Efficient generation of cytotoxic T lymphocytes against cervical cancer cells by adeno-associated virus/human papillomavirus type 16 E7 antigen gene transduction into dendritic cells
The first two authors contributed equally to this work.
Abstract
Adeno-associated virus (AAV) is able to efficiently deliver a cytokine gene into dendritic cells (DC). Improvements in T cell priming by DC might be effected by the delivery of antigen genes into DC, resulting in continuous protein expression, as most proteins have short half-lives. In this study, a recombinant AAV vector containing the human papillomavirus (HPV)-16 E7 gene was used to pulse/infect DC and compared to the pulsing of DC by the lipofection of bacterially produced E7 protein. Pulsing of DC with AAV/antigen (Ag) gene was found to be superior to pulsing with protein in six different assay systems: (1) the level of antigen transfer into DC as determined by intracellular staining; (2) the level of MHC class I-restricted killing in cytotoxic T lymphocyte (CTL) assays;(3) the level of IFN-γ expression; (4) the level of DC-T cell priming clusters generated; (5) the level of CD80 and CD83 expression on DC; and (6) in the resulting CD8:CD4 ratio. Finally, AAV/Ag gene pulsing resulted in strong CTL activity after only 7 days of priming. These data suggest that AAV vectors may offer advantages over the commonly used protein-pulsing technique and that AAV vectors may be useful for the stimulation of CTL activity and adoptive immunotherapy protocols.
Abbreviations:
-
- AAV:
-
Adeno-associated virus
-
- DC:
-
Dendritic cell
-
- Mo:
-
Monocytes
1 Introduction
The manipulation of antigen-presenting cells, such as dendritic cells (DC), is a recognized approach toward developing effective immunotherapeutic protocols. DC are potent, professional antigen-presenting cells that are able to initiate primary immune response to antigens by naive T cells 1. Various protocols for generating DC in vitro from peripheral blood have recently been developed. These new technologies permit in vitro manipulation of DC for clinical studies 2, 3. These protocols include pulsing DC with tumor fragments, antigen peptides, defined tumor antigens, or antigen genes by way of retrovirus and adenovirus vectors 4–13. For cervical cancer, the HPV E7 oncoprotein can serve as a unique antigen for immune targeting 14.
AAV has been shown in various studies to be an effective gene delivery vector for both immortalized tissue culture cells as well as primary hematopoietic cells 15–26. We recently showed that it is possible to successfully transduce, with chromosomal integration, the granulocyte macrophage-colony stimulating factor (GM-CSF) gene into monocytes (Mo) and DC via AAV 15. The delivery of the GM-CSF gene by recombinant AAV (rAAV) resulted in the ability to omit the standard addition of exogenous GM-CSF in the pre-DC cultures. This hasled us to hypothesize that, like GM-CSF gene introduction into DC, antigen gene delivery into DC may be more efficient than if the antigen is delivered as an exogenous protein. In this study, it isshown that AAV was able to successfully transduce the HPV-16 E7 antigen gene into DC to prime and generate CTL that showed MHC class I-restricted killing of cervical cancer cells. This approach was extremely efficient, in that CTL were generated after only 1 week of co-culture.
2 Results
2.1 AAV/E7/Neo-transduced DC express E7
The goal of this study was to observe whether AAV-based antigen gene delivery into DC was able to elicit a significant CTL response. Fig. 1A shows a structural map of the AAV/E7/Neo vector used in this study. In this vector, the E7 gene was expressed from the AAV p5 promoter, which is known to be active in DC 15. A two-step process was used to generate an AAV/E7/Neo virus stock, as previously described 15. However, we found that generating producer cell lines for AAV/E7/Neo was more difficult than for other AAV vectors. Sometimes the clones would spontaneously die (possibly apoptosis). Fig. 1B shows a comparison of various G418 resistant producer cell lines for their ability to produce AAV/E7/Neo virus. Fig. 1C shows that the titer of the virus stock, in encapsidated genomes per milliliter (eg/ml) was about 109 eg/ml.
Protocols for the generation of DC by differentiation of peripheral blood Mo usually involve treating adherent Mo with GM-CSF and interleukin-4 (IL-4). We have modified these protocols in order to promote AAV vector transduction in DC 15 precursor Mo by treating adherent Mo with GM-CSF alone for several days before the addition of IL-4 on day 3. This will allow a brief period of Mo proliferation, which will promote a higher level of AAV transduction. A schematic diagram of the experimental protocol is shown in Fig. 1D. On day 3, after AAV infection and GM-CSF treatment, the cells were finally treated with IL-4 to induce differentiation into DC. The transduction of the Mo/DC population was first observed by measuring poly-adenylated RNA expression of the E7 transgene. At day 5, polyadenylated RNA was isolated from AAV/E7/Neo-infected and mock-infected DC cultures (after IL-4 introduction and differentiation into DC). This mRNA was then analyzed for E7 expression by reverse transcriptase-polymerase chain reaction (RT-PCR). A cellular gene, TFIIB, was also undertaken as a control. As shown in Fig. 2, E7 expression was only taking place in the virally infected DC.
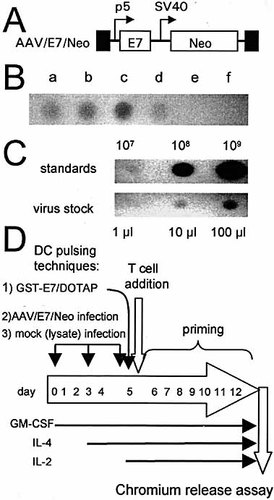
AAV/E7/Neo vector, producer cell lines, and experimental scheme. (A) Structural map of the AAV/E7/Neo (also known as dl6–95/E7p5/NeoSV40) virus with the names of the components at the top. TR (black box) refers to the AAV-terminal repeats. P5 (bent arrow) refers to the AAV p5 promoter. SV40 (bent arrow) refers to the simian virus 40 early enhancer/promoter. The boxes labeled E7 and Neo represent the indicated open reading frames. (B) Analysis of various 293/vector producer cell lines. (C) Titer analysis of the AAV/E7/Neo stock used in these experiments. (D) Graphic depiction of the experimental protocol.
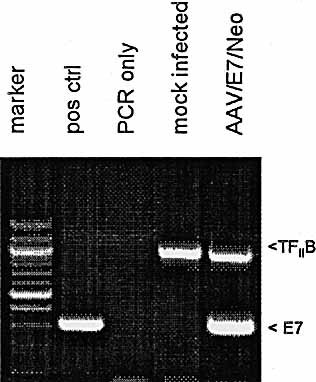
E7 mRNA expression in infected DC. Total RNA was isolated from two cell populations: mock-infected and AAV/E7/Neo-infected adherent Mo at 72 h post-infection. These samples were analyzed by RT-PCR and PCR, as indicated, for the presence of E7 RNA as described in Sect. 4. The positive control was the PCR product resulting from using the AAV/E7/Neo vector plasmid as a template. Another control was RT-PCR analysis for the cellular TFIIB mRNA. Note that only RNA from cells infected with AAV/E7/Neo virus resulted in an appropriate RT-PCR-sized product, whereas mock cells did not do so.
2.2 AAV delivery results in high levels of antigen-positive DC
The efficiency of virus/gene and lipofectin/protein-pulsing of Mo/DC was analyzed and compared by intracellular staining. Our initial attempts at analyzing E7 levels were unsuccessful. We therefore utilized an equivalent AAV/E6-E7/Neo vector under the same conditions and analyzed for E6 protein levels. The cells were analyzed at day 7 of the protocol. The results, shown in Fig. 3, demonstrate that AAV/E6-E7/Neo infection of Mo resulted in a higher percentage of cells containing intracellular E6 protein.
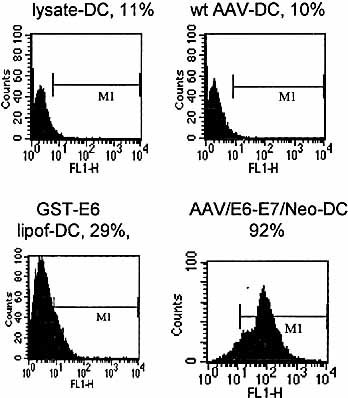
Efficiency of Mo/DC-pulsing analyzed by intracellular staining. Mo were pulsed (infection or lipofection) as indicated, treated with cytokines, and analyzed for E6 protein by intracellular staining on day 7, as described in Sect. 4. Note that AAV/E6-E7/Neo infection/pulsing gave the highest levels of E6-positive cells compared with protein lipofection (92 versus 29%).
2.3 AAV/E7/Neo infection results in chromosomal integration
We next observed chromosomal integration of the AAV/E7/Neo vector in DC. Chromosomal integration, while not essential for gene expression from AAV vectors, does signify a permanent genetic alteration of the DC, and is a desirable "gold standard" for viral transduction. The analysis was carried out by PCR amplification of vector-chromosome junctions using primers complementary to the simian virus 40 (SV40) promoter within the vector and Alu I repetitive chromosomal elements 15. AAV-cell junction products were analyzed by PCR amplification and Southern blot analysis, and probing for the Neo gene sequences. Multiple vector-chromosomal junction products were observed in the AAV/E7/Neo-infected DC, but not in mock-infected DC (Fig. 4). These data indicate that at least a subset of the viral genomes are able to chromosomally integrate in the DC population.
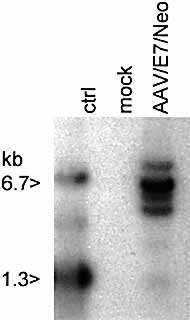
Chromosomal integration by AAV/E7/Neo in DC. Cells, treated as indicated as described in Sect. 4 and sorted for CD83 expression, were further analyzed for chromosomally integrated AAV/E7/Neo viral genomes. Total cellular DNA (0.1 μg) from the infected, CD83+ selected cells and uninfected cells served as a template in PCR amplification assays using primers targeting the SV40 early promoter of the vector and the cellular repetitive Alu I element. The products were Southern-blotted and probed with 32P-Neo DNA. The positive control lane contained 100 ng of BamHI digested AAV/E7/Neo plasmid (6.7 and 1.7 kb). Note that multiple Neo-positive bands resulted from the infected cell population, indicating chromosomal integration by the vector, and that multiple vector-positive cell clones were present in the population.
2.4 AAV/E7/Neo-transduced DC primed and propagated E7-specific CTL
The ability of the AAV/E7/Neo vector to generate CTL was next analyzed. DC were pulsed by three different techniques as shown in Fig. 1D. One interesting nuance observed during the priming process was the formation of cell rosettes or clusters in the AAV/E7/Neo-pulsed cultures 2 days after addition of the T cells (Fig. 5). By studying the morphology of individual cells within these rosettes, we believe they may represent DC-T cell rosettes and may be an evidence of enhanced priming. Similar DC-T cell rosettes or clusters have been reported by others 27–29. To determine the ability of the AAV/E7/Neo-transduced DC to prime and propagate E7-specific CTL, we carried out a standard 6-h 51Cr-release assay on day 7, using the T cell populations in the co-cultures. Fig. 6A and B show that the T cell population derived from co-culture with AAV/E7/Neo-transduced DC was able to lyse HLA class I-matched E7-positive primary cervical tumor cell lines, indicating the ability of AAV/E7/Neo-transduced DC to prime and propagate E7-specific CTL. When we analyzed CTL generated from co-culture with E7 protein or lysate-pulsed DC, CTL primed and generated with the use of AAV/E7/Neo-transduced DC were able to mediate target lysis in a much more efficient manner. HLA class I-restricted target lysis was further confirmed by the ability of a monoclonal antibody directed at the monomorphic HLA class I molecules to block killing. Tumor cell lysis was also not mediated through NK cells because no cytolysis was observed when K562 cells were used as targets. To further address the ability of this technique to specifically recognize the E7 target antigen we performed an addition cytotoxicity assay. In this experiment the target cells were autologous lymphoblastoid cell lines (LCL) either unmodified, modified by the lipofection of GST-E7 protein, or infected with AAV/E7/Neo virus. The primed T cells were generated as shown in Fig. 6A and B. As shown in Fig. 6C, with the use of this fully autologous system, the primed T cells had little activity (10%) against unaltered self-LCL, moderate activity in killing GST-E7-lipofected LCL (25%), and strong activity against AAV/E7/Neo-infected LCL (86%) (average of two experiments). These results confirm the antigen specificity of this technique.
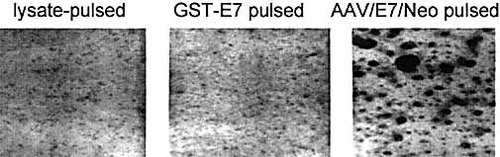
Early appearance of priming rosettes during AAV-mediated priming. Adherent Mo were mock-, GST-E7-, or AAV/E7/Neo virus-pulsed as described in Sect. 4. At day 5, the resulting DC were the incubated with nonadherent peripheral blood lymphocytes. Two days after the addition of T cells representative pictures were taken of the cultures a low and high power. Note that the virus-treated CD-T cell cultures exhibited much higher levels of rosetted cell clusters, suggesting stronger DC-T cell interaction.
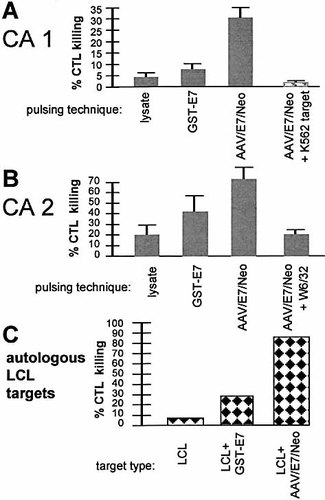
Cytotoxic response resulting from AAV vector and DOTAP-protein lipofection after 7 days of priming. (A) A representative experiment of cytotoxic response resulting from the indicated pulsing techniques using Mo/DC, and T cells from a normal individual against HLA-A-1-matched primary cervical cancer cells (CA1) and K562 cells. Also note lack of killing activity against the K562 cells. (B) A representative experiment of cytotoxic response against a second HLA-A-1-matched primary cervical cancer (CA2). Note that the addition of the class I-blocking antibody W632 greatly inhibits killing. (C) Cytotoxic response, or nonresponse, against autologous LCL targets by virus-primed T cells. The LCL+GST-E7, and LCL+AAV/E7/Neo-positive targets were generated as described in Sect. 4. This experimental system is fully autologous. Primed T cells were generated by pulsing Mo/DC with AAV/E7/Neo virus. Average of two experiments is shown. Note that the unaltered (E7-negative) LCL were not strong targets, whereas LCL+GST-E7 and LCL+AAV/E7/Neo were good to excellent targets, indicating the antigen specificity of the response.
2.5 Immunophenotypes of T cells
Immunophenotyping of the AAV/E7/Neo-pulsed DC-primed T cell population showed that this T cell population expressed predominantly CD8 (Fig. 8) and that the CD8:CD4 ratio was slightly higher than in the protein-pulsed and primed situation. In addition, AAV/E7/Neo-pulsing resulted in only low levels of CD56 marker expression. In sharp contrast, pulsing by the lipofection of GST-E7 resulted in much higher levels of CD56. Although CD56 is generally believed to be a natural killer T cell-specific marker, HLA class I-restricted CD8+ T cells can also express CD56 30.
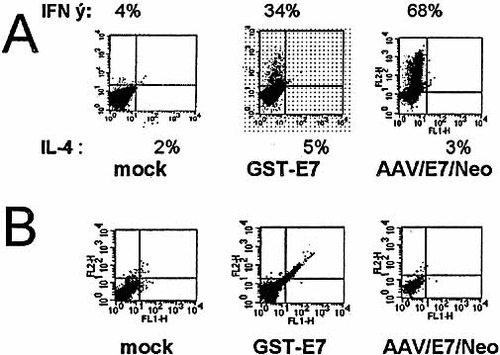
Two-color flow cytometric characterization of intracellular cytokine expression in primed T cell populations. (A) Intracellular prevalence of IFN-γ and IL-4 within primed and stimulated mixed T cell populations resulting from three different techniques as described in Sect. 4. (B) Intracellular prevalence of IFN-γ and IL-4 within unstimulated control T cell populations resulting from the same techniques as in (A).
2.6 Cytokine profile of T cells
To determine the cytokine profile of the T cells generated from co-culture with AAV/E7/Neo-transduced DC, we carried out intracellular staining of these T cells for IFN-γ and IL-4. Fig. 8 demonstrates that most of the T cells expressed IFN-γ and very little IL-4, suggesting that these cells are of the Th1 phenotype. A smaller proportion of IFN-γ-producing T cells were observed in the T cell populations co-cultured with either E7 protein or tumor lysate-pulsed DC.
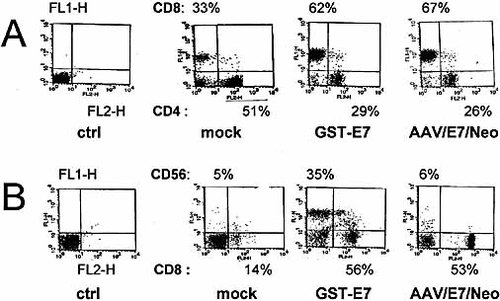
Two-color flow cytometric characterization of surface markers in primed T cell populations. (A) CD8 and CD4 prevalence within the primed T cell populations resulting from three different techniques as indicated (on the right), determined as described in Sect. 4, as well as an FL1-H, FL2-H control (left). (B) CD56 and CD8 ratios in the same experimental situations as in (A).
2.7 Analysis of DC by various manipulations
Finally, we characterized the DC resulting from the various pulsing techniques to observe whether significant differences were discernible. We used flow cytometric analysis to determine the phenotype of untreated, lysate-pulsed, GST-E7-pulsed, and AAV/E7/Neo-pulsed DC populations. The results, shown in Figs. 9 and 10, demonstrate that the DC generated from all four techniques share all of the common DC markers. However, AAV/E7/Neo-pulsed DC did express significantly higher levels of CD80 and CD83, and slightly lower levels of CD86 than those produced by GST-E7-pulsed DC.
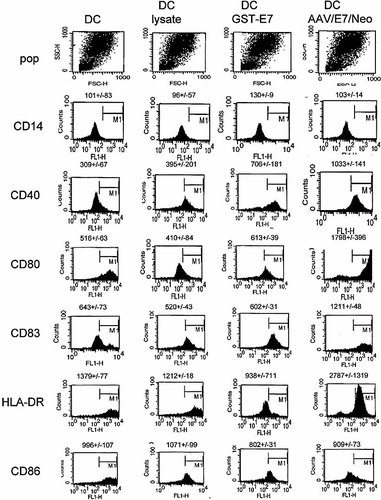
FACS analysis of DC at day 7 under different conditions. Original histograms of the indicated experimental situation are shown with the quantified mean fluorescent intensity shown above each histogram.
3 Discussion
Because more than 90% of cervical cancers contain HPV DNA and the E6 and E7 oncoproteins are always expressed, cervical cancer is a good model system for investigating adoptive immunotherapy protocols. Antigen gene pulsing of DC theoretically may be more efficient than protein pulsing of DC for several reasons. First, due to protein degradation and major histocompatibility complex (MHC) molecule cycling, protein pulsing of DC may be an inefficient way to deliver an antigen. In contrast, gene transfer leads to continuous production of the antigenic protein and may provide the opportunity for repeated rounds of CTL stimulation. Secondly, viral entry into cells is usually more efficient than lipofection delivery of proteins. Thirdly, viral delivery of antigens has been shown to result in higher epitope presentation in the context of class I molecules, which should result in a more efficient activation of the CD8+ CTL than lipofection delivery of antigen proteins 31. Fourthly, epitopes of proteins produced in bacteria may be less immunoreactive, as bacteria lack many of the post-translational modifications seen in mammals. Viral gene delivery, resulting in many epitopes produced and expressed endogenously in DC, should be more representative and more immunoreactive. For these reasons, viral-antigen pulsing of DC seemed a logical approach.
In this study, we demonstrate that AAV is able to transduce freshly adherent Mo/DC precursors with an antigen gene, that some vector-chromosomal integration takes place, and that the transduction of E7 gene into DC results in functionally active E7-epitope presenting DC. The rAAV-transduced DC were able to prime and propagate antigen-specific CTL in an efficient manner resulting in significant cytotoxicity for E7-bearing target cells after only 7 days of priming (12 days for the complete protocol). We are pleased with the effectiveness of our CTL given that our DC do not appear to be fully mature. We believe this protocol may be the shortest protocol yet described for generating strong CTL activity. No cytotoxicity was observed against a non-E7-bearing target. Moreover, this efficiency of AAV/gene pulsing was further supported by higher IFN-γ and DC-T cell rosetting activities observed with this technique.
While improvement in CTL and IFN-γ activity resulting from AAV/gene pulsing of DC was not surprising, for the reasons listed earlier, the rapidity of T cell priming was not anticipated. Only 1 week of DC-T cell incubation was needed to yield T cell populations with significant CTL activity. Most priming protocols require a much longer time. The specific reason for this is not known but may be related to a combination of improved quality of DC (as suggested by up-regulation of CD80) and the use of a viral-based vector that appears to stimulate the proliferation of the CD8+ Th1T cells (Figs. 7, 8). There was little evidence of CD56-positive cells in the AAV/E7-pulsed situation, so that endogenous NK activity cannot account for it. Finally, the higher CD80 expression in the DC pulsed with AAV/E7/Neo is also noteworthy. It has been suggested that CD80 may be a more independent co-stimulatory molecule than CD86, and this may explain the improved capabilities of the vector-transduced DC 32. In any case, we are pleased about the effectiveness of our CTL given that our DC do not appear to be fully mature. Taken together, these data support that the effectiveness of AAV-mediated antigen gene delivery into Mo/DC is quite good for CTL generation and that AAV-based pulsing of DC represents a promising technique for effectively introducing antigens into DC to stimulate specific-CTL response against the transduced antigen. As our DC appear to be immature, it would be interesting to determine whether additional stimuli might improve DC maturation and CTL response even further.
4 Materials and methods
4.1 Materials
The primary cervical cancer cell lines, CA1 (patient 1) and CA2 (patient 2), have previously been described 12. Both contain HPV-16 DNA and were approximately ten passages from initial isolation when used in this study. The cells were grown in Keratinocyte-SFM supplemented with epidermal growth factor and bovine pituitary extract (Gibco BRL/Life Technologies). Both cell lines contain the HLA-A1 haplotype. Mo and DC were derived from peripheral blood mononuclear cells (PBMC). PBMC were obtained from a normal donor with the HLA-A1 haplotype. The lymphoblastoid cellline was generated by transforming donor B cells with Epstein-Barr virus (EBV) as previously described 12.
4.2 Construction of the rAAV/Ag genomes
The AAV/E7/Neo and AAV/E6-E7/Neo genomes were constructed in a similar strategy to that previously described AAV/GM-CSF/Neo viral genome 15. However, instead of the GM-CSF gene, the HPV-16 E7 and E6-E7 open reading frames were cloned by PCR amplification using Pfu polymerase and ligated into the vector, respectively. High-titer rAAV stocks were generated in a two-step process using the complementor plasmid ins96–0.8 and titered as described previously 15, 33.
4.3 Generation and infection of Mo/DC
The PBMC were inoculated into six-well culture plates for 2 h at 37 °C and 5% CO2, and the adherent cells were harvested following three gentle washes. Immediately after the removal of the nonadherent cells, the adherent Mo were infected with 0.5 ml of virus stock (109 eg/ml). After 2 h of incubation the medium/virus solution was removed, and the cells were washed and replaced with fresh AIM-V medium. Altogether, the Mo/DC precursors were infected with the virus stock three times: on days 0, 3, and 5. GM-CSF (LEUKINE®, Immunex Corporation) at a final concentration of 800 IU/ml was included in the medium throughout the culture. To induce the maturation of Mo into DC, human IL-4, (R&D SYSTEMS Co.) at 1,000 IU/ml was added at day 3.
4.4 Generation of autologous LCL targets
Donor LCL (2×106) were lipofected with 50 μg of GST-E7 protein 1 day before the 51Cr release assay to generate one type of autologous target. Donor LCL (2×106) were infected with 0.5 ml of the virus stock 3 days before the 51Cr-release assay to generate a second type of autologous target.
4.5 Generation of bacterial E7 and E6 protein-pulsed Mo/DC
Mo/DC were generated as above and pulsed with 50 to 100 μg of GST-E7 protein on day 5 as previously described 12.
4.6 RT-PCR analysis
E7 mRNA expression was detected by RT-PCR amplification along with a cellular mRNA control. Total RNA was isolated Trizol reagent (Gibco BRL Life Technologies Inc.) and treated with 5 U/μgof RNase-free DNase I (Promega Co.) at 37°C for 2 h. Messenger RNA was separated using the Oligotex mRNA Mini Kit (Qiagen Inc.). First-strand cDNA synthesis was performed using oligo(dT)15primers. PCR amplification for E7 sequence was carried out with the following primer pair: 5′-GAAGATCTATCATGCATGGAGATACACC-3′ and 5-CTAGATCTTTATGGTTTCTGAGAACAGATGG-3′ that amplifythe HPV-16 sequence from nucleotides 139 to 420. A control PCR analysis consisting of amplification of the TFIIB gene segment was included in all reactions. In addition, a control amplification using mRNA without prior RT was also included. PCR products were visualized by ethidium bromide staining on an ultraviolet light transilluminator.
4.7 Detection of viral integration by PCR/Southern blot analysis
Chromosomal integration of the AAV/E7/Neo genome was undertaken by vector-chromosome junction PCR amplification and Southern blot analysis, as previously described 15.
4.8 Generation and testing of E7-specific CTL
Nonadherent PBMC from the same healthy donor were washed and resuspended in AIM-V at 10×106–20×106 cells/well in six-well culture plates (Costar, Cambridge, MA) with rAAV- or GST-E7-pulsed DC (ratios from 20:1, responders:dendritic). The cultures were supplemented with recombinant human GM-CSF (800 U/ml) and recombinant human IL-2 (10 U/ml). At 7 days of the co-culture, the cells were used for cytotoxicity assays in a 6-h 51Cr release assay, as previously described 12.
4.9 Analysis for intracellular cytokines and protein
This protocol is adapted from that described by Pala et al. 34. The mixed T cell population was tested at 2 weeks post-priming, including 7 days of resting, after activationby PMA and ionomycin. Briefly, T cells (7.5×105/ml) were incubated at 37oC for 6 h in AIM-V 5% autologous plasma plus 50 ng/ml PMA and 500 ng/ml ionomycin. Brefeldin A, 10 ng/ml, was added for the final 3 h of incubation. Controls (nonactivated cultures) were incubated in the presence of Brefeldin A only. The cells were harvested, washed and fixed with 2% paraformaldehyde in phosphate buffered saline (PBS) for 20 min at room temperature. The cells were washed and permeabilized with PBS/1% bovine serum albumin (BSA)/0.5% saponin (S-7900, Sigma) for 10 min at room temperature. Activated and control cells were stained with FITC-anti-IFN-γ, and PE-anti-IL-4 and analyzed by flow cytometry. For intracellular E6 protein analysis, AAV/E6-E7/Neo-infected cells were stained with anti-HPV-16/18 E6 (Chemicon Inc., Temecula, CA; Cat MAB874) plus FITC-anti-mouse-Ig (Becton Dickinson Inc., cat no. 554001) and analyzed by flow cytometry.
4.10 Cell surface marker analysis of T cells and DC by fluorescent antibody cell sorting (FACS)
For the analysis of T cells at day 12 of the experiment the primed T cell populations were analyzed for surface markers. Monoclonal antibodies (mAb) recognizing the following antigens were used: anti-CD4, anti-CD8, and anti-CD56 (Pharmingen, San Diego, CA). Control irrelevant isotype-matched FITC- or PE-conjugated mAb were obtained from Becton Dickinson. These cells were >95% viableas assessed by trypan blue exclusion. Cell suspensions were counted and distributed into 12×75 mm tubes. Mouse mAb were diluted in cold assay buffer, and the final pellet was resuspended in a 500-μl volume. Tubes were incubated for 30 min and washed twice with assay buffer, and the final cell pellet was resuspended in 500 μl of assay buffer for subsequent analysis. Cells were analyzed with a fluorescence activated cell sorter (FACS; Becton Dickinson) with a 15-mW argon laser having an excitation of 488 nm. Fluorescent signals were gated on the basis of cell dimension (i.e. forward and right-angle light scattering typical of PBL activated). Gated signals (5,000–10,000) were detected at 585 BP filter and analyzed with Cell Quest software (Becton Dickinson).
For the analysis of DC, a panel of mAb recognizing the following antigens was used: anti-CD40 (Immunotech, Marseille, France); anti-CD14, anti-DR, anti-CD80 (Becton Dickinson), anti-CD86 (Pharmingen, San Diego, CA), and anti-CD83 (Coulter, Miami, FL). Briefly, nonadherent cells were harvested by washing the plates with PBS (pH 7.2, GibcoBRL). Adherent cells were recovered by incubatingthe plates at room temperature for 15–20 min in the presence of Ca2+- and Mg2+-free PBS, followed by gentle scraping. These cells were >95% viable as assessed by trypan blue exclusion. Cell suspensions were counted and distributed into 12×75 mm tubes. Mouse mAb antibodies were diluted in cold assay buffer (PBS, pH 7.2, supplemented with 0.1% fetal bovine serum) andadded in a 50-μl volume. For direct fluorescence, tubes were incubated for 30 min and washed twice with assay buffer, and the final cell pellet was resuspended in 500 μl assay buffer for subsequent analysis.
Acknowledgements
We acknowledge the excellent assistance provided by Teri Fields and Rena Sheffer.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




