Chemotactic agents induce IL-6Rα shedding from polymorphonuclear cells: involvement of a metalloproteinase of the TNF-α-converting enzyme (TACE) type
Abstract
Interleukin (IL)-6 transsignaling plays a pivotal role in the shift from neutrophil to monocyte recruitment at the inflammatory site. Release of neutrophil IL-6 receptor-α (IL-6Rα, CD126) in its soluble form is a key step of IL-6 transsignaling, however, its physiological inducers are poorly known. Here, we observed that the neutrophil chemoattractants IL-8, C5a complement fraction, platelet activating factor, leukotriene B4 and N-formyl-methionyl-leucyl-phenylalanine rapidly decreased IL-6Rα membrane expression and increased soluble IL-6Rα concentrations in the neutrophil supernatants, consistent with a shedding process. IL-6Rα shedding involved a TNF-α-converting enzyme-type metalloproteinase since it was partly decreased in the presence of a specific inhibitor, but not cathepsin G since PMSF or α1 antichymotrypsin had no effect. Neutrophil IL-6Rα shedding may be a common feature of neutrophilic infiltrates in various inflammatory situations, allowing IL-6 transsignaling, decreasing neutrophil infiltration and in the meantime favoring monocyte recruitment, thus the initiation of an immune response and subsequently the resolution of inflammation.
Abbreviations:
-
- α1 ACT:
-
α1 antichymotrypsin
-
- CRP:
-
C-reactive protein
-
- FMLP:
-
N-formyl-methionyl-leucyl-phenylalanine
-
- LTB4:
-
Leukotriene B4
-
- PAF:
-
Platelet-activating factor
-
- TACE:
-
TNF-α-converting enzyme
-
- TAPI:
-
TNF-α protease inhibitor
1 Introduction
IL-6 is a pleiotropic cytokine which has been reported to exert both pro- and anti-inflammatory properties in vivo and in vitro 1, 2. IL-6acts on cells through liaison with a low-affinity receptor IL-6Rα and a transducing protein, gp130 3. Whereas gp130 expression is ubiquitous, IL-6Rα is expressed on a limited number of cell types, apparently rendering other cells unable to respond to IL-6 4. In fact, IL-6Rα can be released from cells in a soluble form, sIL-6Rα, which associates with IL-6 and this IL-6/sIL-6Rα complex can then bind gp130 on cell membranes to form the high-affinity IL-6 receptor 1. Through this mechanism called "transsignaling", virtually all cells expressing gp130 alone, such as endothelial, mesangial or mesothelial cells can respond to IL-6. Thus, IL-6 has been shown to induce chemokine production and adhesion molecule expression and seems to play an unexpected role in leukocyte recruitment 5 – 7. Lastly, two studies have reported that IL-6/IL-6Rα transsignaling induces a switch from neutrophil to monocyte recruitment at the site of inflammation, thus being an intermediate between acute and chronic inflammation 8, 9.
A key event in transsignaling is the source of sIL-6Rα. IL-6Rα is expressed on various cells, notably leukocytes 10. The levels of sIL-6Rα are elevated in inflammatory body fluids, and parallel the number of leukocytes 11. Indeed IL-6Rα can be released from leukocytes in two ways, one is membrane receptor shedding through ectodomain proteolytic cleavage 12, the other is the secretion of an alternatively spliced form of the receptor which lacks the transmembrane domain 13. Shedding from leukocytes, notably neutrophils, appears to be the main mechanism of sIL-6Rα production during inflammation 10, 11. The inducing agents and the mechanisms of IL-6Rα shedding remain however poorly known. Chemical agents such as PMA or calcium ionophores, as well as bacterial products such as N-formyl-methionyl-leucyl-phenylalanine (FMLP) and pore-forming toxins from Streptococcus and E. coli have been shown to induce IL-6Rα shedding 6, 11, 14, 15. To date, however, the only known physiological inducer of IL-6Rα shedding appears to be the C-reactive protein (CRP), as recently reported 16. The mechanism(s) involved in the shedding process has(ve) not been formally identified but metalloproteinase(s) from the ADAM family may play an important role, since inhibitors such as hydroxamates and the TNF-α protease inhibitor (TAPI) have been reported to decrease IL-6Rα shedding 15, 17.
Recently, two groups including ours observed that IL-8-activated human neutrophils and much less potently GROα-activated neutrophils, constitute a source of shed sIL-6Rα 8, 9. In this study, we asked whether IL-6Rα shedding may be a common feature in all neutrophilic infiltrates. We therefore, tested whether other agonist and chemotactic agents can induce neutrophil IL-6Rα shedding and what enzymatic mechanisms are involved in this phenomenon.
2 Results
2.1 Chemotactic agents reduce IL-6Rα expression on neutrophil membranes
Neutrophils were activated for 30 min with physiological concentrations of different chemotactic agents such as IL-8, C5a complement fraction, platelet-activating factor (PFA) or leukotriene B4 (LTB4), then IL-6Rα membrane expression was studied by FACS analysis. All these agents significantly decreased IL-6Rα expression (Figs. 1 and 2). When we analyzed the dose response for each chemoattractant, C5a appeared as potent as FMLP in its ability to decrease IL-6Rα membrane expression, whereas PAF, LTB4 and IL-8 appeared to be consistently less efficient (Fig. 2). A time-course of neutrophil stimulation showed that the effects of the various chemotatic agents were maximum after 30 min of stimulation (data not shown).
Since IL-8 and PAF can be co-expressed on endothelial cells during inflammation 18, we tested the effect of both chemoattractants on IL-6Rα shedding and observed a synergistic effect (percentage of IL-6Rα membrane expression decrease in the presence of 5 ng/ ml IL-8: 4.6 +/− 2.4 %; 10 nM PAF: 4.3 +/− 2.3 %; IL-8 + PAF: 26 +/− 7 %, n = 3).
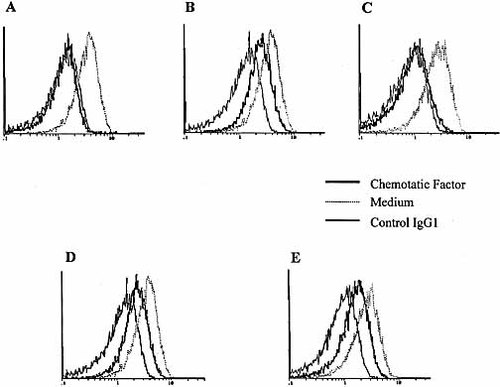
Stimulation of neutrophils with chemotactic agents induces a decrease of IL-6Rα membrane expression (FACS analysis). Neutrophils were cultured for 30 min with medium or different chemotactic agents such as FMLP (10– 6 M, A), IL-8 (100 ng/ml, B), C5a (50 nM, C), PAF (500 nM, D) or LTB4 (100 nM, E), before staining with anti-CD126 mAb or a control IgG1 and FACS analysis. Each panel represents one representative experiment among four performed.
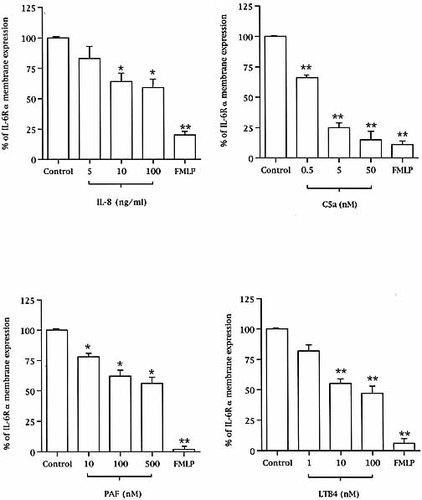
Chemotactic agent-induced membrane IL-6Rα loss of expression. Neutrophils were activated for 30 min with increasing concentrations of the various chemotactic agents before anti-CD126 staining and FACS analysis (*p < 0.05 and **p < 0.01, compared to unstimulated neutrophils, n = 4).
2.2 Stimulation with chemotactic agents increases sIL-6Rα concentrations in neutrophil supernatants
The rapid effect of the chemotactic agents in reducing IL-6Rα membrane expression was consistent with IL-6Rα shedding. To test this hypothesis, we measured sIL-6Rα in the supernatants of neutrophils stimulated for 30 min. Compared to unstimulated neutrophils, the concentrations of sIL-6Rα were significantly higher in the supernatants of neutrophils stimulated with the chemotactic agents (Fig. 3). In agreement with FACS data, C5a appeared to be as potent as FMLP, whereas IL-8, LTB4 and PAF appeared to be less efficient.
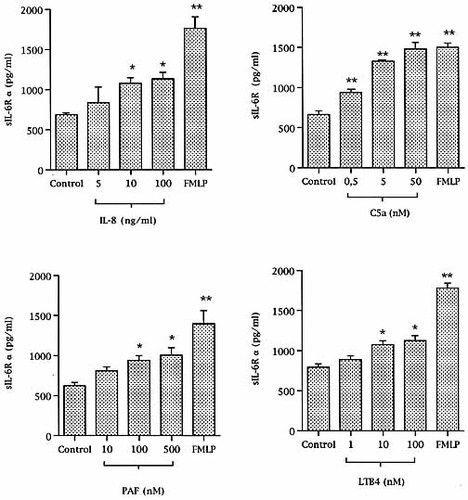
Increased sIL-6Rα concentrations in the supernatants of chemotactic agent-activated neutrophils. sIL-6Rα were measured in the supernatants of neutrophils stimulated for 30 min with increasing concentrations of the various chemotactic factors and compared to unstimulated-neutrophil supernatant (*p < 0.05 and **p < 0.01 compared to control, n = 4).
2.3 Mechanisms involved in chemoattractant-induced IL-6Rα shedding: a role for TACE-like enzyme(s) but not for cathepsin G
To evaluate the role of TNF-α-converting enzyme (TACE)-type enzymes in IL-6Rα shedding, neutrophils were stimulated with the various chemotactic agents in the presence of TAPI, a specific inhibitor of TACE. As a control experiment, we tested the inhibitory effects of TAPI on PMA-induced IL-6Rα shedding. Whereas TAPI 70 %-inhibited PMA-induced IL-6Rα shedding in THP-1 cells, as previously reported 17, TAPI decreased by only 30 % PMA-induced IL-6Rα shedding on neutrophils (data not shown). In the presence of TAPI, membrane IL-6Rα decreased expression induced by chemoattractants was significantly reduced (Fig. 4, upper panel), and in parallel the sIL-6Rα concentrations were also significantly decreased (Fig. 4, lower panel), consistent with a decreased IL-6Rα shedding under these conditions. The inhibitory effects of TAPI appeared to be more important on the shedding properties of FMLP and C5a than on those of the other chemoattractants.
We then evaluate the inhibitory effects of cathepsin G on IL-6Rα shedding by testing the effects of two serine proteinase inhibitors, PMSF, and α1 antichymotrypsin (α1 ACT), a specific cathepsin G inhibitor. Neither PMSF, nor α1 ACT decreased chemoattractant-induced IL-6Rα membrane release (data not shown).

Inhibitory effect of TAPI on chemotactic agent-induced IL-6Rα shedding. Neutrophils were stimulated for 30 min with the different chemotactic agents (100 ng/ml IL-8, 50 nM C5a, 500 nM PAF, 100 nM LTB4 and 10– 6 M FMLP) in the absence or presence of TAPI (TAPI 100 μM), before studying IL-6Rα membrane expression by FACS analysis and sIL-6Rα concentrations in neutrophils supernatants by ELISA (sIL-6Rα concentrations in the absence of TAPI representing 100 % for each chemoattractant, open bars). (* p < 0.05 and ** p < 0.01, compared to respective control, n = 4).
3 Discussion
Elevated concentrations of sIL-6Rα have been reported in various inflammatory diseases such as rheumatoid arthritis, polyarticular juvenile rheumatoid arthritis or asthma 11, 19, 20 and may originate from shedding of the receptor from the neutrophil membrane 10, 11. Recently, the CXC chemokines IL-8 and GROα have been shown to induce IL-6Rα shedding from neutrophils 8, 9, whereas CC chemokines MCP-1 or RANTES did not induce IL-6Rα shedding from monocytes or lymphocytes, respectively (14 and personal unpublished observation). Therefore, the shedding properties of chemokines on IL-6Rα appear to be limited to neutrophils and to be mainly mediated by the CXCR1 receptor 8. In the present study, we observed that several other molecules with known chemotactic and agonist properties such as C5a, IL-8, LTB4 and PAF are able to induce IL-6Rα release from neutrophil membrane with a rapid kinetic, consistent with an enzymatic shedding process 13. Chemoattractants induce neutrophil membrane IL-6Rα shedding at concentrations corresponding to optimal chemotactic functions except for IL-8 and C5a, which induce IL-6Rα shedding at higher concentrations 21, 22. C5a consistently appeared to be the most potent shedding agonist, whereas PAF and LTB4 seemed less efficient and equivalent to IL-8. Interestingly, IL-8 and PAF which can be coexpressed by inflamed cells such as endothelial cells, and which costimulate neutrophils 18, have a synergistic effect on IL-6Rα shedding.
The IL-6Rα shedding enzyme(s) has(ve) not been identified, but may belong to the TACE subgroup of metalloproteases 15, 17. In agreement with previous reports, we observed that TAPI inhibited potently PMA-induced IL-6Rα shedding on THP-1 cell line 12 – 14. However, TAPI was much less efficient on PMA-activated neutrophils. TAPI also inhibited very significantly C5a- and FMLP-induced IL-6Rα shedding, but demonstrated only 30 – 40 % inhibition of the effects of IL-8, LTB4 or PAF. These data suggest that other enzymes may be involved during neutrophil IL-6Rα shedding. Since cathepsin G, an azurophil granule serine proteinase has been shown to be involved in IL-6Rα shedding in myeloid cell line 23, we evaluated the potential involvement of this enzyme in our experiments. Two inhibitors, PMSF and α1 ACT did not show any inhibitory effect on chemoattractant-induced neutrophil IL-6Rα shedding. Therefore, cathepsin G is not likely to play a role in this phenomenon. Nevertheless, these experiments do not totally rule out the possibility that other neutrophilic enzymes may be involved in neutrophil IL-6Rα shedding. It should be noted, however, that at the concentrations used in our experiments, the various chemoattractants are very weak inducers of neutrophil degranulation, rendering the involvement of serine proteinases in this process unlikely 21, 24.
Usually, soluble cytokine receptors behave as antagonists of cytokine functions and chemoattractant-induced IL-1R type II and TNFR shedding from neutrophil membranes may provide anti-inflammatory mechanisms 25, 26. Despite its agonist activity on IL-6, chemoattractant-induced neutrophil IL-6Rα shedding may also provide a safeguard mechanism to limit local inflammation. First, binding of sIL-6Rα to IL-6 protects IL-6 from enzymatic degradation 27. IL-6/sIL-6Rα complex may induce IL-1 receptor antagonist secretion and p55 TNFR shedding 28, thus favoring a negative control of the proinflammatory functions of IL-1 and TNF-α. This effect may be amplified by the ability of IL-6 to decrease monocyte IL-1 and TNF-α synthesis 29. In addition, sIL-6Rα may complex with locally produced IL-6 to induce transsignaling activation of resident cells such as endothelial, mesothelial or mesangial cells, decreasing IL-8 secretion and increasing MCP-1 production 8, 9. This chemokine profile may decrease local neutrophil recruitment while favoring mononuclear cell recruitment, the initiation of an efficient immune response and the subsequent resolution of acute inflammation. The observation that the various chemotactic agents found during infections, immune injuries or allergic challenges can induce IL-6Rα shedding from neutrophil membranes suggests that in vivo IL-6Rα shedding may be a common negative feedback of all neutrophilic infiltrates, whatever the initial pro-inflammatory injury could be.
4 Materials and methods
4.1 Materials
The following materials were purchased: FMLP, C5a, LTB4, polymyxin B, α1 ACT, PMSF, PAF (Sigma Saint-Quentin Fallavier, France), recombinant IL-8 (R & D Systems, Abingdon, GB), TAPI-1 (Peptides International-Louisville, KY).
4.2 Neutrophil polymorphonuclear cells preparation and stimulation
Neutrophils were prepared from freshly drawn heparinized blood obtained from healthy donors, as previously reported 30. Cells were adjusted to 4 × 106 cells/ml in RPMI containing 10 % heat-inactivated FCS and 7 μg/ml polymyxin B, then stimulated for 30 min or 2 h with either IL-8, C5a, LTB4, PAF, FMLP (10– 6 M), PMA (2.5 ng/ml), or cultured in control medium, before FACS analysis. In each experiment, cell culture supernatants were also collected and stored at − 80 °C, before sIL-6Rα assay. In order to study the role of TACE and serine proteinases, the same experiments were performed on neutrophils preincubated at 37 °C for 10 min with TAPI (100 μM), PMSF (1 mM) or α1 ACT (200 nM).
4.3 FACS analysis
To study IL-6Rα surface expression, neutrophils were directly stained with phycoerythrin-conjugated anti-IL-6Rα mAb (anti-CD126, clone 91) or with a control IgG1 (Beckmann-Coulter, Villepinte, France). Fluorescence was measured on a FACS analyzer (XL; Coultronics, Margency, France). Cell surface IL-6Rα expression was studied by comparing staining with anti-CD126 mAb to that obtained with the control IgG1. Cell surface IL-6Rα expression was expressed as the percent of median fluorescence, as follows: [(median experimental anti-CD126 fluorescence – median experimentalIgG1 fluorescence) / (median control anti-CD126 fluorescence – median control IgG1 fluorescence)] × 100. In experiments using TAPI, since TAPI decreased the spontaneous IL-6Rα release from neurophils, we calculated IL-6Rα expression under the different conditions, using median fluorescence observed with anti-CD126 in unstimulated neutrophils + TAPI, as 100 %.
4.4 sIL-6Rα assay
For each condition of neutrophil stimulation, sIL-6Rα concentrations were measured in the supernatants, using a specific ELISA, from Beckmann-Coulter. For experiments using TAPI, since TAPI reduced the spontaneous IL-6Rα release from neutrophils, we calculated the effect of TAPI on various chemoattractant-induced sIL-6Rα concentrations as follows:
% of sIL-6Rα released = (sIL-6Rα with chemoattractant and TAPI – sIL-6Rα with TAPI) / (sIL-6Rα with chemoattractant – sIL-6Rα with medium) × 100
4.5 Statistical analysis
Cell surface IL-6Rα expression and sIL-6Rα concentrations were expressed as the mean ± SEM of results obtained from at least three individual experiments. The data were compared using paired Student's t-test.




