosine (and so often evident on the crude media available to earlier workers), and often formerly called “Bacillus subtilis var. niger.”
Endospores are very widespread in soil, dust and on vegetation, and in many other environments. The vegetative organisms participate in the early stages of the breakdown of organic matter. Causative agent of ropy (slimy) bread.
-
Bacillus subtilis subsp. subtilis Nakamura, Roberts and Cohan 1999, 1214VP
Description is that given above for the species.
Phenotypically similar to Bacillus atrophaeus and distinguishable from that species only by the pigmentation of the latter. Not distinguishable from Bacillus mojavensis, Bacillus subtilis subsp. spizizenii and Bacillus vallismortis by conventional phenotypic tests.
DNA G + C content (mol%): 41.5–47.5 (T m) for 31 strains, 41.8–46.3 (Bd) for 34 strains, and 42.9 (T m) for the type strain.
Type strain : ATCC 6051, IAM 12118, CCM 2216, DSM 10, IFO 12210, NCIMB 3610, NCTC 3610, NRRL NRS-744.
EMBL/GenBank accession (16S rRNA gene): AB042061 (IAM 12118).
Additional remarks: Strains designated by Gibson (1944) and Smith et al. (1946) as synonyms of Bacillus subtilis included Bacillus aterrimus, Bacillus mesentericus, Bacillus natto, Bacillus niger, Bacillus nigrificans and Bacillus panis. “Bacillus natto” is a name given to Bacillus subtilis strains associated with natto, a Japanese food made by fermenting soybeans with these organisms. Strains formerly designated “Bacillus amyloliquefaciens” or “Bacillus subtilis var. amyloliquefaciens” are now accommodated within Bacillus amyloliquefaciens.
-
Bacillus subtilis subsp. spizizenii Nakamura, Roberts and Cohan 1999, 1214VL
spi.zi.ze'ni.i. L. gen. n. spizizenii named after the American bacteriologist J. Spizizen.
Phenotypically indistinguishable from Bacillus subtilis subsp. subtilis, and separated from that taxon only by DNA relatedness values of 58–68% with 12 strains of that subspecies, by the presence of ribitol in the cell wall, and by transformation studies.
The type strain was isolated from Tunisian soil.
Type strain : NRRL B-23049, DSM 15029, LMG 19156, KCTC 3705.
EMBL/GenBank accession number (16S rRNA gene): AF074970 (NRRL B-23049).
-
Bacillus aeolius Gugliandolo, Maugeri, Caccamo and Stackebrandt 2003b, 1701VP (Effective publication: Gugliandolo, Maugeri, Caccamo and Stackebrandt 2003a, 175.)
ae.o'li.us. L. adj. aeolius pertaining to the Eolian Island (Insulae Aeoliae) where the organism was isolated from a shallow marine hydrothermal vent.
Aerobic, Gram-positive, motile rods, 0.5 µm by 2.0 µm, forming terminal, oval endospores. Description is based upon a single isolate. Catalase-negative, oxidase-positive. Growth temperature range is 37–65°C, with an optimum growth temperature of 55°C. pH range for growth 7–9, with an optimum of pH 8.0. Grows in the range 0.5–5% NaCl, with an optimum of 2% NaCl. Acid is produced from glucose and a wide range of other carbohydrates. The following may be utilized as carbon sources: arabinose, N-acetylglucosamine, citrate, glucose, gluconate, malate, maltose, mannitol, mannose, phenylacetate. Produces acetoin but not H2S or indole. Nitrate is not reduced. Casein, gelatin and starch are hydrolyzed, but esculin and urea are not. Arginine dihydrolase, and lysine and ornithine decarboxylases negative. Exopolysaccharides are produced in mineral medium supplemented with sucrose. See Table 3.
Source: a shallow marine hydrothermal vent, Vulcano Island, Eolian Islands, Italy.
DNA G + C content (mol%): 40.8 (T m) (for methods, see Maugeri et al., 2001).
Type strain : 4–1, DSM 15804, and CIP 107628.
EMBL/GenBank accession number (16S rRNA gene): AJ504797 (4–1).
-
Bacillus agaradhaerens Nielsen, Fritze and Priest 1995b, 879VP (Effective publication: Nielsen, Fritze and Priest 1995a, 1758.)
a.gar.ad'hae.rens. Malayan n. agar gelling polysaccharide from brown algae; L. adj. adhaerens adherent; N.L. adj. agaradhaerens adhering to the agar.
Strictly alkaliphilic organisms forming ellipsoidal spores which lie subterminally in swollen sporangia. Cells 0.5–0.6 by 2.0–5.0 µm. Colonies are adherent, white and rhizoid with filamentous margins. Growth temperature range 10–45°C. Optimal growth at pH 10.0 or above; no growth at pH 7.0. Grows (sometimes only weakly) in presence of up to 16% NaCl. Nitrate is reduced to nitrite. Casein, cellulose, gelatin, starch, Tween 40 and xylan are hydrolyzed. Tween 60 is hydrolyzed by most strains. Hippurate, 4-methylumbelliferone glucuronide, Tween 20 and 80 are not hydrolyzed; phenylalanine is not deaminated. Glucose and a range of other carbohydrates can be utilized as sole sources of carbon. See Table 2.
Source: soil.
DNA G + C content (mol%): 39.3–39.5 (HPLC).
Type strain : PN-105, ATCC 700163, DSM 8721, LMG 17948.
EMBL/GenBank accession number (16S rRNA gene): X76445 (DSM 8721).
-
Bacillus alcalophilus Vedder 1934, 141AL (emend. Nielsen, Fritze and Priest 1995a, 1758.)
al.cal.o.phil'us. N.L. alcali En. alkali from the Arabic al the; qaliy soda ash; Gr. adj. philos loving; N.L. adj. alcalophilus liking alkaline (media).
Alkaliphilic organisms forming ellipsoidal spores which lie subterminally in unswollen sporangia. Cells 0.5–0.7 by 3.0–5.0 µm. Colonies are white, circular, smooth and shiny, sometimes with darker centers. Growth temperature range 10–40°C. Optimal growth at pH 9.0–10.0; no growth at pH 7.0. Maximum NaCl concentration tolerated ranges from less than 5% up to 8%. Nitrate is usually not reduced to nitrite. Casein, gelatin, pullulan, starch, and Tween 40 and 60 are hydrolyzed. Hippurate, 4-methylumbelliferone glucuronide, Tween 20 (usually) and 80 are not hydrolyzed; phenylalanine is not deaminated. Glucose and a range of other carbohydrates can be utilized as sole sources of carbon. See Table 2.
Source: a variety of materials after enrichment at pH 10.
DNA G + C content (mol%): 36.2–38.4 (HPLC analysis) and 37.0 (T m), 36.7 (Bd) for the type strain.
Type strain : Vedder 1, ATCC 27647, DSM 485, JCM 5262, LMG 17938, NCIMB 10436.
EMBL/GenBank accession number (16S rRNA gene): X76436 (DSM 485).
-
Bacillus algicola Ivanova, Alexeeva, Zhukova, Gorshkova, Buljan, Nicolau, Mikhailov and Christen 2004b, 1425VP (Effective publication: Ivanova, Alexeeva, Zhukova, Gorshkova, Buljan, Nicolau, Mikhailov and Christen 2004a, 304.)
al.gi'co.la. L. fem. n. alga -ae, alga; L. suff. -cola (from L. masc. n. incola -ae inhabitat, dweller); N.L. masc. n. algicola algaedweller.
Gram-positive cells (0.5–0.9 µm in diameter and 1.8–5.0 µm long) are aerobic, filamentous with “cross-like” branching, and produce subterminally located ellipsoidal spores (0.5–0.7 µm by 0.7–1.0 µm). Description is based upon a single isolate. Colonies are semitransparent, creamy, and slightly yellowish in color. Growth occurs between 10°C and 45°C with optimum at 28–30°C. No growth is detected at 4°C and at 50°C. Alkalitolerant, growing at pH 7–10. Growth occurs at 0–3% NaCl. Anaerobic growth and oxidase are negative. Catalase and nitrate reduction are weak. Urea, alginate, starch, and gelatin are hydrolyzed. Do not decompose agar and casein. According to Biolog, utilizes dextrin, cellobiose, D-fructose, α-D-glucose, maltose, D-mannose, sucrose, D-trehalose, pyruvic acid methyl ester, β-hydroxybutyric acid, α-ketobutyric acid, inosine, uridine, thymidine, glycerol, and DL-α-glycerol phosphate. The predominant cellular fatty acids are C-14:0 iso, C-15:0 iso, C-15:0 anteiso, C-16:0 iso; and C-17:0 anteiso. See Table 2.
Source: degraded thallus of brown alga Fucus evanescens collected from Kraternaya Bight, Pacific Ocean.
DNA G + C content (mol%): 37.4 (T m).
Type strain : KMM 3737, CIP 107850.
GenBank/EMBL accession number (16S rRNA gene): AY228462 (KMM 3737).
-
Bacillus amyloliquefaciens Priest, Goodfellow, Shute and Berkeley 1987, 69VP
am.yl.o.li.que.fac'i.ens. L. n. amylum starch; L. part. adj. liquefaciens dissolving; N.L. part. adj. amyloliquefaciens starchdigesting.
Strictly aerobic, Gram-positive, motile rods, 0.7–0.9 by 1.8–3.0 µm, often occurring in chains, and forming ellipsoidal spores (0.6–0.8 by 1.0–1.4 µm) which lie centrally, paracentrally and subterminally in unswollen sporangia. No growth below 15°C or above 50°C; optimum growth temperature 30–40°C. Casein, elastin, esculin, gelatin, starch and Tween 20, 40 and 60 are degraded, but adenine, cellulose, guanine, hypoxanthine, pectin, testosterone, tyrosine, urea and xanthine are not. Nitrate is reduced to nitrite. Voges–Proskauer-positive. Citrate is utilized as sole carbon source, propionate is not. Growth occurs in presence of 5% NaCl, and most strains tolerate 10% NaCl. Acid without gas is produced from glucose and a range of other carbohydrates. This species is important as a source of α-amylase and protease for industrial applications. See Table 7.
Source: soil and industrial amylase fermentations.
DNA G + C content (mol%): 44.35 ± 0.38 (T m) for eight strains; 44.2 ± 0.7 (Bd) with a range of 44–46; the mol% G + C of the type strain is 44.6.
Type strain : Fukumoto strain F, ATCC 23350, DSM 7, LMG 9814, NCIMB 12077, NRRL B-14393.
EMBL/GenBank accession number (16S rRNA gene): X60605 (ATCC 23350).
-
Bacillus anthracis Cohn 1872, 177AL
an'thra.cis. Gr. n. anthrax charcoal, a carbuncle; N.L. n. anthrax the disease anthrax; N.L. gen. n. anthracis of anthrax.
Phenotypically similar to Bacillus cereus (see below and Table 8) except in the characters undernoted. Colonies of Bacillus anthracis (Figure 2a) are similar to those of Bacillus cereus, but those of the former are generally smaller, non-hemolytic, may show more spiking or tailing along the lines of inoculation streaks, and are very tenacious as compared with the usually more butyrous consistency of Bacillus cereus and Bacillus thuringiensis colonies, so that they may be pulled into standing peaks with a loop. Nonmotile. Usually susceptible to penicillin. Susceptible to gamma phage (see Logan and Turnbull, 2003, Logan et al., 2007). Produces a glutamyl-polypeptide capsule in vivo and when grown on nutrient agar containing 0.7% sodium bicarbonate incubated overnight under 5–7% CO2. Colonies of the capsulate Bacillus anthracis appear mucoid, and the capsule can be visualized by staining smears with M'Fadyean's polychrome methylene blue or India Ink (Turnbull et al., 1998). An isolate showing the characteristic phenotype but unable to produce capsules may be an avirulent form lacking either or both capsule or toxin genes (Turnbull et al., 1992). Virulent and avirulent strains may be distinguished from other members of the Bacillus cereus group using tests in the API System (Logan et al., 1985). Virulence genes are carried by plasmids pX01 (toxins) and pX02 (capsule); these plasmids may be transmissible to other members of the Bacillus cereus group (Turnbull et al., 2002). Primer sequences are now available for confirming the presence of the toxin and capsule genes, and hence the virulence of an isolate. Genetically very closely related to Bacillus cereus and other members of the Bacillus cereus group (Turnbull, 1999); Bacillus anthracis may be distinguished from other members of the Bacillus cereus group by amplified fragment length polymorphism (AFLP) analysis (Keim et al., 2000; Turnbull et al., 2002). A 277bp DNA sequence (Ba813) has been described as a specific chromosomal marker for Bacillus anthracis and in combination with sequencing of parts of lef and cap genes its sequence allows the identification of virulent strains (application note 209 of Pyrosequencing AB). Isolates of Bacillus anthracis show considerable molecular homogeneity, and the species may derive from a relatively recent common ancestor.
Table 8. Differentiation of Bacillus cereus from closely related Bacillus speciesa, b 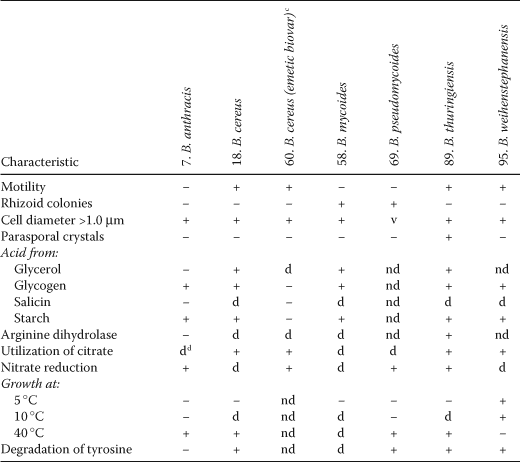
- a Symbols: +, >85% positive; d, different strains give different reactions (16–84% positive); −, 0–15% positive; v, variation within strains; nd, no data are available.
- b Compiled from Gordon et al. (1973); Logan and Berkeley (1984); Logan et al. (1985); Claus and Berkeley (1986); Lechner et al. (1998); Nakamura (1998).
- c Strains of Bacillus cereus of serovars 1, 3, 5 and 8, which are particularly associated with outbreaks of emetic-type food poisoning.
- d Citrate test results may vary according to the test method used; Gordon et al. (1973) found citrate utilization to be a variable property among 23 strains of Bacillus anthracis, while Logan and Berkeley (1984) and Logan et al. (1985) obtained negative results for 37 strains using the API 20E test method.
Causative agent of the disease anthrax in herbivorous and other animals and man. Widely studied and developed as a biological weapon. Generally considered to be an obligate pathogen; if it ever multiplies in the environment, it probably only does so rarely. Spores remain viable in soil for many years and their persistence does not depend on animal reservoirs.
Source: blood of animals and humans suffering from anthrax, from anthrax carcasses, and from animal products and soil contaminated with spores of the organism.
DNA G + C content (mol%): 32.2–33.9 (T m) for five strains, and 33.2 (T m) for the type strain.
Type strain : Vollum strain, ATCC 14578, NCIB 9377, NCTC 10340.
The 16S rRNA (or rDNA) gene sequence of the type strain is not available in the EMBL/GenBank database. However, 16S rDNA sequences of 98 strains of this species in EMBL are nearly all identical. Accession number AF176321 corresponds with the strain “Sterne.”
-
Bacillus aquimaris Yoon, Kim, Kang, Oh and Park 2003a, 1301VP
a.qui.ma'ris. L. n. aqua water; L. gen. n. maris of the sea; N.L. gen. n. aquimaris of the water of the sea.
Aerobic, Gram-variable rods, 0.5–0.7 by 1.2–3.5 µm, motile by means of peritrichous flagella. Description is based on a single isolate. Ellipsoidal endospores are borne centrally in large, swollen sporangia. Colonies are pale orange-yellow, circular to slightly irregular, slightly raised, and 2–4 mm in diameter after 3 d at 30°C on marine agar. Optimal growth temperature is 30–37°C. Growth occurs at 10 and 44°C, but not at 4 or above 45°C. Optimal growth pH is 6.0–7.0, and no growth is observed at pH 9.0 or 4.5. Optimal growth occurs in the presence of 2–5% (w/v) NaCl. Growth is poor in the absence of NaCl, but occurs in the presence of up to 18% (w/v) NaCl. Catalase-positive, oxidase- and urease-negative. Casein, starch and Tween 80 are hydrolyzed. Esculin, hypoxanthine, tyrosine and xanthine are not hydrolyzed. Acid is produced from D-fructose, D-glucose, glycogen, 5-ketogluconate, maltose, D-ribose, starch, sucrose and D-trehalose. The cell-wall peptidoglycan contains meso-diaminopimelic acid. The predominant menaquinone is MK-7. The major fatty acids are C15:0 iso and C15:0. anteiso
Source: sea water of a tidal flat of the Yellow Sea in Korea.
DNA G + C content (mol%) of the type strain is: 38 (HPLC).
Type strain : TF-12, JCM 11545, KCCM 41589.
GenBank accession number (16S rRNA gene): AF483625 (TF-12).
-
Bacillus arseniciselenatis (nom. corrig. Bacillus arsenicoselenatis [sic]) Switzer Blum, Burns Bindi, Buzzelli, Stolz and Oremland 2001, 793VP (Effective publication: Switzer Blum, Burns Bindi, Buzzelli, Stolz and Oremland 1998, 28.)
ar.se.ni.ci.se.le.na'tis. L. n. arsenicum arsenic; N.L. n. selenasatis selenate; N.L. gen. n. arseniciselenatis of arsenic (and) selenate.
Strictly anaerobic, nonmotile, spore-forming, Gram-positive rods which show respiratory growth with Se(VI) (selenate), As(V) (arsenate), Fe(III), nitrate and fumarate as electron acceptors. Cells are 0.5–1.0 by 3–10 µm. Description is based upon a single strain. Catalase- and oxidase-positive. Colonies are formed on lactate/selenate/yeast extract-supplemented lakewater medium incubated anaerobically at 20°C. Grows fermentatively on fructose. Uses lactate, malate, fructose, starch and citrate as electron donors. Moderately halophilic, with optimum salinity of 60 g/l NaCl, and a requirement for NaCl for growth. Moderately alkaliphilic, with optimum growth in the range pH 8.5–10. See Table 2.
Source: arsenic-rich sediment of Mono Lake, California.
DNA G + C content (mol%) of the type strain: 40.0% (T m).
Type strain : E1H, ATCC 700614, DSM 15340.
EMBL/GenBank accession number (16S rRNA gene): AF064705 (E1H).
-
Bacillus asahii Yumoto, Hirota, Yamaga, Nodasaka, Kawasaki, Matsuyama and Nakajima 2004c, 1999VP
as.a.hi'i. N.L. gen. n. asahii of Asahi; named after Asahi Kasei Co., a researcher from which isolated the bacterium.
Cells are Gram-positive peritrichously flagellated straight rods (1.4–3.0 × 0.4–0.8 µm) and produce terminally or centrally located ellipsoidal spores. Description is based upon a single isolate. Utilizes butyrate as carbon source for growth. Spores do not cause swelling of sporangium. Colonies are circular and white. Catalase and oxidase reactions are positive. Nitrate reduction to nitrite is weakly positive. Negative for indole production, Voges–Proskauer test, methyl red test, growth on MacConkey agar and H2S production. Trypsin, esterase (C4) and esterase/lipase (C8) are positive. Alkaline phosphatase, valine arylamidase, cystine arylamidase, chymotrypsin, acid phosphatase, β-glucosidase, β-glucosidase and N-acetyl-β-glucosaminidase are negative. Growth occurs at pH 6–9; growth at pH 5 is variable. Growth occurs at 0–1% NaCl but not at 2% NaCl. Growth occurs at 15–45°C, but not above 50°C. No acid is produced carbohydrates. Hydrolysis of casein, DNA, and Tween 20, 40 and 60 is observed but hydrolysis of gelatin is not. Hydrolysis of starch is weak. C15:0 iso (39.0%) and C15:0 anteiso (27.8%) represent the main fatty acids produced during growth in PY-1 medium.
Source: a soil sample obtained from Tagata-gun, Shizuoka, Japan.
DNA G + C content (mol%): 39.4 (HPLC).
Type strain : FERM BP-4493, MA001, JCM 12112, NCIMB 13969, CIP 108638.
GenBank/EMBL accession number (16S rRNA gene): AB109209 (FERM BP-4493).
-
Bacillus atrophaeus Nakamura 1989, 299VP
a.tro.phae'us. L. adj. ater black; Gr adj. phaeus brown; N.L. adj. atrophaeus dark brown.
Aerobic, Gram-positive, motile rods, forming ellipsoidal spores which lie centrally or paracentrally in unswollen sporangia. Cells 0.5–1.0 by 2.0–4.0 µm, occurring singly and in short chains. Colonies are opaque, smooth circular and entire and up to 2 mm in diameter after 2 d at 28°C, and form a dark brownish-black soluble pigment in 2–6 d on media containing tyrosine or other organic nitrogen source. Optimum growth temperature 28–30°C, with minimum of 5–10°C and maximum of 50–55°C. Catalase-positive, oxidase-negative.
Includes strains formerly called “Bacillus subtilis var. niger”; some strains so designated are used for autoclave sterility testing. Phenotypically distinguishable from Bacillus mojavensis, Bacillus subtilis subsp. spizizenii and Bacillus subtilis subsp. subtilis only by pigment production and a negative oxidase reaction. Phenotypically indistinguishable from Bacillus vallismortis. See Table 7.
Source: isolated mainly from soil.
DNA G + C content (mol%): 41.0–43.0 (Bd).
Type strain : NRRL NRS-213, ATCC 49337, DSM 7264, JCM 9070, LMG 17795, NCIMB 12899.
EMBL/GenBank accession number (16S rRNA gene): AB021181 (JCM 9070).
-
Bacillus azotoformans Pichinoty, de Barjac, Mandel and Asselineau 1983, 660VP
a.zo.to.for'mans. Fr. n. azote nitrogen; L. part. adj. formans forming; N.L. part. adj. azotoformans nitrogen-forming.
Gram-negative, peritrichously motile rods (0.5–0.8 µm by 3–7 µm), forming ellipsoidal, subterminal and terminal spores which swell the sporangia. Nitrate, nitrite and nitrous oxide are denitrified with the production of N2. For anaerobic growth, nitrate, nitrite, nitrous oxide, tetrathionate and fumarate act as terminal electron acceptors. Growth requirements are complex; non-fermentative, carbohydrates are not attacked, a range of organic acids is utilized as carbon sources. Colonies circular and partially translucent, with entire margins, on yeast extract agar. Oxidase-positive, catalase-negative. Gelatin, starch and Tween 80 not hydrolyzed. Maximum growth temperature 42–46°C.
Source: garden soil by enrichment culture in peptone broth under N2O.
DNA G + C content (mol%): 39.0–43.9 (mean of 39.8 for 17 strains) (Bd) and 39.0 for the type strain.
Type strain : Pichinoty 1, ATCC 29788, NRRL B-14310, DSM 1046, LMG 9581, NCIMB 11859.
EMBL/GenBank accession number (16S rRNA gene): D78309 (DSM 1046). This sequence seems to be somewhat more reliable than the one reported for ATCC 29788. Both sequences differ and contain inadequately determined bases.
-
Bacillus badius Batchelor 1919, 23AL
ba.di'us. L. adj. badius chestnut brown.
Aerobic, Gram-positive, motile rods, cells 0.8–1.2 by 2.5–5.0 µm, occurring singly and in pairs and chains, forming ellipsoidal spores which are located subterminally, and sometimes paracentrally or terminally, and which do not swell the sporangia. Growth occurs between 15°C and 50°C, with the optimum around 30°C. Catalase-positive. Casein and gelatin are hydrolyzed; starch is not hydrolyzed. Tyrosine is degraded. Grows in presence of up to 7% NaCl. Citrate may be utilized as sole carbon source. Nitrate is not reduced. Acid is not produced from glucose and other carbohydrates. Assimilates certain amino acids and organic acids.
Source: feces, dust, marine sources, foods, antacids and gelatin production plant.
DNA G + C content (mol%): 43.8 (T m) and 43.5 (Bd) for the type strain.
Type strain : ATCC 14574, DSM 23, LMG 7122, NCIMB 9364, NRRL NRS-663, IAM 11059.
EMBL/GenBank accession number (16S rRNA gene): X77790 (ATCC 14574).
-
Bacillus barbaricus Taubel, Kämpfer, Buczolits, Lubitz and Busse 2003, 729VP
bar.ba'ri.cus. L. adj. barbaricus strange, foreign, referring to the strange behavior towards growth at different pH levels.
Facultatively anaerobic, Gram-positive, nonmotile rods, 0.5 µm wide and 4–5 µm long. Oval endospores are borne subterminally in swollen sporangia. Colonies are brownish, opaque, circular, flat and 3–7 mm in diameter when grown on peptone-yeast extract-succinate (PYES) agar. Catalase-positive, oxidase- and urease-negative. Nitrate is not reduced. Indole and H2S are not produced. Alkalitolerant; growth is weak at pH 6.0, but strong at pH 7.2, 8.0 and 9.5 in PYES medium adjusted with HCl or NaOH before autoclaving, but no growth in buffered media at pH 7.0–11.0. Good growth occurs at temperatures ranging from 18°C to 37°C, with no growth at 4 or 47°C. Weak growth occurs in presence of 2% NaCl, and no growth in 5% NaCl. Hippurate is decomposed. Esculin is not hydrolyzed. Citrate is not utilized. Acid is produced from D-glucose, N-acetyl-glucosamine, maltose, trehalose, starch and glycogen. Acid production is variable from D-fructose (type strain weakly positive), galactose, methyl-D-glucoside, lactose, sucrose and D-turanose (type strain negative). The cell-wall diamino acid is diaminopimelic acid and MK-7 is the predominant menaquinone. The polar lipid profile is composed of the major compounds phosphatidylethanolamine, phosphatidylglycerol and diphosphatidylglycerol. The fatty acid profile consists of the predominant compounds C15:0 anteiso and C15:0 iso; C14:0 iso and C16:0 iso are present in moderate amounts.
Source: an experimental wall painting exposed in the Virgilkapelle in Vienna, Austria.
DNA G + C content (mol%): not reported.
Type strain : V2-BIII-A2, DSM 14730, CCM 4982.
EMBL/GenBank accession number (16S rRNA gene): AJ422145 (V2-BIII-A2).
-
Bacillus bataviensis Heyrman, Vanparys, Logan, Balcaen, Rodríguez-Díaz, Felske and De Vos 2004, 55VP
ba.ta.vi.en'sis. L. adj. bataviensis pertaining to Batavia, the name with which Julius Caesar described The Netherlands.
Facultatively anaerobic, Gram-positive or Gram-variable (at 24 h), motile, slightly tapered rods, 0.7–1.2 µm in diameter, occurring singly, and in pairs and short chains. Endospores are mainly ellipsoidal but may be spherical, and lie centrally, paracentrally and occasionally subterminally in slightly swollen sporangia. Colonies on TSA are butyrous, cream-colored, and produce a soft-brown pigment that diffuses in the agar; they are slightly raised and umbonate, have regular margins and smooth or rough, eggshell-textured surfaces. The optimum temperature for growth is 30°C, and the maximum growth temperature lies between 50°C and 55°C. The optimum pH for growth is 7.0–8.0, and the pH range for growth is from 4.0–6.0 to 9.5–10.0. Casein is not hydrolyzed. In the API 20E strip, o-nitrophenyl-β-D-galactopyranoside hydrolysis is positive, gelatin is hydrolyzed by most strains, and nitrate reduction is positive; Voges–Proskauer reaction is negative, and reactions for arginine dihydrolase (one strain positive), lysine decarboxylase, ornithine decarboxylase, citrate utilization, hydrogen sulfide production, urease, tryptophan deaminase, indole production are negative. Hydrolysis of esculin is positive. Acid without gas is produced from the following carbohydrates in the API 50 CH gallery using the CHB suspension medium: N-acetyl-D-glucosamine, D-cellobiose, D-fructose, galactose, β-gentiobiose, D-glucose, glycerol (weak), lactose, maltose, D-mannitol, D-mannose, D-melezitose, raffinose, ribose (weak), salicin (weak), D-trehalose and D-turanose. The following reactions are variable between strains and, when positive, are usually weak: amygdalin, arbutin, L-fucose, inulin, D-melibiose, starch and sucrose; type strain is positive but weak for: arbutin, L-fucose, inulin, D-melibiose, methyl α-D-glucoside, methyl α-D-mannoside and sucrose. The major cellular fatty acids are C15:0 iso and C15:0 anteiso, present at a level of about 37 and 21%, respectively, while C16:1 ω11c accounts for about 11% of the total fatty acids.
Source: soil in the Drentse A agricultural research area, The Netherlands.
DNA G + C content (mol%): 39.6–40.1 (type strain 40.1) (HPLC).
Type strain : LMG 21833, DSM 15601.
EMBL/GenBank accession number (16S rRNA gene): AJ542508 (LMG 21833).
-
Bacillus benzoevorans Pichinoty, Asselineau and Mandel 1987, 179VP (Effective publication: Pichinoty, Asselineau and Mandel 1984, 215.)
ben.zo.e.vor'ans. L. part. adj. acidum benzoicum. benzoic acid; L. vorans devouring; N.L. part. adj. benzoevorans devourer of benzoic acid.
Prototrophic, facultatively anaerobic, Gram-variable, large (1.8 µm diameter) filaments and rods which use aromatic acids and phenols, but not carbohydrates and amino acids (except glycine) as carbon and energy sources. Do not grow in media containing only peptone or tryptone; grow rapidly in media containing yeast extract and sodium acetate or benzoate. Filamentous growth on solid and in stationary liquid media, with motile rods appearing in shaken liquid culture. Form ellipsoidal spores which do not swell the sporangia. Colonies circular, flat, off-white and opaque with matt surface. Nitrate, but not nitrite, reduced. Optimum growth temperature 32°C; maximum 39–45°C.
Source: pasteurized soil by aerobic enrichment in minimal medium containing benzoate, p-hydroxybenzoate or cyclohexane carboxylate.
DNA G + C content (mol%) of the type strain: 41.3% (T m).
Type strain : Pichinoty strain B1, ATCC 49005, DSM 5391, LMG 20225, NCIMB 12555, NRRL B-14535, CCM 3364.
EMBL/GenBank accession number (16S rRNA gene): X60611 (NCIMB 12555).
-
Bacillus carboniphilus Fujita, Shida, Takagi, Kunugita, Pankrushina and Matsuhashi 1996, 118VP
car.bo.ni'phi.lus. L. n. carbo coal, carbon; Gr adj. philos loving; N.L. adj. carboniphilus carbon-loving.
Aerobic, Gram-positive, peritrichously motile rods, forming ellipsoidal spores which lie centrally or terminally in unswollen sporangia. Cells 0.5–0.9 by 3.0–5.0 µm. Growth promoted by activated carbon and graphite. Colonies on nutrient agar are circular, flat, smooth, and grayish yellow; brown-red pigment is produced on trypto-soya agar. Growth temperature range 17–47°C. Strictly aerobic; nitrate not reduced. Grows in presence of 7% NaCl. Catalase- and oxidase-positive; casein, gelatin, hippurate, starch and Tween 80 are hydrolyzed. Acid and gas are not produced from glucose and a range of other carbohydrates.
Source: air, using antibiotic-containing medium spotted with sterile graphite.
DNA G + C content (mol%): 37.8–38.1 (T m), and 37.9 for the type strain.
Type strain : strain Matsuhashi Kasumi 6, JCM 9731, ATCC 700100, LMG 18001, NCIMB 13460.
EMBL/GenBank accession number (16S rRNA gene): AB021182 (JCM 9731).
-
Bacillus cereus Frankland and Frankland 1887, 257AL
ce're.us. L. adj. cereus waxen, wax-colored.
Facultatively anaerobic, Gram-positive, usually motile rods 1.0–1.2 by 3.0–5.0 µm, occurring singly and in pairs and long chains, and forming ellipsoidal, sometimes cylindrical, subterminal, sometimes paracentral, spores which do not swell the sporangia (Figure 3b); spores may lie obliquely in the sporangia. Cells grown on glucose agar produce large amounts of storage material, giving a vacuolate or foamy appearance. Colonies are very variable in appearance, but nevertheless distinctive and readily recognized: they are characteristically large (2–7 mm in diameter) and vary in shape from circular to irregular, with entire to undulate, crenate or fimbriate edges; they usually have matt or granular textures, but smooth and moist colonies are not uncommon (Figure 2b). Colonies are usually whitish to cream in color, but some strains may produce a pinkish brown pigment, and some strains produce a yellow diffusible pigment or a yellowish-green fluorescent pigment. Fresh plate cultures commonly have a “mousy” smell. Minimum temperature for growth is usually 10–20°C, and the maximum 40–45°C, with the optimum about 37°C. Psychrotolerant strains growing at 6°C have been isolated. Egg yolk reaction is positive. Catalase-positive, oxidase-negative. Casein, gelatin and starch are hydrolyzed. Voges–Proskauer-positive. Citrate is utilized as sole carbon source. Nitrate is reduced by most strains. Tyrosine is decomposed. Phenylalanine is not deaminated. Resistant to 0.001% lysozyme. Acid without gas is produced from glucose and a limited range of other carbohydrates. Most strains produce acid from salicin and starch, but strains of serovars 1, 3, 5 and 8 (which include strains associated with emetic food poisoning) do not produce acid from these substrates. Extracellular products include hemolysins, enterotoxins, heat-stable emetic toxin, cytotoxin, proteolytic enzymes and phospholipase; psychrotolerant strains may produce toxins (Stenfors and Granum, 2001).
Bacillus cereus has been divided into serovars on the basis of H-antigens (Kramer and Gilbert, 1992); 42 serovars are presently recognized (Ripabelli et al., 2000). Plasmid banding patterns and amplified fragment length polymorphism analysis may be of value in distinguishing between strains of the same serotype (Nishikawa et al., 1996; Ripabelli et al., 2000).
Endospores are very widespread in soil, in milk and other foods, and in many other environments. The vegetative organisms may multiply readily in a variety of foods and may cause diarrheal and emetic food poisoning syndromes. Growth in milk may result in “bitty cream defect”. Occasionally causes opportunistic infections in man and other animals. Certain endospore-forming, trichome-forming bacteria that occur in the alimentary tracts of animals, some of which have been called “Arthromitus”, have been identified as Bacillus cereus; see Cell morphology and Habitats, in Further descriptive information, above.
DNA G + C content (mol%): 31.7–40.1 (T m) for 11 strains, 34.7–38.0 (Bd), and 35.7 (T m), 36.2 (Bd) for the type strain.
Type strain : ATCC 14579, DSM 31, JCM 2152, LMG 6923, NCIMB 9373, NRRL B-3711, IAM 12605.
EMBL/GenBank accession number (16S rRNA gene): D16266 (IAM 12605).
Additional remarks: Phenotypically similar to other members of the Bacillus cereus group: Bacillus anthracis, Bacillus mycoides, Bacillus thuringiensis and Bacillus weihenstephanensis. For distinguishing characters see the individual species descriptions and Table 8. Another member of the group, Bacillus pseudomycoides, is separated from Bacillus cereus only by DNA relatedness and some differences in fatty acid composition. Genetic evidence supports the recognition of members of the Bacillus cereus group as one species, given that differentiation often relies on the presence of virulence characters which are carried by extrachromosomal mobile genetic elements (Turnbull et al., 2002), but practical considerations argue against such a move.
-
Bacillus circulans Jordan 1890, 821AL
cir'cu.lans. L. part. adj. circulans circling.
For many years this species accommodated a wide variety of phenotypically unrelated strains. It was referred to by Gibson and Topping (1938) as a complex rather than a species, and later investigators agreed with this description. Strains were frequently allocated to this species on account of their distinctive motile microcolonies (Figure 2i); however, Jordan named his isolate for the circular motion that he saw in the interior of colonies observed under low magnification, rather than because of motile microcolonies. Jordan's original strain is considered lost, but Ford's isolate 26, that he believed to be of the same species as Jordan's strain, is available. Smith and Clark (1938) observed the rotary motion within the colonies of Ford's strain and noted also the production of motile microcolonies. Despite a few discrepancies between Jordan's and Ford's descriptions of their strains, Smith et al. (1952) considered that Ford's strain 26 could be accepted as authentic and this became the type strain. The production of motile microcolonies is more characteristic of strains now allocated to Paenibacillus (see Further descriptive information, Colony characteristics, above).
Further grounds for the allocation of later isolates to this species were the production of sporangia swollen by subterminal to terminal ellipsoidal spores, and their being very active in the production of acid from a very wide range of carbohydrates. DNA relatedness studies revealed at least 10 homology groups among strains labeled Bacillus circulans, and it became clear that the phenotypic and genotypic heterogeneity of the complex had resulted from the allocation of unrelated strains to the species (Nakamura and Swezey, 1983). This work led to the allocation of members of several of the homology groups to new or revived species which were subsequently assigned to Paenibacillus: Paenibacillus amylolyticus, Paenibacillus lautus, Paenibacillus pabuli and Paenibacillus validus. A further group of strains previously assigned to Bacillus circulans was proposed as the new species Bacillus (now Paenibacillus) glucanolyticus on the basis of a numerical taxonomic study (Alexander and Priest, 1989). However, many misnamed strains remain allocated to Bacillus circulans and await reallocation, and authentic strains of this species are in the minority in most collections.
The description which follows is based upon the type strain and several other strains which have been shown by amplified rDNA restriction analysis, polyacrylamide gel electrophoresis of whole-cell proteins, and various phenotypic characters (De Vos, Logan and colleagues, unpublished data) to be closely related to the type strain. Phylogenetic studies indicate that Bacillus circulans, Bacillus firmus and Bacillus lentus are related.
Facultatively anaerobic, motile, straight, round-ended, occasionally slightly tapered and curved rods 0.6–0.8 µm in diameter, appearing singly or in pairs and occasionally short chains. Endospores are ellipsoidal and lie terminally or subterminally in swollen sporangia (Figure 2c). Colonies grown for 2 d on TSA at 30°C are 1–3 mm in diameter, opaque, cream-colored, slightly convex, with eggshell surface textures and irregular margins that may spike along the streak lines. Optimum temperature lies between 30°C and 37°C; maximum temperature for growth lies between 50°C and 55°C. The optimum pH for growth is 7.0. Minimum pH for growth lies between 4.0 and 5.0. The maximum pH lies between 9 and 10. Casein and starch are weakly hydrolyzed. In the API 20E strip, o-nitrophenyl-β-D-galactopyranoside hydrolysis is positive and urease production, hydrolysis of gelatin and nitrate reduction are occasionally positive. Arginine dihydrolase, lysine decarboxylase and ornithine decarboxylase production, citrate utilization, hydrogen sulfide, tryptophan deaminase and indole production and Voges–Proskauer reaction are negative. In the API 50CH gallery using the CHB suspension medium, hydrolysis of esculin is positive, and acid without gas is produced from a very wide range of carbohydrates: Production of acid without gas is variable for: adonitol, D-arabitol, 2-keto- and 5-keto-D-gluconate, rhamnose and ribose; the type strain is positive for adonitol, rhamnose and ribose. Acid production is negative for the following substrates: D-arabinose, dulcitol, erythritol, D-fucose, L-fucose, L-sorbose, D-tagatose and L-xylose. In the variable results, the type strain scores positive for: adonitol, rhamnose and ribose. Occasional strains may produce acid without gas from D-lyxose.
Source: sewage, soil, food and infant bile.
DNA G + C content (mol%): 35.7 (T m), 36.2 (Bd) for the type strain.
Type strain : ATCC 4513, DSM 11, JCM 2504, LMG 13261, IAM 12462.
EMBL/GenBank accession number (16S rRNA gene): D78312 (IAM 12462).
-
Bacillus clarkii Nielsen, Fritze and Priest 1995b, 879VP (Effective publication: Nielsen, Fritze and Priest 1995a, 1758.)
clar'ki.i. N.L. gen. n. clarkii of Clark, named after the American bacteriologist Francis E. Clark.
Strictly alkaliphilic and moderately halophilic organisms forming ellipsoidal spores which lie subterminally. The sporangia of the type strain are distinctly swollen, those of the other two strains characterized by Nielsen et al. were not swollen. Cells 0.6–0.7 by 2.0–5.0 µm. Colonies are circular and smooth, creamy-white to pale yellow or (with age) dark yellow, and with entire margins. Growth temperature range 15–45°C. Optimal growth at pH 10.0 or above; no growth at pH 7.0. Grows in presence of up to 16% NaCl; unable to grow in the absence of sodium ions. Nitrate is reduced to nitrite. Casein, hippurate, gelatin, and Tween 40 and 60 are hydrolyzed. Pullulan, starch, and Tween 20 and 80 are not hydrolyzed; phenylalanine is not deaminated. See Table 2.
Source: mud and soil.
DNA G + C content (mol%): 42.4–43.0 (HPLC analysis).
Type strain : PN-102, ATCC 700162, DSM 8720, LMG 17947.
EMBL/GenBank accession number (16S rRNA gene): X76444 (DSM 8720).
-
Bacillus clausii Nielsen, Fritze and Priest 1995b, 879VP (Effective publication: Nielsen, Fritze and Priest 1995a, 1759.)
clau'si.i. N.L. gen. n. clausii of Claus, named after the German bacteriologist Dieter Claus.
Alkalitolerant organisms forming ellipsoidal spores which lie paracentrally to subterminally in sporangia which may be slightly swollen. Cells 0.5–0.7 by 2.0–4.0 µm. Colonies are white and filamentous with filamentous margins. Growth temperature range 15–50°C. Optimal growth at pH 8.0; good growth at pH 7.0. Grows in presence of up to 8–10% NaCl. Nitrate is reduced to nitrite. Casein, gelatin and starch are hydrolyzed. Hippurate, pullulan, and Tween 20, 40, 60 and 80 are not hydrolyzed; phenylalanine is not deaminated. Glucose and a wide range of other carbohydrates can be utilized as sole sources of carbon. Strains in this species were formerly assigned to Bacillus lentus type II by Gordon and Hyde (1982).
Source: clay and soil.
DNA G + C content (mol%): 42.8–45.5 (HPLC analysis).
Type strain : PN-23, ATCC 700160, DSM 8716, LMG 17945, NCIMB 10309.
EMBL/GenBank accession number (16S rRNA gene): X76440 (DSM 8716).
-
Bacillus coagulans Hammer 1915, 119AL
co.a'gu.lans. L. part. adj. coagulans curdling, coagulating.
Moderately thermophilic, aciduric, facultativelyanaerobic, Gram-positive, motile rods. The cell diameter is 0.6–1.0 µm. Spores are ellipsoidal but sometimes appear spherical; they lie subterminally and occasionally paracentrally or terminally in slightly swollen sporangia; some strains do not sporulate readily. After 2d incubation on TSA at 40°C, colonies are <1 to 3 mm in diameter, white, convex with entire margins and smooth surfaces; they become cream-colored with age. Growth occurs at 30°C, the optimum growth temperature lies between 40°C and 57°C, and the maximum temperature for growth lies between 57°C and 61°C. The optimum pH for growth is 7.0; cells are able to grow at pH 4 and the maximum pH for growth lies between 10.5 and 11. Does not grow in presence of 5% NaCl. Minimal nutritional requirements are variable, and may include several amino acids and vitamins. Catalase-positive. Starch is hydrolyzed. Tyrosine is not decomposed. Casein is not hydrolyzed. In the API 20E strip, strains give variable results for arginine dihydrolase, gelatin liquefaction (type strain positive), nitrate reduction, ONPG (type strain positive) and the Voges–Proskauer test (type strain weak positive); all the strains are negative for lysine decarboxylase and ornithine decarboxylase reactions, citrate utilization, hydrogen sulfide production, urease, tryptophan deaminase, and indole production. In the API 50CH gallery using the CHB suspension medium, hydrolysis of esculin is variable (most strains positive), and acid without gas is produced from the following carbohydrates by more than 85% of strains: D-galactose, D-fructose, D-glucose, glycerol, maltose, D-mannose, D-melibiose, N-acetylglucosamine, starch and D-trehalose. Acid production from the other substrates varies between strains (see Tables 5 and 6, and De Clerck et al., 2004b).
Bacillus coagulans is economically important as a food spoilage agent, as a producer of commercially valuable products such as lactic acid, thermostable enzymes, and the antimicrobial peptide coagulin, and as a probiotic for chickens and piglets, but several taxonomic studies revealed considerable diversity within the species. De Clerck et al. (2004b) carried out a polyphasic taxonomic study of 30 strains, and found that although individual characterization methods revealed subgroups of strains, these intraspecies groupings were not sufficiently consistent among the different methods to support the proposal of subspecies, nor were there any features to suggest such a division, and DNA–DNA relatedness data and 16S rDNA sequence comparisons upheld the accommodation of all the strains in one species.
Source: soil, canned foods, tomato juice, gelatin, milk, medical preparations and silage.
DNA G + C content (mol%): 44.3–50.3 (T m) for seven strains, 45.4–56.0 (Bd) for three strains, and 47.4 (HPLC), 47.1 (T m), 44.5 (Bd) for the type strain.
Type strain : ATCC 7050, DSM 1, JCM 2257, LMG 6326, NCIMB 9365, NRRL NRS-609, IAM 12463.
EMBL/GenBank accession number (16S rRNA gene): D16267 (IAM 12463).
-
Bacillus cohnii Spanka and Fritze 1993, 155VP
coh'nii. N.L. gen. n. cohnii of Cohn; named after the German bacteriologist Ferdinand Cohn.
Alkaliphilic, Gram-positive, peritrichously motile rods, forming ellipsoidal spores which lie subterminally to terminally in swollen sporangia. In the cell wall, diaminopimelic acid is replaced by ornithine, and aspartic acid forms the interpeptide bridge. Colonies are creamy white, and 1–2 mm in diameter after 2 d at 45°C. Growth temperature range 10–47°C. Growth in presence of 5% but not 10% NaCl. Grows at pH 9.7. Nitrate is reduced. Catalase- and oxidase-positive. Gelatin, hippurate, starch and Tween 60 are hydrolyzed; hydrolysis of casein, pullulan and Tween 80 usually positive. Urea is not hydrolyzed, phenylalanine is not deaminated. See Table 2.
Source: soil and feces.
DNA G + C content (mol%): 33.9–35.0, and that of the type strain is 34.6 (T m).
Type strain : RSH, ATCC 51227, DSM 6307, LMG 16678, IFO 15565.
EMBL/GenBank accession number (16S rRNA gene): X76437 (DSM 6307).
-
Bacillus decolorationis Heyrman, Balcaen, Rodríguez-Díaz, Logan, Swings and De Vos 2003a, 462VP
de.co.lo.ra.ti.on'is. L. gen. n. decolorationis of discoloration.
Aerobic, Gram-variable, motile, rods and coccoid rods, 0.5–0.8 µm wide and 1.0–4.0 µm long, that occur singly, in pairs or short chains. Spores are produced slowly and in small numbers in culture; they are ellipsoidal, sometimes nearly spherical, central to subterminal and swell the sporangia slightly. Colonies on TSA are cream-colored to beige, circular with a smooth to slightly irregular margin, low-convex with a glistening and rough surface. Oxidase- and catalase-positive. The temperature range for growth is 5–40°C with optimal growth at 25–37°C. The NaCl concentration for growth is 0–10% (w/v), with an optimum of 4–7% (w/v). Casein hydrolysis is positive within 4 d incubation. In the API 20E strip, conversion of nitrates to nitrite and dinitrogen is positive and gelatin hydrolysis occurs with or without added salt, but only with large inocula. Reactions are negative for arginine dihydrolase, lysine decarboxylase, ornithine decarboxylase, citrate utilization, hydrogen sulfide production, urease, tryptophan deaminase, indole production and Voges-Proskauer. The ONPG reaction is negative without added NaCl and variable (type strain positive) when supplemented with 7% NaCl. Acid is produced weakly and without gas from the following carbohydrates in the API 50 CH gallery using the CHB suspension medium supplemented with 7% NaCl: cellobiose, D-fructose, gentiobiose, D-glucose, 5-keto-D-gluconate, maltose, D-mannose, N-acetylglucosamine, ribose, salicin, sucrose and trehalose. Esculin hydrolysis is positive with or without added NaCl. Results are variable amongst strains for weak acid production from arbutin, galactose, glycerol, lactose and D-mannitol; type strain is positive for arbutin, glycerol and D-mannitol. The major fatty acid is C15:0 anteiso, present at about 68%; C17:0 anteiso accounts for about 11% of the total.
Source: mural paintings, discolored by microbial growths.
DNA G + C content (mol%) of the type strain: 39.8 (HPLC).
Type strain : LMG 19507, DSM 14890.
EMBL/GenBank accession number (16S rRNA gene): AJ315075 (LMG 19507).
-
Bacillus drentensis Heyrman, Vanparys, Logan, Balcaen, Rodríguez-Díaz, Felske and De Vos 2004, 56VP
dren.ten'sis. N.L. adj. drentensis of Drente, a province in The Netherlands.
Facultatively anaerobic, Gram-positive or Gram-variable, motile, tapered rods, 1.5–3.5 µm in diameter, occurring singly and in pairs. Cells show pleomorphism (narrow and broad cells, the latter showing swellings) and produce intracellular storage products (possibly PHB) on TSA. Endospores are spherical or ellipsoidal and lie in paracentral or occasionally subterminal positions in swollen sporangia. Colonies are slightly convex with regular margins when small, and sometimes wrinkled with irregular margins and prominent centers when larger. Colonies are cream-colored and produce a brownish soluble pigment; consistency is butyrous, with an eggshell-like surface texture. The optimum temperature for growth is 30°C, and the maximum growth temperature lies between 50°C and 55°C. The optimum pH for growth is 7.0–8.0, and growth occurs from pH 5.5–6.0 to 9.5–10.0. Casein is not hydrolyzed. In the API 20E strip, o-nitrophenyl-β-D-galactopyranoside hydrolysis is positive, Voges–Proskauer reaction is variable (most strains negative, positive strains weak), and nitrate reduction is variable; reactions for arginine dihydrolase, lysine decarboxylase, ornithine decarboxylase, citrate utilization, hydrogen sulfide production, urease, tryptophan deaminase, indole production and gelatin hydrolysis are negative. Hydrolysis of esculin is positive. Acid without gas is produced from the following carbohydrates in the API 50 CH gallery using the CHB suspension medium: N-acetyl-D-glucosamine, D-fructose, D-glucose (some strains, including the type strain, weak), lactose, maltose, D-melibiose and salicin (some strains, including the type strain, weak). The following reactions are variable between strains and, when positive, are usually weak: amygdalin, arbutin, galactose, gluconate, inulin, D-mannose, D-melezitose, α-methyl-D-glucoside, raffinose, ribose, starch, sucrose, D-trehalose, D-turanose and D-xylose; type strain is positive for inulin, D-mannose, D-melezitose, sucrose and weak for raffinose, ribose, starch and D-turanose. The major cellular fatty acids are C15:0 iso and C15:0 anteiso, present at a level of about 32 and 22%, respectively, while C16:1 ω11c accounts for about 13% of the total fatty acids.
Source: soil in the Drentse an agricultural research area, The Netherlands.
DNA G + C content (mol%): 39.3–39.4 (HPLC) and 39.4 for the type strain.
Type strain : LMG 21831, DSM 15600.
EMBL/GenBank accession number (16S rRNA gene): AJ542506 (LMG 21831).
-
Bacillus endophyticus Reva, Smirnov, Pettersson and Priest 2002, 106VP
en.do.phy'ti.cus. Gr. endo within; Gr. n. phyton plant.; L. masc. suff. -icus adjectival suffix used with the sense of belonging to; N.L. adj. endophyticus within plant; originally isolated from plant tissues.
Strictly aerobic, Gram-positive, nonmotile rods, forming ellipsoidal spores which lie subterminally or terminally in unswollen sporangia. Cells 0.5–1.5 by 2.5–3.5 µm, occurring singly and in short or long chains, the latter appearing filamentous. Vacuoles are formed in the cytoplasm of cells grown on media containing 2% glucose. Colonies are circular, 1–3 mm in diameter, with entire or slightly indented margins, and may be slimy or rough; they are usually white, but pink and red pigmentation is occasionally seen. Growth temperature range 10–45°C; optimum about 28°C. Grows in presence of 10% NaCl. Nitrate is not reduced to nitrite. Catalase- and oxidase-positive. Casein, gelatin, starch and urea are not hydrolyzed. Acid without gas produced from D-glucose and a range of other carbohydrates. Citrate and gluconate are utilized; acetate, propionate and tartrate are not.
Source: inner tissues of healthy cotton plants.
DNA G + C content (mol%): not reported.
Type strain : 2DT, ATCC 29604, NRRL NRS-1705, LMG 7124, NCIMB 11326, CIP 106778, JCM 9331.
EMBL/GenBank accession number (16S rRNA gene): AF295302 (2DT).
-
Bacillus farraginis Scheldeman, Rodríguez-Díaz, Goris, Pil, De Clerck, Herman, De Vos, Logan and Heyndrickx 2004, 1362VP
far.ra.gin'is. L. gen. fem. n. farraginis from mixed fodder for cattle, referring to feed concentrate for dairy cattle as the principal isolation source.
Cells are long, straight, round-ended, motile, strictly aerobic, Gram-negative rods, occurring singly, in pairs or filaments. Cell diameter is 0.5–0.8 µm and cell length 1.2–4 µm. Spores are ellipsoidal and occur paracentrally or subterminally in occasionally slightly swollen sporangia. Colonies grown for 3 d at 30°C on nutrient agar are cream-colored or translucent, slightly raised, with irregular margins and granular, glossy surfaces. Colony diameter is no greater than 1 mm. Good growth occurs at 30 and 45°C and weak growth occurs at 20°C. Some strains are capable of growth at pH 9 but none grows at pH 5. Growth is not inhibited by 7% (w/v) NaCl. Hydrolysis of starch and casein is not observed within 7 d of incubation at 30°C, and growth in casein agar is poor or negative. Catalase- and oxidase are positive. All strains are unreactive in the API 20E and API 50CHB test kits. In the Biotype100 kit using the Biotype 2 medium, nearly all strains (>83%) belonging to the species are able to use the following substrates as sole carbon sources: 4-aminobutyrate, 5-aminovalerate, D- and L-alanine, fumarate, L-glutamate, glutarate, L-histidine, 3-hydroxybutyrate, 2-oxoglutarate, D- and L-malate, DL-lactate, L-proline, putrescine, succinate, L-tryptophan and L-tyrosine. Many strains (>45%) are able to use L-aspartate, dulcitol, m-hydroxybenzoate, malonate, D-mannitol, D-ribose and D-sorbitol. Other substrates are used less frequently (17–44%): L-arabinose, D-galactose, gentisate, D-glucuronate, p-hydroxybenzoate, myo-inositol and α-L-rhamnose.
The type strain utilizes the following substrates as sole carbon sources: 4-aminobutyrate, 5-aminovalerate, D- and L-alanine, L-aspartate, dulcitol, fumarate, D-glucosamine, L-glutamate, glutarate, histamine, L-histidine, m-hydroxybenzoate, 3-hydroxybutyrate, 2-oxoglutarate, D- and L-malate, malonate, DL-lactate, L-proline, putrescine, D-ribose, D-sorbitol, succinate, meso-tartrate, L-tryptophan and L-tyrosine. The major cellular fatty acids (>5% of total cellular fatty acids) are C15:0 iso, C15:0 anteiso, C17:0 anteiso, C16:0 iso and C16:1 ω7c alcohol.
Source: cattle feed concentrate, milking clusters, hay, silage, grass, lucerne and green fodder.
DNA G + C content (mol%): 43.7 (HPLC).
Type strain : R-6540, MB 1885, LMG 22081, DSM 16013.
GenBank/EMBL accession number (16S rRNA gene): AY443034 (R-6540).
-
Bacillus fastidiosus den Dooren de Jong 1929, 344AL
fas.tid'i.os.us. L. adj. fastidiosus disdainful, fastidious.
Strictly aerobic rods about 1.3 µm in diameter, forming ellipsoidal spores which usually lie centrally, paracentrally and subterminally, occasionally terminally, in unswollen sporangia. Colonies on 1% uric acid agar become opaque and are usually unpigmented but may become yellowish; margins are often ragged and have hair-like outgrowths, or the colonies may be rhizoid. Colonies are surrounded by zones of clearing and the reaction becomes strongly alkaline. Growth occurs at 10°C and 40°C, but not at 5°C or 50°C. Does not grow at pH 6.8 or below. Grows in presence of 5% NaCl. Grows on allantoic acid, allantoin or uric acid as sole carbon, nitrogen and energy sources. Some strains will grow on certain peptones, especially at high concentrations. Growth factors not required, Nitrate is not reduced to nitrite. Catalase- and oxidase-positive. Urea is hydrolyzed; casein, gelatin and starch are not hydrolyzed. Acid and gas are not produced from D-glucose and other carbohydrates; there is no growth in the media used to test these characters. Citrate and propionate are not utilized.
Source: soil and poultry litter.
DNA G + C content (mol%): 34.3–35.1 (T m) for 17 strains, and 35.1 (T m), 35.1 (Bd) for the type strain.
Type strain : Delft LMD 29–14, ATCC 29604, DSM 91, LMG 7124, NCIMB 11326, NRRL NRS-1705, KCTC 3393.
EMBL/GenBank accession number (16S rRNA gene): X60615 (DSM 91).
-
Bacillus firmus Bredemann and Werner in Werner 1933, 446AL
fir'mus. L. adj. firmus strong, firm.
This species has for long been genetically heterogeneous, and many strains have been incorrectly assigned to it. Strains received as Bacillus firmus show phenotypic profiles that appear to overlap with those of strains assigned to Bacillus lentus, so that Gordon et al. (1977) raised the question of whether Bacillus firmus–Bacillus lentus represented a single species or a series of strains.
The description which follows is based upon the type strain and 17 other strains which have been shown by amplified rDNA restriction analysis, polyacrylamide gel electrophoresis of whole-cell proteins, and various phenotypic characters (De Vos, Logan and colleagues, unpublished data) to be closely related to the type strain. Phylogenetic studies indicate that Bacillus circulans, Bacillus firmus and Bacillus lentus are related.
Facultatively anaerobic, straight, round-ended, motile rods, 0.8–0.9 µm in diameter, that occur singly, in pairs, or occasionally as short chains. Endospores are ellipsoidal or cylindrical, lie subterminally, paracentrally or centrally, and may swell the sporangia slightly. Colonies grown for 3 d on TSA at 30°C are 1–12 mm in diameter, creamy-yellow to pale orangey-brown in color, are of butyrous consistency, have margins that vary from entire to finely rhizoidal and surface appearances that are egg-shell to glossy, sometimes with granular or zoned areas in center. Maximum growth temperature is 40–50°C, the optimum temperature lies between 30°C and 40°C, and growth occurs at 20°C. The optimum pH for growth is 7.0–9.0; the minimum is 6.0–7.0, and the maximum lies between 11 and 11.5. Grows in presence of 7% NaCl. Catalase-positive. Casein is weakly hydrolyzed and a pale to dark honey-brown diffusible pigment is produced on it. Starch is hydrolyzed, but strength of reaction varies among strains. Citrate and propionate are not utilized. In the API 20E strip, gelatin is partially or completely hydrolyzed by most strains and nitrates are totally or partially reduced. o-nitrophenyl-β-D-galactopyranoside is not hydrolyzed, arginine dihydrolase, lysine decarboxylase and ornithine decarboxylase are not produced, citrate is not utilized, hydrogen sulfide, urease, tryptophan deaminase and indole are not produced and Voges–Proskauer reaction is negative. In the API 50CH gallery using the CHB suspension medium, hydrolysis of esculin is variable and acid without gas is positive or weakly positive from the following carbohydrates: D-glucose, maltose, mannitol, starch and sucrose. In the API Biotype 100 kit the following substrates are utilized as sole carbon sources: D- and L-alanine, D-gluconate, D-glucosamine, α-D-glucose, L-glutamate, glycerol, 2-oxoglutarate, DL-lactate, L-malate, maltose, maltotriose, D-mannitol, N-acetyl-D-glucosamine, L-proline, sucrose, L-serine, succinate and D-trehalose. The type strain and some other strains are positive or weak for: glycerol, N-acetylglucosamine and D-trehalose.
According to their patterns of acid production from other carbohydrates, and use of other substrates as sole carbon sources, 2 bioypes may be recognized, with Biovar 1 containing the type strain; Biovar 2 strains may represent a distinct species and are distinct from Biovar 1 strains in their slightly stronger acid production from the above-mentioned carbohydrates and their acid production from: D-fructose, glycogen and, although variable among Biovar 2 strains, D-xylose. Only Biovar 2 strains are able to utilize: cis-aconitate, citrate, β-D-fructose, DL-glycerate, 2-keto-D-gluconate, maltitol, 3-methyl-D-glucopyranose, methyl α-D-glucopyranoside, tricarballylate, trigonelline, L-tryptophan and D-xylose. In assimilation tests giving variable results, the type strain is positive for: L-histidine and 3-hydroxybutyrate.
Source: soil and other environments.
DNA G + C content (mol%): 41.4 (T m), 40.7 (Bd).
Type strain : ATCC 14575, DSM 12, JCM 2512 (D78314), LMG 7125, NCIMB 9366 NRRL B-14307, IAM 12464.
EMBL/GenBank accession number (16S rRNA gene): D16268 (IAM 12464).
-
Bacillus flexus (ex Batchelor 1919) Priest, Goodfellow and Todd 1989, 93VP (Effective publication: Priest, Goodfellow and Todd 1988, 1878.)
fle'xus. L. adj. flexus flexible.
Strictly aerobic, Gram-variable rods, forming ellipsoidal spores which lie centrally or paracentrally in unswollen sporangia. Description is based upon two strains. Mean cell width 0.9 µm. Colonies are opaque and smooth. Growth occurs at 17–37°C, but not at 5°C or 50°C. Grows between pH 4.5 and 9.5. Grows in presence of 10% NaCl. Nitrate is not reduced to nitrite. Oxidase-positive. Casein, elastin, gelatin, pullulan, starch and urea are hydrolyzed; esculin is not. Acid without gas is produced from D-glucose and a range of other carbohydrates; acid is not produced from pentoses. Acetate, citrate, formate and succinate are utilized; gluconate, lactate and malonate are not.
Source: feces and soil.
DNA G + C content (mol%): 35 and 36 (T m) for two strains.
Type strain : ATCC 49095, DSM 1320, LMG 11155, NCIMB 13366, NRRL NRS-665, IFO 15715.
EMBL/GenBank accession number (16S rRNA gene): AB021185 (IFO 15715).
-
Bacillus fordii Scheldeman, Rodríguez-Díaz, Goris, Pil, De Clerck, Herman, De Vos, Logan and Heyndrickx 2004, 1363VP
for'di.i. N.L. gen. n. fordii named after W. W. Ford, an American microbiologist working on aerobic spore-forming bacteria at the beginning of the twentieth century.
Cells are long, straight, round-ended, motile, strictly aerobic, Gram-negative rods, occurring singly or in pairs. Cell diameter is 0.6–0.8 µm and length 1.6–3.5 µm. Spores are ellipsoidal and occur paracentrally or subterminally in, occasionally, slightly swollen sporangia. Colonies grown on nutrient agar at 30°C for 3 d are cream-colored, raised, with entire margins and smooth glossy surfaces. Their maximum diameter is 2 mm. Good growth occurs at 30 and 45°C and weak growth occurs at 20°C. Growth occurs at pH 9 and some strains grow at pH 5. Growth is not inhibited by 7% (w/v) NaCl. Hydrolysis of starch and casein is not observed within 7 d of incubation at 30°C. Growth on casein agar is colored faint pink. Catalase and oxidase are positive. All strains are unreactive in the API 20E and API 50CHB test kits. In the Biotype100 kit using the Biotype 2 medium, all strains show very good production of biomass using malonate and L-tyrosine as sole carbon sources. Variable results with good production of biomass are obtained for L-histidine, 2-oxoglutarate, glutarate, DL-lactate, 5-aminovalerate and L-tryptophan. All or most strains are capable of weak growth from the following carbon sources: esculin, gentisate, protocatechuate, meso-tartrate, D-glucosamine, DL-glycerate, quinate, ethanolamine, D-glucuronate, p-hydroxybenzoate, hydroxyquinoline β-glucuronide and 2- and 5-keto-D-gluconate. Growth occurs seldom and weakly from the following substrates: adonitol, L-alanine, L-arabinose, L-arabitol, benzoate, fumarate, D-galacturonate, D-gluconate, α-D-glucose, histamine, 3-hydroxybutyrate, D-lyxose, D-malate, methyl α-galactopyranoside, methyl β-D-glucopyranoside, N-acetyl d-glucosamine, phenylacetate, propionate, putrescine, D-raffinose, D-ribose, D-saccharate, L-sorbose, succinate, D-tagatose, L-tartrate, tricarballylate, trigonelline and D-xylose.
Where results are variable, the type strain uses the following substrates: esculin, 5-aminovalerate, L-arabinose, benzoate, ethanolamine, D-galacturonate, gentisate, D-glucuronate, glutarate, DL-glycerate, histamine, p-hydroxybenzoate, 3-hydroxybutyrate, hydroxyquinoline-β-glucuronide, 2- and 5-keto-D-gluconate, 2-oxoglutarate, DL-lactate, D-lyxose, N-acetyl D-glucosamine, phenylacetate, protocatechuate, putrescine, quinate, D-raffinose, meso-tartrate, tricarballylate, L-tryptophan and D-xylose. The major cellular fatty acids (>5% of total cellular fatty acids) are C15:0 iso, C15:0 anteiso, C17:0 anteiso, C16:1 ω11c and C17:0 iso.
Source: cattle feed concentrate, milking clusters, filter cloths, and raw milk.
DNA G + C content (mol%): 41.9 (HPLC).
Type strain : R-7190, MB 1878, LMG 22080, DSM 16014.
GenBank/EMBL accession number (16S rRNA gene): AY443034 (R-7190).
-
Bacillus fortis Scheldeman, Rodríguez-Díaz, Goris, Pil, De Clerck, Herman, De Vos, Logan and Heyndrickx 2004, 1362VP
for'tis. L. adj. fortis strong, referring to the fact that the strains were isolated after heat treatment for 30 min at 100°C.
Cells are straight, round-ended, motile, strictly aerobic, Gram-negative rods, occurring singly or in pairs. Cell diameter is 0.6–0.8 µm and length 1.0–3.5 µm. Spores are oval and occur centrally or paracentrally in slightly swollen sporangia. Colonies grown on nutrient agar at 30°C for 3 d are cream-colored, raised with entire margins and smooth, glossy surfaces. Their maximum diameter is 1 mm. Good growth occurs at 30 and 45°C and weak growth occurs at 20°C. Growth does not occur at pH 9 or 5. Growth is not inhibited by 7% (w/v) NaCl. Hydrolysis of starch and casein is not observed within 7 d of incubation at 30°C, and growth on casein agar is poor or negative. Catalase and oxidase are positive. All strains are unreactive in the API 20E and API 50CHB test kits. In the Biotype100 kit using the Biotype 2 medium, strains use L-tryptophan and L-histidine as sole carbon sources. Most strains (>50%) use the following substrates as sole carbon sources: 4-aminobutyrate, 5-aminovalerate, esculin, ethanolamine, glutarate, hydroxyquinoline β-glucuronide, 2-oxoglutarate, DL-lactate, malonate, phenylacetate, L-proline, putrescine, D-ribose and L-tyrosine. Some strains (<50%) use the following substrates: citrate, erythritol, D-gluconate, D-glucosamine, α-D-glucose, L-glutamate, DL-glycerate, histamine, m-hydroxybenzoate, 2- and 5-keto-D-gluconate, L- and D-malate, α-D-melibiose, protocatechuate, L-sorbose and L-tartrate. Where results are variable, the type strain uses the following substrates: esculin, D-gluconate, D-glucosamine, D-glucuronate, DL-glycerate, hydroxyquinoline-β-glucuronide, 2-oxoglutarate, 2- and 5-keto-D-gluconate, DL-lactate, malonate, phenylacetate, protocatechuate, L-tartrate and L-tyrosine. The major cellular fatty acids (>5% of total cellular fatty acids) are C15:0 iso, C15:0 anteiso, C16:0 iso, C15:0 and C17:0 anteiso.
Source: cattle feed concentrate, milking clusters, soy, and raw milk.
DNA G + C content (mol%): 44.3 (HPLC).
Type strain : R-6514, LMG 22079, DSM 16012.
GenBank/EMBL accession number (16S rRNA gene): AY443034 (R-6514).
-
Bacillus fumarioli Logan, Lebbe, Hoste, Goris, Forsyth, Heyndrickx, Murray, Syme, Wynn-Williams and De Vos 2000, 1751VP
fum.a.rio'li. nemt. L. gen. n. fumariolum a smoke hole; L. gen. n. fumarioli of a smoke hole, whence fumarole, a hole emitting gases in a volcanic area.
Moderately thermoacidophilic and strictly aerobic, feebly motile, Gram-positive organisms growing and sporulating best at pH 5.5 and 50°C on nutrient-weak media such as BFA (Logan et al., 2000) and BFA at half nutrient-strength, but also growing and sporulating weakly on trypticase soy agar containing 5 mg/l MnSO4. Colonies 3–10 mm in diameter, low convex, circular and slightly irregular, glossy, creamy-brown, and butyrous. Spores ellipsoidal to cylindrical, lying paracentrally and subterminally, and not swelling the sporangia. Temperature limits for growth: 25–30°C and 55°C; optimum temperature is about 50°C. Limits of pH for growth: 4–5 and 6–6.5. Catalase-positive. Nitrate is not reduced. Gelatin is hydrolyzed, but esculin and casein are not. Acid without gas produced from D-fructose, D-glucose, mannitol, D-mannose, N-acetylglucosamine (weak), sucrose, D-trehalose (weak). Acid production from galactose, glycerol, lactose, maltose, D-melibiose, D-melezitose, methyl-α-D-glucoside, D-raffinose, ribose and D-turanose varies between strains. See Table 3.
Source: geothermal soils and active and inactive fumaroles in continental and maritime Antarctica, and from gelatin production plants in Belgium, France, and the USA.
DNA G + C content (mol%): 40.7% (T m).
Type strain : Logan B1801, LMG 19448 (replaces LMG 17489), NCIMB 13771, KCTC 3851.
EMBL/GenBank accession number (16S rRNA gene): AJ250056 (LMG 17489).
-
Bacillus funiculus Ajithkumar, Ajithkumar, Iriye and Sakai 2002, 1143VP
fu.ni'cu.lus. L. masc. n. funiculus string, rope; referring to the filamentous appearance of the cells.
Aerobic, Gram-variable, motile rods 0.8–2.0 by 4.0–6.0 µm, which form filamentous trichomes by cellular binding. Description is based upon a single isolate. Ellipsoidal spores lie centrally in unswollen sporangia. On prolonged incubation heat-resistant, spore-like resting cells, which outgrow by budding, are formed. Colonies are round, opaque, and off-white to colorless. Growth temperature range 20–40°C, optimum about 30°C. The pH range for growth is 5.0–9.0 with optimum at 7.0–8.0. Catalase-positive, oxidase-negative. Voges–Proskauer reaction is positive. Citrate utilization negative. Nitrate is reduced to nitrite. Esculin, starch and urea are hydrolyzed; casein, gelatin and Tween 80 are not hydrolyzed. Acid without gas is produced from glucose. Glucose and a range of other carbohydrates are utilized as sole carbon sources.
Source: activated sewage sludge.
DNA G + C content (mol%): 37.2 (HPLC).
Type strain : NAF001, DSM 15141, JCM 11201, CIP 107128, KCTC 3796.
EMBL/GenBank accession number (16S rRNA gene): AB049195 (NAF001).
-
Bacillus fusiformis (ex Smith, Gordon and Clark 1946) comb. nov. Bacillus sphaericus var. fusiformis Smith, Gordon and Clark 1946), Priest, Goodfellow and Todd 1989, 93VP (Effective publication: Priest, Goodfellow and Todd 1988, 1878.)
fus.i.form'is. L. n. fusus spindle; L. suff. -formis of the shape of; N.L. adj. fusiformis spindle-shaped.
Strictly aerobic, Gram-variable rods, forming spherical spores which lie centrally or terminally in swollen sporangia. Mean cell width ≤0.9 µm. Colonies are opaque and smooth. Growth occurs at 17–37°C, but not at 5°C or 50°C. Grows between pH 6.0 and 9.5. Growth in presence of 2–5% NaCl varies. Nitrate is not reduced to nitrite. Casein and gelatin are hydrolyzed; urea hydrolysis varies; esculin is not hydrolyzed. Acid and gas are not produced from D-glucose or other carbohydrates. Acetate, citrate, formate, lactate and succinate are utilized; gluconate and malonate utilization varies between strains.
Phenotypically similar to Bacillus sphaericus, and according to Priest et al. (1988) distinguishable from that organism by urease positivity, ability to grow in presence of 7% NaCl, and sensitivity to 1 µg/ml tetracycline; however, the data reported in that study indicated that only three of the four strains assigned to this species were urease-positive, and only one strain could grow at 2 or 5% NaCl. In a study of 12 strains belonging to the Bacillus fusiformis DNA homology group (Krych et al., 1980), all strains could grow in 7% NaCl and only one strain failed to degrade urea, but members of other Bacillus sphaericus homology groups were also positive for these characters. See Table 9.
Table 9. Differentiation of spherical-spored Bacillus speciesa, b 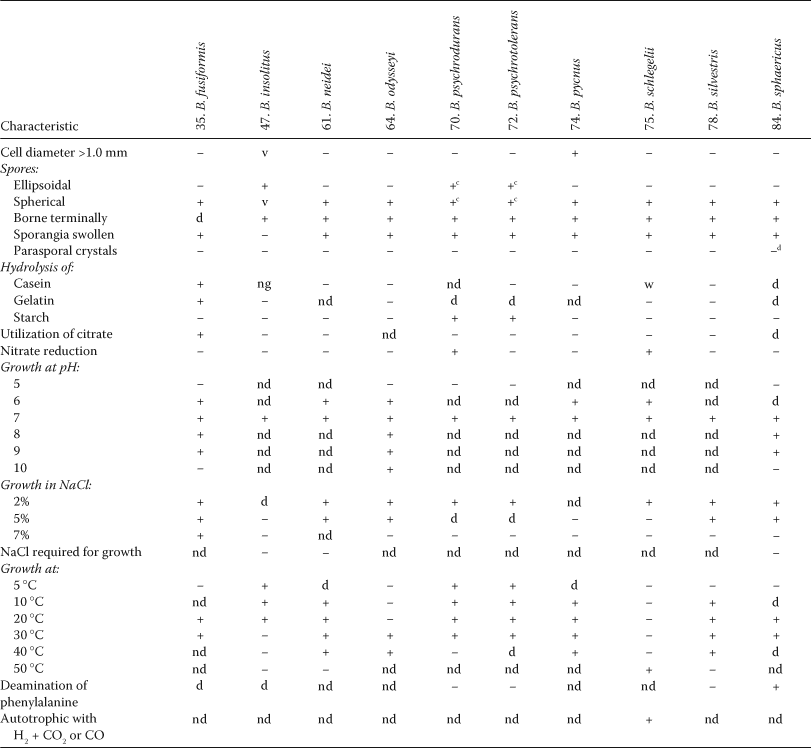
- a Symbols: +, >85% positive; d, different strains give different reactions (16–84% positive); −, 0–15% positive; d/w, different strains give different reactions, but positive reactions are weak; v, variation within strains; w, weak reaction; ng, no growth in test medium; nd, no data are available.
- b Compiled from Larkin and Stokes (1967); Schenk and Aragno (1979); Logan and Berkeley (1984); Claus and Berkeley (1986); Priest et al. (1988); Fritze (1996a); Rheims et al. (1999); Abd El-Rahman et al. (2002); Nakamura et al. (2002); Priest (2002); La Duc et al. (2004).
- c Spores of Bacillus psychrodurans and Bacillus psychrotolerans are rarely formed; on casein-peptone soymeal-peptone agar spores are predominantly spherical, but on marine agar they are predominantly ellipsoidal.
- d Mosquitocidal strains of Bacillus sphaericus produce parasporal toxin crystals which are smaller than those produced by Bacillus thuringiensis, but which are nonetheless visible by phase-contrast microscopy.
Ahmed et al. (2007c) proposed the transfer of this species to the new genus Lysinibacillus.
Source: soil.
DNA G + C content (mol%): 35–36 (T m).
Type strain : ATCC 7055, DSM 2898, LMG 9816, NRRL NRS-350, IFO 15717.
EMBL/GenBank accession number (16S rRNA gene): AJ310083 (DSM 2898).
-
Bacillus galactosidilyticus Heyndrickx, Logan, Lebbe, Rodríguez-Díaz, Forsyth, Goris, Scheldeman and De Vos 2004,*** 619VP
ga.lac.to.si.di.ly'ti.cus N.L. neut. n. galactosidum galactoside, N.L. adj. lyticus lysing, dissolving; N.L. adj. galactosidilyticus referring to positive ONPG test revealing β-galactosidase activity.
Facultatively anaerobic, Gram-positive or Gram-variable, small, plump, round-ended rods 0.7–0.9 µm by 2–5 µm, with tumbling motility, occurring singly and in pairs, and occasionally in short chains. Ellipsoidal endospores are borne in central, paracentral and subterminal positions within slightly swollen sporangia. After 2 d on TSA, the creamy or off-white colonies have opaque centers and are approximately 1 mm in diameter, smooth, flat and butyrous; the margins are usually irregular with pointed projections that may spread and become rhizoid in older cultures. Catalase-positive. Growth occurs at 30 and 40°C but not at 50°C. Alkalitolerant; growth occurs between pH 6 and 10.5, but not at pH 5 or below. Casein hydrolysis is very weak. In the API 20E strip, o-nitrophenyl-β-D-galactopyranoside is hydrolyzed, nitrate is reduced to nitrite, urease production is variable (type strain is negative), arginine dihydrolase, lysine decarboxylase and ornithine decarboxylase are negative, citrate is not utilized, hydrogen sulfide is not produced, the Voges–Proskauer reaction is negative, indole is not produced, and gelatin is not hydrolyzed. Hydrolysis of esculin is positive. In the API 50 CHB gallery, acid without gas is produced, often weakly, from N-acetylglucosamine, D-fructose and D-glucose. Acid production from the following carbohydrates is variable, and when positive is usually very weak: amygdalin, L-arabinose, arbutin, D-cellobiose, galactose, gentiobiose, inulin, lactose, maltose, mannitol, D-mannose, D-melezitose, D-melibiose, methyl-D-glucoside, D-raffinose, rhamnose, ribose, salicin, starch, sucrose, D-trehalose, D-turanose and D-xylose; the type strain is positive for arbutin, D-cellobiose, D-melibiose, D-melezitose, D-raffinose, starch, sucrose and D-trehalose. The major cellular fatty acids are: C15:0 anteiso (33% of total), C16:0 (27%), C15:0 iso (13%) and C14:0 (8%).
Source: raw milk, partially decomposed wheat grain and infant bile.
DNA G + C content (mol%): 35.7–38.2 (HPLC), and for the type strain is 37.7.
Type strain : LMG 17892, DSM 15595.
EMBL/GenBank accession number (16S rRNA gene): AJ535638 (LMG 17892).
-
Bacillus gelatini De Clerck, Rodríguez-Díaz, Vanhoutte, Heyrman, Logan and De Vos 2004c, 944VP
ge.la.ti'ni. N.L. gen. neut. n. gelatini from gelatin.
Strictly aerobic, Gram-variable, feebly motile, round-ended, straight rods, 0.5–0.9 µm by 4–10 µm, which form long chains and occasionally appear singly. Endospores are oval, lie paracentrally and subterminally, and do not swell the sporangia. Colonies on TSA incubated at 30°C for 4 d are smooth, cream-colored but darker in the center, have slightly irregular borders, and are waxy in appearance, with eggshell-textured surfaces. Colonies are slightly convex, but older colonies are flatter with concave, transparent centers, and diameters range from 1 to 4 mm. The maximum temperature for growth lies between 58°C and 60°C and the optimum temperature lies between 40°C and 50°C. Good growth occurs at pH 5–8; the minimum pH for growth is 4–5 and the maximum is 9–10. Good growth occurs in nutrient broth with 15% NaCl added. Catalase-positive, oxidase-negative. Casein is hydrolyzed. In the API 20E strip, hydrolysis of gelatin is positive. All the strains are negative for o-nitrophenyl-β-D-galactopyranoside hydrolysis, arginine dihydrolase, lysine decarboxylase and ornithine decarboxylase reactions, citrate utilization, hydrogen sulfide production, urease, tryptophan deaminase, indole production, Voges–Proskauer reaction, and nitrate reduction. In the API 50CH gallery using the CHB suspension medium, hydrolysis of esculin is positive, and acid without gas is produced, often weakly, from the following carbohydrates: D-fructose, D-glucose, glycerol, mannitol, D-mannose, D-trehalose and D-xylose. Most strains show a very weak production of acid from N-acetylglucosamine, maltose, and ribose. The more reactive strains may also produce acid from D-cellobiose, D-galactose, 5-keto-D-gluconate, D-melezitose, meso-inositol, methyl D-glucoside and D-turanose. The major cellular fatty acids are C15:0 iso, C17:0 iso and C17:0 anteiso (respectively representing about 60, 13 and 10% of total fatty acid). The following fatty acids are present in smaller amounts: C15:0 anteiso, C16:0 iso and C16:0 (respectively representing about 9, 4 and 2% of total fatty acid).
Source: gelatin production plants.
DNA G + C content (mol%): 41.5% (HPLC).
Type strain : LMG 21880, DSM 15865.
EMBL/GenBank accession number (16S rRNA gene): AJ551329 (LMG 21880).
-
Bacillus gibsonii Nielsen, Fritze and Priest 1995b, 879VP (Effective publication: Nielsen, Fritze and Priest 1995a, 1759.)
gib.so'ni.i. N.L. gen. gibsonii of Gibson, named after the British bacteriologist Thomas Gibson.
Alkalitolerant organisms forming ellipsoidal spores which lie subterminally and, in ageing cultures paracentrally and occasionally laterally, in unswollen sporangia. Cells 0.6–1.0 by 2.0–3.0 µm. Colonies are, yellow, smooth, shiny and circular. Growth temperature range 10–30–37°C. Optimal growth at pH 8.0; growth occurs at pH 7.0. Grows in presence of up to 9–12% NaCl. Nitrate reduction varies between strains. Casein, and gelatin are hydrolyzed. Hippurate, pullulan, starch and Tween 20 are not hydrolyzed; phenylalanine is not deaminated. Glucose and a range of other carbohydrates can be utilized as sole sources of carbon.
Source: soil.
DNA G + C content (mol%): 40.6–41.7 (HPLC analysis).
Type strain : Nielsen PN-109, ATCC 700164, DSM 8722, LMG 17949.
EMBL/GenBank accession number (16S rRNA gene): X76446 (DSM 8722).
-
Bacillus halmapalus Nielsen, Fritze and Priest 1995b, 879VP (Effective publication: Nielsen, Fritze and Priest 1995a, 1759.)
hal.ma'pa.lus. Gr. n. halme brine; Gr adj. hapalos delicate; N.L. adj. halmapalus sensitive to brine.
Alkalitolerant organisms forming ellipsoidal spores which lie paracentrally to subterminally in unswollen sporangia. Description is based upon two isolates. Cells 0.6–1.0 by 3.0–4.0 µm. Colonies are small, circular, shiny and creamy-white with entire margins. Growth temperature range 10–40°C. Optimal growth at pH 8.0; growth occurs at pH 7.0. No growth in presence of 5% NaCl. Nitrate is not reduced. Casein, gelatin, hippurate, pullulan and starch are hydrolyzed. Tween 20, 40, 60 and 80 are not hydrolyzed; phenylalanine is not deaminated. Glucose and a narrow range of other carbohydrates can be utilized as sole sources of carbon. It is distinguished from Bacillus horikoshii by its larger cell size, lower salt tolerance, and DNA relatedness.
Source: soil.
DNA G + C content (mol%): 38.6 (HPLC analysis).
Type strain : Nielsen PN-118, ATCC 700165, DSM 8723, LMG 17950.
EMBL/GenBank accession number (16S rRNA gene): X76447 (DSM 8723).
-
Bacillus halodurans Nielsen, Fritze and Priest 1995b, 879VP (Effective publication: Nielsen, Fritze and Priest 1995a, 1759.)
ha.lo.du'rans. Gr. n. hals salt; L. pres. part. durans enduring; N.L. adj. halodurans salt-enduring.
Alkaliphilic and moderately halotolerant organisms forming ellipsoidal spores which lie subterminally in slightly swollen sporangia. Cells 0.5–0.6 by 3.0–4.0 µm, and occur in long chains even when sporulated. Colonies are white and circular with slightly filamentous margins. Growth temperature range 15–55°C. Optimal growth at pH 9–10; most strains grow at pH 7.0. Growth in presence of up to 12% NaCl. Most strains do not reduce nitrate. Casein, gelatin pullulan, starch, and Tween 40 and 60 are hydrolyzed, but most strains do not hydrolyze Tween 20, and Tween 80 is not hydrolyzed. Most strains do not hydrolyze hippurate. Phenylalanine is not deaminated. Glucose and a wide range of other carbohydrates can be utilized as sole sources of carbon. The type strain was previously named “Bacillus alcalophilus subsp. halodurans” (Boyer et al., 1973). Strains in this species were formerly assigned to Bacillus lentus type III by Gordon and Hyde (1982). See Table 2.
Source: soil.
DNA G + C content (mol%) ranges from 42.1–43.9 (HPLC).
Type strain : PN-80, ATCC 27557, DSM 497, LMG 7121, NRRL B-3881.
EMBL/GenBank accession number (16S rRNA gene): AJ302709 (DSM 497).
-
Bacillus halophilus Ventosa, García, Kamekura, Onishi and Ruiz-Berraquero 1990a, 105VP (Effective publication: Ventosa, García, Kamekura, Onishi and Ruiz-Berraquero 1989, 164.)
hal.o.phi'lus. Gr. n. hals salt; Gr. adj. philos loving: N.L. adj. halophilus salt-loving.
Halophilic, strictly aerobic, Gram-positive, motile rods, 0.5–1.0 by 2.5–9.0 µm, occurring singly and in pairs or chains, and forming ellipsoidal spores which lie centrally in unswollen sporangia. Description is based upon a single isolate. Colonies on 15% NaCl medium are circular, smooth, entire, opaque and unpigmented. Grows at between 3% and 30% total salts with optimal growth at about 15% salts. Growth occurs between 15°C and 50°C, and is optimal at 37°C. The pH range for growth is 6.0–8.0, with the optimum at 7.0. Catalase- and oxidase-positive. Chemo-organotroph. Acid is produced without gas from glucose and a range of other carbohydrates. Nitrate is not reduced. Esculin, DNA and urea are hydrolyzed; casein, gelatin, starch and tyrosine are not. Negative for arginine dihydrolase, lysine and ornithine decarboxylases, Voges–Proskauer, and indole. Utilizes a range of amino acids, carbohydrates and organic acids as sole carbon and energy sources, and utilizes a small range of amino acids as sole carbon, nitrogen and energy sources.
Source: rotting wood on seashore.
DNA G + C content (mol%): 51.5 (T m).
Type strain : Kamekura N23–2, ATCC 49085, DSM 4771, LMG 17942, KCTC 3566.
EMBL/GenBank accession number (16S rRNA gene): AB021188 (DSM 4771).
-
Bacillus horikoshii Nielsen, Fritze and Priest 1995b, 879VP (Effective publication: Nielsen, Fritze and Priest 1995a, 1760.)
ho.ri.ko'shi.i. N.L. gen. n. horikoshii of Horikoshi; named after the Japanese microbiologist Koki Horikoshi.
Alkalitolerant organisms forming ellipsoidal spores which lie subterminally in sporangia which may be slightly swollen. Cells 0.6–0.7 by 2.0–4.0 µm. Colonies are small, circular, shiny and creamy-white with entire margins. Growth temperature range 10–40°C. Optimal growth at pH 8.0; growth occurs at pH 7.0. 8–9% NaCl is tolerated. Nitrate is not reduced. Casein, gelatin, hippurate, pullulan, starch and Tween 80 are hydrolyzed. Tween 40 and 60 are hydrolyzed by most strains. Tween 20 is not hydrolyzed; phenylalanine is not deaminated. Glucose and a narrow range of other carbohydrates can be utilized as sole sources of carbon. Distinguished from Bacillus halmapalus by having a smaller cell size, higher salt tolerance, and by DNA relatedness.
Source: soil.
DNA G + C content (mol%): 41.1–42.0 (HPLC).
Type strain : Nielsen PN-121, ATCC 700161, DSM 8719, LMG 17946.
EMBL/GenBank accession number (16S rRNA gene): AB043865 (DSM 8719).
-
Bacillus horti Yumoto, Yamazaki, Sawabe, Nakano, Kawasaki, Ezura and Shinano 1998, 570VP
hor'ti. L. masc. n. hortus garden; L. gen. n. horti from the garden.
Alkaliphilic, strictly aerobic, Gram-negative, motile rods, 0.6–0.8 by 1.5–6.0 µm, forming ellipsoidal, subterminal spores in swollen sporangia. Description is based upon two isolates. Colonies on complex medium at pH 10 are white. Grow occurs at pH 7, with optimum growth at pH 8–10. Grows in presence of 3–11% NaCl but not at 12% NaCl. Growth occurs between 15°C and 40°C; no growth at 10 and 45°C. Catalase- and oxidase-positive. Nitrate is reduced to nitrite, o-nitrophenyl-β-D-galactopyranoside is hydrolyzed and H2S is produced at pH 7. Acid is produced without gas from glucose and a narrow range of other carbohydrates. Casein, gelatin, starch and DNA are hydrolyzed; Tween 20, 40, 60 and 80 and urea are not. See Table 2.
Source: garden soil in Japan.
DNA G + C content (mol%): 40.9% for the type strain and 40.2 for another strain (HPLC).
Type strain : K13, ATCC 700778, DSM 12751, JCM 9943, LMG 18497.
EMBL/GenBank accession number (16S rRNA gene): D87035 (K13).
-
Bacillus hwajinpoensis Yoon, Kim, Kang, Oh and Park 2004b, 807VP
hwa.jin.po.en'sis. N.L. adj. hwajinpoensis of Hwajinpo, a beach of the East Sea in Korea, where the type strain was isolated.
Aerobic, nonmotile rods, 1.0–1.3 µm in diameter and 2.5–4.0 µm long. Gram-positive, but Gram-variable in older cultures. Description is based on a single isolate. Ellipsoidal endospores are borne centrally or terminally in swollen sporangia. Colonies are smooth, circular to slightly irregular, slightly raised, light yellow in color and 2–4 mm in diameter after 3d cultivation at 30°C on marine agar. Optimum growth temperature is 30–35°C. Growth occurs at 10 and 40°C but not at 4°C or above 41°C. Optimum pH for growth is 6.0–7.0. Growth is observed at pH 5.0, but not at pH 4.5. NaCl is required for growth. Optimal growth occurs in the presence of 2–5% NaCl. Growth occurs in the presence of 19% NaCl but is inhibited by 20% NaCl. No anaerobic growth on marine agar. Esculin is hydrolyzed. Hypoxanthine, tyrosine, urea and xanthine are not hydrolyzed. Acid is produced from D-mannitol and stachyose. Cell-wall peptidoglycan contains meso-diaminopimelic acid. Predominant menaquinone is MK-7. Major fatty acid is C15:0 anteiso.
Source: sea water of the East Sea in Korea.
DNA G + C content (mol%): 40.9 (HPLC).
Type strain : SW-72, KCCM 41641, JCM 11807.
EMBL/GenBank accession number (16S rRNA gene): AF541966 (SW-72).
-
Bacillus indicus Suresh, Prabagaran, Sengupta and Shivaji 2004, 1374VP
in'di.cus. L. masc. adj. indicus pertaining to India, Indian.
Cells are aerobic, Gram-positive, nonmotile rods measuring approximately 0.9–1.2 µm wide and 3.3–5.3 µm long. Description is based upon a single isolate. Produces subterminal endospores in a slightly swollen sporangium. Colonies on nutrient agar are yellowish-orange pigmented, circular, raised, smooth, convex and 3.0–4.0 mm in diameter. The pigment in acetone exhibits three absorption maxima at 404, 428 and 451 nm, characteristic of carotenoids. Grows in the range of 15–37°C (optimum 30°C) but not at 40°C. Grows between pH 6 and 7 and tolerates up to 2.0% (w/v) NaCl. Positive for catalase, gelatinase, amylase, arginine dihydrolase and esculin. Does not hydrolyze Tween 20 or urea. Does not reduce nitrate to nitrite and is negative for indole production, Voges–Proskauer test and citrate utilization. Utilizes D-cellobiose, meso-erythritol, inositol, lactose, D-melibiose, D-maltose, D-mannose, sucrose, L-rhamnose, D-ribose, raffinose, L-arginine, L-tryptophan and L-tyrosine as sole carbon sources. The major fatty acids are C14:0 iso (10.9%), C15:0 iso (33.5%), C15:0 anteiso (19.3%), C16:0 iso (11.0%), C16:0 (5.9%) and C17:0 iso (10.8%). The main proportion of the polar lipids consists of phosphatidylglycerol, diphosphatidylglycerol and phosphatidylethanolamine. The major respiratory quinone is MK-7. The cell wall is an A4β-murein with ornithine as the diamino acid and aspartic acid as the interpeptide bridge.
Source: sand of an arsenic-contaminated aquifer in West Bengal, India.
DNA G + C content (mol%): 41.2 (T m).
Type strain : Sd/3, MTCC 4374, DSM 15820.
GenBank/EMBL accession number (16S rRNA gene): AJ583158 (Sd/3).
-
Bacillus infernus Boone, Liu, Zhao, Balkwill, Drake, Stevens and Aldrich 1995, 447VP
in.fer'nus. N.L. adj. infernus that which comes from below (the ground).
Strictly anaerobic, thermophilic, nonmotile rods 0.7–0.8 by 4–8 µm. Cell-wall morphology is Gram-positive but the Gram reaction is ambiguous. Endospores not observed, but their presence has been inferred from the survival of heat-treated cultures. Growth occurs at 45–60°C but not at 40 or 65°C; optimum temperature for growth is about 61°C. Optimum pH for growth is about 7.3; grows well at pH 8.1 but does not grow at pH 9.2. The type strain is halotolerant; other strains have not been tested for this property. Grows fermentatively with glucose as substrate, but not with a range of other carbohydrates, alcohols and organic acids. Respiratory growth uses formate or lactate as electron donors and MnO2, Fe3+, trimethylamine oxide, and nitrate as electron acceptors. Nitrate is reduced to nitrite but not to ammonia or dinitrogen. Sulfate and thiosulfate are not reduced. Casein, gelatin and starch are not hydrolyzed. See Table 3.
Source: a shale core taken from 2.7 km below the land surface in the Taylorsville Triassic Basin in Virginia, USA.
DNA G + C content (mol%): not reported.
Type strain : Boone TH-23, DSM 10277, SMCC/W 479.
EMBL/GenBank accession number (16S rRNA gene): U20385 (Boone TH-23).
-
Bacillus insolitus Larkin and Stokes 1967, 891AL
in.so.li'tus. L. adj. insolitus unusual.
Strictly aerobic, Gram-positive, nonmotile cocci and motile rods and coccoid rods 1.0–1.5 by 1.6–2.7 µm on nutrient agar, and 0.7–0.9 by 2.4–5.3 µm on trypticase soy agar, occurring singly and in pairs. Description is based upon two isolates. Spores vary in shape from spherical to cylindrical, and from 0.7 to 1.4 µm in diameter, depending on the growth medium; terminal ellipsoidal and cylindrical spores are formed in rod-shaped cells. Sporangia are not appreciably swollen. Motile at 5°C and 20°C by one polar and one subpolar flagellum. Colonies on nutrient agar are small, soft, off-white and irregular. Optimum growth temperature about 20°C, minimum below 0°C, maximum 25°C; sporulates and germinates at 0°C. Tolerance of 2% NaCl varies; 4% NaCl is not tolerated. Catalase- and oxidase-positive. Gelatin and starch are not hydrolyzed; one of the two strains hydrolyzes urea (Logan and Berkeley, 1984). No growth on milk agar. Nitrate is not reduced to nitrate. Citrate not utilized as sole carbon source. No acid or gas produced from glucose or a range of other carbohydrates. See Table 9.
Source: normal and marshy soil.
DNA G + C content (mol%): 35.9 (T m), 36.1 (Bd) for the type strain and 41.0 (T m) for another strain.
Type strain : W 16B, ATCC 23299, DSM 5, LMG 17757, NCIMB 11433, KCTC 3737.
EMBL/GenBank accession number (16S rRNA gene): X60642 (DSM 5). This sequence displays 41 nucleotides as N-hits, indicating the weak quality of the sequence analysis. A further partial sequence of ATCC 23299 is available under accession number AF478084.
-
Bacillus jeotgali Yoon, Kang, Lee, Kho, Choi, Kang and Park 2001a, 1091VP
je.ot.ga'li. N.L. gen. n. jeotgali of jeotgal, Korean traditional fermented seafood.
Facultatively anaerobic, Gram-variable, motile rods 0.8–1.1 by 4.0–6.0 µm, forming ellipsoidal spores in swollen sporangia. Description is based upon two isolates. Colonies are cream-yellow or light orange-yellow, smooth and flat with irregular margins. Growth occurs at 10 and 45°C but not at 55°C; optimum growth temperature 30–35°C. Growth occurs at pH 7.0–8.0. Tolerates up to 13% NaCl. Growth poor on nutrient agar and trypticase soy agar without added salts. Catalase-positive, oxidase-negative. Esculin, gelatin, starch and urea are hydrolyzed; casein, hypoxanthine, tyrosine and xanthine are not hydrolyzed. Acid without gas is produced from glucose and a narrow range of other carbohydrates.
Source: jeotgal, a Korean traditional fermented seafood.
DNA G + C content (mol%): 41.0 (HPLC).
Type strain : YKJ-10, AF221061, JCM 10885, CIP 107104, KCCM 41040.
EMBL/GenBank accession number (16S rRNA gene): AF221061 (YKJ-10).
-
Bacillus krulwichiae Yumoto, Yamaga, Sogabe, Nodasaka, Matsuyama, Nakajima and Suemori 2003, 1534VP
krul.wich.i'ae. N.L. fem. gen. n. krulwichiae of Krulwich; named after American microbiologist Terry A. Krulwich who made fundamental contributions to the study of alkaliphilic bacteria.
Alkaliphilic, facultatively anaerobic, Gram-positive, peritrichously flagellated straight rods, 0.5–0.7 by 1.5–2.6 µm. Ellipsoidal endospores are borne subterminally and do not cause swelling of sporangia. Description is based on two isolates. Colonies are circular and colorless. Catalase and oxidase reactions are positive. Negative for indole production, ONPG hydrolysis, and H2S production. Growth occurs at pH 8–10, but no growth occurs at pH 7. Grows in presence of 14% NaCl, but not at higher concentrations. Nitrate is reduced to nitrite. Acid, but no gas, is produced from D-xylose, D-glucose, D-fructose, D-galactose, D-ribose, maltose, sucrose, trehalose, glycerol and mannitol when grown at pH 10. Positive for hydrolysis of starch, DNA, hippurate and Tween 20, 40, 60 and 80. Hydrolysis of casein and gelatin is variable among strains. Utilizes benzoate and m-hydroxybenzoate as sole carbon sources. The major isoprenoid quinones are menaquinone-5, -6 and -7. The main fatty acids produced during growth in an alkaline medium (pH 10) are C15:0 iso (17.1–19.2%) and C15:0 anteiso (45.6–49.0%). See Table 2.
Source: a soil sample obtained from Tsukuba, Ibaraki, Japan.
DNA G + C content (mol%): 40.6–41.5 (HPLC).
Type strain : AM31D, IAM 15000, NCIMB 13904, JCM 11691.
EMBL/GenBank accession number (16S rRNA gene): AB086897 (AM31D).
-
Bacillus lentus Gibson 1935, 364AL
len'tus. L. adj. lentus slow.
As with Bacillus firmus, strains allocated to this species are genetically heterogeneous, and many strains have been incorrectly assigned to it. Strains received as Bacillus lentus show phenotypic profiles that appear to overlap with those of strains assigned to Bacillus firmus, so that Gordon et al. (1977) raised the question of whether Bacillus firmus-Bacillus lentus represented a single species or a series of strains.
The description which follows is based upon the type strain and a small number of other strains which have been shown by amplified rDNA restriction analysis, polyacrylamide gel electrophoresis of whole-cell proteins, and various phenotypic characters (Logan, De Vos and colleagues, unpublished data) to be closely related to the type strain. Phylogenetic studies indicate that Bacillus circulans, Bacillus firmus and Bacillus lentus are related.
Strictly aerobic, Gram-positive, straight or slightly curved, round-ended, motile rods 0.7–0.8 µm in diameter that occur singly, in pairs and occasionally in short chains. Endospores are ellipsoidal, lie subterminally or paracentrally, and may swell the sporangia slightly. After 2 d on TSA at 30°C, colonies are 1–2 mm in diameter, whitish, opaque and flat, with glossy surfaces and entire margins. Optimum growth temperature is 30°C, minimum temperature is 10°C and maximum lies below 40°C. The optimum pH is 8.0; the minimum pH lies between 5.0 and 6.0 and the maximum between 9.5 and 10. Catalase-positive. Grows in presence of 5% NaCl. Starch hydrolysis is positive but casein is not hydrolyzed. In the API 20E strip, o-nitrophenyl-β-D-galactopyranoside hydrolysis, urease production and nitrate reduction are positive. Citrate utilization is variable. Arginine dihydrolase, lysine decarboxylase, ornithine decarboxylase, hydrogen sulfide production, tryptophan deaminase, indole production, gelatin hydrolysis, and Voges–Proskauer reaction are negative. Acid without gas is produced, often weakly, from D-glucose and from a wider range of other carbohydrates than is attacked by strains of Bacillus firmus. In the API 50CH gallery using the CHB suspension medium, hydrolysis of esculin is positive, and production of acid without gas is positive for lactose, N-acetylglucosamine, sucrose, and D-trehalose. Acid production is weak or positive for amygdalin, L-arabinose, arbutin, D-cellobiose, D-fructose, galactose, gentiobiose, D-glucose, maltose, mannitol, D-mannose, D-melezitose, D-melibiose, methyl-xyloside, D-raffinose, rhamnose, ribose, salicin, starch and D-xylose. In the API Biotype 100 kit, hydroxyquinoline-β-glucuronide is hydrolyzed and D-glucosamine, D-glucuronate and 2-keto-D-gluconate are assimilated.
Source: soil.
DNA G + C content (mol%): 36.3 (T m), 36.4 (Bd).
Type strain : ATCC 10840, AF478107, DSM 9, JCM 2511, LMG 16798, NCIMB 8773.
EMBL/GenBank accession number (16S rRNA gene): AB021189 (NCIMB 8773).
-
Bacillus licheniformis (Weigmann 1898) Chester 1901, 287AL (Clostridium licheniforme Weigmann 1898, 822)
li.che.ni.for'mis. Gr. n. lichen lichen; L. adj. suff. -formis -like, in the shape of; N.L. adj. licheniformis lichen-shaped.
Facultatively anaerobic, Gram-positive, motile rods, forming ellipsoidal to cylindrical spores which lie centrally, paracentrally and subterminally in unswollen sporangia (Figure 3d). Cells grown on glucose agar stain evenly. Cells 0.6–0.8 by 1.5–3.0 µm, occurring singly and in pairs, and chains. Colonial morphology is variable, within and between strains, and, as with Bacillus subtilis, may give the appearance of a mixed culture. Colonies are round to irregular in shape and of moderate (2–4 mm) diameter, with margins varying from undulate to fimbriate; they become opaque, with surfaces that are dull and which may become wrinkled; color is whitish, and may become creamy or brown (perhaps red on carbohydrate media containing sufficient iron); textures range from moist and butyrous or mucoid, through membranous with an underlying mucoid matrix, with or without mucoid beading at the surface, to rough and crusty as they dry; these “licheniform” colonies tend to be quite adherent to the agar. Minimum growth temperature 15°C, maximum 50–55°C; an isolate from a geothermal environment with a maximum growth temperature of 68°C has been reported (Llarch et al., 1997). Growth occurs at pH 5.7 and 6.8, but limits have not been reported. Grows in presence of up to 7% NaCl. Catalase-positive, oxidase variable. Casein, esculin, gelatin and starch are hydrolyzed; occasional strains will hydrolyze urea; phenylalanine is not deaminated. Usually arginine dihydrolase-positive. Pectin and polysaccharides of plant tissues are decomposed. Dextran and levan are formed extracellularly from sucrose. Citrate and propionate are utilized as sole carbon sources by most strains. Nitrate is reduced to nitrite. Voges–Proskauer-positive. Acid without gas is produced from glucose and from a wide range of other carbohydrates.
Widely distributed in soil and many other environments, including milk and other foods, and clinical and veterinary specimens. Vegetative growth may occur readily in foods held at 30–50°C. Occasionally reported as an opportunistic pathogen in man and other animals, and as a cause of food poisoning.
DNA G + C content (mol%): 42.9–49.9 (T m) for 12 strains, 44.9–46.4 (Bd) for 19 strains, and 46.4 (T m), 44.7 (Bd) for the type strain.
Type strain : ATCC 14580, CCM 2145, DSM 13, LMG 12363, IFO 12200, NCIMB 9375.
EMBL/GenBank accession number (16S rRNA gene): X68416 (DSM 13).
Additional remarks: Gibson (1944) considered Bacillus globigii to be a synonym of Bacillus licheniformis, but as Gordon et al. (1973) pointed out, strains of Bacillus circulans, Bacillus pumilus and Bacillus subtilis labeled Bacillus globigii have been widely circulated, so that the name is meaningless. Strains named Bacillus globigii were formerly popular for tracing studies, including those associated with the development of biological weapons.
-
Bacillus luciferensis Logan, Lebbe, Verhelst, Goris, Forsyth, Rodríguez-Díaz, Heyndrickx and De Vos 2002b, 1988VP
lu.cif.er.en'sis. N.L. adj. luciferensis referring to Lucifer Hill, a volcano on Candlemas Island, South Sandwich Islands, the soil of which yielded the organism.
Motile rods (0.4–0.8 by 3–6 µm) occurring singly and in pairs and showing pleomorphism. Gram-positive, but becoming Gram-negative within 24 h of culture at 30°C. Ellipsoidal endospores lie subterminally and occasionally terminally, and may swell the sporangia slightly. Colonies are 1–5 mm in diameter, creamy-gray, raised, translucent, glossy, moist and loosely butyrous, with irregular margins and surfaces. The growth temperature range lies between 15–20°C and 35–45°C, with an optimum of 30°C. The pH range for growth is from 5.5–6.0 to 8.0–8.5, with an optimum of 7.0. Organisms are facultatively anaerobic and weakly catalase-positive. Esculin and gelatin are hydrolyzed, casein is weakly hydrolyzed. Nitrate is not reduced. Acid without gas is produced from glucose and a range of other carbohydrates. The major cellular fatty acids are C15:0 anteiso and C15:0 iso (representing about 25% and 50% of total fatty acid, respectively).
Source: geothermal soil taken from an active fumarole on Lucifer Hill, a volcano on Candlemas Island, South Sandwich archipelago.
DNA G + C content (mol%): 33.0 (T m) for the type strain.
Type strain : Logan SSI061, LMG 18422, CIP 107105.
EMBL/GenBank accession number (16S rRNA gene): AJ419629 (LMG 18422).
-
Bacillus macyae Santini, Streimann and vanden Hoven 2004, 2244VP
ma.cy'ae. N.L. fem. gen. n. macyae of Macy, named after the late Joan M. Macy, La Trobe University, Australia, in tribute to her research in the area of environmental microbiology.
Cells are Gram-positive, motile rods (2.5–3 µm long and 0.6 µm wide) and produce subterminally located ellipsoidal spores. Spores do not cause swelling of sporangia. Colonies are round and white. Catalase reaction is positive and oxidase is negative. Strict anaerobe that respires with arsenate and nitrate as terminal electron acceptors. Arsenate is reduced to arsenite and nitrate to nitrite. The electron donors used for anaerobic respiration are acetate, lactate, pyruvate, succinate, malate, glutamate and hydrogen (with acetate as carbon source). Growth occurs at 28–37°C, pH 7–8.4 and 0.12–3% NaCl.
Source: arsenic-contaminated mud from a gold mine in Bendigo, Victoria, Australia.
DNA G + C content (mol%): 37 (HPLC).
Type strain : JMM-4, DSM 16346, JCM 12340.
GenBank/EMBL accession number (16S rRNA gene): AY032601 (JMM-4).
-
Bacillus marisflavi Yoon, Kim, Kang, Oh and Park 2003a, 1301VP
ma.ris.fla'vi. L. gen. neut. n. maris of the sea; L. masc. adj. flavus yellow; N.L. gen. masc. n. marisflavi of the Yellow Sea.
Aerobic rods, 0.6–0.8 µm by 1.5–3.5 µm, motile by means of a single polar flagellum. Gram-positive, but Gram-variable in older cultures. Description is based on a single isolate. Ellipsoidal endospores lie centrally or subterminally in swollen sporangia. Colonies are pale yellow, smooth, circular to slightly irregular, slightly raised, and 2–4 mm in diameter after 3 d at 30°C on marine agar. Optimal growth temperature is 30–37°C. Growth occurs at 10 and 47°C, but not at 4 or above 48°C. Optimal growth pH is 6.0–8.0. Growth is observed at pH 4.5, but not at pH 4.0. Growth occurs in the presence of 0–16% (w/v) NaCl, and optimal growth occurs at 2–5% (w/v) NaCl. Catalase-positive, oxidase- and urease-negative. Esculin and casein are hydrolyzed. Hypoxanthine, starch, Tween 80, tyrosine and xanthine are not hydrolyzed. Acid is produced from arbutin, D-cellobiose, D-fructose, gentiobiose, D-glucose, glycerol, maltose, D-mannitol, D-mannose, melibiose, methyl-D-mannoside, D-ribose, salicin, stachyose, sucrose, D-trehalose and D-xylose and produced weakly from D-galactose and D-raffinose. The cell-wall peptidoglycan contains meso-diaminopimelic acid. The predominant menaquinone is MK-7. The major fatty acids are C15:0 anteiso and C15:0 iso.
Source: sea water of a tidal flat of the Yellow Sea in Korea.
DNA G + C content (mol%): 49 (HPLC).
Type strain : TF-11, KCCM 41588, JCM 11544.
EMBL/GenBank accession number (16S rRNA gene): AF483624 (TF-11).
-
Bacillus megaterium de Bary 1884, 499AL
me.ga.te'ri.um. Gr. adj. mega large; Gr. n. teras, teratis monster, beast; N.L. n. megaterium big beast.
Aerobic, Gram-positive, motile rods, large cells 1.2–1.5 by 2.0–5.0 µm, occurring singly and in pairs and chains, forming ellipsoidal and sometimes spherical spores which are located centrally, paracentrally or subterminally, and which do not swell the sporangia (Figure 3e). Cells grown on glucose agar produce large amounts of storage material, giving a vacuolate or foamy appearance. Colonies are glossy, round to irregular, and have entire to undulate margins (Figure 2c). Minimum temperature for growth 3–15°C, maximum 35–45°C, with the optimum around 30°C. The temperature range of a water isolate from an Antarctic geothermal island was 17–63°C, with an optimum of 60°C (Llarch et al., 1997). Catalase-positive. Casein, gelatin and starch are hydrolyzed. Phenylalanine is deaminated by most strains; tyrosine degradation is variable. Most strains grow in presence of 7% NaCl, but none grow at 10% NaCl. Citrate is utilized as sole carbon source. Most strains do not reduce nitrate. Acid without gas is produced from glucose and a wide range of other carbohydrates.
Source: soil, cow feces, foods and clinical specimens.
DNA G + C content (mol%): 37.0–38.1 (T m) for 12 strains, and 37.2 (T m) for the type strain.
Type strain : ATCC 14581, CCM 2007, DSM 32, NCIMB 9376, NCTC 10342, LMG 7127, IAM 13418.
EMBL/GenBank accession number (16S rRNA gene): D16273 (IAM 13418).
Additional remarks: Gordon et al. (1973) found that their 60 cultures of Bacillus megaterium formed two merging aggregates of strains, and Hunger and Claus (1981) recognized three DNA relatedness groups among 21 strains labeled as Bacillus megaterium, with the type strain lying within relatedness group A; Priest et al. (1988) revived the names Bacillus simplex for strains of DNA relatedness group B and Bacillus flexus for two strains which showed low homology with these two relatedness groups.
-
Bacillus methanolicus Arfman, Dijkhuizen, Kirchhof, Ludwig, Schleifer, Bulygina, Chumakov, Govorhukina, Trotsenko, White and Sharp 1992, 444VP
me.tha'noli.cus. N.L. n. methanol methanol; L. masc. suff. -icus adjectival suffix used with the sense of belonging to; N.L. masc. adj. methanolicus relating to methanol.
Methylotrophic, thermotolerant, strictly aerobic, nonmotile, Gram-positive rods, usually occurring singly. Filamentous cells may be seen, especially in older cultures. Ellipsoidal endospores lie centrally to subterminally and swell the sporangia. Grows on methanol, some strains also grow on ethanol. Colonies on tryptone soya agar are circular, and usually have rough surfaces and crenated, undulating margins. The growth temperature range lies between 35°C and 60°C, with an optimum of 55°C. Catalase- and oxidase-positive. Casein and hippurate are not hydrolyzed; starch hydrolysis varies between strains. Nitrate is not reduced. Acid without gas is produced from glucose and a narrow range of other carbohydrates. See Table 3.
Source: soil, aerobic (and thermophilic) wastewater treatment systems and volcanic hot springs.
DNA G + C content (mol%): 48–50 (T m).
Type strain : Dijkhuizen PB1, NCIMB 13113, LMG 16799, KCTC 3735.
EMBL/GenBank accession number (16S rRNA gene): X64465; this is for strain C1 (=NCIMB 13114) which is not the type strain.
-
Bacillus mojavensis Roberts, Nakamura and Cohan 1994, 263VP
mo.hav.en'sis. N.L. masc. adj. mojavensis from the Mojave Desert.
Aerobic, Gram-positive, motile rods, forming ellipsoidal spores which lie centrally or paracentrally in unswollen sporangia. Cells 0.5–1.0 by 2.0–4.0 µm, occurring singly and in short chains. Colonies are opaque, smooth, circular and entire and 1.0–2.0 mm in diameter after 2 d at 28°C. Optimum growth temperature 28–30°C, with minimum of 5–10°C and maximum of 50–55°C. Catalase-positive, oxidase-positive. Casein, gelatin and starch are hydrolyzed; Tween 80, tyrosine and urea are not. Nitrate is reduced to nitrite. Acid without gas is produced from glucose and a range of other carbohydrates.
Phenotypically indistinguishable from Bacillus subtilis subsp. subtilis and Bacillus subtilis subsp. spizizenii and distinguished from those organisms principally by DNA relatedness and resistance to transformation. Phenotypically indistinguishable from Bacillus vallismortis, and distinguished from that organism by DNA relatedness, restriction digestion analysis, and fatty acid analysis.
Phenotypically distinguishable from Bacillus atrophaeus only by failure to produce dark brown pigmented colonies on media containing tyrosine or other organic nitrogen source.
Source: desert soils.
DNA G + C content (mol%): 43.0 (T m).
Type strain : Cohan RO-H-1, ATCC 51516, NRRL B-14698, DSM 9205, LMG 17797, NCIMB 13391, IFO 15718.
EMBL/GenBank accession number (16S rRNA gene): AB021191 (IFO 15718).
-
Bacillus mycoides Flügge 1886, 324AL
my.co.i'des. Gr. n. myces fungus; Gr. eidus form, form, shape; N.L. adj. mycoides fungus-like.
Facultatively anaerobic, Gram-positive, nonmotile organisms forming ellipsoidal spores which lie paracentrally to subterminally in unswollen sporangia. Cells 1.0–1.2 by 3.0–5.0 µm, occurring singly and in chains. Cells grown on glucose agar produce large amounts of storage material, giving a vacuolate or foamy appearance. Colonies are white to cream, opaque, and characteristically rhizoid; this ability to form rhizoid colonies may be lost. Minimum growth temperature 10–15°C, maximum 35–40°C. Grows at pH 5.7, and in 0.001% lysozyme. Ability to grow in presence of 7% NaCl varies between strains. Catalase-positive, oxidase-negative. Lecithinase and Voges–Proskauer reactions are positive. Citrate utilization variable; propionate not utilized. Nitrate reduction is variable. Casein and starch are hydrolyzed; decomposition of tyrosine variable. Acid without gas is produced from glucose and a limited range of other carbohydrates.
Phenotypically similar to other members of the Bacillus cereus group: Bacillus anthracis, Bacillus cereus, Bacillus thuringiensis and Bacillus weihenstephanensis. Bacillus mycoides is distinguished by its characteristic rhizoid colonies Figure 2d) and absence of motility. Smith et al. (1952) and Gordon et al. (1973) considered Bacillus mycoides to be a variety of Bacillus cereus. For distinguishing characters within the Bacillus cereus group, see the individual species descriptions and Table 8. Those strains which have been proposed as Bacillus pseudomycoides can only be separated from Bacillus mycoides by DNA relatedness and some differences in fatty acid composition.
Source: mainly from soil.
DNA G + C content (mol%): 32.5–38.4 (T m) for nine strains, 35.2–39.0 (Bd) for four strains, and 34.2 (T m), 34.1 (Bd) for the type strain.
Type strain : ATCC 6942, DSM 2048, LMG 7128, NRRLB-14811, NRS 273, NCIMB 13305, KCTC 3453.
EMBL/GenBank accession number (16S rRNA gene): AB021192 (ATCC 6462).
-
Bacillus naganoensis Tomimura, Zeman, Frankiewicz and Teague 1990, 124VP
na.ga.no.en'sis. N.L. masc. adj. naganoensis of the Japanese Prefecture Nagano.
Aerobic, moderately acidophilic, Gram-positive, nonmotile rods, forming ellipsoidal spores which lie subterminally in swollen sporangia. Description is based upon a single isolate. Cells are 0.5–1.0 by 2.1–10.0 µm, have rounded or square ends, and occur singly or in chains. Colonies are opaque, smooth, glistening, circular and entire, and reach 2.0–3.0 mm in diameter. Optimum growth temperature 28–33°C, with minimum above 20°C and maximum below 45°C. The pH range for growth is about 4.0–6.0. Catalase-positive. A thermostable pullulanase is produced. Casein, gelatin and starch are hydrolyzed. Hippurate and tyrosine are not decomposed. Citrate and propionate are not utilized as sole sources of carbon. Nitrate is not reduced. Acid without gas is produced from glucose and a small range of other carbohydrates after extended (>14 d) incubation. Not pathogenic to mice.
Source: soil by selection using a pullulan-containing medium.
DNA G + C content (mol%): 45 ± 2 (T m).
Type strain : ATCC 53909, DSM 10191, LMG 12887, KCTC 3742.
EMBL/GenBank accession number (16S rRNA gene): AB021193 (ATCC 53909).
Additional remark: this species has recently (Hatayama et al., 2006) been classified as Pullulanibacillus naganoensis.
-
Bacillus nealsonii Venkateswaran, Kempf, Chen, Satomi, Nicholson and Kern 2003, 171VP
neal'son.i.i. N.L. gen. n. nealsonii referring to Kenneth H. Nealson, an American microbiologist.
Facultatively anaerobic, Gram-positive, motile rods 1.0 by 4.0–5.0 µm. The ellipsoidal spores are 0.5 by 1.0 µm, and bear an additional extraneous layer similar to an exosporium. Description is based upon a single isolate. Young colonies on TSA are 3–4 mm in diameter, irregular, rough and umbonate with undulate or lobate edges and are of a beige color. Sodium ions are not essential for growth; up to 8% NaCl is tolerated. Growth occurs at pH 6–10, with an optimum of pH 7. Optimum growth temperature is 30–35°C, with minimum of 25°C and maximum of 60°C. Catalase and β-galactosidase are produced, but gelatinase, arginine dihydrolase, lysine and ornithine decarboxylases, lipase, amylase and alginase are not. H2S is not produced from thiosulfite. Denitrification does not occur. Acid is produced from glucose and a wide range of other carbohydrates.
Source: dust particles collected at a spacecraft-assembly facility.
DNA G + C content (mol%): not reported.
Type strain : FO-92, ATCC BAA-519, DSM 15077.
EMBL/GenBank accession number (16S rRNA gene): AF234863 (FO-92).
-
Bacillus neidei Nakamura, Shida, Takagi and Komagata 2002, 504VP
nei'de.i. N.L. gen. n. neidei of the early microbiologist Neide.
Aerobic, Gram-positive, motile rods, forming spherical spores which lie terminally in swollen sporangia. Cells are about 1.0 by 3.0–5.0 µm. Colonies are translucent, thin, smooth, circular and entire, and reach 1 mm in diameter after 24 h of incubation at 28°C. Optimum growth temperature 28–33°C, with minimum 5–10°C and maximum 40–45°C. Catalase-positive. Biotin, thiamin and cystine are required for growth. Casein, starch, Tween 40 and 80, tyrosine and urea are not hydrolyzed. L-alanine, citrate β-hydroxybutyrate, propionate and pyruvate are not oxidized. Grows in presence of 5% NaCl, but sensitive to 0.001% lysozyme. Nitrate is not reduced to nitrite. No acid or gas produced from glucose and other common carbohydrates. Cell-wall peptidoglycan type is L-Lys-D-Glu. Phenotypically similar to Bacillus fusiformis, Bacillus pycnus and Bacillus sphaericus, and separable from these species by growth factor requirements, several substrate oxidation and decomposition tests, and differences in fatty acid compositions. See Table 9.
Source: soil.
DNA G + C content (mol%): 35 (T m).
Type strain : NRRL BD-87, JCM 11077, LMG 21635.
EMBL/GenBank accession number (16S rRNA gene): AF169520 (NRRL BD-87).
Additional remark: this species has recently been classified as Viridibacillus neidei by Albert et al. (2007).
-
Bacillus niacini Nagel and Andreesen 1991, 137VP
ni.a.ci'ni. N.L. n. niacinum niacin or nicotinic acid; N.L. gen. n. niacini of nicotinic acid.
Aerobic rods 0.9–1.4 by 3–5.6 µm, forming central, and sometimes subterminal, ellipsoidal spores which may swell the sporangia slightly. Cells may be pleomorphic and increase in width. Long chains may be formed when grown on complex media. Gram-variable when grown in nutrient broth, and Gram-positive when grown on nicotinate agar. Some strains motile. Colonies are smooth and have light beige centers surrounded by translucent areas of variable extension, and are about 3–5 mm in diameter. In the presence of molybdate, nicotinate can be used as sole source of carbon, nitrogen and energy. Grows at 10–40°C. Catalase-positive or weakly positive; oxidase usually positive. Some strains are indole-positive. Gelatin is hydrolyzed, sometimes weakly; starch is hydrolyzed by some strains, urease is usually negative; casein, phenylalanine and tyrosine are not decomposed. Utilization of aspartate, citrate, formate and lactate as sole carbon sources varies between strains. Optimum pH for growth between 7 and 8. Nitrate is usually reduced to nitrite. Acid without gas is produced from glucose and from a number of other carbohydrates, depending upon the strain.
Source: soil.
DNA G + C content (mol%): 37–39 (T m).
Type strain : IFO 15566, DSM 2923, LMG 16677.
EMBL/GenBank accession number (16S rRNA gene): AB021194 (IFO 15566).
-
Bacillus novalis Heyrman, Vanparys, Logan, Balcaen, Rodríguez-Díaz, Felske and De Vos 2004, 52VP
no.va'lis. L. gen. n. novalis of fallow land.
Facultatively anaerobic, Gram-positive, motile, slightly curved, round-ended rods, 0.6–1.2 µm in diameter, occurring singly and in pairs, and occasionally in short chains or filaments. Endospores are mainly ellipsoidal, and lie in subterminal and occasionally paracentral positions in slightly swollen sporangia. When grown on TSA, colonies are raised, butyrous, cream-colored, produce a soft brown pigment that diffuses in the agar, and have slightly irregular margins and smooth or eggshell-textured surfaces; they sometimes have iridescent centers when viewed by low-power microscopy. Optimal growth occurs at 30–40°C, and the maximum growth temperature lies between 50°C and 55°C. Growth occurs from pH 4.0–5.0–9.5–10.0, and the optimum pH for growth is 7.0–9.0. Casein is hydrolyzed. In the API 20E strip, Voges–Proskauer reaction is negative, gelatin is hydrolyzed by most strains, and nitrate reduction is positive (sometimes weakly); reactions for o-nitrophenyl-β-D-galactopyranoside hydrolysis, arginine dihydrolase, lysine decarboxylase, ornithine decarboxylase, citrate utilization, hydrogen sulfide production, urease, tryptophan deaminase, indole production, are negative. Hydrolysis of esculin positive. Acid without gas is produced (weakly by some strains) from the following carbohydrates in the API 50 CH gallery using the CHB suspension medium: N-acetyl-D-glucosamine, D-fructose, galactose (always weak), D-glucose, maltose, D-mannose, D-trehalose. The following reactions are variable between strains and, when positive, are usually weak: amygdalin, arbutin, D-cellobiose, β-gentiobiose, gluconate, glycerol, 5-keto-D-gluconate, D-lyxose, D-mannitol, ribose, sorbitol, D-xylose; the type strain is positive for sorbitol and D-xylose and weakly positive for: amygdalin, D-cellobiose, β-gentiobiose, D-mannitol. The major cellular fatty acids are C15:0 iso and C15:0 anteiso, present at a level of about 44 and 31% of the total fatty acid content, respectively.
Source: soil in the Drentse A agricultural research area, The Netherlands.
DNA G + C content (mol%): 40.0–40.5 (HPLC) and 40.5 for the type strain.
Type strain : LMG 21837, DSM 15603.
EMBL/GenBank accession number (16S rRNA gene): AJ542512 (LMG 21837).
-
Bacillus odysseyi La Duc, Satomi and Venkateswaran 2004, 200VP
o.dys.se'yi. L. n. Odyssea the Odyssey; N.L. gen. n. odysseyi pertaining to the Mars Odyssey spacecraft, from which the organism was isolated.
Strictly aerobic, Gram-positive motile rods, 4–5 µm in length and 1 µm in diameter. Forms spherical endospores which are borne terminally and swell the sporangia. Spores show an additional exosporium layer. Description is based upon a single isolate. Colonies on TSA are round, smooth, flat with entire edges and beige in color. Sodium ions are not essential for growth; growth occurs in 0–5% NaCl. Grows at pH 6–10 (optimum at pH 7) and 25–42°C (optimum 30–35°C). With the exception of arabinose, breakdown of sugars to acids does not occur following prolonged incubation. Glucose is not utilized as sole carbon source. Pyruvate, amino acids, purine or pyrimidine bases and related compounds are preferred as carbon and energy sources. Catalase-positive, but does not produce gelatinase, arginine dihydrolase, lysine or ornithine decarboxylase, lipase, amylase or alginase. Does not produce H2S from thiosulfite and is not involved in denitrification. Closely related to species that have been transferred to the novel genus Lysinibacillus (Ahmed et al., 2007c), but data on peptidoglycan composition and polar lipids are not available for Bacillus odysseyi, and so it has not been transferred to the new genus. See Table 9.
Source: the surface of the Mars Odyssey spacecraft.
DNA G + C content (mol%): not reported.
Type strain : 34hs-1, ATCC PTA-4993, NRRL B-30641, NBRC 100172.
EMBL/GenBank accession number (16S rRNA gene): AF526913 (34hs-1).
-
Bacillus okuhidensis Li, Kawamura, Shida, Yamagata, Deguchi and Ezaki 2002, 1208VP
o.ku.hid.en'sis. N.L. masc. adj. okuhidensis referring to Okuhida in Gifu, Japan, where the strains were originally isolated.
Alkaliphilic, weakly Gram-positive rods, 0.5–1.0 by 5–7 µm, forming ellipsoidal, subterminal spores that may swell the sporangia slightly. Description is based upon two isolates. Motile by means of peritrichous flagella. Cells stain slightly Gram-positive in the exponential growth phase and Gram-negative in the stationary phase. Colonies are circular, convex, smooth, and yellowish. Growth temperature range 30–60°C; optimum 45–50°C. Optimal growth at pH 10.5; pH range 6.0–11.0. Grows in presence of 10% NaCl. Catalase- and oxidase-positive. Casein, starch and gelatin are hydrolyzed; hippurate, and Tween 20, 40 and 60 are not. Phenylalanine is not deaminated. Nitrate is reduced to nitrite. A range of carbohydrates can be utilized as sole sources of carbon. The major cellular fatty acids are C15:0 iso (43.75% ± 0.7%) and C15:0 anteiso (25.8% ± 0.6%). See Table 2.
Source: hot spa water.
DNA G + C content (mol%): 40.0–41.1 (HPLC).
Type strain : GTC 854, JCM 10945, DSM 13666.
EMBL/GenBank accession number (16S rRNA gene): AB047684 (GTC 854).
-
Bacillus oleronius Kuhnigk et al. 1996, 625VP (Effective publication: Kuhnigk et al. 1995, 704.)
o.le.ro'ni.us. N.L. adj. oleronius of Îsle de Oléron, France, where the termite host thrives.
Cells are nonmotile, Gram-negative, medium-sized rods, that occur singly and in pairs, and sometimes form short chains of 3–4 cells. They bear ellipsoidal endospores that lie in subterminal and paracentral positions within swollen sporangia. After 2 d on TSA colonies are approximately 1–2 mm diameter, circular, entire, shiny, beige or cream and butyrous with slightly translucent edges. Organisms are strictly aerobic and catalase-positive. Growth may occur between 30°C and 50°C, with an optimum of 37°C. Casein is not hydrolyzed and starch is sometimes hydrolyzed weakly. Nitrate is reduced to nitrite, the Voges–Proskauer reaction is variable (type strain positive), citrate is not utilized, hydrogen sulfide and indole are not produced, and the ONPG reaction is negative. Esculin is hydrolyzed, gelatin is weakly hydrolyzed and urea is not hydrolyzed. Acid without gas is produced from N-acetylglucosamine, D-cellobiose, D-fructose, D-glucose, mannitol and D-tagatose. Acid production from the following carbohydrates is variable, and when positive it is weak: galactose, glycerol, maltose, D-mannose, ribose salicin, starch and D-trehalose; the type strain produces acid from: glycerol, maltose, ribose, starch and D-trehalose. The major cellular fatty acids (mean percentage + standard deviation of total fatty acids) after 24 h growth on brain heart infusion supplemented with vitamin B12 at 37°C are: C15:0 iso (39.24 ± 1.38), C15:0 anteiso (22.89 ± 2.22) and C17:0 anteiso (20.78 ± 0.85).
Source: the hindgut of termite Reticulitermes santonensis (Feytaud), and from raw milk and cattle feed concentrate.
DNA G + C content (mol%): 35.2–34.7 (HPLC) and 35.2 for the type strain.
Type strain : Kuhnigk RT 10, DSM 9356, ATCC 700005, LMG 17952, CIP 104972.
EMBL/GenBank accession number (16S rRNA gene): X782492 (DSM 9356).
-
Bacillus pseudalcaliphilus Nielsen, Fritze and Priest 1995b, 879 (Effective publication: Nielsen, Fritze and Priest 1995a, 1760.)
pseu.dal.ca.li'phi.lus. Gr. adj. pseudes false; N.L. adj. alcalophilus a specific epithet; N.L. adj. pseudalcaliphilus false alcalophilus because it is phenotypically closely related to Bacillus alcalophilus but phylogenetically distinct.
Alkaliphilic organisms forming ellipsoidal spores which lie paracentrally to subterminally in swollen sporangia. Cells 0.5–0.6 by 2.0–4.0 µm. Colonies are white and circular with undulate margins. Growth temperature range 10–40°C. Optimal growth at about pH 10.0; no growth at pH 7.0. Maximum NaCl concentration tolerated is 10%. Nitrate is not reduced. Casein, gelatin, pullulan and starch are hydrolyzed. Hippurate and Tween 20 are not hydrolyzed; phenylalanine is not deaminated. Glucose and a range of other carbohydrates can be utilized as sole sources of carbon.
Phenotypically similar to Bacillus alcalophilus, but phylogenetically distinct. See Table 2.
Source: soil.
DNA G + C content (mol%): 38.2–39.0 (HPLC).
Type strain : Nielsen PN-137, DSM 8725, ATCC 700166, LMG 17951, CIP 105304.
EMBL/GenBank accession number (16S rRNA gene): X76449 (DSM 8725).
-
Bacillus pseudofirmus Nielsen, Fritze and Priest 1995b, 879VP (Effective publication: Nielsen, Fritze and Priest 1995a, 1760.)
pseu.do.fir'mus. Gr. adj. pseudes false; L. adj. firmus a specific epithet; N.L. adj. pseudofirmus false firmus referring to physiological similarities to Bacillus firmus.
Alkaliphilic and halotolerant organisms forming ellipsoidal spores which lie paracentrally to subterminally in unswollen sporangia. Cells 0.6–0.8 by 3.0–6.0 µm. Colonies are yellow and circular with irregular margins. Growth temperature range 10–45°C. Optimal growth at about pH 9.0; no growth at pH 7.0 for most strains. Maximum NaCl concentration tolerated is 16–17%. Nitrate is not reduced. Casein, gelatin, starch and Tween 40 and 60 are hydrolyzed; some strains can hydrolyze pullulan. Hippurate and Tween 20 are not hydrolyzed. Phenylalanine is deaminated. Glucose and a range of other carbohydrates can be utilized as sole sources of carbon.
Phenotypically similar to Bacillus firmus, but alkalophilic and phylogenetically distinct. See Table 2.
Source: soil and animal manure.
DNA G + C content (mol%): 39.0–40.8 (HPLC).
Type strain : PN-3, DSM 8715, NCIMB 10283, LMG 17944, ATCC 700159.
EMBL/GenBank accession number (16S rRNA gene): X76439 (DSM 8715).
-
Bacillus pseudomycoides Nakamura 1998, 1035VP
pseu.do.my.co.i'des. Gr adj. pseudes false; N.L. adj. mycoides fungus-like; N.L. adj. pseudomycoides false fungus-like.
Facultatively anaerobic, Gram-positive, nonmotile organisms forming ellipsoidal spores which lie paracentrally to subterminally in unswollen sporangia. Cells 1.0 by 3.0–5.0 µm, occurring singly and in short chains. Cells grown on glucose agar produce large amounts of storage material, giving a vacuolate or foamy appearance. Colonies are white to cream, opaque, and usually rhizoid. Growth temperature range 15–40°C, optimum 28°C. Grows at pH 5.7, in 7% NaCl, and in 0.001% lysozyme. Catalase-positive, oxidase-negative. Lecithinase and Voges–Proskauer reactions are positive. Citrate utilization variable; propionate not utilized. Nitrate is reduced to nitrite. Casein, starch and tyrosine are hydrolyzed. Acid without gas is produced from glucose and a limited range of other carbohydrates.
Phenotypically similar to Bacillus cereus and indistinguishable from Bacillus mycoides by conventional characters; distinguished from them by DNA relatedness and some differences in fatty acid composition. For distinguishing characters within the Bacillus cereus group, see the individual species descriptions and Table 8.
Source: mainly from soil.
DNA G + C content (mol%): 34.0–36.0 (T m).
Type strain : NRRL B-617, DSM 12442, LMG 18993.
EMBL/GenBank accession number (16S rRNA gene): AF013121 (NRRL B-617).
-
Bacillus psychrodurans Abd El-Rahman, Fritze, Spröer and Claus 2002, 2132VP
psy.chro.dur'ans. Gr. adj. psychros cold; L. pres. part. durans enduring; N.L. part. adj. psychrodurans cold-enduring.
Aerobic, Gram-positive, psychrotolerant rods 0.5–0.6 by 2.0–5.0 µm. Sporulation infrequently observed; spores lie terminally in swollen sporangia and are predominantly spherical in casein-peptone soymeal-peptone agar cultures and predominantly ellipsoidal in marine agar cultures. No growth or very poor growth in/on nutrient agar/broth. Minimum growth temperature −2–0°C and maximum 30–35°C. Catalase-positive. Starch, Tween 20, 40, 60 and 80 (type strain negative for Tween 80) are hydrolyzed. Gelatin usually hydrolyzed. Esculin, casein and urea not hydrolyzed. Grows in presence of 3 and usually 5%, but not 7% NaCl. Sensitive to 0.001% lysozyme. Will grow anaerobically with KNO3. Nitrate is reduced to nitrite. No acid or very weak acid production from glucose and other common carbohydrates; no gas produced. Cell-wall peptidoglycan type is L-Orn-D-Glu. Phenotypically similar to Bacillus insolitus and Bacillus psychrotolerans, and separable from these species by anaerobic growth with KNO3, nitrate reduction, and NaCl tolerance. See Table 9.
Source: garden soil in Egypt.
DNA G + C content (mol%): 36–37 (Bd) and 36.3 for the type strain.
Type strain : 68E3, DSM 11713, NCIMB 13837, KCTC 3793.
EMBL/GenBank accession number (16S rRNA gene): AJ277984 (DSM 11713).
-
Bacillus psychrosaccharolyticus (ex Larkin and Stokes 1967) Priest, Goodfellow and Todd 1989, 93VP (Effective publication: Priest, Goodfellow and Todd 1988, 1879.)
psy.chro.sacch'ar.o.lyt.ic.us. Gr. adj. psychros cold; Gr. n. sakchâron sugar; Gr. adj. lutikos able to dissolve; N.L. adj. psychrosaccharolyticus cold (adapted), sugar-fermenting.
Facultatively anaerobic, Gram-positive or variable, peritrichously motile, pleomorphic rods, which vary from coccoid to elongate, forming ellipsoidal spores which lie centrally or paracentrally in swollen sporangia; the spore may fill most of the sporangium and may lie laterally. Description is based on three strains. Cells range from 0.6–1.5 µm by 1.5–3.5 µm; normal size for cells grown on nutrient agar is 0.9–1.0 µm by 2.5–3.0 µm. Colonies are opaque and smooth. Growth occurs at 0–30°C; sporulates and germinates at 0°C. Grows between pH 6.0–7.2 and 9.5. Growth in presence of 2–5% NaCl varies. Nitrate is reduced. Casein, esculin, gelatin, pullulan, starch and urea are hydrolyzed. Acid without gas is produced from D-glucose and some other carbohydrates. Acetate, citrate, gluconate, malonate and succinate are not utilized.
Source: soil and lowland marshes.
DNA G + C content (mol%): 43–44 (T m).
Type strain : NRRL-B3394, DSM 13778, LMG 9580, NCIMB 11729, ATCC 23296, KCTC 3399.
EMBL/GenBank accession number (16S rRNA gene): AB021195 (ATCC 23296).
-
Bacillus psychrotolerans Abd El-Rahman, Fritze, Spröer and Claus 2002, 2131VP
psy.chro.tol'er.ans. Gr. adj. psychros cold; L. pres. part. tolerans tolerating; N.L. part. adj. psychrotolerans cold-tolerating.
Strictly aerobic, Gram-positive, psychrotolerant rods 0.4–1.0 by 2.0–7.0 µm. Sporulation infrequently observed; spores lie terminally in swollen sporangia and are predominantly spherical in cultures on casein-peptone soymeal-peptone agar containing Mn+ and predominantly ellipsoidal in marine agar cultures. No growth or very poor growth in/on nutrient agar/broth. Minimum growth temperature −2–0°C and maximum 30–40°C. Catalase-positive. Starch, and Tween 20, 40 and 60 are hydrolyzed. Tween 80 usually hydrolyzed. Gelatin usually not hydrolyzed. Esculin, casein and urea not hydrolyzed. Grows in presence of 3, usually not at 5%, and not 7% NaCl. Sensitive to 0.001% lysozyme. Will not grow anaerobically with KNO3. Nitrate is not reduced to nitrite. No acid or very weak acid production from glucose and other common carbohydrates; no gas produced. Cell-wall peptidoglycan type is L-Orn-D-Glu. Phenotypically similar to Bacillus insolitus and Bacillus psychrotolerans, and separable from these species by strict aerobic growth, inability to reduce nitrate, and NaCl tolerance. See Table 9.
Source: field soil in Germany.
DNA G + C content (mol%): 36–38 (Bd) and 36.5 for the type strain.
Type strain : 3H1, DSM 11706, NCIMB 13838, KCTC 3794.
EMBL/GenBank accession number (16S rRNA gene): AJ277983 (DSM 11706).
-
Bacillus pumilus Meyer and Gottheil in Gottheil 1901, 680AL
pu'mi.lus. L. adj. pumilus little.
Aerobic, Gram-positive or Gram-variable, motile, small rods 0.6–0.7 by 2.0–3.0 µm, occurring singly and in pairs, and forming cylindrical to ellipsoidal spores which lie centrally, paracentrally and subterminally in unswollen sporangia (Figure 3a). Cells grown on glucose agar stain evenly. Colonial morphology is variable; colonies may be wrinkled and irregular (Figure 2e), and they are unpigmented and most are smooth and opaque. Minimum growth temperature >5–15°C, maximum 40–50°C. Growth occurs at pH 6.0 and 9.5; some strains will grow at pH 4.5. Grows in presence of 10% NaCl. Catalase-positive. Casein, esculin and gelatin are hydrolyzed; starch is not hydrolyzed. Phenylalanine is not deaminated. Citrate is utilized as sole carbon source; propionate is not. Nitrate is not reduced. Voges–Proskauer-positive. Acid without gas is produced from glucose and from a wide range of other carbohydrates.
Source: soil and many other environments, including foods, and clinical and veterinary specimens.
DNA G + C content (mol%): 39.0–45.1 (T m) for 12 strains, 40.0–46.9 (Bd) for 25 strains, and to be 41.9 (T m), 40.7 (Bd) for the type strain.
Type strain : NCDO 1766, ATCC 7061, DSM 27, JCM 2508, NCIMB 9369.
EMBL/GenBank accession number (16S rRNA gene): X60637 (NCDO 1766).
Isolates of Bacillus pumilus from Antarctic soils and penguin rookeries show some phenotypic distinction from other strains of the species, including the production of a diffusible yellow pigment by some strains on initial culture (Logan and Forsyth, unpublished observations).
-
Bacillus pycnus Nakamura, Shida, Takagi and Komagata 2002, 504VP
pyc'nus. Gr adj. pyknos thick; N.L. adj. pycnus thick, referring to thick cells.
Aerobic, Gram-positive, motile rods, forming spherical spores which lie terminally in swollen sporangia. Cells are about 1.0–1.5 by 3.0–5.0 µm. Colonies are translucent, thin, smooth, circular and entire, and reach 1 mm in diameter after 24 h of incubation at 28°C. Optimum growth temperature 28–33°C, with minimum 5–10°C and maximum 40–45°C. Catalase-positive. Biotin, thiamin and cystine are not required for growth. Casein, starch, Tween 40 and 80, tyrosine and urea are not hydrolyzed. β-Hydroxybutyrate and pyruvate are oxidized; L-alanine, citrate and propionate are not oxidized. Does not grow in the presence of 5% NaCl or 0.001% lysozyme. Nitrate is not reduced to nitrite. No acid or gas produced from glucose and other common carbohydrates. Cell-wall peptidoglycan type is L-Lys-D-Glu. Phenotypically similar to Bacillus fusiformis, Bacillus neidei and Bacillus sphaericus, and separable from these species by growth factor requirements, several substrate oxidation and decomposition tests, and differences in fatty acid compositions. See Table 9.
Source: soil.
DNA G + C content (mol%): 35 (T m).
Type strain : NRRL NRS-1691, JCM 11075, DSM 15030, LMG 21634.
EMBL/GenBank accession number (16S rRNA gene): AF169531 (NRS-1691).
-
Bacillus schlegelii Schenk and Aragno 1981, 215VP (Effective publication: Schenk and Aragno 1979, 338.)
schle.gel'i.i. N.L. gen. n. schlegelii of Schlegel, named after H. G. Schlegel, a German bacteriologist.
Facultatively chemolithoautotrophic, thermophilic, strictly aerobic, motile, Gram-variable rods 0.6 by 2.5–5 µm, forming terminally located, spherical spores which swell the sporangia. Colonies are cream-colored, circular or spreading. No growth factors are required. Optimum growth temperature about 70°C; no growth at 37 or 80°C. Optimum pH for growth 6–7. Grows in presence of 3% but not 5% NaCl. Strictly respiratory, with oxygen as terminal electron acceptor. Nitrate is reduced to nitrite, but nitrate respiration does not occur. Catalase-positive and oxidase weakly positive. Grows chemolithoautotrophically, using H2 as electron donor and CO2 as carbon source, or CO which satisfies both requirements, or chemoorganoheterotrophically. Can also grow autotrophically on thiosulfate (Hudson et al., 1988). Hydrogenase is constitutive and has a temperature optimum between 70°C and 75°C. Carbohydrates are not metabolized. Utilizes acetate, butyrate, fumarate, propionate, succinate, phenol, 1-propanol and a small number of amino acids as sole carbon sources. Ammonium ions, asparagine and urea can be utilized as sole nitrogen sources. Casein is weakly hydrolyzed; gelatin, starch and urea are not hydrolyzed. It is unclear from phylogenetic studies as to whether this species still belongs in the genus Bacillus. See Tables 3 and 9.
Source: lake sediment, geothermal soils, and sugar factory sludge.
DNA G + C content (mol%): 62.3–65.4 (T m) on the basis of two studies, and 67.1–67.7 (Bd) in one study; that of the type strain is 64.4 (T m), 67.7 (Bd).
Type strain : Aragno MA-48, DSM 2000, LMG 7133, ATCC 43741, NCIMB 13107.
EMBL/GenBank accession numbers (16S rRNA gene): Z26934 (DSM 2000) and AB042060 (ATCC 43741); these 16S rDNA sequences differ considerably from each other, showing only 98.2% similarity.
-
Bacillus selenitireducens Switzer Blum, Burns Bindi, Buzzelli, Stolz and Oremland 2001, 29VP
se.le.ni.ti.re.du'cens. M.L. masc. gen. n. selenitis of selenite; L. part. adj. reducens reducing; M.L. part. adj. selenitireducens reducing selenite.
Facultatively anaerobic, nonmotile, non-spore-forming, Gram-positive rods 2–6 µm by 0.5 µm, which show weak microaerobic growth and anaerobic respiratory growth with Se(IV) (selenite), As(V) (arsenate), nitrate, nitrite, trimethylamine oxide and fumarate as electron acceptors. Description is based upon a single isolate. Quantitatively reduces Se(IV) to Se (0) (elemental selenium) during growth. Red colonies formed on lactate/selenite/yeast extract-supplemented lakewater medium incubated anaerobically at 20°C. Grows fermentatively with fructose, glucose or starch. Uses lactate, glucose and pyruvate as electron donors. Moderately halophilic, with salinity optimum of 24–60 g/l NaCl. Moderately alkaliphilic, with optimum growth in the range pH 8.5–10. See Table 2.
Source: arsenic-rich sediment of Mono Lake, California.
DNA G + C content (mol%) of the type strain: 49.0 (T m).
Type strain : MLS10, ATCC 700615, DSM 15326.
EMBL/GenBank accession number (16S rRNA gene): AF064704 (MLS10).
-
Bacillus shackletonii Logan, Lebbe, Verhelst, Goris, Forsyth, Rodríguez-Díaz, Heyndrickx and De Vos 2004b, 375VP
sha.ckle.ton'i.i. N.L. adj. shackletonii of Shackleton, referring to R.R.S. Shackleton, the ship used by the first British scientific expedition to visit Candlemas Island, the vessel being named in honor of the celebrated Antarctic explorer Sir Ernest Shackleton.
Aerobic, Gram-variable, motile, round-ended rods 0.7–0.9 by 2.5–4.5 µm occurring singly. Gram-positive reactions are only seen in cultures of 18 h or less at 30°C. Endospores are ellipsoidal, lie subterminally and occasionally paracentrally, and usually swell the sporangia. After 2 d on TSA colonies are 2–5 mm in diameter, have a granular appearance and butyrous texture, with opaque, cream-colored centers and translucent irregular margins. Minimum temperature for growth lies between 15°C and 20°C, the optimum temperature for growth is 35–40°C, and the maximum growth temperature is 50–55°C. Minimum pH for growth lies between 4.5 and 5.0, the optimum pH for growth is 7.0, and the maximum pH for growth lies between 8.5 and 9.0. Catalase-positive. Do not grow readily on casein agar, but when they do grow on it they may hydrolyze the casein. Starch is not hydrolyzed. At 30°C in the API 20E strip, o-nitrophenyl-β-D-galactopyranoside is hydrolyzed slowly, reactions for arginine dihydrolase, lysine decarboxylase, ornithine decarboxylase, citrate utilization, hydrogen sulfide production, urease, tryptophan deaminase, indole production, Voges–Proskauer reaction, gelatin hydrolysis, and nitrate reduction are negative. (In the API 20E strip incubated at 40°C, citrate may be utilized slowly, gelatin may be hydrolyzed slowly, and the Voges–Proskauer reaction may be positive). In the API 50 CH gallery hydrolysis of esculin is positive. Acid without gas is produced from the following carbohydrates: amygdalin, cellobiose, D-glucose, N-acetylglucosamine and salicin; weak acid reactions were detected for arbutin, D-fructose, galactose, β-gentiobiose, lactose, maltose, D-mannitol, D-mannose, ribose, D-tagatose and D-trehalose. The major cellular fatty acids are C15:0 anteiso, C15:0 iso, C16:0 iso and C17:0 anteiso (respectively representing about 35, 31, 6 and 18% of total fatty acids).
Source: unheated volcanic soil taken from the eastern lava flow of Candlemas Island, South Sandwich archipelago.
DNA G + C content (mol%): 35.4 (type strain) to 36.8 (HPLC).
Type strain : Logan SSI024, LMG 18435, CIP 107762.
EMBL/GenBank accession number (16S rRNA gene): AJ250318 (LMG 18435).
-
Bacillus silvestris Rheims, Frühling, Schumann, Rohde and Stackebrandt 1999, 800VP
sil.ves'tris. L. masc. adj. Silvestris of or belonging to a wood or forest, isolated from a forest.
Aerobic, Gram-positive, motile rods 0.5–0.7 by 0.9–2.0 µm, forming spherical spores which lie terminally in swollen sporangia. Description is based upon a single isolate. Colonies are whitish and shiny. Optimum growth temperature 20–30°C, with minimum of 10°C and maximum of 40°C. Catalase-positive, oxidase-negative. Casein, esculin, gelatin, starch and Tween 80 and tyrosine are not hydrolyzed. Citrate and propionate are not utilized as sole carbon sources. Grows in the presence of up to 5% NaCl. Does not grow in the presence of lysozyme. Nitrate is not reduced to nitrite. No acid or gas produced from, and no utilization of, glucose and other common carbohydrates. Cell-wall peptidoglycan contains lysine, glutamic acid and alanine. This cell-wall composition differentiates this species from members of the novel genus Lysinibacillus that has been proposed to accommodate Bacillus fusiformis, Bacillus sphaericus, and the novel species Lysinibacillus boronitolerans (Ahmed et al., 2007c). See Table 3.
Source: forest soil.
DNA G + C content (mol%): 39.3 (HPLC).
Type strain : HR3-23, DSM 12223, LMG 18991.
EMBL/GenBank accession number (16S rRNA gene): AJ006086 (HR3-23).
-
Bacillus simplex (ex Priest, Goodfellow and Todd 1989) Heyrman, Logan, Rodríguez-Díaz, Scheldeman, Lebbe, Swings, Heyndrickx and De Vos 2005a, 129VP
sim'plex. L. adj. simplex simple.
Rods are straight, 0.7–0.9 µm in diameter, round-ended or occasionally slightly tapered and occur in chains and sometimes singly or in pairs. Motile. Endospores are ellipsoidal, occasionally spherical, lie centrally, paracentrally or subterminally, and do not obviously swell the sporangia. Gram reaction is variable. Colonies on nutrient agar at 30°C, are 3–6 mm in diameter after 2 d, cream-colored, glossy, with irregular margins, slightly raised and umbonate. Most strains are strictly aerobic, although some strains may grow weakly on nutrient agar in anaerobic conditions. They grow at 20° and 30°C but are not able to grow at 45°C. Strains grow well at pH 7 and pH 9; growth at pH 5 is variable. Casein hydrolysis is variable and the medium becomes tinted brown. Starch is hydrolyzed. Tolerance of 5% NaCl (w/v) is variable and no growth occurs with 7% NaCl (w/v). Oxidase-negative, catalase-positive. ONPG, arginine dihydrolase, lysine and ornithine decarboxylase, hydrogen sulfide production, urease, indole and Voges–Proskauer are negative; citrate utilization is negative, but type strain is positive in API Biotype 100 citrate assimilation test. Gelatin hydrolysis variable. Nitrate is reduced to nitrite. Hydrolysis of esculin is variable and weak. Acid without gas is produced weakly, from D-fructose, N-acetylglucosamine, D-glucose, inulin, D-trehalose and sucrose. Acid is produced weakly and variably from salicin. Two biovars may be recognized: strains belonging to Bacillus simplex Biovar 1 produce acid weakly and variably from L-arabinose, mannitol, D-raffinose, ribose and sorbitol, while acid production is always negative from D-cellobiose, glycerol, maltose, meso-inositol, and D-xylose; strains of Bacillus simplex Biovar 2, produce acid weakly from L-arabinose, mannitol, D-raffinose, ribose, sorbitol, and D-xylose, and are variable for weak acid production from D-cellobiose, glycerol, maltose and meso-inositol. For the variable characters the type strain shows: weak or moderate acid production for L-arabinose, mannitol, ribose and sorbitol, and no acid from D-raffinose. The major cellular fatty acids are C15:0 anteiso and C15:0 iso, present at on mean 59.03 (±5.88) and 15.55 (±2.95)% of the total fatty acids, respectively.
Heyrman et al. (2005a) considered that strains of “Bacillus carotarum” and its suggested synonyms “Bacillus capri,” “Bacillus cobayae” and “Bacillus musculi,” and strains of “Bacillus maroccanus” and “Bacillus macroides” NCIMB 8796 (=NCDO=LMG 18508), should be reclassified as Bacillus simplex.
Source: soil.
DNA G + C content (mol%): 39.5–41.8 (T m).
Type strain : ATCC 49097, DSM 1321, LMG 11160, NRRL-NRS 960, IFO 15720.
EMBL/GenBank accession number (16S rRNA gene): AJ439078 (DSM 1321).
-
Bacillus siralis Pettersson, de Silva, Uhlén and Priest 2000, 2186VP
si.ra'lis. L. masc. n. sirus grain pit, silo; N.L. adj. siralis belonging to the silo.
Aerobic, Gram-positive rods 0.5–0.8 by 2.0–3.0 µm, forming ellipsoidal spores which lie subterminally to terminally in swollen sporangia. Colonies on brain heart infusion agar after 24 h are 3–5 mm in diameter, and are circular and entire, light brown to brown in color, with shiny, glistening and granular surfaces; on nutrient agar the colonies are smaller, pale and opaque. Maximum growth temperature 50°C. Catalase- and oxidase-positive. Grows in presence of 7% NaCl but not 10%. Nitrate is reduced to nitrite but not to dinitrogen; nitrate respiration positive. Casein, esculin and gelatin are hydrolyzed; starch is not. Citrate is not used as sole carbon source. Acid is not produced from glucose and other carbohydrates. Contains characteristic inserts of 49 bases in the distal region of the 16S rRNA genes.
Source: silage.
DNA G + C content (mol%): not reported.
Type strain : 171544, NCIMB 13601, CIP 106295, DSM 13140.
EMBL/GenBank accession number (16S rRNA gene): AF071856 (171544).
-
Bacillus smithii Nakamura, Blumenstock and Claus 1988, 70VP
smi'thi.i. N.L. gen. n. smithii named after Nathan R. Smith, American bacteriologist and Bacillus taxonomist.
Facultatively anaerobic, facultatively thermophilic, Gram-positive, motile rods 0.8–1.0 by 5.0–6.0 µm, forming ellipsoidal to cylindrical spores which lie terminally or subterminally in unswollen or slightly swollen sporangia. Colonies are unpigmented, translucent, thin, smooth, circular, entire, and about 2 mm in diameter. Growth temperature range 25–60 or 65°C. Catalase- and oxidase-positive. No growth in presence of 3% NaCl or 0.001% lysozyme. Nitrate is not reduced to nitrite. DNA and hippurate are hydrolyzed; starch is weakly hydrolyzed; esculin and pullulan hydrolysis is variable; casein, chitin, gelatin, tyrosine and urea are not hydrolyzed. Utilization of citrate and propionate as sole carbon sources is variable. Acid without gas is produced from glucose and a variable range of other carbohydrates.
Source: evaporated milk, canned foods, cheese, and sugar beet juice.
DNA G + C content (mol%): 38.1–40.4 (Bd), 38.7–39.7 (T m); that of the type strain is 40.2 (Bd).
Type strain : NRRL NRS-173, JCM 9076, LMG 12526, DSM 4216.
EMBL/GenBank accession number (16S rRNA gene): Z26935 (DSM 4216).
-
Bacillussoli Heyrman, Vanparys, Logan, Balcaen, Rodríguez-Díaz, Felske and De Vos 2004, 55VP
so'li. L. gen. n. soli of soil.
Facultatively anaerobic, Gram-positive or Gram-variable, motile, round-ended rods 0.6–1.2 µm in diameter, sometimes curved, occurring singly and in pairs and chains. Ellipsoidal endospores are borne paracentrally, and may swell the sporangia. On TSA, colonies are butyrous, cream-colored, low, slightly umbonate, and have entire margins and glossy or eggshell textured surfaces. The optimum temperature for growth is 30°C, and the maximum growth temperature lies between 40°C and 45°C. The optimum pH for growth is 7.0–8.0, and growth occurs from pH 5.0–4.0 to 9.0–9.5. Hydrolysis of casein is positive. In the API 20E strip, gelatin is hydrolyzed and nitrate reduction is positive; reactions for o-nitrophenyl-β-D-galactopyranoside hydrolysis, arginine dihydrolase, lysine decarboxylase, ornithine decarboxylase, citrate utilization, hydrogen sulfide production, urease, tryptophan deaminase, indole production, and Voges–Proskauer are negative. Hydrolysis of esculin positive. Acid without gas is produced from the following carbohydrates in the API 50 CH gallery using the CHB suspension medium: N-acetyl-D-glucosamine, D-fructose, D-glucose, glycogen, maltose (weak), D-mannose, ribose (weak), starch and D-trehalose (weak). Acid production from galactose and sucrose is variable, and weak when positive; type strain is weakly positive for galactose and negative for sucrose. The major cellular fatty acids are C15:0 iso and C15:0 anteiso, present at a level of about 43 and 34%, respectively.
Source: soil of the Drentse A agricultural research area, The Netherlands.
DNA G + C content (mol%): 40.1–40.4 (type strain, 40.1) (HPLC).
Type strain : LMG 21838, DSM 15604.
EMBL/GenBank accession number (16S rRNA gene): AJ542513 (LMG 21838).
-
Bacillus sonorensis Palmisano, Nakamura, Duncan, Istock and Cohan 2001, 1678VP
so.no.ren'sis. N.L. adj. sonorensis of the Sonoran, named after the Sonoran Desert, where the organism was found.
Facultatively anaerobic, Gram-positive, motile rods, forming ellipsoidal spores which lie subterminally in unswollen sporangia. Cells 1.0 by 2.0–5.0 µm, occurring singly and in pairs and short chains. Colonies are yellowish cream, with mounds and lobes of amorphous slime, and 2–4 mm in diameter after 2 d at 30°C; colonies on tyrosine agar are brown. Minimum growth temperature about 15°C and maximum about 55°C. Growth is inhibited by 5% NaCl and by 0.001% lysozyme. Citrate and propionate are utilized. Catalase-positive. Casein and starch are hydrolyzed. Nitrate is reduced to nitrite. Acid without gas is produced from glucose and other carbohydrates.
Phenotypically similar to Bacillus licheniformis and distinguishable from that species mainly by pigment production on tyrosine agar, certain gene sequences, enzyme electrophoresis, and DNA relatedness.
Source: desert soil.
DNA G + C content (mol%): 46.0 (T m).
Type strain : L87-10, NRRL B-23154, DSM 13779.
EMBL/GenBank accession number (16S rRNA gene): AF302118 (NRRL B-23154).
-
Bacillus sphaericus Meyer and Neide in Neide 1904, 337AL
sphae'ri.cus. L. adj. sphaericus spherical.
Aerobic, Gram-positive, motile rods, forming spherical spores which lie terminally in swollen sporangia (Figure 3f). Cells are about 1.0 by 1.5–5.0 µm. Colonies are opaque, unpigmented, smooth and often glossy, and usually entire. Minimum growth temperature 10–15°C and maximum 30–45°C. Grows at pH 7.0–9.5; some strains grow at pH 6.0. Catalase- and oxidase-positive. Biotin and thiamin are required for growth; cystine is not required. Tween 20 is hydrolyzed; casein, gelatin, Tween 80 and urea hydrolysis variable; starch, and tyrosine are not hydrolyzed. Phenylalanine is deaminated. Citrate is utilized as sole carbon source. Grows in the presence of 5% NaCl, but not in 7% NaCl. Nitrate is not reduced to nitrite. No acid or gas produced from glucose and other common carbohydrates. Cell-wall peptidoglycan type is L-Lys-D-Asp. See Table 9.
Ahmed et al. (2007c) proposed the transfer of this species to the new genus Lysinibacillus.
Source: soil and water, and a variety of other environments including foods, clinical specimens and mosquitoes.
DNA G + C content (mol%): 37.3 (T m), 38.3 (Bd) for the type strain.
Type strain : IAM 13420, ATCC 14577, CCM 2120, DSM 28, NCIMB 9370, LMG 7134.
EMBL/GenBank accession number (16S rRNA gene): AJ310084 (DSM 28). Two other 16S rDNA sequences in the EMBL/GenBank database, L14010 (ATCC 14577) and X60639 (NCDO 1767), are of poor quality.
Additional remarks: Nucleic acid studies have shown that Bacillus sphaericus is genetically heterogeneous, and have revealed six DNA relatedness groups (Krych et al., 1980; Rippere et al., 1997) and seven 16S rDNA sequence similarity groups (Nakamura et al., 2002); strains of three groups have been allocated to the species Bacillus fusiformis (Priest et al., 1988), Bacillus neidei and Bacillus pycnus (Nakamura et al., 2002). Bacillus sphaericus is phenotypically similar to Bacillus fusiformis, Bacillus neidei and Bacillus pycnus, and only separable from these species by growth factor requirements, several substrate oxidation and decomposition tests, and differences in fatty acid compositions. It is this lack of diagnostic characters that has hindered the recognition of the various molecularly defined groups as taxa of species rank.
Strains insecticidal for mosquitoes are found in DNA homology group IIA of Krych et al. (1980) (Rippere et al., 1997), and other taxonomic studies (see Priest, 2002) have confirmed the distinctness of the group. Serotyping (de Barjac et al., 1985) and phage typing (Yousten, 1984) schemes have been developed for group IIA. It must be emphasized that many members of the group are not mosquitocidal. Although strains in this group represent a distinct taxon, the lack of defining phenotypic characters has discouraged the proposal of a new species, and they remain allocated to Bacillus sphaericus; however, the name “B. culicivorans” has been suggested for the group (Priest, 2002). Recently, this species has been reclassified as Lysinibacillus sphaericus Ahmed et al. (2007c).
-
Bacillus sporothermodurans Pettersson, Lembke, Hammer, Stackebrandt and Priest 1996, 763VP
spo.ro.ther.mo.du'rans. Gr. n. sporos seed, spore; Gr. adj. thermos warm, hot; L. adj. part. durans resisting. N.L. adj. part. sporothermodurans with heat-resisting spores.
Aerobic, Gram-positive cells that usually occur as motile, thin rods in chains. Strains require vitamin B12 (cyanocobalamin) for satisfactory growth. After 2 d on brain heart infusion (BHI) agar supplemented with 5 mg/l MnSO4 and with 1 mg/l vitamin B12, colonies are 1–2 mm diameter, flat, circular, entire, beige or cream and smooth or glossy in appearance. They bear spherical to ellipsoidal endospores which lie in paracentral and subterminal, sometimes terminal, positions within slightly swollen and unswollen sporangia; the spores of the type strain, though scanty, are ellipsoidal, terminal, and do not swell the sporangia. Sporulation is infrequent but can be enhanced by using BHI-soil extract agar supplemented with vitamin B12 and MnSO4. Strains isolated from ultrahigh-temperature (UHT) treated (135–142°C for several seconds) milk grow poorly and sporulate poorly, but their spores show very high heat resistance and have the ability to survive ultraheat treatment. This very high heat resistance may decrease upon subculture. Isolates from farm environments may grow more readily than UHT milk isolates but be less heat resistant. Oxidase- and catalase-positive. Casein and starch are not hydrolyzed. Nitrate is reduced to nitrite, the Voges–Proskauer reaction is variable (type strain positive), citrate utilization is variable (type strain negative), hydrogen sulfide and indole are not produced, and the ONPG reaction is negative. Gelatin and esculin are hydrolyzed, urea is not. Growth may occur between 20°C and 55°C, with an optimum of about 37°C. Growth occurs between pH 5 and 9, and NaCl is tolerated up to 5%. Acid without gas is produced from N-acetylglucosamine, D-glucose, D-fructose, maltose, and from sucrose and D-trehalose by most strains, but reactions may be weak. Acid production from the following carbohydrates is variable: amygdalin, arbutin, D-cellobiose, gentiobiose, glycerol, mannitol, D-mannose, D-melezitose, methyl-D-glucoside, salicin, starch, D-tagatose, D-turanose and xylitol (weak). The type strain produces acid without gas from arbutin, D-cellobiose, glycerol, mannitol, D-melezitose, salicin, D-tagatose, D-turanose and xylitol, but not from amygdalin, gentiobiose, D-mannose, methyl-D-glucoside and starch.
Source: UHT-treated milk and dairy farm environments.
DNA G + C content (mol%): 36 (HPLC).
Type strain : M215, DSM 10559, LMG 17894, NCIMB 13600, KCTC 3777.
EMBL/GenBank accession number (16S rRNA gene): U49078 (M215).
-
Bacillus subterraneus Kanso, Greene and Patel 2002, 873VP
sub.ter.ra'ne.us. L. adj. subterraneus underground, subterranean, referring to the isolation source.
Facultatively anaerobic, Gram-negative, non-spore-forming, motile, curved rods, 0.5–0.8 by 2.0–25.0 µm, occurring singly and also in pairs and chains. Description is based upon a single isolate. After 2 d incubation at 40°C, colonies on nutrient agar are 0.5–1.2 mm in diameter, translucent and convex, with undulating irregular edges, while on tryptic soy agar they are dark yellow to orange, mucoid and rhizoid. Optimum growth temperature 37–40°C, temperature range for growth about 20–45°C. pH range for growth 6.5–9.0. Utilizes amorphous iron (III), manganese (IV), nitrate, nitrite and fumarate as electron acceptors in the presence of yeast extract, or certain carbohydrates, ethanol or lactate. Electron acceptors are not required for growth, but growth is better in the presence of nitrate. Yeast extract can be used as sole carbon and energy source. Growth occurs in the presence of up to 9% NaCl. Catalase-positive, oxidase-negative. Esculin, gelatin and starch are hydrolyzed; casein and urea are not hydrolyzed.
Source: deep subterranean waters of the Great Artesian Basin of Australia.
DNA G + C content (mol%): 43 ± 1 (T m).
Type strain : COOI3B, ATCC BAA-136, DSM 13966.
EMBL/GenBank accession number (16S rRNA gene): AY672638 (COOI3B).
-
Bacillus thermoamylovorans Combet-Blanc, Ollivier, Streicher, Patel, Dwivedi, Pot, Presnier and Garcia 1995, 15VP
ther.mo.a.my.lo.vo'rans. Gr. adj. thermos hot; Gr. n. amylum starch; L. v. vorare to devour; N.L. adj. thermoamylovorans utilizing starch at high temperature.
Facultatively anaerobic, moderately thermophilic, Gram-positive, slightly motile rods, 0.45–0.5 µm by 3.0–4.0 µm. Description based upon a single isolate. Endospores have not been detected; cells killed by heating at 80°C for 5 min. Colonies are white and lenticular, and 2–3 mm in diameter after 2 d. Optimum growth temperature about 50°C; maximum 58°C. Grows between pH 5.4 and 8.5, with optimum pH 6.5–7.5. Catalase-positive, oxidase-negative. Amylolytic. Nitrate is not reduced to nitrite. Vitamins and nucleic acid derivatives will stimulate growth, but are not essential. Acid without gas is produced from glucose, starch and a range of other carbohydrates; heterolactic fermentation of hexoses yields acetate, formate, lactate and ethanol. See Table 3.
Source: Senegalese palm wine.
DNA G + C content (mol%): 38.8 ± 0.2 mol% (HPLC).
Type strain : CNCM I-1378, strain DKP, LMG 18084.
EMBL/GenBank accession number (16S rRNA gene): L27478 (CNCM I-1378).
-
Bacillus thermocloacae Demharter and Hensel 1989a, 495 (Effective publication: Demharter and Hensel 1989b, 274.)
ther.mo.clo'a.cae. Gr. n. therme heat; L. n. cloaca sewer; N.L. gen. n. thermocloacae of a heated sewer.
Aerobic, moderately alkaliphilic and thermophilic, Gram-positive, nonmotile rods, 0.5–0.8 µm by 3.0–8.0 µm. Description is based upon three isolates. Spore formation only detected in one strain; ellipsoidal spores lie subterminally and terminally in swollen sporangia. Colonies are flat to convex, pale, transparent to opaque, and circular with entire or slightly lobed margins, and reach 2–5 mm in diameter after 1–2 d at 60°C. Optimum growth temperature 55–60°C; minimum 37°C and maximum 70°C. Optimum pH 8–9; no growth at pH 7. Grows in presence of up to 5% NaCl, but growth with 5% NaCl is weak. Catalase- and oxidase-positive. Casein, esculin, gelatin, starch and tributyrin not hydrolyzed. Voges–Proskauer-negative. Nitrate is not reduced to nitrite. No acid or gas are produced from glucose and other carbohydrates. See Tables 2 and 3.
Source: heat-treated sewage sludge.
DNA G + C content (mol%): 42.8–43.7 (T m), 41.7–42.1 (HPLC).
Type strain : S 6025, DSM 5250.
EMBL/GenBank accession number (16S rRNA gene): Z26939 (DSM 5250).
-
Bacillus thuringiensis Berliner 1915, 29AL
thur.in.gi.en'sis. N.L. masc. adj. thuringiensis of Thuringia, the German province from where the organism was first isolated.
Facultatively anaerobic, Gram-positive, usually motile rods 1.0–1.2 by 3.0–5.0 µm, occurring singly and in pairs and chains, and forming ellipsoidal, sometimes cylindrical, subterminal, sometimes paracentral, spores which do not swell the sporangia; spores may lie obliquely in the sporangia. Sporangia carry parasporal bodies adjacent to the spores; these crystalline protein inclusions (Figure 3h) may be bipyramidal, cuboid, spherical to ovoid, flat-rectangular, or heteromorphic in shape. They are formed outside the exosporium and readily separate from the liberated spore. They are known as δ-endotoxins or insecticidal crystal proteins, and are protoxins which may be toxic for certain insects and other invertebrates including flatworms, mites, nematodes and protozoa. The ability to synthesize parasporal bodies is plasmid borne, has been transferred to strains of Bacillus cereus and even to Bacillus pumilus (Selinger et al., 1998), and may be lost on subculture. Cells grown on glucose agar produce large amounts of storage material, giving a vacuolate or foamy appearance. Like those of Bacillus cereus, colonies are very variable in appearance, but nevertheless distinctive and readily recognized (Figure 2h): they are usually whitish to cream in color, large (2–7 mm in diameter), and vary in shape from circular to irregular, with entire to undulate, crenate or fimbriate edges; they usually have matt or granular textures, but smooth and moist colonies are not uncommon. Minimum temperature for growth is 10–15°C, and the maximum 40–45°C. Egg yolk reaction is positive. Catalase-positive, oxidase-negative. Casein, gelatin and starch are hydrolyzed. Voges–Proskauer-positive. Citrate is utilized as sole carbon source. Nitrate is reduced. Tyrosine is decomposed. Phenylalanine is not deaminated. Resistant to 0.001% lysozyme. Acid without gas is produced from glucose and a limited range of other carbohydrates. Some strains can produce diarrheal enterotoxin.
Phenotypically similar to other members of the Bacillus cereus group: Bacillus anthracis, Bacillus cereus, Bacillus mycoides, Bacillus pseudomycoides and Bacillus weihenstephanensis. Bacillus thuringiensis is distinguished by its characteristic parasporal crystals. Smith et al. (1952) and Gordon et al. (1973) considered Bacillus thuringiensis to be a variety of Bacillus cereus. For distinguishing characters within the Bacillus cereus group, see the individual species descriptions and Table 8.
Endospores are very widespread in soil and many other environments, and this organism has been isolated from all continents, including Antarctica. Although numerous strains are toxic to invertebrates, this property has not been demonstrated in many other strains. Natural epizootics do not seem to occur, and it has been suggested that the natural habitat of this organism is soil.
DNA G + C content (mol%): 33.5–40.1 (T m) for two strains; 35.7–36.7 (Bd) for four strains, and 33.8 (T m), 34.3 (Bd) for the type strain.
Type strain : IAM 12077, ATCC 10792, NRRL NRS-996, DSM 2046, LMG 7138, NCIMB 9134.
EMBL/GenBank accession number (16S rRNA gene): D16281 (IAM 12077).
Additional remarks: Bacillus thuringiensis has been divided on the basis of flagellar (H) antigens into 69 serotypes with 13 subantigenic groups, giving a total of 82 serovars (Lecadet et al., 1999); see also Antigenic Structure, above), but there is little correlation between serotype and insecticidal toxicity, the latter being mainly encoded by plasmids. Ribotyping data have shown good correlation with serotypes for 10 well known serovars (Priest et al., 1994); other approaches to subspecies analysis of Bacillus thuriengiensis are discussed by Lecadet et al. (1999).
-
Bacillus tusciae Bonjour and Aragno 1985, 223VP (Effective publication: Bonjour and Aragno 1984, 400.)
tus'cia.e. L. gen. n. tusciae from Tuscia, the Roman name for the region of central Italy where the organism was found.
Facultatively chemolithoautotrophic, moderately thermophilic, strictly aerobic, motile (by one lateral flagellum), Gram-positive rods 0.8 by 4–5 µm, forming subterminal, ellipsoidal spores which swell the sporangia. Description is based upon two isolates. The spreading colonies are creamy-white and chalky. Heavy autotrophic cultures form a yellow, water-soluble pigment. No growth factors are required. Strictly respiratory, with oxygen as terminal electron acceptor. Nitrate is reduced to nitrite, but nitrate respiration does not occur. Grows chemolithoautotrophically, using H2 as electron donor and CO2 as carbon source, or chemoorganoheterotrophically. Optimum growth temperature about 55°C; no growth at 35 or 65°C. Optimum pH for growth 4.2–4.8; weak growth at pH 3.5 and 6.0. No growth in presence of 1% NaCl. Catalase weakly positive and oxidase-positive. Carbohydrates are not metabolized. Starch is not hydrolyzed. Utilizes a few alcohols, amino acids and organic acids as sole carbon sources, with ammonium as the nitrogen source. Ammonium ions, asparagine and urea can be utilized as sole nitrogen sources. See Table 3.
Source: an acidic pond in a solfatara in Italy.
DNA G + C content (mol%): 57–58 (T m), and for the type strain 57.5 (T m).
Type strain : Aragno T2, DSM 2912, LMG 17940, IFO 15312.
EMBL/GenBank accession number (16S rRNA gene): AB042062 (IFO 15312).
-
Bacillus vallismortis Roberts, Nakamura and Cohan 1996, 474VP
val.lis.mor'tis. L. n. vallis valley; L. fem. n. mors death; N.L. gen. fem. n. vallismortis of Death Valley.
Aerobic, Gram-positive, motile rods, forming ellipsoidal spores which lie centrally or paracentrally in unswollen sporangia. Cells 0.8–1.0 by 2.0–4.0 µm, occurring singly and in short chains. Colonies are opaque, smooth, circular and entire and 1.0–2.0 mm in diameter after 2 d at 28°C. Optimum growth temperature 28–30°C, with minimum of 5–10°C and maximum of about 50°C. Catalase-positive, oxidase-positive. Citrate is utilized as a sole carbon source; propionate is not. Casein and starch are hydrolyzed. Tween 80 is decomposed weakly, phenylalanine and tyrosine are not decomposed. Nitrate is reduced to nitrite. Acid without gas is produced from glucose and a range of other carbohydrates.
Indistinguishable from Bacillus mojavensis, Bacillus subtilis subsp. subtilis and Bacillus subtilis subsp. spizizenii by conventional phenotypic tests, and distinguished from those organisms principally by DNA relatedness, by data from restriction digestion analyses of certain genes, and by fatty acid analysis. Phenotypically distinguishable from Bacillus atrophaeus only by failure to produce dark brown pigmented colonies on media containing tyrosine or other organic nitrogen source. See Table 7.
Source: desert soil.
DNA G + C content (mol%): 43.0 (T m).
Type strain : DV1-F-3, NRRL B-14890, DSM 11031, LMG 18725, KCTC 3707.
EMBL/GenBank accession number (16S rRNA gene): AB021198 (DSM 11031).
-
Bacillus vedderi Agnew, Koval and Jarrell 1996, 362 (Effective publication: Agnew, Koval and Jarrell 1995, 229.)
ved'deri. M.L. gen. n. vedderi of Vedder, named after A. Vedder, the Dutch microbiologist who described Bacillus alcalophilus in 1934.
Alkaliphilic, facultatively anaerobic, Gram-positive, motile, narrow rods forming ellipsoidal to spherical spores which lie terminally in swollen sporangia. Description is based upon a single isolate. Colonies are white, flat and circular, and 1.5 mm in diameter after 2 d growing on alkaline oxalate medium at 37°C. Optimum growth temperature 40°C; maximum 45–50°C. Optimal growth at pH 10.0; pH range 8.9–10.5. Grows in presence of 7.5% NaCl, but not 10% NaCl. Growth stimulated by presence of vitamins (in yeast extract). Catalase- and oxidase-positive. Pectin and birchwood xylan are hydrolyzed; gelatin and carboxymethylcellulose are weakly hydrolyzed; casein, starch and oakwood xylan are not hydrolyzed. Glucose and a small range of other carbohydrates can be utilized as sole sources of carbon. See Table 2.
Source: red mud bauxite-processing waste, using alkaline oxalate enrichment.
DNA G + C content (mol%): 38.3 (T m).
Type strain : JaH, DSM 9768, ATCC 7000130, LMG 17954, NCIM B 13458.
EMBL/GenBank accession number (16S rRNA gene): Z48306 (JaH).
-
Bacillus vietnamensis Noguchi, Uchino, Shida, Takano, Nakamura and Komagata 2004, 2119VP
vi.et.nam.en'sis. N.L. adj. vietnamensis referring to Vietnam, the country where the type strain was isolated.
Cells are rod-shaped, measuring 0.5–1.0 by 2.0–3.0 µm, Gram-positive and aerobic. They are motile with peritrichous flagella. Ellipsoidal spores develop centrally in the cells and sporangia are not swollen. Catalase and oxidase are produced. Nitrate reduction, indole production, arginine dihydrolase and urease are negative. Growth occurs in the presence of lysozyme. Casein, starch, DNA, esculin, gelatin, p-nitrophenyl β-D-galactopyranoside and tyrosine are hydrolyzed. Production of hydrogen sulfide is not detected on trypticase soy agar. Acid is produced from glycerol, D-ribose, D-glucose, D-fructose, mannitol, N-acetyl D-glucosamine, maltose, sucrose, trehalose, inulin, starch and glycogen; no acid is produced from erythritol, D-arabinose, L-arabinose, D-xylose, L-xylose, adonitol, methyl β-D-xyloside, galactose, D-mannose (NRRL B-14850 produces acid from this sugar), L-sorbose, rhamnose, dulcitol, inositol, sorbitol, methyl α-D-mannoside, methyl β-D-glucoside, amygdalin, arbutin, salicin, cellobiose, lactose, melibiose, melezitose, D-raffinose, xylitol, β-gentiobiose, D-turanose, D-lyxose, D-tagatose, D-fucose, L-fucose, D-arabitol, L-arabitol, D-gluconate, 2-ketogluconate and 5-ketogluconate. Assimilation is positive for glucose, D-mannitol, N-acetyl D-glucosamine, maltose, gluconate and DL-malic acid, and negative for L-arabinose, D-mannose, n-capric acid, citrate and adipic acid. Growth occurs at 0–15% (w/v) NaCl (optimum at 1%). The isolates are regarded as moderately halotolerant bacteria. Growth occurs at 10–40°C (optimum at 30–40°C) (16–3 and NRRL B-14850 grow at 50°C). Growth occurs at pH 6.5–10.0 but not at pH 6.0. DNA G + C content is 43–44 mol% (HPLC). The major fatty acid is C15:0 anteiso (48.3 ± 11.9%), with lesser C15:0 iso (16.2 ± 4.4%). The major quinone is MK-7. meso-Diaminopimelic acid is found in the cell walls. Strains have been isolated from Vietnamese fish sauce and from the Gulf of Mexico. Major cellular fatty acids are C15:0 anteiso (51.4%) and C15:0 iso (19.8%).
Source: Vientnamese fish sauce.
DNA G + C content (mol%): 43 (HPLC).
Type strain 15-1, JCM 11124, NRIC 0531, NRRL 23890.
GenBank/EMBL accession number (16S rRNA gene): AB099708.
-
Bacillus vireti Heyrman, Vanparys, Logan, Balcaen, Rodríguez-Díaz, Felske and De Vos 2004, 54VP
vi.re'ti. L. gen. n. vireti of a field.
Facultatively anaerobic, Gram-negative, motile, slightly curved, round-ended rods, 0.6–0.9 µm in diameter, occurring singly and in pairs. Do not produce endospores on TSA supplemented with 5 mg/l MnSO4, but sporulate on Bacillus fumarioli agar at pH 7 after 48 h. Endospores are ellipsoidal, lie in central, paracentral, or sometimes subterminal positions, and may swell the sporangia slightly; the ends of the sporangia may be slightly tapered. After 3 d of growth on TSA, colonies are dark cream-colored, circular, raised and up to 4 mm in diameter, with entire edges. Colonies have loose biomass and egg-shell textured surfaces. The optimum temperature for growth is 30°C, and the maximum growth temperature lies between 40°C and 45°C. Growth occurs from pH 4.0–5.0 to 7.0–7.5; the optimum lies at the upper end of this range. Casein is hydrolyzed. In the API 20E strip, gelatin is hydrolyzed and nitrate reduction is positive; o-nitrophenyl-β-D-galactopyranoside hydrolysis is variable, reactions for arginine dihydrolase, lysine decarboxylase, ornithine decarboxylase, citrate utilization, hydrogen sulfide production, urease, tryptophan deaminase, indole production, and Voges–Proskauer are negative. Hydrolysis of esculin positive. Acid without gas is produced from the following carbohydrates in the API 50 CH gallery using the CHB suspension medium: N-acetyl-D-glucosamine, D-fructose, L-fucose (weak), galactose (weak), D-glucose, glycogen, maltose, D-mannitol, D-mannose, methyl α-D-glucoside (weak), ribose (weak), starch, sucrose and D-trehalose. The following reactions are variable between strains and, when positive, are usually weak: gluconate, meso-inositol, methyl α-D-mannoside, rhamnose; type strain is weak for gluconate and methyl α-D-mannoside and negative for meso-inositol and rhamnose. The major cellular fatty acids are C15:0 iso and C15:0 anteiso, present at a level of about 47 and 34%, respectively.
Source: soil of Drentse A agricultural research area, The Netherlands.
DNA G + C content (mol%): 39.8–40.3 (type strain, 40.2) (HPLC).
Type strain : LMG 21834, DSM 15602.
EMBL/GenBank accession number (16S rRNA gene): AJ542509 (LMG 21834).
-
Bacillus weihenstephanensis Lechner, Mayr, Francis, Prüss, Kaplan, Wiessner-Gunkel, Stewart and Scherer 1998, 1380VP
we'ihen.ste'phan.en'sis. N.L. masc. adj. weihenstephanensis referring to Freising-Weihenstephan in Southern Germany, where the type strain was isolated.
Phenotypically similar to Bacillus cereus and distinguished from it by ability to grow at 7°C, inability to grow at 43°C, and by certain 16S rDNA signature sequences. Distinguished from Bacillus anthracis, Bacillus mycoides, B, pseudomycoides and Bacillus thuringiensis by the same characters that differentiate those species from Bacillus cereus. For distinguishing characters within the Bacillus cereus group, see the individual species descriptions and Table 8.
Source: pasteurized milk.
DNA G + C content (mol%): not reported, but can be expected to lie within the range reported for Bacillus cereus.
Type strain : DSM 11821, WSCB 10204, LMG 18989.
EMBL/GenBank accession number (16S rRNA gene): AB021199 (DSM 11821).
Additional remarks: Although pychrotolerance is an important distinguishing character of Bacillus weihenstephanensis, it must be appreciated that not all psychrotolerant organisms resembling Bacillus cereus are Bacillus weihenstephanensis (Stenfors and Granum, 2001), and the practical value of recognizing this close relative of Bacillus mycoides may be questioned.
Species Incertae Sedis
Claus and Berkeley (1986) listed 26 species incertae sedis in the First Edition of this Manual. 12 of these have been revived since then and, in some cases, transferred to other genera; either at the times of their revivals or later. Details are given in the species listings of the appropriate genera as follows: Bacillus amyloliquefaciens (Priest et al., 1987); Bacillus flexus (Priest et al., 1989); Bacillus laevolacticus (Andersch et al., 1994); Bacillus psychrosaccharolyticus (Priest et al., 1989); Aneurinibacillus aneurinilyticus (Heyndrickx et al., 1997; Shida et al., 1996); Anoxybacillus flavithermus (Pikuta et al., 2000a); Geobacillus thermocatenulatus (Golovacheva et al., 1975; Nazina et al., 2001); Geobacillus thermodenitrificans (Manachini et al., 2000; Nazina et al., 2001); Paenibacillus agarexedens (Uetanabaro et al., 2003); Paenibacillus apiarius (Nakamura, 1996); Paenibacillus larvae subsp. pulvifaciens (Heyndrickx et al., 1996c; Nakamura, 1984c); Paenibacillus thiaminolyticus (Nakamura, 1990; Shida et al., 1997). Many other names that have been proposed in the past for Bacillus species were discussed by Smith et al. (1952) and Gordon et al. (1973), and many were considered by these authors to be synonyms of established species. For comprehensive listings of such names the reader is referred to Index Bergeyana (Buchanan et al., 1966; Gibbons et al., 1981).
White et al. (1993) proposed the merger of the two species “Bacillus caldotenax” and “Bacillus caldovelox” (both Heinen and Heinen, 1972) within a revived Bacillus caldotenax, but found “Bacillus caldolyticus” (Heinen and Heinen, 1972) to show low homology with these two species. Sunna et al. (1997b) identified “Bacillus caldolyticus”, “Bacillus caldotenax” and “Bacillus caldovelox,” as members of Bacillus thermoleovorans on the basis of DNA homology studies, but this proposal has not been validated. In any case these species belong in the genus Geobacillus (see the chapter on Geobacillus). Polyphasic studies of “Bacillus longisporus,” “Bacillus nitritollens” and “Bacillus similibadius” (all Delaporte, 1972) have not revealed homogeneous groupings among strains inherited from Delaporte (Logan and De Vos, unpublished). Heyrman et al. (2005a) found “Bacillus carotarum” and “Bacillus maroccanus” to be synonyms of Bacillus simplex.
-
“Bacillus agrestis” Werner 1933, 468
The species was accepted as a synonym of Bacillus megaterium by Gordon et al. (1973) but differs from typical members of that species in that its cells are smaller (mean diameter <1.0 µm), poly-β -hydroxybutyrate is not formed, esculin is not hydrolyzed, and phenylalanine is not deaminated. This strain is not closely related to Bacillus megaterium sensu stricto (Hunger and Claus, 1981).
DNA G + C content (mol%): 37.4 (T m).
Representative strain: NRS 602 (DSM 1316).
-
“Bacillus aminovorans” den Dooren de Jong 1926, 157
Strictly aerobic, motile rods, 0.8–1.5 × 1.5–5.0 µm, Gram-positive in young cultures. Spherical endospores are borne centrally and paracentrally, and do not swell the sporangia. Maximum growth temperature 37°C. Feeble growth on nutrient agar, and better growth on trypticase peptone medium. Utilizes mono-, di- and trimethylamine and glucose. Fructose and maltose used by some strains. No growth with other carbohydrates, organic acids, or amino acids, except gluconate, glutamate, 3-hydroxybenzoate and citrate. Some strains utilize betaine. Farrow et al. (1994) did not find a specific relationship between this organism and the spherical spore-formers of Group 2 of Ash et al. (1991).
Source: Soil.
DNA G + C content (mol%): 40.4–41.8 (T m) for about 20 strains.
Original strain: ATCC 7046 (DSM 1314).
-
“Bacillus freudenreichii” (Miquel) Chester 1898, 110
This species was described by Claus and Berkeley (1986) as very similar to Bacillus (now Brevibacillus) brevis in nutritional requirements (Bornside and Kallio, 1956), morphology and physiology, but differing in that it produces a considerable titratable alkalinity in urea broth and is less tolerant of acid. Additionally, growth occurs in 5% NaCl broth and phenylalanine is deaminated.
Source: Soil, river water and sewage.
Representative strain: ATCC 7053.
-
“Bacillus macroides” Bennett and Canale-Parola 1965, 204
Originally described as “Lineola longa” (Pringsheim, 1950). This is organism is unreactive in routine phenotypic tests, and Claus and Berkeley (1986) considered that its characters conform to those of Bacillus sphaericus (with the one exception that its spore is frankly oval and scarcely distends the sporangium) and to Bacillus badius. Minimal nutritional requirements are a carbon energy source, NH4-N, thiamine, biotin and, in one strain, guanine. Carbon sources include various amino acids and C2–C5 n-fatty acids, but not sugars. Proteolytic action is not detected within 3 weeks. By comparing the sequences of the 16S–23S internal transcribed spacer region of representatives of 27 Bacillus species and 19 strains representing five other endospore-forming genera, Xu and Cote (2003) found that “Bacillus macroides” strain ATCC 12905 (=LMG 18508=NCDO 1661) showed phylogenetic relationships with Bacillus fusiformis and Bacillus sphaericus (now both reclassified in Lysinibacillus; Ahmed et al., 2007c). Heyrman et al. (2005a) reclassified “Bacillus macroides” strain NCIMB 8796 (=DSM 54=LMG 18474) as a strain of Bacillus simplex, and on the basis of subsequent work Heyrman et al. (unpublished) propose the revival of “Bacillus macroides” as Lysinibacillus macroides, for the single strain ATCC 12905.
Source: Cow dung, plant material decaying in water.
DNA G + C content (mol%): 37.6–38.9 (T m).
Representative strain: ATCC 12905, LMG 18474, DSM 54.
-
“Bacillus pacificus” Delaporte 1967, 3071
Cells oval, exceptionally large, measuring 1.5–2.1 × 2.7–3.4 µm, with capsules and lipid inclusions. Motile, with one or two flagella inserted at or near one pole, or both poles. Spores are ellipsoidal and 1.3–1.5 × 2.7–3.4 µm in size. Best medium reported is 0.1% tryptone in sea water; no growth occurs on ordinary nutrient agar. Growth good at 28–40°C but none at 4°C.Glucose broth reaches pH 6 in 10 d; no acetoin is formed. Gelatin is slowly liquefied. Nitrate is reduced to nitrite. Catalase-positive. Grows in 10% NaCl.
Source: Shore sand, Pacific ocean, California.
Original strain: ATCC 25098 NCIMB 1862.
-
“Bacillus xerothermodurans” Bond and Favero 1977, 159
Strictly aerobic rods, 0.7–1.2 by 1.6–2.8 µm, which are pleomorphic, especially in older cultures. Spores are spherical to oval, and swell the sporangia. Scanning electron microscopy of spores revealed a surface honeycomb pattern of polygonal depressions surrounded by straight ridges. Unusual ultrastructure with an irregular, thick outer spore coat composed of globular subunits and laminated inner spore coat containing up to nine distinct layers. Catalase-positive. Growth occurs in 10% NaCl broth. Potato starch is hydrolyzed. Reactions in other properties studied are negative. Cleaned spore preparations show extreme resistance to dry heat, the strain was isolated after heating dry samples at 125°C for 48 h.
Source: Sandy soil, Cape Kennedy, Florida.
Original strain: ATCC 27380, DSM 520.
Misclassified species
16S rRNA gene sequence comparison studies indicate that the following species are currently misclassified within Bacillus. They await formal proposals of transfer to other genera:
To transfer to Geobacillus
Bacillus thermantarcticus (nom. corrig. Bacillus thermoantarcticus [sic]) Nicolaus, Lama, Esposito, Manca, di Prisco and Gambacorta (1996). corrig. Bacillus thermantarcticus Nicolaus, Lama, Esposito, Manca, di Prisco and Gambacorta 2002, 3VP therm'ant.arct'ic.us. Gr. n. therme heat, N.L. adj. antarcticus from Antarctica, from Antarctic geothermal soil.
Aerobic, Gram-positive, motile rods, 0.6–2.0 µm wide and 3.0–5.0 µm long, with oval endospores which are borne terminally. Description is based on a single isolate. Colonies are opaque, flat and circular with entire margins. In stationary phase of growth an exopolysaccharide is produced. Catalase-negative, oxidase-positive. Temperature range for growth is 37–65°C, and optimal growth occurs at 63°C. Growth occurs in the pH range 5.5–9.0, and optimum is pH 6.0. Growth is weak in the presence of 2% NaCl but inhibited by 5% NaCl. Growth occurs on yeast extract. Glucose, trehalose and xylose can be utilized as sole carbon sources. Citrate and propionate are not utilized. Nitrate is not reduced. Gelatin and starch are hydrolyzed, but casein is not hydrolyzed. Hippurate and tyrosine are not degraded. Exo- and endocellular α-glucosidases, an intracellular alcohol dehydrogenase and an exocellular xylanase are produced. The major fatty acids at 60°C are C17:0 anteiso (36% of total), C17:0 iso (27%), C15:0 iso (15%) and C16:0 iso (13%) (Nicolaus et al., 1995).
DNA G + C content (mol%): 53.7.
Type strain: DSM 9572, strain M1.
EMBL/GenBank accession number (16S rRNA gene): not available.
To transfer to Paenibacillus
Bacillus edaphicus Shelobolina et al. 1998, 631VP (Effective publication: Shelobolina et al. 1997, 688.)
e.daph'ic.us Gr. n. edaphos ground; L. masc. suff. -icus adjectival suffix used with the sense of belonging to; N.L. adj. edaphicus living in soil.
Strictly aerobic, chemo-organotrophic, nonmotile regular rods 1–1.5 by 4–10 µm in size. Produce mucous, smooth, transparent, convex colonies with even edges, 0.8–2.0 cm in diameter, on synthetic media with carbohydrates but devoid of nitrogen sources. Cells grown on such media are surrounded by mucous capsules 7–12 µm thick. Colonies are smooth, moist, light and flat, but raised in the center, and 0.5 cm in diameter, and with even edges when grown on potato agar and on synthetic media with carbohydrates and ammonium nitrogen. No growth occurs on nutrient agar or in nutrient broth. On media containing ammonium nitrogen, ellipsoidal endospores (1–1.2 × 1.7–2.0 µm) with eight to ten longitudinal ridge-like protusions are formed. Cell-wall structure is of Gram-positive type. Catalase-positive. Glucose is not fermented, and nitrate is not reduced. Starch and tyrosine are hydrolyzed but gelatin is not liquefied. Sugars and polyols are used as carbon and energy sources, and acid is produced from a range of carbohydrates. Cells are resistant to lysozyme. Palmitic, anteisopentadecanoic and stearic acids are the most frequent in the cellular lipids. Temperature range for growth is 7–45°C.
Source: soil.
DNA G + C content (mol%): 54.6–56.5 (type strain, 56.4) (T m).
Type strain: DSM 12974, VKPM B-7517, strain T7.
EMBL/GenBank accession number (16S rRNA gene): AF006076.
Bacillus mucilaginosus Avakyan et al. 1998, 631VP (Effective publication: Avakyan et al. 1986, 480; emend. Shelobolina et al. 1998, 631VP; effective publication: Shelobolina et al. 1997, 688.)
mu.ci.la.gi.no'sus. L. masc. adj. mucilaginosus slimy.
Strictly aerobic, chemo-organotrophic, nonmotile, regular, round-ended rods, borne singly and 1–1.2 by 4–7 µm in size; they are surrounded by capsules. On potato agar colonies are light gray, smooth, even-edged, wet and shiny; they do not exceed 0.5 cm in diameter. On Ashby sucrose agar and on synthetic media with carbohydrates and ammonium nitrogen, colonies are convex, semitransparent, mucous, even-edged, of viscous consistency, and 0.5–1 cm in diameter. No growth occurs on nutrient agar or nutrient gelatin, or in nutrient broth. On media containing ammonium nitrogen, and on potato agar, oval endospores (1–1.2 × 1.7–2.0 µm) with nine longitudinal ridge-like protusions are formed. Spores are borne centrally and subterminally to give a fusiform appearance to sporangia. Sporulation does not occur on media lacking a nitrogen source. Cell-wall structure is of Gram-positive type, but Gram staining may yield varying results. Catalase-positive. Glucose is not fermented, and nitrate is not reduced. Starch is hydrolyzed, but tyrosine is not, and gelatin is not liquefied. Carbohydrates, polyols, and some organic acids are used as carbon and energy sources, and acid is produced from a range of carbohydrates. Cells are resistant to lysozyme. Anteisopentadecanoic, palmitic and stearic acids are the most frequent in the cellular lipids. Temperature range for growth is 10–45°C.
Source: soil.
DNA G + C content (mol%): 55.8 (T m).
Type strain: VKM B-1480D, VKPM B-7519.
EMBL/GenBank accession number (16S rRNA gene): AF006077.
Note added in proof
Between the completion of the manuscript and tables (in late 2004) and the time of going to press (late 2007), the following new Bacillus species names were validly published, and with one exception (on account of a short sequence for Bacillus tequilensis) they have been included in Figure 1:
Bacillus acideceler Peak et al. 2007, 2035VP
a.ci.de'ce.ler. N.L. neut. n. acidum, acid, L. masc. adj. celer, fast, N.L. masc. adj. acidiceler, fast-growing in acid.
Gram-positive, endospore-forming rod, isolated from a forensic specimen considered a credible threat of harboring anthrax.
DNA G + C content (mol%): 37.3.
Type strain: strain CBD 119, DSM 18954 and NRRL B-41736.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): DQ374637.
Bacillus acidicola Richard, Archambault, Rosselló-Mora and Tindall 2005, 2129VP
a.ci.di'co.la. N.L. n. acidum an acid; L. suff. -cola an inhabitant of a place, a resident; N.L. masc. n. acidicola an inhabitant of acidic environments.
Cells occur singly or in chains, and in liquid culture can form filamentous rods that are 1.0–1.3 µm wide.
Source: acidic Sphagnum peat bog.
DNA G + C content (mol%): 42.3.2 (HPLC).
Type strain: 10, DSM 14745, ATCC BAA-366, NRRL B-23453.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AF547209 (10).
Bacillus aerius Shivaji, Chaturvedi, Suresh, Reddy, Dutt, Wainwright, Narlikar and Bhargava 2006, 1471VP
ae'ri.us. L. masc. adj. aerius pertaining to the air, aerial.
Shows high 16S rRNA gene sequence similarity with Bacillus aerophilus, Bacillus licheniformis, Bacillus sonorensis and Bacillus stratosphericus.
Source: air sample collected at high altitude.
DNA G + C content (mol%): 45 T m.
Type strain 24K, MTCC 7303, JCM 13348.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AJ831843 (24K).
Bacillus aerophilus Shivaji, Chaturvedi, Suresh, Reddy, Dutt, Wainwright, Narlikar and Bhargava 2006, 1471VP
ae.ro.phi'lus. Gr. n. aêr air; Gr. adj. philos loving; N.L. masc. adj. aerophilus air-loving.
Shows high 16S rRNA gene sequence similarity with Bacillus aerius, Bacillus licheniformis, Bacillus sonorensis and Bacillus stratosphericus.
Source: air sample collected at high altitude.
DNA G + C content (mol%): 44 T m.
Type strain 28K, MTCC 7304, JCM 13347.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AJ831844 (28K).
Bacillus akibai Nogi, Takami and Horikoshi 2005, 2314VP
a.ki.ba'i. N.L. gen. n. akibai of Akiba, named after the Japanese microbiologist Teruhiko Akiba, who made fundamental contributions to the study of alkaliphilic bacteria.
Related to Bacillus krulwichiae.
Source: preparation of carboxymethyl cellulase.
DNA G + C content (mol%): 34.4 (HPLC).
Type strain 1139, JCM 9157, ATCC 43226.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AB043858 (1139).
Bacillus altitudinis Shivaji, Chaturvedi, Suresh, Reddy, Dutt, Wainwright, Narlikar and Bhargava 2006, 1472VP
al.ti'tu.di.nis. L. fem. n. altitudo altitude; L. fem. gen. n. altitudinis of altitude.
Shows high 16S rRNA gene sequence similarity with Bacillus pumilus.
Source: air sample collected at high altitude.
DNA G + C content (mol%): 43 (T m).
Type strain 41KF2b, MTCC 7306, JCM 13350.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AJ831842 (41KF2b).
Bacillus alveayuensis Bae, Lee and Kim 2005, 1214VP
al.ve.a.yu.en'sis. L. n. alveus trough; N.L. masc. adj. ayuensis pertaining to Ayu (as a locality); N.L. masc. adj. alveayuensis pertaining to the Ayu Trough in the Pacific Ocean.
Thermophile, growing at up to 65°C and growing optimally at 3% NaCl but inhibited by 5% NaCl.
Source: deep-sea sediment.
DNA G + C content (mol%): 38.7 (T m).
The type strain is TM1, KCTC 10634, JCM 12523.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AY605232 (TM1).
Bacillus arenosi Heyrman, Rodríguez-Díaz, Devos, Felske, Logan and De Vos 2005b, 115VP
ar.en.o'si. L. gen. n. arenosi of a sandy place.
Closely related to Bacillus arvi. Spherical endospores are borne terminally and swell the sporangia slightly; largely unreactive in routine biochemical tests.
Source: soil.
DNA G + C content (mol%): 35 (HPLC).
Type strain LMG 22166, DSM 16319.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AJ627212 (LMG 22166).
Comment: this species has recently been reclassified as Viridibacillus arenosi (Albert et al., 2007).
Bacillus arsenicus Shivaji, Suresh, Chaturvedi, Dube and Sengupta 2005, 1126VP
ar.sen.i'cus. N.L. masc. adj. arsenicus pertaining to arsenic.
Grows in the presence of 20 mM arsenate and 0.5 mM arsenite.
Source: arsenic ore.
DNA G + C content (mol%): 35 (T m).
Type strain Con a/3, MTCC 4380, DSM 15822, JCM 12167.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AJ606700 (Con a/3).
Bacillus arvi Heyrman, Rodríguez-Díaz, Devos, Felske, Logan and De Vos 2005b, 115VP
ar'vi. L. gen. n. arvi of a field.
Closely related to Bacillus arenosi. Spherical endospores are borne terminally and swell the sporangia slightly; acid is produced from few carbohydrates.
Source: soil.
DNA G + C content (mol%): 35 (HPLC).
Type strain LMG 22165, DSM 16317.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AJ627211 (LMG 22165).
Comment: this species has recently been reclassified as Viridibacillus arvi (Albert et al., 2007).
Bacillus axarquiensis Ruiz-Garcia, Quesada, Martínez-Checa, Llamas, Urdaci and Béjar 2005b, 1282VP
a.xar.qui.en'sis. N.L. adj. masc. axarquiensis pertaining to Axarquia, the Arabic name for the region surrounding the city of Málaga in Southern Spain.
Halotolerant, biosurfactant producer.
Source: brackish river sediment.
According to Wang et al. (2007b), Bacillus axarquiensis and Bacillus malacitensis are later heterotypic synonyms of Bacillus mojavensis.
DNA G + C content (mol%): 42.5 (T m).
Type strain CR-119, CECT 5688, LMG 22476.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AY603657 (CR-119).
Bacillus bogoriensis Vargas, Delgado, Hatti-Kaul and Mattiasson 2005, 901VP
bo.gor.i.en'sis. N.L. adj. bogoriensis pertaining to Lake Bogoria, a soda lake in Kenya.
Grows in pH range 1 and tolerates 2 M NaCl.
Source: soda lake.
DNA G + C content (mol%): 37.5 (HPLC).
Type strain: LBB3, ATCC BAA-922, LMG 22234.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AY376312 (LBB3).
Bacillus boroniphilus Ahmed, Yokota and Fujiwara 2007a, 893VP (Effective publication: Ahmed, Yokota and Fujiwara 2007c, 222.)
boron.i.phi'lus. N.L. n. boron -onis boron; Gr. adj. philos loving; N.L. masc. adj. boroniphilus boron-loving.
Boron is required for growth and more than 450 mM is tolerated. Also tolerates up to 7.0% (w/v) NaCl in the presence of 50 mM B in agar medium but grows optimally without NaCl.
From naturally boron-containing soil of Hisarcik area in the Kutahya Province, Turkey.
DNA G + C content (mol%): 41.1–42.2.
Type strain: T-15Z, ATCC BAA-1204, DAM 17376, IAM 15287, JCM 21738.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AB198719 (T-15Z).
Bacillus cellulosilyticus Nogi, Takami and Horikoshi 2005, 2314VP
cell.u.lo.si.ly'ti.cus. N.L. neut. n. cellulosum cellulose; Gr. adj. lutikos able to loosen, able to dissolve; N.L. masc. adj. cellulosilyticus cellulose-dissolving.
Grows at pH 0 with optimum of pH 0, and tolerates up to 12% NaCl.
Source: a cellulase preparation.
DNA G + C content (mol%): 39.6 (HPLC).
Type strain: N4, DSM 2522, JCM 9156, ATCC 21833, CCRC 15439, CIP 109017.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AB043852 (N4).
Bacillus cibi Yoon, Lee and Oh 2005c, 735VP
ci'bi. L. n. cibus -i food; L. gen. n. cibi of food.
Produces orange-yellow colonies, and ellipsoidal endospores are borne centrally or subterminally in swollen sporangia.
Source: a fermented seafood.
DNA G + C content (mol%): 45 (HPLC).
Type strain: JG-30, KCTC 3880, DSM 16189.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AY550276 (JG-30).
Bacillus chagannorensis Carrasco, Marquez, Xue, Ma, Cowan, Jones, Grant and Ventosa 2007, 2087VP
N.L. masc. adj. chagannorensis pertaining to Lake Chagannor.
A Gram-positive, moderately halophilic, spore-forming bacterium isolated from a soda lake, Lake Chagannor, in the Inner Mongolia Autonomous Region, China.
DNA G + C content (mol%): 53.8.
Type strain: CG-15, CCM 7371, CECT 7153, CGMCC 1.6292 and DSM 18086.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AM492159.
Bacillus decisifrondis Zhang, Xu and Patel 2007, 977VP
de.ci.si.fron'dis. L. part. adj. decisus thrown off, dead, died; L. n. frons frondis of/from foliage; N.L. gen. n. decisifrondis from thrown off decayed foliage.
Produces cream, round, smooth colonies, and cells are motile rods, producing subterminal spherical spores in swollen sporangia.
Source: soil underlying the decaying leaf litter of a slash pine forest located in south east Queensland, Australia.
DNA G + C content (mol%): 41±1 (T m).
Type strain: E5HC-32, DSM 17725, JCM 13601.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): DQ465405 (E5HC-32).
Bacillus foraminis Tiago, Pires, Mendes, Morais, da Costa and Veríssimo 2006, 2573VP
fo.ra'mi.nis. L. n. foramen -inis a hole; L. gen. n. foraminis from a hole.
Spores not observed and cells do not exhibit resistance to 80°C for 8 min.
Source: highly alkaline, non-saline groundwater.
DNA G C content (mol%): 43.1 (HPLC).
Type strain: CV53, LMG 23174, CIP 108889.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AJ717382 (CV53).
Bacillus hemicellulosilyticus Nogi, Takami and Horikoshi 2005, 2312VP
hem.i.cell.u.lo.si.ly'ti.cus. N.L. neut. n. hemicellulosum hemicellulose; Gr. adj. lutikos able to loosen, able to dissolve; N.L. masc. adj. hemicellulosilyticus hemicellulose-dissolving.
Grows at pH 1 with optimum of pH 10, and tolerates up to 12% NaCl.
Source: a hemicellulase preparation.
DNA G + C content (mol%): 36.8 (HPLC).
Type strain: C-11, JCM 9152, DSM 16731.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AB043846 (C-11).
Bacillus herbersteinensis Wieser, Worliczek, Kämpfer and Busse 2005, 2122VP
her.ber.stein'en.sis. N.L. masc. adj. herbersteinensis pertaining to Castle Herberstein in Styria, in which the chapel with the medieval wall painting is located from which the type strain was isolated.
Wide ranges of carbohydrates and organic acids are assimilated, but many amino acids are not assimilated and acid is not produced from most carbohydrates.
Source: medieval wall painting.
DNA G + C content (mol%): 36.2–36.9 (HPLC).
Type strain: D-1,5a, DSM 16534, CCM 7228.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AJ781029 (D-1,5a).
Bacillus humi Heyrman, Rodríguez-Díaz, Devos, Felske, Logan and De Vos 2005b, 116VP
hu'mi. L. gen. n. humi of earth, soil.
Ellipsoidal and sometimes spherical endospores are borne terminally and swell the sporangia slightly; acid is produced from a few carbohydrates.
Source: soil.
DNA G + C content (mol%): 37.5 (HPLC).
Type strain: LMG 22167, DSM 16318.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AJ627210 (LMG 22167).
Bacillus idriensis Ko, Oh, Lee, Lee, Lee, Peck, Lee and Song 2006, 2543VP
id.ri.en'sis. N.L. masc. adj. idriensis arbitrary specific epithet pertaining to IDRI, the Infectious Disease Research Institute, where this study was performed.
Related to Bacillus cibi.
Source: blood of a neonate with sepsis.
DNA G + C content (mol%): 41.2 (T m).
Type strain SMC 435, KCCM 90024, JCM 13437.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AY904033 (SMC 435).
Bacillus infantis Ko, Oh, Lee, Lee, Lee, Peck, Lee and Song 2006, 2543VP
in.fan'tis. L. gen. n. infantis of an infant, baby, the putative source of the type strain.
Related to Bacillus firmus.
Source: blood of a neonate with sepsis.
DNA G + C content (mol%): 40.8 (T m).
Type strain: SMC 435, KCCM 90025, JCM 13438.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AY904032 (SMC 435).
Bacillus koreensis Lim et al. 2006b, 62VP
ko.re.en'sis. N.L. masc. adj. koreensis pertaining to Korea.
Closest relative is Bacillus flexus.
Source: rhizosphere of willow.
DNA G + C content (mol%): 36 (HPLC).
Type strain BR030, KCTC 3914, DSM 16467.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AY667496 (BR030).
Bacillus kribbensis Lim et al. 2007, 2914VP
krib.ben'sis. N.L. masc. adj. kribbensis arbitrary name formed from the acronym of the Korea Research Institute of Bioscience and Biotechnology, KRIBB, where taxonomic studies on this species were performed.
Source: a field used for potato cultivation in Jeju, Korea.
DNA G + C content (mol%): 43.3 (HPLC).
Type strain: BT080, KCTC 13934, DSM 17871).
GenBank/EMBL/DDBJ accession number (16S rRNA gene): DQ280367 (BT080).
Bacillus lehensis Ghosh, Bhardwaj, Satyanarayana, Khurana, Mayilraj and Jain 2007, 241VP
le.hen'sis. N.L. masc. adj. lehensis pertaining to Leh, in India, where the type strain was isolated.
Colonies are circular, convex, smooth and pigmented creamish-yellow, and cells are aerobic, Gram-positive, motile rods producing subterminal oval spores in unswollen sporangia.
Source: soil collected from Leh, India.
DNA G + C content (mol%): 41, 4 (T m).
Type strain: MLB2, MTCC 7633, JCM 13820.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AY793550 (MLB2).
Bacillus litoralis Yoon and Oh 2005, 1947VP
li.to.ra'lis. L. masc. adj. litoralis of the shore.
Optimal growth in 2–3% NaCl; no growth without NaCl or with >11% NaCl.
Source: tidal sediment of Yellow Sea in Korea.
DNA G + C content (mol%): 35.2 (HPLC).
Type strain: SW-211, KCTC 3898, DSM 16303.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AY608605 (SW-211).
Bacillus macauensis Zhang, Fan, Hanada, Kamagata and Fang 2006, 352VP
ma.cau.en'sis. N.L. masc. adj. macauensis pertaining to Macau, the city where the type strain was isolated. Forms long, unbranched chains of cells; related to unnamed deep-sea isolates, Bacillus barbaricus and Bacillus megaterium.
Source: a drinking water treatment plant.
DNA G + C content (mol%): 40.8 (HPLC).
Type strain: ZFHKF-1, JCM 13285, DSM 17262.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AY373018 (ZFHKF-1).
Bacillus malacitensis Ruiz-Garcia, Quesada, Martínez-Checa, Llamas, Urdaci and Béjar 2005b, 1282VP
ma.la.ci.ten'sis. L. adj. masc. malacitensis pertaining to Flavia Malacita, the Roman name for Málaga in southern Spain.
Halotolerant; surfactant producing.
Source: brackish river sediment.
DNA G + C content (mol%): 41 (T m).
Type strain: CR-95, CECT 5687, LMG 22477.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AY603656 (CR-95).
Comment: this species is considered as a hetrotypic synonym of Bacillus mojavensis (Wang et al., 2007b).
Bacillus mannanilyticus Nogi, Takami and Horikoshi 2005, 2314VP
mann.an.i.ly'ti.cus. N.L. neut. n. mannanum mannan; Gr. adj. lutikos able to loosen, able to dissolve; N.L. masc. adj. mannanilyticus mannan-dissolving.
Produces yellow colonies; pH range for growth is 0 with optimum of pH 9.
Source: a β-mannosidase and β-mannanase preparation.
DNA G + C content (mol%): 37.4 (HPLC).
Type strain: AM-001, JCM 10596, DSM 16130.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AB043864 (AM-001).
Bacillus massiliensis Glazunova, Raoult and Roux 2006, 1487VP
mas.si.li.en'sis. L. masc. adj. massiliensis of Massilia, the ancient Greek and Roman name for Marseille, France, where the type strain was isolated.
Member of Bacillus sphaericus group, forming terminal spherical spores that swell the sporangia. Closely related to species that have been transferred to the novel genus Lysinibacillus (Ahmed et al., 2007c), but data on peptidoglycan composition and polar lipids are not available for Bacillus massiliensis, and so it has not been transferred to the new genus.
Source: cerebrospinal fluid.
DNA G + C content (mol%) not reported.
Type strain: 4400831, CIP 108446, CCUG 49529.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AY677116 (4400831).
Bacillus muralis Heyrman, Logan, Rodríguez-Díaz, Scheldeman, Lebbe, Swings, Heyndrickx and De Vos 2005a, 129VP
mu.ra'lis. L. masc. adj. muralis pertaining or belonging to walls.
Related to Bacillus simplex.
Source: mural painting in a church in Germany.
DNA G + C content (mol%): 41.2 (HPLC).
Type strain: LMG 20238, DSM 16288.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AJ628748 (LMG 20238).
Bacillus murimartini Borchert, Nielsen, Graber, Kaesler, Szewyck, Pape, Antrnikian and Schäfer 2007, 2892VP
mu.ri.mar.ti'ni. L. n. murus wall; N.L. gen. n. martini of Martin (masc. name of a saint); N.L. gen. n. murimartini from the wall of the (St) Martin church in Greene-Kreiensen, Germany.
Source: a church wall mural painting in Germany.
DNA G + C content (mol%): 39.6.
Type strain: type strain LMG 21005 and NCIMB 14102.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AJ880003.
Bacillus niabensis Kwon, Lee, Kim, Weon, Kim, Go and Lee 2007, 1910VP
niab.en'sis. N.L. masc. adj. niabensis arbitrary name formed from NIAB, the acronym for the National Institute of Agricultural Biotechnology, Korea, where taxonomic studies on this species were performed.
Colonies are yellowish-white, 2–3 mm in diameter, and circular with clear margins, and cells are motile, by means of single polar flagella. Forms ellipsoidal or oval spores that lie subterminally or terminally in swollen sporangia.
Source: cotton-waste composts in Suwon, Korea.
DNA G + C content (mol%): 37.7–40.9 (HPLC).
Type strain: 4T19, KACC 11279, DSM 17723.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AY998119 (4T19).
Bacillus okhensis Nowlan, Dodia, Singh and Patel 2006, 1076VP
ok.hen'sis. N.L. masc. adj. okhensis pertaining to Port Okha, a port of the Dwarka region in India, where the type strain was isolated.
Halotolerant and related to Bacillus krulwichiae; bears a subterminal tuft of flagella, but spores have not been detected.
Source: soil of natural saltpan.
DNA G + C content (mol%): 41 (T m).
Type strain: Kh101, JCM 13040, ATCC BAA-1137.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): DQ026060 (ATCC BAA-1137).
Bacillus oshimensis Yumoto, Hirota, Goto, Nodasaka and Nakajima 2005a, 910VP
o'shi.men.sis. N.L. masc. adj. oshimensis from Oshima, the region where the micro-organism was isolated.
Grows in 0–20% NaCl, with 7% NaCl optimal; grows from pH 7, with pH 10 optimal.
Source: soil.
DNA G + C content (mol%): 40.8 (HPLC).
Type strain: K11, JCM 12663. NCIMB 14023.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AB188090 (K11).
Bacillus panaciterrae Ten, Baek, Im, Liu, Aslam and Lee 2006, 2864VP
pa.na.ci.ter'rae. N.L. n. Panax -acis scientific name for ginseng; L. n. terra soil; N.L. gen. n. panaciterrae of soil of a ginseng field.
Utilizes a wide range of carbohydrates, amino acids and organic acids, and hydrolyzes chitin; forms ellipsoidal endospores centrally in swollen sporangia.
Source: soil.
DNA G + C content (mol%): 47.8 (HPLC).
Type strain: Gsoil 1517, KCTC 13929, CCUG 52470, LMG 23408.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AB245380 (Gsoil 1517).
Bacillus patagoniensis Olivera et al. 2005, 446VP
pa.ta.go'ni.en.sis. N.L. masc. adj. patagoniensis pertaining to Patagonia, in Argentina, where the type strain was isolated.
Alkalitolerant and halotolerant.
Source: desert soil rhizosphere.
DNA G + C content (mol%): 39.7 (HPLC).
Type strain: PAT 05, DSM 16117, ATCC BAA-965.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AY258614 (PAT 05).
Bacillus plakortidis Borchert, Nielsen, Graber, Kaesler, Szewyck, Pape, Antranikian and Schäfer 2007, 2892VP
Source: material from the sponge Plakortis simplex that was obtained from the Sula-Ridge, Norwegian Sea.
DNA G + C content (mol%): 41,1.
Type strain: P203,DSM 19153 and NCIMB 14288.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AJ880003.
Bacillus pocheonensis Ten, Baek, Im, Larin, Lee, Oh and Lee 2007, 2535VP
N.L. masc. adj. pocheonensis pertaining to Pocheon Province in South Korea.
A Gram-positive, nonmotile, endospore-forming rod.
Source: soil of a ginseng field in Pocheon Province, South Korea.
DNA G + C content (mol%): 44.9.
Type strain: Gsoil 420, KCTC 13943 and DSM 18135.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AB245377.
Bacillus qingdaonensis Wang, Li, Liu, Cao, Li and Guo 2007c, 1146VP
qing.da.o.nen'sis. N.L. masc. adj. qingdaonensis pertaining to Qingdao, the name of the place from which the type strain was isolated.
A moderately haloalkaliphilic, aerobic, rod-shaped, nonmotile, Gram-positive organism capable of growth at salinities of 2.5–20% (w/v) NaCl. Spores were not observed.
Source: a crude sea-salt sample collected near Qingdao in eastern China.
DNA G + C content (mol%): 48 (HPLC).
Type strain: CM1, CGMCC 1.6134, JCM 14087.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): DQ115802 (CM1).
Bacillus ruris Heyndrickx, Scheldeman, Forsyth, Lebbe, Rodríguez-Díaz, Logan and De Vos 2005, 2553VP
ru'ris. L. neut. n. rus the country, the farm; L. gen. n. ruris from the country, the farm.
Related to Bacillus galactosidilyticus.
Source: raw milk and dairy cattle feed concentrate.
DNA G + C content (mol%): 39.2 (HPLC).
Type strain: LMG 22866. DSM 17057.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AJ535639 (LMG 22866).
Bacillus safensis Satomi, La Duc and Venkateswaran 2006, 1739VP
sa.fen'sis. N.L. masc. adj. safensis arbitrarily derived from SAF, the spacecraft-assembly facility at the Jet Propulsion Laboratory, Pasadena, CA, USA, from where the organism was first isolated.
Closely related to Bacillus pumilus on basis of 16S rRNA and gyrB gene sequences.
Source: a spacecraft-assembly plant.
DNA G + C content (mol%): 41.1.4 (HPLC).
Type strain: FO-36b, ATCC BAA-1126, NBRC 100820.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AF234854 (FO-36b).
Bacillus salarius Lim et al. 2006c, 376VP
sa.la'ri.us. L. masc. adj. salarius of or belonging to salt.
Member of the alkaliphilic group (Group 6 of Nielsen et al., 1994) of Bacillus; grows at 0% NaCl, with optimum of 12% NaCl, and at pH 6.5 with optimum pH of 8.
Source: sediment of a salt lake.
DNA G + C content (mol%): 43 (HPLC).
Type strain: BH169, KCTC 3912, DSM 16461.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AY667494 (BH169).
Bacillus saliphilus Romano, Lama, Nicolaus, Gambacorta and Giordano 2005a, 162VP
sal.i.phi'lus. L. n. sal salt; Gr. adj. philos loving; N.L. masc. adj. saliphilus salt-loving.
A coccoid member of the alkaliphilic group (Group 6 of Nielsen et al., 1994) of Bacillus; grows at 5% NaCl, with optimum of 16% NaCl, and at pH 0 with optimum pH of 9. Spores not reported.
Source: green algal mat in a mineral pool.
DNA G + C content (mol%): 48.4 (HPLC).
Type strain: 6AG, DSM 15402, ATCC BAA-957.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AJ493660 (6AG).
Bacillus selenatarsenatis Yamamura, Yamashita, Fujimoto, Kuroda, Kashiwa, Sei, Fujita and Ike 2007, 1063VP
se'le.nat.ar.se.na'tis. N.L. gen. n. selenatis of selenate; N.L. gen. n. arsenatis of arsenate; N.L. gen. n. selenatarsenatis of selenate and arsenate.
Gram-positive, spore-forming, motile rods. Colonies are round and white. Selenate is reduced to elemental selenium via the intermediate selenite, arsenate to arsenite and nitrate to ammonia via the intermediate nitrite.
Source: an effluent drain in a glass-manufacturing plant in Japan.
DNA G + C content (mol%): 42.8 (HPLC).
Type strain: SF-1, JCM 14380, DSM 18680.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AB262082 (SF-1).
Bacillus seohaeanensis Lee, Lim, Park, Jeon, Li and Kim 2006a, 1896VP
seo.hae.an.en'sis. N.L. masc. adj. seohaeanensis of Seohaean, the Korean name for the west coast of Korea, where the type strain was isolated.
Related to Bacillus aquimaris and Bacillus marisflavi.
Source: a solar saltern.
DNA G + C content (mol%): 39 (HPLC).
Type strain: BH724, KCTC 3913, DSM 16464.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AY667495 (BH724).
Bacillus stratosphericus Shivaji, Chaturvedi, Suresh, Reddy, Dutt, Wainwright, Narlikar and Bhargava 2006, 1471VP
stra.to.sphe.ri'cus. N.L. fem. n. stratosphera stratosphere; L. suff. -icus adjectival suffix used with the sense of belonging to; N.L. masc. adj. stratosphericus belonging to the stratosphere.
Shows high 16S rRNA gene sequence similarity with Bacillus aerius, Bacillus aerophilus, Bacillus licheniformis and Bacillus sonorensis.
Source: air sample collected at high altitude.
DNA G + C content (mol%): 44 (T m).
Type strain: 41KF2a, MTCC 7305, JCM 13349.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AJ831841 (41KF2a).
Bacillus taeanensis Lim, Jeon and Kim 2006a, 2905VP
tae.an.en'sis. N.L. masc. adj. taeanensis belonging to Taean, where the organism was isolated.
Neutrophilic and halotolerant, with optimum growth at 2–5% NaCl.
Source: solar saltern.
DNA G + C content (mol%): 36 (HPLC).
Type strain: BH030017, KCTC 3918, DSM 16466.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AY603978 (BH030017).
Bacillus tequilensis Gatson, Benz, Chandrasekaran, Satomi, Venkateswaran and Hart 2006, 1481VP
te.qui.len'sis. N.L. masc. adj. tequilensis referring to Tequila, Mexico.
Member of the Bacillus subtilis group.
Source: Mexican shaft tomb sealed in approximately 74 AD.
DNA G + C content (mol%): not reported.
Type strain: 10b, ATCC BAA-819, NCTC 13306.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AY197613: AY197613 (10b); the sequence contains only 549 bp and was therefore not included in the phylogenetic tree (Figure 1).
Bacillus thioparans Pérez-Ibarra, Flores and Garica-Varela 2007a, 1933VP (Effective publication: Pérez-Ibarra, Flores and Garica-Varela 2007b, 295.)
thi.o'parus. Gr. n. thios sulfur; L. v. paro to produce; M.L. adj. thioparus sulfur-producing.
Source: a continuous wastewater treatment culture system operating with a bacterial consortium. Gram-variable, aerobic, moderately halotolerant, motile and endospore-forming rods.
DNA G + C content (mol%): 43.8 (T m).
Type strain: BMP-1, BM-B-436 and CECT 7196.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): DQ371431.
Bacillus velezensis Ruiz-Garcia, Béjar, Martínez-Checa, Llamas and Quesada 2005a, 195VP
vel.e.zen'sis. N.L. adj. masc. velezensis pertaining to Vélez, named thus for being first isolated from the river Vélez in Málaga, southern Spain.
Member of the Bacillus subtilis group.
Source: mouth of River Vélez, Spain.
DNA G + C content (mol%): 46.6.4 (T m).
Type strain: CR-502, CECT 5686, LMG 22478.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AY603658 (CR-502).
Bacillus wakoensis Nogi, Takami and Horikoshi 2005, 2312VP
wa.ko.en'sis. N.L. masc. adj. wakoensis of Wako, a city in Japan.
Related to Bacillus krulwichiae.
Source: preparation of cellulase.
DNA G + C content (mol%): 38.1 (HPLC).
Type strain: N-1, JCM 9140, DSM 2521.
GenBank/EMBL/DDBJ accession number (16S rRNA gene): AB043851 (N-1).



