The phosphatidylinositol phosphatase PTEN is under control of costimulation and regulates proliferation in human T cells
Abstract
The phosphatidylinositol phosphatase gene PTEN is a dualspecific phosphatase acting on phospho amino acids but also on three phosphorylated inositol phospholipids. Present results demonstrate that PTEN is inducible by costimulatory signals in human CD4+ T cells. PTEN expression was up-regulated on RNA and protein level in freshly isolated human CD4+ T cells following stimulation with CD28 or CD2. In contrast, PTEN expression was high but remained CD28 and CD2 unresponsive in lymphoma cells. Intracellular staining revealed PTEN expression in CD4+ T cell populations stimulated with anti-CD28 or anti-CD28 / anti-CD3. Stimulation with anti-CD3 alone did not induce PTEN expression. Inhibition of PTEN expression by antisense oligonucleotides in CD4+ T cells stimulated with non-mitogenic anti-CD28 resulted in massively increased proliferation, which was sensitive to the phosphatidylinositol 3-kinase (PI3 K) inhibitor wortmannin. Although CD28 and CD2 induce PI3 K signal transduction, wortmannin did not block PTEN up-regulation by CD28 or CD2 indicating that PTEN gene expression is PI3 K independent. These results demonstrate that PTEN negativelycontrols costimulatory signals by antagonizing PI3 K activity in the absence of TCR engagement.
Abbreviations:
-
- PI3 K:
-
Phosphatidylinositol 3-kinase
-
- PTEN:
-
Phosphatase and tensin homologue
-
- PIP2:
-
Phosphatidylinositol (4,5)-bisphosphate
-
- PIP3:
-
Phosphatidylinositol (3,4,5)-trisphosphate
-
- ICOS:
-
Inducible costimulator protein
1 Introduction
Molecular interactions of CD28 and CD2 are required to induce full activation of T cells in costimulation with the T cell receptor (TCR). In addition, CD28 is assumed to be important for the formation of the immunological synapse prior to TCR engagement (for review see 1). Both receptors activate the phosphatidylinositol 3-kinase (PI3 K) catalyzing the phosphorylation of phosphatidylinositol (4,5)-bisphosphate (PIP2) into phosphatidylinositol (3,4,5)-trisphosphate (PIP3), which in turn plays an important role for the cell-cycle, -survival and -migration (for review see 2).
The tumor suppressor gene PTEN (phosphatase and tensin homologue) encodes a 55 kDa phosphatase, which can dephosphorylate phosphotyrosine and phosphothreonine in vitro; however, acidic substrates such as PIP3 are presumably the main targets of this enzyme 3, 4. By dephosphorylating PIP3, PTEN antagonizes the activity of PI3 K. Accordingly, overexpression of PTEN reduces insulin-induced increase of PIP3 without affecting the expression of PI3 K 5.
The critical role of PTEN in PIP homeostasis is demonstrated by the fact that PTEN is the most frequently mutated human tumor suppressor gene 4. This clinical aspect is underlined by the fact that PTEN-deficient mice die at embryonic stage and even heterozygous mice lacking one allele die from multiorgan failure caused by autoimmune disease 6. Therefore, PTEN is not only a critical regulator of cell-cycle progression but is also involved in the regulation of the immune system. PTEN blocks cell-cycle progression from the G1/0 phase into the S phase in somatic cells by regulating the PKB/AKT pathway 7, 8. The PKB/AKT gene is an important regulator of apoptosis and proliferation 9, 10. It could be demonstrated that PTEN can be actived in the regulation of T cell lymphoma apoptosis and TCR-induced activation of mitogen-activated protein kinase ERK2 (extracellular signal-related kinase 2; 11). Moreover it has been shown that PTEN can regulate the activity of the PIP3 binding kinases such as ITK in Jurkat T cells 12. In addition, recent studies showed that mice deficient in PTEN expression only in T cells are defective in central and peripheral tolerance 13. To further investigate the role of PTEN in normal T cells, we analyzed whether immunological signals regulating the cell cycle such as TCR stimulation and cytokines can regulate PTEN expression and whether changes of PTEN expression affect proliferation in human peripheral blood CD4+ T cells.
2 Results and discussion
2.1 PTEN mRNA expression is up-regulated by CD28 in primary CD4+ T cells but not in T cell lymphomas
To examine the role of PTEN in primary T cells, we tested signals that are known to affect cell-cycle progression of T cells. T cell lymphomas (HUT78, 6TCEM 20, Jurkat) and freshly isolated CD4+ T cells were incubated on plate-bound CD28, immediately lysed and subjected to cDNA synthesis. PTEN mRNA expression could be detected only at very low levels in resting, unstimulated cells (Fig. 1A, lane 1). These traces may result from in vivo activated cells (Fig. 3). Upon stimulation with anti-CD28 PTEN mRNA expression increased (Fig. 1A, lane 2) and was found to be increased as early as 1 h (Fig. 1D, lane 5) and remained stable thereafter (Fig. 1D, 3 h = lane 6, 6 h = lane 7, 24 h = lane 8, 72 h = data not shown), despite removing the cells from plate-bound anti-CD28 antibody. The expression kinetics of PTEN were surprising in that expression lasted for a long time; in fact, even in 3-day cultures no decrease in PTEN expression could be detected. Anti-CD3 treatment did not induce PTEN mRNA expression (data not shown) and anti-CD3 together with anti-CD28 did not enhance PTEN mRNA expression above the level of anti-CD28 stimulation alone (Fig. 1A, lane 3). The degree of expression was determined using real time RT-PCR which revealed a 2.8-fold difference between unstimulated and CD28 triggered cells following a 6-h incubation period (Fig. 1D). These findings are important since the current knowledge of PTEN biology in human T cells is exclusively based on experiments using the Jurkat lymphoma line. In contrast to primary CD4+ T cells, Jurkat and 6-TCEM 20 cells do both express high levels of CD28, but do not respond to CD28 stimulation with increased levels of PTEN mRNA expression (Fig. 1F, lanes 10–13). PTEN mRNA expression was high in HUT78 T cells (Fig. 1F, lane 9), which do not express CD28 (data not shown). The expression of β-actin mRNA is shown in Fig. 1B and G, lanes 1–3 and 9–13. For the time-course and real-time PCR, GAPDH was used as additional house keeping gene (Fig. 1E, lanes 4–8). These results demonstrate for the first time that PTEN is transcriptionally regulated by costimulation and that this regulation is not operative in lymphoma cells. Since all lymphoma cells show high PTEN expression it is unlikely that the uncontrolled cell cycle is the result of aberrant PTEN regulation. We hypothesize that high levels of PTEN expression represent an attempt to limit cell-cycle progression in lymphoma cells.
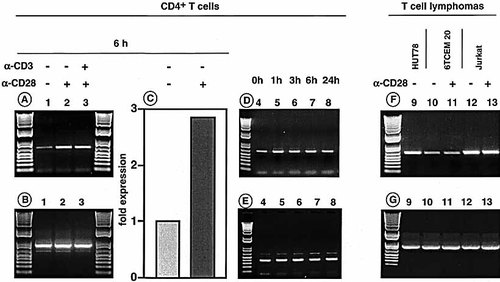
PTEN-specific PCR amplification of cDNA from CD4+ T cells stimulated for 6 h with plate-bound anti-CD28 (mAb 15E8, 2 μg/ml; A, lane 2) and plate-bound anti-CD3 (OKT3, 1 μg/ml; lane 3) as indicated by crosses above the lanes. Quantitative differences based on Δ ΔCT values of the same cDNA (lanes 1 and 2) were tested using real time PCR (C). The Δ ΔCT value was calculated on the basis of GAPDH. PCR amplification of cDNA from CD4+ T cells stimulated for increasing time points are shown in lanes 4–8 (D) along with the corresponding GAPDH expression (E). The PTEN-specific amplification of cDNA of T cell lymphomas are shown on the right panel (F) including control amplification of a housekeeping gene (β-actin, G). A molecular weight standard was loaded on the borders of the 1% agarose gel. The data shown in Fig. 1 are representative of three independent experiments.
2.2 PTEN protein is induced by CD28 and CD2
In analogy to PTEN mRNA expression anti-CD28 induced PTEN protein expression. In contrast to mRNA data, we could detect PTEN expression without any stimulation only in very few cases. CD28 induced PTEN protein expression was in average fivefold (n =3) higher than background levels (Fig. 2A, lane 7). Therefore, mRNA levels reflect very well protein levels of the PTEN gene product. CD28-induced expression was detectable as early as 3 h following CD28 stimulation and persisted up to 24 h (Fig. 2D). Expression of PTEN was even observed up to 72 h (data not shown). This remarkable abundance raises the question about inactivation by PTEN. We tested several stimuli such as anti-CD3 stimulation, which could reduce PTEN expression, but we could not observe a decrease in PTEN content of the cells. The C-terminal domain of PTEN including the PDZ binding motif has been shown to be involved in regulation of PTEN stability 14, 15; however, none of PTEN-PDZ interacting molecules have been described in T cells.
Neither IL-2 nor IL-4 induced any PTEN protein production (Fig. 2A, lanes 2 and 3). This result demonstrates that molecules, which involve PI3 K signaling such as cytokines utilizing the common gamma chain, do not induce PTEN. Mitogens such as ionomycin or PMA or both together induce no or only very minor amounts of PTEN protein (Fig. 2A, lanes 4–6).
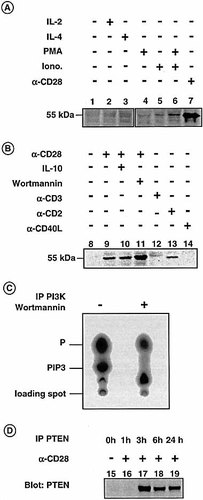
Anti-PTEN blots of whole-cell lysates of CD4+ T cells stimulated for 12 h with medium, IL-2 (50 U/ml) and IL-4 (25 ng/ml), ionomycin (1 μM), PMA (10 nM), both together, or anti-CD28 (2 μg/ml, plate bound) and separated by SDS PAGE using 8–16% gels. (B) Anti-PTEN blots of CD4+ T cells stimulated for 12 h with medium, anti-CD28, anti-CD28 + IL-10 (50 ng/ml), anti-CD28 + wortmannin (50 nM), anti-CD3 (1 μg/ml plate bound), anti-CD2 (2 μg/ml, plate bound) or sCD40L (5 μg/ml, plate bound) are shown. (C) TLC analysis of lipids incubated with immunoprecipitated PI3 K of cells treated as described for cells of panel B, lane 11. (D) Immunoprecipitated PTEN of T cells stimulated with anti-CD28 for increasing time spans as indicated. The immunoprecipitates were detected with a polyclonal anti-PTEN serum. The data shown are representative of three independent experiments.
2.3 The role of PI3 K activating costimulators on PTEN expression
A major mechanism of CD28-induced costimulation is the activation of PI3 K 16, 17, which is also shared by CD2 18, inducible costimulator protein (ICOS) 19 and possibly also by other costimulators 20. To test the capability of CD2, CTLA-4, ICOS and CD40L to induce PTEN expression, CD4+ T cells were stimulated with plate-bound antibodies against these costimulatory antigens. Surprisingly, PTEN up-regulation was also observed for CD2 (Fig. 2B, lane 13) although to a lower degree than CD28. It was not observed for CD40L (Fig. 2B, lane 14), CTLA-4 or ICOS (data not shown). Consistent with mRNA analysis anti-CD3 stimulation did not up-regulate PTEN protein expression (Fig. 2B, lane 12).
2.4 PTEN induction is PI3K independent
If PTEN antagonizes the PI3 K activity it would be tempting to speculate that PI3 K directly induces its own phosphatase to create a negative feedback. To test this hypothesis we loaded CD4+ T cells with the PI3 K inhibitor wortmannin (50 nM) and stimulated them for 12 h with anti-CD28. Wortmannin did clearly not suppress PTEN up-regulation (Fig. 2B, lane 11). In fact, a slight increase in PTEN expression was observed following wortmannin treatment, which, however, could not be observed on mRNA levels (data not shown). However, immunoprecipitated PI3 K, derived from cells treated with wortmannin, appeared enzymatically inactive when tested using a PI3 K assay (Fig. 2C). IL-10 is known to abrogate PI3 K association with the CD28 cytoplasmatic tail 21, 22. Therefore, cells were incubated with IL-10 on plate-bound anti-CD28. In analogy to the experiment with wortmannin, we could not observe any reduction of PTEN protein levels (Fig. 2B, lane 10). These results demonstrate that the PTEN gene is not a direct target of the PI3 K signaling cascade, since PTEN expression is neither wortmannin nor IL-10 sensitive. Although PI3 K activation appears to be a common feature in costimulation, the induction of the antagonistic PTEN gene represents a differential feature of CD28 and CD2. Further research is required to analyze which signals induce PTEN gene expression. Interesting candidates are the EMT/ITK/TSK 23, 24 and the p59fyn pathway 25, as these kinases are involved in signaling of CD2 and CD28.
2.5 PTEN expression in CD4+ T cells
PTEN expression was analyzed in different T cell populations by intracellular staining of PTEN following stimulation of CD4+ cells with anti-CD28. Anti-CD28 stimulation is sufficient to induce PTEN, increasing the PTEN-positive cells to 52% (Fig. 3). Interestingly, CD3 further increased the number of PTEN-positive cells, without increasing the extend of PTEN expression, confirming the Western blot results, which are based on an equal cell load. The increase of cells is likely to be a result of the first cells completing the initial cell division. Preliminary results indicate that PTEN is regulated to the same extend in α β, γ δ TCR+ T cells and in CD8+ cells.
As expected from Western blots, PTEN was low or undetectable both in CD3-treated cells as well as in nonstimulated, resting T cells (Fig. 3).
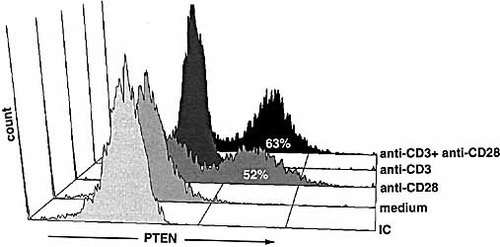
FACS analysis of CD4+ T cells. Cells were incubated overnight with medium, anti-CD28 (2 μg/ml, plate bound), anti-CD3 (1 μg/ml) or anti-CD3 and anti-CD28. Histograms show intracellular PTEN staining (x-axis), which was performed with Texas Red-conjugated anti-PTEN or isotype-matched, Texas Red-conjugated Ig (mouse IgG1; IC). The data shown are representative of three independent experiments.
2.6 PTEN suppresses CD28-induced proliferation
Since PTEN antagonizes the activity of the PI3 K, it can be hypothesized that CD28-induced PTEN expression represents a negative feedback to control PI3 K-mediated costimulation. To further support this theory we inhibited PTEN expression by antisense oligonucleotides to prove the functional role of PTEN in T cells. CD28 and CD2 both induce PIP3 production by immediate phosphorylation of the receptor enabling enzymatic activation of the PI3 K. Therefore CD4+ T cells were stimulated with plate bound anti-CD28 mAb 15E8 in the presence or absence of PTEN antisense oligonucleotides. The anti-CD28 mAb effectively costimulate CD3-induced proliferation and rescue the cells from CD3-induced activation induced cell death (data not shown), but does itself not induce proliferation (Fig. 4A, medium). However, when CD4+ T cells were stimulated with anti-CD28 mAb and anti-PTEN antisense oligonucleotides (AS), proliferation was observed at day 7 following stimulation (Fig. 4A, AS) in the absence of additional TCR engagement. In parallel we tested anti-CD2 triggering, which however, did not induce proliferation, possibly because of the antibodies, which are less efficient than the anti-CD28 mAb. In contrast, CD4+ T cells stimulated with anti-CD28 and sense oligonucleotides (Fig. 4A, ConS) did not show increased proliferation, demonstrating that antisense oligonucleotides specifically targeted PTEN mRNA. The antisense and anti-CD28-induced proliferation was completely inhibited by wortmannin, a PI3 K inhibitor (Fig. 4A, AS+Wort). In all conditions viability was higher than 95% as tested by trypan blue exclusion (data not shown). This experiment suggests that PTEN activity directly counteracts PI3 K activity.
Active cell division under antisense treatment was monitored using CSFE labeling prior CD28 stimulation. The majority of cells treated with anti-PTEN oligonucleotides and stimulated with anti-CD28 divided one to four times within 7 days (Fig. 4D), whereas cells treated with sense or no oligonucleotide did not show a response to anti-CD28 stimulation (Fig. 4B and C). Therefore PTEN appears to control cell cycle control of T cells. This finding is also supported by experiments performed with PTEN-deficient T cells, which show proliferative responses in the syngeneic control of mixed lymphocyte reactions 13. It is likely that PTEN is expressed to control cell-cycle progression as it has been formulated above in context of high PTEN expression of lymphoma cells.
Uncontrolled PI3 K activity gives rise to TCR independent proliferation and is likely to promote expansion of autoreactive T cells in vivo, as it has been demonstrated for PTEN–/+ mice 6. PTEN mutations are most frequent in patients with Cowden Syndrome 26 and in fact, one of these patients was demonstrated to have abnormalities in T cell function 27. It is therefore possible that some patients with mutations inactivating PTEN suffer from autoimmune diseases. They would probably profit from immunosuppressive medications 28, 29.
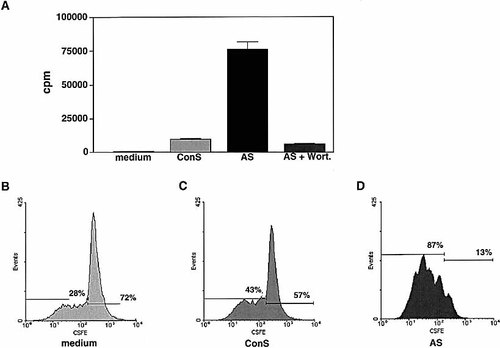
[3H]Thymidine incorporation of CD4+ T cells stimulated with anti-CD28 (2 μg/ml, plate bound) and pulsed during the last 16 h with 10 μCi [3H]thymidine. The cells were incubated for 5 days with medium (open bars, not visible for most conditions) PTEN antisense (AS), sense (ConS) phosphothioate oligonucleotides or AS together with wortmannin (50 nM). Cells labeled with CSFE prior to stimulation are depicted in (B) and (D). All cells were stimulated with plate-bound anti-CD28 for 7 days. Only anti-PTEN phosphothioate oligonucleotides induced up to four cell divisions, visible by decreased CSFE content. The data shown are representative of three independent experiments.
2.7 The role of costimulators in cell-cycle control
To test whether costimulatory molecules are generally involved in the regulation of cell-cycle regulators (e.g. tumor suppressor genes), we stimulated resting T cells as described above with costimulatory receptors and analyzed the expression of cell-cycle regulators (p130, Rb, p107, p53, p57, p27, p21, p19, p16, p14/15) by RNase protection assay after 6 h of incubation (Fig. 5). Two of these genes were predominantly expressed, p53 and p27. All other genes were low or absent. However, the expression of p53 mRNA was unchanged under CD28, CD2, CD40L, CTLA-4 or ICOS stimulation. Although it would be tempting to speculate that different costimulators trigger different cell-cycle regulators, the present results show rather minor effects on cell cycle regulators other than PTEN. CD28-induced up-regulation of PTEN represents a negative signal for T cell activation and is therefore surprising in the context of costimulation. We interpret this mechanism on the basis of the second signal hypothesis postulated by Jenkins and Schwartz 30: as anti-CD3 alone can induce anergy or activation-induced cell death, costimulation without TCR engagement has a negative feedback on the cell via PTEN and the PIP3 levels. This feedback is delayed by the transcription process, allowing CD28 to transiently elevate PIP3 levels. During this time window of elevated PIP3 the T cell might contact and search APC for a matching Ag-MHC complex. If the APC does not carry any matching Ag-MHC complex PTEN activity would be responsible to reduce PIP3 levels to steady state levels. Current investigations are now focussing on the effect of TCR engagement on PTEN enzymatic activity. Preliminary results suggest that TCR engagement post-transcriptionally modifies PTEN to make it enzymatically inactive.
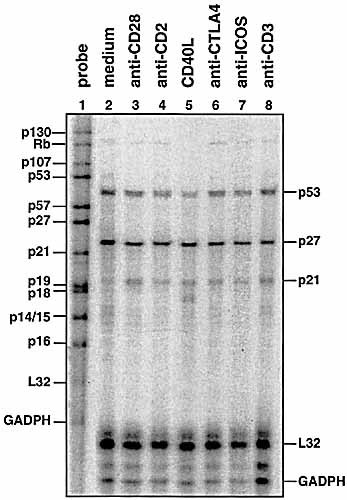
RNase protection assay of CD4+ T cells. Overnight rested CD4+ T cells were stimulated with plate-bound mAb against CD28, CD2, CTLA4, ICOS (all 2 μg/ml, plate bound) and CD3 (1 μg/ml, plate bound). Plate-bound sCD40 was used in lane 5 and medium in lane 2. Lane 1 shows the undigested RNA probe. The gel shown is representative of three experiments.
3 Concluding remarks
The present study demonstrates for the first time that PTEN is expressed in human primary CD4+ T cells and is up-regulated by costimulatory molecules. In addition, PTEN keeps CD28 induced proliferation under control to prevent expansion in the absence of Ag. This negative signaling mechanism complements the two-signal-hypothesis of T cell activation 30. The costimulatory molecules mobilizing PI3 K activity generate a negative feedback regulation by inducing PI3 K antagonizing PTEN expression. The new aspects of PTEN biology are not only important for T cell immunology but may also contribute to the understanding of the regulative capacity of PIP.
4 Materials and methods
4.1 Cell culture
PBMC were isolated from blood of healthy volunteers. The CD4+ subset was purified using CD4-Dynal magnetic beads and Detach-a-Bead mAb (Dynal, Hamburg, Germany) according to the protocol of the manufacturer. The efficiency of purification was initially tested by FACS and was ≥95% and cells were rested overnight. Jurkat T cells (E6–1) and 6T-CEM 20 cells were purchased from ATCC (Manassas, VA). All cultures were carried out in RPMI 1640 supplemented with 1 mM L-glutamine, sodium pyruvate, nonessential amino acids, 2-mercaptoethanol and 10% FCS (all from Life Technologies, Basel, Switzerland). Wortmannin (Calbiochem, Lucerne, Switzerland) was used at 50 nM as indicated in the figure legends. For Western blots 2×106 CD4+ T cells were incubated with the stimuli described in Fig. 2. Cells were lysed with RIPA buffer (20 mM MOPS pH7, 150 mM NaCl, 1 mM EDTA, 1% NP40, 1% sodiumdeoxycholate, 0.1% SDS), containing protease inhibitors (20 ng/ml aprotinin, 10 ng/ml leupeptin; both Roche, Rotkreuz, Switzerland) and phosphatase inhibitor 1 μM orthovanadate (Sigma, Buchs, Switzerland). For RNA analysis 5×106 cells were lysed with RNeasy lysis buffer (Qiagen, Hamburg, Germany) after 6 h as described in the figures. The 15E8 mAb was purchased from CLB (Amsterdam, The Netherlands). Of note, plate-bound anti-CD28 mAb does not induce Ca2+ influx (C.B. Schmidt-Weber, unpublished results) which is in contrast to other anti-CD28 mAb 31. The OKT3 clone was purchased from ATCC. Following purification of cell culture supernatants, OKT3 was tested for its ability to induce T cell proliferation. Half-maximal concentration proliferation induced by plate-bound anti-CD3 was observed at 0.1 μg/ml.
4.2 RT-PCR
RNA was isolated as previously described 32. The RNA was eluted in 30 μl water and subjected to reverse transcription. Approximately 5 μg total RNA (10 μl) was incubated with 500 μg/ml oligo dT12 primer (Roche) and Omniscript transcriptase (Qiagen) for 1 h at 37°C. The cDNA was denatured and used for PCR amplification. PTEN primer (sense: TGA AGA CCA TAA CCC ACC; antisense: ACG CTC TAT ACT GCA AAT GC; Microsynth, Balgach, Switzerland) amplified a cDNA fragment of 655 bp. The housekeeping gene β-actin was used as control (sense: ACT TGG CAC CAC ACC TTC TAC AAT GAG CTG CG; antisense: CGT CAT ACT CCT GCT TGC TGA TCC ACA TCT GC; both Clontech). The PCR products were separated on 1% agarose gel next to a standard (1kbplus, Life Technologies). Following electrophoresis the gel was scanned on an FLA-3000 imager (Raytest, Urdorf, Switzerland) and quantitated using the AIDA software (Raytest).
4.3 Real time RT-PCR
The PCR-primer and Taqman probes to amplify and detect PTEN and GAPDH mRNA were designed using the Primer Express Software version 1.0 (Perkin Elmer / Applied Biosystems, Foster City, CA) based on the sequence reported in GenBank as follows: PTEN forward 5′-AAA GGC ACA AGA GGC CCT AG; PTEN probe 5′-6FAM-CTG AGG ATT GCA AGT TCC GCC ACT GA-TAMRA; PTEN reverse ACC TTT AGC TGG CAG ACC ACA; GAPDH: forward: GAA GGG TGA AGG TCG GAG TC, GAPDH probe: 6FAM-CAA GCT TCC CGT TCT CAG CC-TAMRA; GAPDH reverse: GAA GAT GGT GAT GGG ATT TC. The oligonucleotides were purchased from TIB MolBiol (Berlin, Germany). The cDNA were prepared as described for regular RT-PCR and amplified using the universal PCR mastermix (Perkin Elmer / Applied Biosystems) according to the recommendations ofthe manufacturer. Accumulation of the PCR products was detected in real time directly by monitoring the probe cleavage-induced increase in fluorescence of the reporter dye with the ABI PRISM 7700 Sequence detector. Relative quantitation was performed using the comparative CT method as described by the manufacturer 33.
4.4 Western blots
Whole-cell lysates of CD4+ cells were mixed with denaturating loading buffer (0.1 M Tris base, 20% Glycerol, 0.001% Bromphenol Blue, 2% SDS and 2% β-mercaptoethanol, pH 6.8) and boiled for 5 min. For the immunoprecipitations 2×106–3×106 cells CD4+ T cells were lysed in RIPA buffer (20 mM MOPS, 150 mM NaCl, 1 mM EDTA, 1%NP-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate) supplemented with 1 mM sodium orthovanadate, 10 μg/ml aprotinin, and 10 μg/ml leupeptin. These lysates were incubated overnight with 2 μg anti-PTEN and precipitated with protein-G agarose beads (Sigma) and washed three times with PBS. Samples were loaded next to a protein-mass ladder (Benchmark, Life Technologies) on a 8–16% PAA gel (Novex, Wiesbaden, Germany). Proteins were electroblotted onto a nitrocellulose membrane (Amersham Life Science, Dübendorf, Switzerland) and PTEN peptides detected with 1 μg/ml anti-PTEN mAb (Oncogene, Cambridge, MA). The blot was developed with an anti-mouse HRP mAb using the ECL reagent (Amersham Life Science). The gel was photographed using the LAS 1000 camera (Raytest). For estimation of protein quantity incremental photographs were taken and the accumulated signal was measured using AIDA software (Raytest).
4.5 Flow cytometry
Following stimulation, 106 cells were subjected to permeabilization and fixation by a paraformaldehyde/saponin solution (Permeafix; Ortho Diagnostic Systems, Raritan, NJ). Permeabilized cells were stained with anti-PTEN (A2b1, Chemicon International, Temecula, CA), which was previously conjugated with Texas Red (Molecular Probes). Subsequently cells were subjected to FACS (EPICS XL-MCL, Coulter, Miami, FL). For cell-cycle analysis T cells were stained for 8 min at room temperature with 5-(and-6)-carboxyfluorescein diacetate succinimidyl ester (CSFE; Molecular Probes, Leiden, The Netherlands) which was diluted to a final concentration of 0.5 μM in PBS containing 5% FCS.
4.6 Antisense treatment
These studies were designed following the guidelines of Stein et al. 34 and previous anti-PTEN antisense studies 35. PTEN antisense phosphothioate oligonucleotides were used AS (GCC GCC GCC GTC TCT CAT CTC), as well as control oligonucleotides Con18 (TGG ATC CGA CAT GTC AGA), Con21 (ATG GAA GTT TGA GAG AGT TGA) and ConS (GAG ATG AGA GAC GGC GGC GGC).One microgram of antisense and control oligonucleotides were added to 3×106 CD4+ T cells, which were previously washed with PBS and resuspended in 100 μl Nucleofector® reagent in a electroporation cuvette (Amaxa Biosystems, Köln, Germany). Nucleofection was performed with program U-14 using the Nucleofector electroporation apparatus (Amaxa). The cells were immediately transferred into pre-warmed culture medium. Viability was tested and was >90%. The cells were stimulated as described in the figure legends.
4.7 PI3 K assay
PI3 K was immunprecipitated as described above following 1-h preincubation with or without 50 nM wortmannin as described in the legend of Fig. 2. The PI3 K assays was performed as previously described 36, except that PIP2 instead of PIP and PIP2 was used as substrate. Unlabeled, non-radioactive standards were visualized by treatment of the thin layer chromatography (TLC) plates with resublimed iodine and used to determine the running length of PIP3.
4.8 RNase protection assay
RNA was isolated as described above and precipitated with isopropanol. Total RNA was hybridized with 32P-labeled RNA template (CC-2 from PharMingen). The unprotected RNA was digested using an RNase T1/A mix according to the suggestions of the manufacturer. The digested RNA was purified and loaded on a 6% urea gel. Radioactivity was visualized using the FLA-3000 Phospho-Imager (Raytest) and analyzed using the AIDA software.
Acknowledgements
This work was supported by the Swiss National Foundation Grant no.: 31.52986.97 and the Gebert Rüf Foundation Grant G-074/99.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




