The role of selectins and integrins in adenovirus vector-induced neutrophil recruitment to the liver
Abstract
Adenovirus vectors for human gene therapy induce early host inflammatory responses in transduced tissues that limit gene transfer efficiency and can result in significant morbidity. The present study aimed to elucidate the cellular mechanisms underlying the acute inflammation induced by adenovirus vectors in the liver. Leukocyte rolling and adhesion in response to an intravenously administered adenovirus vector was examined by intravital microscopy in mouse liver. Adenovirus vectors significantly increased leukocyte rolling and adhesion in the postsinusoidal venules within minutes of transduction. Unlike other inflammatory states in the liver, no leukocyte retention was seen in the sinusoids in response to adenovirus vector administration. Inhibition of P-selectin, α4-integrin, and E-selectin was necessary to completely block leukocyte rolling and subsequent adhesion. The administration of an anti-α4-integrin antibody alone significantly reduced leukocyte adhesion. In contrast, adenovirus vector-induced leukocyte adhesion was unchanged in CD18-knockout mice. Depletion of circulating neutrophils eliminated leukocyte rolling and adhesion in response to adenovirus vector transduction in the liver. In conclusion, adenovirus vectors induce rapid neutrophil-mediated inflammation in the post-sinusoidal venules by selectins and α4-integrin but surprisingly not by CD18.
Abbreviation:
-
- AdGFP:
-
Serotype 5 adenovirus vector encoding green fluorescent protein
1 Introduction
A key pathological event during acute inflammation is the recruitment of leukocytes to afflicted sites. The recruitment of leukocytes involves the movement of these cells from the intravascular compartment to the extravascular space and is mediated by three sequential events. First, circulating leukocytes in the bloodstream make initial contact with the endothelium that manifests as tethering and rolling along the length of the venule. Second, rolling leukocytes are activated to firmly adhere to the endothelium by various pro-inflammatory mediators. Once firmly adherent, leukocytesare able to perform the third and final step, emigration out of the vasculature. The selectins (L-, P-, and E-selectin) mediate the initial tethering and rolling of leukocytes to the vascular endothelium, whereas firm adhesion of leukocytes to the endothelial wall is mediated by interaction of leukocyte integrins, such as CD18 (β2-integrin), with cell surface molecules such as ICAM-1 or ICAM-2 present on the endothelium 1, 2. These interactions are followed by transendothelial migration and chemotaxis of leukocytes to the site of inflammation 1, 2. α4-Integrin expressed on leukocytes is unique among the integrins in its ability to support both leukocyte rolling and leukocyte adhesion, in vitro and in vivo 3, 4. While α4-integrin is generally thought to mediate monocyte, lymphocyte and eosinophil-endothelial cell interactions, neutrophil adhesion via α4-integrin occurs only in chronic or systemic inflammatory states 4, 5.
Neutrophils participate in inflammatory responses by phagocytosing and killing pathogenic organisms. There are a number of situations where liver injury results from inappropriate neutrophil recruitment and activation 6, 7, 8. In these states, the recruitment of neutrophils to the liver is in part dependent upon selectins and integrins. P-selectin expression is found in the portal veins and postsinusoidal venules and mediates the neutrophil margination in these vasculatures in murine endotoxin shock 9. CD18 (β2-integrin) mediates neutrophil adhesion and plays an important role in the subsequent neutrophil-induced liver cell injury in endotoxin shock 8. In contrast to the postsinusoidal venules, neutrophil recruitment in the liver sinusoids appears to occur independent of selectins and integrins 9, 10. An understanding of the biology of leukocyte recruitment into the different vascular compartments of the liver is therefore essential to develop potential for anti-adhesive therapies for hepatic inflammatory diseases.
Adenoviridae are being developed as agents for gene and cancer therapies in humans. Recombinant adenovirus vectors have several advantages including broad cell tropism, ease of production in high titer and a capacity to transfer large amounts of DNA. However, host inflammatory and immune responses limit the efficacy of adenovirus vectors resulting in transient transgene expression and ultimate vector loss 11, 12. Adenovirus vectors activate both innate and adaptive arms of the immune system. Adaptive immune responses to adenovirus vectors occur several days following transduction and are largely dependent on viral gene transcription 12. On the other hand, adenovirus-induced innate responses are triggered early and occur independent of virus transcription 13. The liver is the major target of intravenously administered adenovirus vectors 14, 15. Thus, the innate arm of the immune system is efficiently activated in this organ following transduction with adenovirus vectors in vivo inducing the expression of numerous cytokines/chemokines and the recruitment of immune effector cells (neutrophils, natural killer cells and monocyte-macrophages) to the liver 13. Activation of the innate system leads to the rapid elimination of the delivered adenovirus vector and the accompanying inflammation results in acute hepatic toxicity 16. Although many inflammatory genes are up-regulated following adenovirus vector transduction of the liver, little is known regarding the cellular mechanisms that mediate the recruitment of effector leukocytes to this organ in response to these agents. Understanding the biology of the early host innate response to adenovirus vectors is essential to improve the safety and effectiveness of adenovirus-mediated gene therapy. The present study aimed to define the role of selectins and integrins in the biology of the innate response to adenovirus vectors in the liver.
2 Results
2.1 Adenovirus vector induced leukocyte-endothelial interactions in the liver
Adenovirus vectors induce acute inflammation in the liver; however, little is known about the biology of leukocyte-endothelial interactions following transduction with these agents. To directly visualize the effect of adenovirus vector administration on leukocyte recruitment to the liver we employed intravital microscopy in C57BL/6 mice. Under baseline condition (0 min), we observed a low basal level of rolling (Fig. 1A) but not adhering (Fig. 1B) leukocytes in postsinusoidal venules of the liver. No leukocytes were seen in liver sinusoids under basal conditions. The intravenous administration of virus vehicle alone did not increase basal leukocyte rolling or adhesion over 120 min of observation. Administration of serotype 5 adenovirus vector encoding green fluorescent protein (AdGFP; 2.5×1011 particles/animal) very rapidly (in minutes) and significantly increased leukocyte rolling in the postsinusoidal venules over 120 min compared with either baseline values (0 min) or the corresponding vehicle control (p<0.01; Fig. 1A). AdGFP also increased leukocyte adhesion in the postsinusoidal venules in a time-dependent manner over the 120 min experiment. This increase was significantly higher compared with either baseline (0 min) or the corresponding controls (Fig. 1B).
Surprisingly, no increase in leukocyte sequestration was noted in sinusoids following the administration of AdGFP. This was in direct contrast to the response to tumor necrosis factor-alpha (TNF-α), which induced a significant increase in leukocyte number in the liver sinusoids (p<0.001; Fig. 2). Since leukocyte trapping in the sinusoids is seen in most inflammatory models studied to date 9, 10, 17, the lack of this phenomenon appears to be unique to the inflammatory response induced by adenovirus vectors.
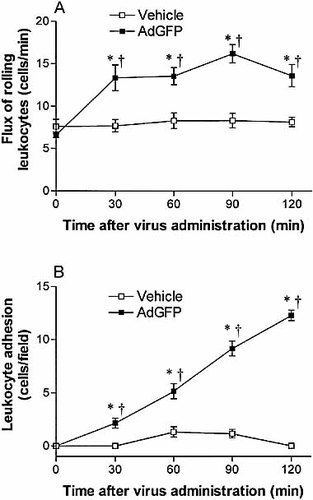
Flux of rolling neutrophils (A) and number of adherent neutrophils (B) in response to AdGFP administration in the liver postsinusoidal venules of C57BL/6 mice (wild type) over 120 min. Data are expressed as mean ± SEM of five animals. *;p<0.05 compared with 0 min. †p<0.05 compared with corresponding vehicle controls.
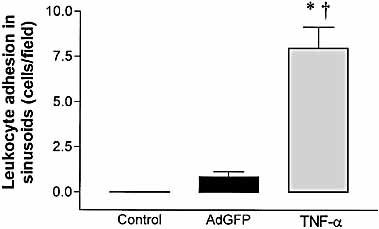
Number of adherent leukocytes in response to AdGFP or TNF-α in the liver sinusoids of C57BL/6 mice (wild type). Data are expressed as mean ± SEM of five animals. *;p<0.05 compared with control. †p<0.05 compared with AdGFP.
2.2 The role of P-selectin in adenovirus vector-induced leukocyte-endothelial interactions in the liver
P-selectin is known to mediate early leukocyte rolling in numerous inflammatory conditions. The administration of anti-P-selectin antibody 10 min prior to the intravenous injection of 2.5×1011 particles of AdGFP significantly decreased leukocyte rolling in the postsinusoidal venules. At 30 and 60 min, the number of rolling leukocytes was significantly lower than the corresponding values in mice receiving isotype control antibody and AdGFP (Fig. 3A). At 90 and 120 min, leukocyte rolling began to increase and approach the degree of rolling seen in mice that received control antibody and AdGFP (Fig. 3A).
Pretreatment with anti-P-selectin antibody also affected adenovirus vector-induced leukocyte adhesion. At 30 and 60 min, anti-P-selectin antibody significantly reduced AdGFP-induced leukocyte adhesion compared to mice pretreated with isotype control IgG1 (Fig. 3B). At 90 min, and coinciding with the observed increase in leukocyte rolling, leukocyte adhesion increased, and by 120 min, was similar to the leukocyte adhesion observed in control mice (Fig. 3B).
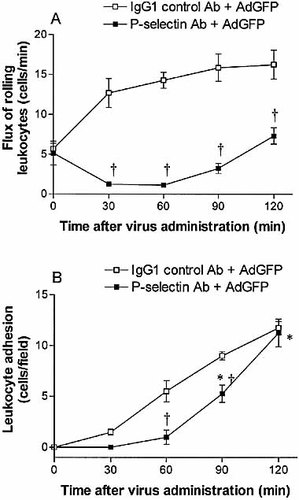
Flux of rolling neutrophils (A) and number of adherent neutrophils (B) in postsinusoidal venules of C57BL/6 mice (wild type) following AdGFP administration. Mice were treated with anti-P-selectin or isotype control antibody 10 min prior to AdGFP challenge. Data are expressed as mean ± SEM of five animals. *;p<0.05 compared with 0 min. †p<0.05 compared with corresponding control.
2.3 Role of E-selectin in adenovirus vector-induced leukocyte-endothelial interactions in the liver
Inhibition of leukocyte rolling and adhesion was only delayed by blocking P-selectin suggesting that other molecules were involved in adenovirus vector-induced leukocyte rolling. E-selectin also mediates leukocyte rolling but its effects are delayed compared to P-selectin 18. To address the role of E-selectin in adenovirus vector-induced leukocyte rolling, experiments were performed in E-selectin-deficient mice. The administration of 2.5×1011 particles of AdGFP to E-selectin-deficient mice increased leukocyte rolling in the postsinusoidal venules similar to the effect seen in wild-type C57BL/6 mice (data not shown). Pretreatment of E-selectin-deficient mice with anti-P-selectin antibody significantly reduced leukocyte rolling following AdGFP administration compared to E-selectin-deficient mice receiving IgG1 control antibody and AdGFP (Fig. 4A). The number of rolling leukocytes at 60 and 90 min was reduced further in E-selectin-deficient mice pretreated with anti-Pselectin antibody versus wild-type mice receiving P-selectin antibody (Fig. 4A). At 120 min, the number of rolling leukocytes once again began to increase; however, the number (3.8±0.9) was considerably less than in wild-type mice receiving AdGFP and anti-P-selectin antibody (7.4±1.0). Similar to the effect seen in wild-type mice, the administration of anti-P-selectin antibody to E-selectin-deficient mice only delayed AdGFP-induced leukocyte adhesion in the liver (Fig. 4B).
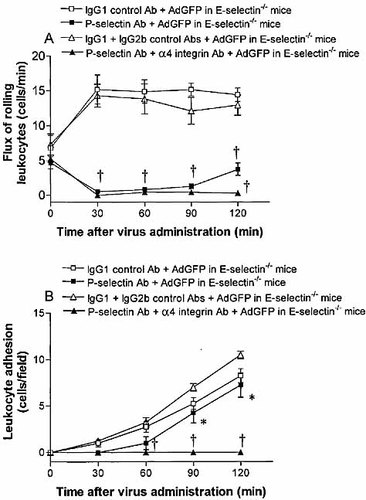
Flux of rolling neutrophils (A) and number of adherent neutrophils (B) in postsinusoidal venules of E-selectin-deficient (E-selectin–/–) mice in response to AdGFP administration. Mice were treated with anti-P-selectin, both anti-P-selectin and anti-α4-integrin, or isotype control antibodies 10 min prior to AdGFP challenge. Data are expressed as mean ± SEM of five animals. *;p<0.05 compared with 0 min. †p<0.05 compared with corresponding control.
2.4 Role of α4-integrin in adenovirus vector-induced leukocyte-endothelial interactions in the liver
Adenovirus vector-induced rolling was not completely abolished in anti-P-selectin antibody-treated, E-selectin-deficient mice. Experiments were therefore performed to examine the role of α4 integrin, a leukocyte-derived molecule that has been shown to contribute to leukocyte rolling in the liver 17. Pretreatment of E-selectin-deficient mice with anti-P-selectin antibody and anti-α4-integrin antibody (R1–2) completely inhibited AdGFP-induced leukocyte rolling at all time points (Fig. 4A). Furthermore, the complete lack of rolling seen in these animals was accompanied by a complete lack of leukocyte adhesion for the duration of the experimental time period following AdGFP administration (2.5×1011 particles/animal; Fig. 4B). In contrast, the administration of isotype control IgG1 and IgG2b antibodies did not affect AdGFP-induced rolling and adhesion in the E-selectin-deficient mice compared to AdGFP alone.
The α4-integrin supports rolling and adhesion both in vitro and in vivo 3, 4. The findings in the preceding experiments led us to examine further the independent role of α4-integrin in adenovirus vector-induced leukocyte rolling and adhesion in liver. For this purpose, two functionally similar blocking α4-integrin antibodies, R1–2 and PS/2, were used 19, 20. In wild-type C57BL/6 mice, R1–2 pretreatment partially reduced the flux of rolling leukocytes in the postsinusoidal venules. This effect started at 30 min, and persisted to 120 min following the intravenous administration of AdGFP. The number of rolling leukocytes at 30, 60, 90 or 120 min was significantly lower than in mice receiving IgG2b control and AdGFP (Fig. 5A). R1–2 pretreatment also significantly decreased AdGFP-induced leukocyte adhesion in the postsinusoidal venules at every time point (Fig. 5B). Pretreatment of animals with PS/2 antibody had the same effect on AdGFP-induced leukocyte rolling and adhesion as pretreatment with R1–2 antibody (Fig. 5A, B).
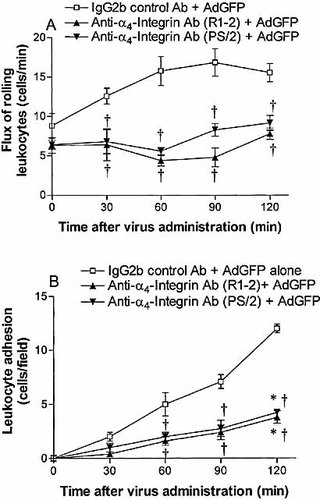
Flux of rolling neutrophils (A) and number of adherent neutrophils (B) in the liver postsinusoidal venules of C57BL/6 mice (wild type) following AdGFP administration. Mice were treated with anti-α4-integrin or isotype control antibody 10 min prior to AdGFP challenge. Data are expressed as mean ± SEM of five animals. *;p<0.05 compared with 0 min. †p<0.05 compared with corresponding control.
2.5 Role of CD18 in adenovirus vector-induced leukocyte-endothelial interactions in the liver
CD18 is known to play an important role in leukocyte adhesion to endothelium. The effect of CD18 in AdGFP-induced leukocyte rolling and adhesion was investigated using a blocking anti-CD18 antibody and CD18-deficient mice. Pretreatment of C57BL/6 mice with an anti-CD18 antibody did not reduce AdGFP-induced leukocyte rolling in the postsinusoidal venules. Interestingly, the flux of rolling leukocytes at 30, 60, 90 and 120 min was significantly higher in mice that received anti-CD18 antibody and AdGFP than in animals that received IgG1 control and AdGFP (Fig. 6A). Although the reason for increased rolling is not immediately apparent, the further enhanced rolling was not seen in CD18-deficient mice and suggests that this observation is related to the antibody per se. Anti-CD18 antibody did not prevent leukocyte adhesion in response to AdGFP (Fig. 6B). To probe further the role of CD18 in adenovirus vector-induced leukocyte rolling and adhesion, experiments were performed in CD18-deficient mice. Leukocyte rolling and adhesion in response to AdGFP were similar to the effects induced in wild-type C57BL/6 mice. Intravenous administration of 2.5×1011 particles of AdGFP still caused a time-dependent increase in rolling and adherent leukocytes in the postsinusoidal venules of CD18-deficient mice (Fig. 6A, B).
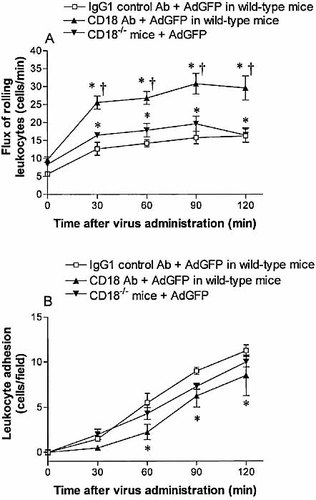
Flux of rolling neutrophils (A) and number of adherent neutrophils (B) in postsinusoidal venules of C57BL/6 mice (CD18+/+) or CD18-deficient mice (CD18–/–) following AdGFP administration. CD18+/+ mice were treated with anti-CD18 antibody or isotype control antibody 10 min prior to AdGFP challenge. Data are expressed as mean ± SEM of five animals. *;p<0.05 compared with 0 min. †p<0.05 compared with corresponding IgG1 control antibody.
2.6 Involvement of neutrophils in adenovirus vector-induced leukocyte recruitment to the liver
Herein, we have demonstrated predominately α4-integrin-dependent and CD18-independent leukocyte adhesion following AdGFP administration. Since neutrophils have been shown to use CD18 for adhesion to endothelium, it remained unclear whether neutrophils were the dominant cell type recruited in this experimental model. C57BL/6 mice were treated with a neutrophil-depleting antibody 24 h prior to the administration of 2.5×1011 particles of AdGFP. Antibody effectiveness was monitored by peripheral circulating neutrophil and total leukocyte counts. Treatment with the anti-neutrophil antibody decreased total peripheral leukocyte number by 40% (Fig. 7A) and completely depleted circulating neutrophils in vehicle-treated mice by 90% (Fig. 7B). The administration of 2.5×1011 particles of AdGFP significantly increased circulating leukocyte number and neutrophil percentage when compared to vehicle-treated animals. Treatment with the anti-neutrophil antibody blocked the elevation of circulating leukocyte number and neutrophil percentage induced by AdGFP (Fig. 7A, B). Interestingly, a complete inhibition of circulating neutrophils was not observed, perhaps due to mobilization of neutrophils from bone marrow following AdGFP injection.
Neutrophil-depleted mice displayed reduced leukocyte rolling in the postsinusoidal venules in response to AdGFP. The flux of rolling leukocytes remained at basal levels 30, 60, 90 and 120 min following vector administration (Fig. 8A). AdGFP-induced leukocyte adhesion in the postsinusoidal venules was also significantly diminished at all time points following neutrophil depletion (Fig. 8B). These results suggest that the early leukocyte recruitment to the liver in response to adenovirus vectors is primarily composed of neutrophils.
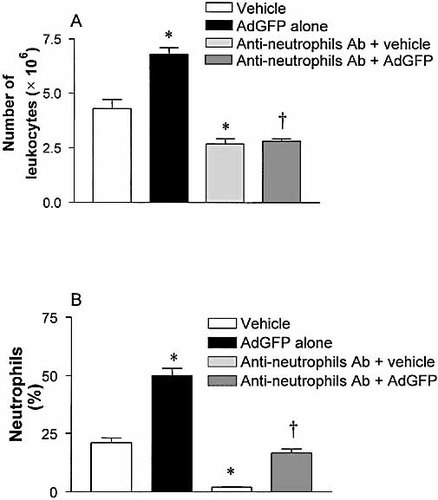
Peripheral circulating leukocytes counts and percentage of neutrophils in mice receiving vehicle, AdGFP alone, anti-neutrophil antibody plus vehicle, and anti-neutrophil antibody plus AdGFP. Anti-neutrophil antibody was given 24 h before the experiments. Data are expressed as mean ± SEM of five animals. *;p<0.05 compared with vehicle control. †p<0.05 compared with AdGFP alone.
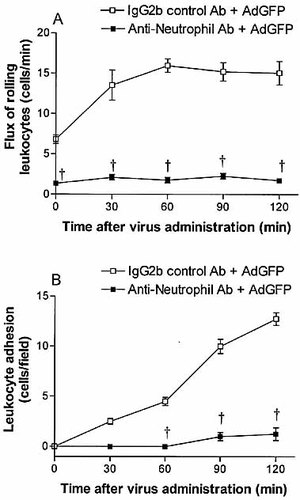
Flux of rolling neutrophils (A) and number of adherent neutrophils (B) in the postsinusoidal venule of C57BL/6 mice (wild type) following AdGFP administration. Mice were treated with anti-neutrophil or isotype control antibody 24 h prior to AdGFP challenge. Data are expressed as mean ± SEM of five animals. †p<0.05 compared with corresponding control.
3 Discussion
Adenovirus vectors and adenovirus-based therapies will potentially be used to treat a variety of genetic and non-genetic human diseases. Host inflammatory and immune responses limit the effective application of these agents in humans. Our results demonstrate that adenovirus vectors cause rapid neutrophil-endothelial interactions in mouse liver characterized by increased rolling and adhesion of neutrophils in the postsinusoidal venules. P-selectin and α4-integrins mediate early neutrophil rolling. E-selectin is also required for optimal neutrophil rolling, but as expected, the effect is delayed compared to P-selectin. Surprisingly, α4-integrin but not CD18 mediates neutrophil adhesion to the postsinusoidal venules in response to adenovirus vectors.
The acute inflammation of AdGFP-transduced liver demonstrated in the present study is consistent with previous findings of innate immune activation 13, 21. Innate immune activation not only dramatically reduces vector persistence and transgene expression but also results in acute inflammation and tissue injury 16, 22. Since adenovirus activation of the innate immune system is dose-dependent, strategies to improve gene transfer efficiency by increasing vector titers only exacerbates the inflammatory response 23. This problem has been highlighted by the death of a patient undergoing gene therapy for the ornithine transcarbamylase deficiency after intrahepatic arterial injection of an adenovirus vector carrying a wild-type version of the defective enzyme 24. Since the liver is the major target of intravenous administered adenovirus vectors, a complete understanding of the inflammatory consequences in this organ is required to improve the effectiveness and safety of adenovirus-based therapies for humans. Our results show, for the first time, the molecular adhesion mechanism by which effector cells of the innate immune system respond to adenovirus vectors.
In this study, AdGFP stimulated an immediate inflammatory response manifested by neutrophil rolling and adhesion in the postsinusoidal venules. It is known that P-selectin is constitutively synthesized in endothelial cells and packaged in secretory granules (Weibel-Palade bodies). Stimuli such as thrombin, histamine, hydrogen peroxide, and other secretagogues rapidly induce translocationof P-selectin to the cell surface during fusion of granular membranes with the plasma membranes 25, 26. Our data show that P-selectin is essential for the very early neutrophil rolling in the postsinusoidal venules immediately after AdGFP administration. Although previous studies 9, 10, 17 have demonstrated that anti-P-selectin therapy can prevent leukocyte rolling in postsinusoidal venules, these same approaches did not reduce sequestration in sinusoids. The fact that AdGFP only induced leukocytes in postsinusoidal vessels is encouraging in terms of therapeutically blocking leukocyte recruitment.
Our data show that E-selectin is responsible for delayed neutrophil rolling induced by adenovirus. Unlike P-selectin, E-selectin requires de novo protein synthesis following stimulation. The delayed role of E-selectin in adenovirus-induced neutrophil rolling is consistent with previous work examining E-selectin inducibility in response to TNF-α, LPS and IL-1 27. Although adenovirus-induced responses in E-selectin-deficient mice are not different from responses in wild-type mice, this is entirely consistent with previous work demonstrating the requirement to inhibit P-selectin to uncover the overlapping role of E-selectin 28. Labow et al. found that in two models of inflammation, thioglycollate-induced peritonitis and delayed-type hypersensitivity in the skin, E-selectin-deficient mice displayed no significant change in trafficking of neutrophils. While neutrophil accumulation at early times was dependent on P-selectin, neutrophil accumulation at later time points was blocked by an anti-P-selectin antibody only in E-selectin-deficient mice 28. Our present data confirm that P-selectin mediates the initial neutrophil rolling, and both P-selectin and E-selectin are involved in the delayed neutrophil rolling induced by adenovirus vectors in the postsinusoidal venules.
At first glance it is somewhat surprising that P-selectin and E-selectin could inhibit 90–95% of neutrophil rolling, and yet neutrophil adhesion was only affected modestly. However, this observation is consistent with previous findings that selectin-dependent rolling must be inhibited by at least 90% to significantly affect adhesion 29. Furthermore, the inhibition of selectins in slower flowing vessels had absolutely no effect on leukocyte adhesion 29. Since the liver blood flow is quite low, bypassing selectins or a greater reliance on integrins for adhesion is quite likely. In this study, we found that anti-α4-integrin plus anti-P-selectin antibodies administered to E-selectin-deficient mice completely abolishes both neutrophil rolling and adhesion elicited by AdGFP in the postsinusoidal venules. These data demonstrate that α4-integrin accounts for the remaining selectin-independent rolling. When α4-integrin alone was blocked, only 50% of the leukocyte rolling was inhibited but a dramatic decrease in adhesion was seen. These data suggest that α4-integrin is important in primarily mediating the leukocyte adhesion caused by AdGFP in the postsinusoidal venules. Clearly, α4-integrin contributed to both leukocyte rolling as well as leukocyte adhesion, a unique characteristic attributed to α4-integrin 3, 4. The rapidity of α4-integrin-mediated rolling and adhesion is consistent with theexpression of its ligand vascular adhesion molecule-1 (VCAM-1). Essani and co-workers found that in livers of normal control mice, only a very weak expression of VCAM-1 was observed in sinusoidal lining cells; however, VCAM-1 was moderately expressed in postsinusoidal blood vessels 30. Furthermore, they also found that increase in VCAM-1 expression on sinusoidal lining cells could be seen only at 4 h after endotoxin administration 30. Our results of predominant leukocyte rolling and adhesion in postsinusoidal venules within 2 h after AdGFP administration are comparable to their findings.
To exclude a role for CD18 in adenovirus-induced leukocyte recruitment, we also performed experiments in CD18-deficient mice. Our data suggest that α4-integrin but not CD18 is involved in the leukocyte recruitment induced by AdGFP. This adhesion profile would infer that mononuclear cells rather than neutrophils were the leukocytes involved. However, our previous histologicalassessments 13 and the neutrophil depletion studies herein clearly show that neutrophils were the predominate infiltrating cells. Although rare, there is a precedent for α4-integrin-dependent neutrophil recruitment in rodent models of inflammation. First, α4-integrin was found to be able to mediate CD18-independent neutrophil recruitment in endotoxin- or KC-induced lung inflammation 31, 32. Second, α4-integrin expression was observed on resting and activated mouse neutrophils 33. The novelty of our work is that AdGFP caused neutrophil adhesion in the postsinusoidal venules through an exclusive and rapid α4-integrin-dependent, but CD18-independent pathway not reported previously. Whether this is a direct effect of AdGFP on leukocytes or on endothelium remains to be determined.
Our data demonstrate that adenovirus vectors induce rapid neutrophil recruitment restricted to the postsinusoidal venules of the liver. Although overt liver injury is not seen until 16–24 h post-administration, our data provide insight to the early mechanism of liver injury induced by adenovirus vectors. Anti-adhesion therapy can reduce early leukocyte recruitment into the liver in response to adenovirus vectors but a combination of P-selectin, E-selectin and α4-integrin inhibition will at a minimum be required. Tandem α4-integrin, P-selectin and E-selectin inhibition does not affect leukocyte recruitment into the liver in other models of inflammation due to sequestration of leukocytes in sinusoids 9, 10, 17. Therefore, the lack of recruitment of leukocytes into sinusoids in this model system bodes well for anti-adhesion therapy. The early modulation of leukocyte recruitment to the liver provides a strategy to improve the safety and effectiveness of adenovirus-based therapies for humans.
4 Materials and methods
4.1 Animals
Male C57BL/6 mice (Charles River Laboratories, Quebec, Canada) were used throughout the study. Mice deficient in E-selectin and CD18 integrin were originally generated by gene targeting in embryonic stem cells as previously described 34, 35, then backcrossed on to C57BL/6 for at least seven generations. These mice were housed in specific pathogen-free facilities. All animals used weighed 22–30 g. Animal protocols were approved by the University of Calgary Animal Care Committee and met the Canadian Guidelines for Animal Research.
4.2 Intravital microscopy in the mouse liver
Mice were anesthetized with a mixture of 200 mg/kg ketamine (Rogar/STB Inc., Montreal, Quebec, Canada) and 10 mg/kg xylazine (MTC Pharmaceuticals, Cambridge, Ontario, Canada) injected intraperitoneally (i.p.). The right jugular vein was cannulated to maintain anesthesia and for injection of the adenovirus vector and the monoclonal antibodies. The abdomen was opened via a midline incision, the skin and peritoneum were removed close to the costal margin, and the hepatoform ligament was carefully released from the gallbladder. Mice were then placed in a left supine position on a Plexiglas form (Universal Plastic, Calgary, Canada) and the left lobe of the liver was gently placed onto the stage. The liver was covered with Saran Wrap (Dow Brands, Ontario, Canada) to hold the organ in position and the intestines were kept close to the abdomen and covered with saline-soaked gauze. Animals were kept warm with an infrared heat lamp.
After the liver was isolated and placed under the intravital microscope, a separate postsinusoidal venule and eight to ten draining sinusoids were located. A microscope (Axiovert S100; Zeiss, Germany) with a ×40 objective lens (Plan-Neofluar; Zeiss) and a ×10 eyepiece was used to observe the microcirculation events of the liver. A video camera (NC—70; DAGE MTI, USA) was mountedon the microscope and projected the image onto the monitor and the images were recorded for playback analysis using a videocassette recorder (SLV-975HF; Sony, Japan). The numbers of rolling and adherent leukocytes were determined offline during video playback analysis. Leukocytes were considered adherent to the venular endothelium if they remained stationary for 30 s. Rolling leukocytes were defined as those moving at a velocity less than that of erythrocytes within a given vessel. Flux of rolling leukocytes was counted as the leukocyte number rolled along a 100 μm distance in 1 min.
4.3 Adenovirus vector
The E1-deleted, E3-defective AdGFP (Quantum, Montreal, Canada) under the control of the cytomegalovirus promoter was propagated in 293 cells as previously described 36. Virus vehicle (10 mM Tris pH 8.0, 1 mM MgCl2, 10% glycerol) was used for control.
AdGFP concentration was determined by measuring the optical density at 260 nm 37. Vectors were screened for replication-competent adenovirus by plaque assay on HeLa cells and remained consistently <1:1010 particles. In the present study, 2.5×1011 particles/animal of AdGFP was given to mice in a volume of 100 μl/animal through injection into the jugular vein.
4.4 Experimental protocol
Immediately after finding an appropriate vessel the image was recorded for 5 min. This served as a baseline assessment. After AdGFP was injected via jugular vein, recordings were taken at 30 min interval for 2 h. Virus vehicle (100 μl/animal) was used as the control.
To study the role of selectins, integrins, and the involvement of neutrophils in this model of adenovirus vector-induced leukocyte recruitment, we administered 20 μg/animal of a monoclonalanti-P-selectin antibody (RB40.34; IgG1; PharMingen), 75 μg/animal of a monoclonal anti-α4-integrin antibody (R1–2; IgG2b; PharMingen), 200 μg/animal of a monoclonal anti-α4-integrin antibody (PS/2; IgG2b; Merck, USA), or 30 μg/animal of a monoclonal anti-CD18 antibody (GAME-46; IgG1; PharMingen) intravenously 10 min before AdGFP challenge in C57BL/6 mice. Mice deficient in CD18 or E-selectin were directly challenged with AdGFP. To study the additive role of adhesion molecules in this response, mice deficient in E-selectin were treated with anti-P-selectin antibody or the combination of anti-P-selectin and anti-α4-integrin (R1–2) antibodies at the dose described above. To identify the involvement of neutrophils in the adenoviral response, neutrophil depletion experiments were performed. C57BL/6 mice were treated by i.p. injection of 150 μg/animal of an anti-neutrophil antibody (RB6–8C5; IgG2b) as described previously 38 24 h prior to AdGFP administration. Neutropenia was confirmed by peripheral blood smear. IgG1 or IgG2b antibodies were used as isotype controls.
4.5 Circulating leukocyte counts
At the end of experiments, whole blood was drawn via cardiac puncture. Total leukocyte counts were performed using a hemocytometer (Bright-line; Hausser Scientific, Horsham, PA). Neutrophil number was counted by blood smear.
4.6 Statistical analysis
The data are presented as the mean ± SEM. ANOVA and Student's t-test with Bonferroni's correction were used for multiple comparisons. Statistical significance was set at p<0.05.
Acknowledgements
Dr. Kubes is an Alberta Heritage Foundation for Medical Research (AHFMR) scientist and a Canada Research Chair. Dr. Li is supported by a Canadian Association of Gastroenterology (CAG) Fellowship. Dr. Muruve is a Canadian Institutes of Health Research (CIHR) Scholar and AHFMR Clinical Investigator. Dr. Lee is an AHFMR Senior Scholar. This study was funded by research operating grants from the Canadian Liver Foundation, Canadian Institutes of Health Research (CIHR), and a CIHR Group Grant.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




