Magnesium Bromide
Abstract
(MgBr2) [2923-28-6] Br2Mg (MW 184.11) InChI = InChI=1S/2BrH.Mg/h2*1H;/q;;+2/p-2 InChIKey = OTCKOJUMXQWKQG-UHFFFAOYSA-L (MgBr2·6H2O) [13446-53-2] H12Br2MgO6 (MW 292.23) InChI = 1S/2BrH.Mg.6H2O/h2*1H;;6*1H2/q;;+2;;;;;;/p-2 InChIKey = LGLXXNHIGIJYQQ-UHFFFAOYSA-L (MgBr2·OEt2) [29858-07-9] C4H10Br2MgO (MW 258.25) InChI = 1S/C4H10O.2BrH.Mg/c1-3-5-4-2;;;/h3-4H2,1-2H3;2*1H;/q;;;+2/p-2 InChIKey = JGZKUKYUQJUUNE-UHFFFAOYSA-L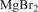
(Lewis acid capable of catalyzing selective nucleophilic additions,1 cycloadditions,2 rearrangements,3 coupling reactions;4 effective brominating agent5)
Physical Data: mp 165 °C (dec) (etherate >300 °C; fp 35 °C).
Solubility: sol alcohol, H2O; etherate sol common organic solvents.
Form Supplied in: white solid, widely available; etherate gray solid.
Preparative Method: the etherate is easily prepared from reacting a slight excess of Magnesium turnings with 1,2‐Dibromoethane in anhydrous diethyl ether.13
Handling, Storage, and Precautions: etherate is flammable and moisture sensitive; freshly prepared material is most reactive and anhydrous. Irritant.



