Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells
Abstract
Following encounter with pathogens, dendritic cells (DC) mature and migrate from peripheral tissues to the T cell areas of secondary lymphoid organs, where they produce regulatory cytokines and prime naive T lymphocytes. We investigated in two subsets of human peripheral blood DC the expression of Toll-like receptors (TLR1 through TLR9) and the regulation of chemokine receptors and cytokine production in response to different maturation stimuli. Myeloid DC express all TLR except TLR7 and TLR9, which are selectively expressed by plasmacytoid DC. Myeloid and plasmacytoid DC respond to pathogen-associated molecular patterns according to their TLR expression. In response to the appropriate stimuli both DC types up-regulate CCR7, a receptor that drives DC migration to the T cell areas. Type I IFN was produced only by plasmacytoid DC and at early time points after stimulation. Furthermore, its production was elicited by some of the maturation stimuli tested. These results reveal a remarkable specialization and complementarity in microbial molecule recognition as well as a flexibility in effector function among myeloid and plasmacytoid DC.
Abbreviations:
-
- pDC:
-
Plasmacytoid dendritic cells
-
- mDC:
-
Myeloid dendritic cells
-
- moDC:
-
Monocyte-derived dendritic cells
-
- TLR:
-
Toll-like receptors
-
- CpG:
-
Nonmethylated CP oligonucleotides
-
- PG:
-
Peptideglycan
-
- PAMP:
-
Pathogen-associated molecular patterns
1 Introduction
Dendritic cells (DC) are professional antigen-presenting cells (APC) that play an essential role in the induction of immune responses 1. In their immature form they express receptors for inflammatory chemokines and migrate to sites of inflammation where they capture antigens and are stimulated by pathogens, inflammatory cytokines or tissue damage to initiate the maturation process. This process involves the up-regulation of major histocompatibility complex (MHC) molecules, costimulatory molecules and CCR7, a receptor for SLC/CCL21 and ELC/CCL19 that drives DC migration into the lymphatic vessels and their final localization to the T cell areas of draining lymph nodes 2.
Cells of the innate immune system recognize pathogen-associated molecular patterns (PAMP) using Toll-like receptors (TLR) 3. The responsiveness to a given PAMP has been linked to the expression of a particular TLR. For instance TLR4 is required for the response to LPS 4, TLR2 to peptidoglycan (PG) and mycobacteria 5–8, TLR5 to flagellin 9 and TLR9 to CpG oligonucleotides and bacterial DNA 10. Furthermore, TLR can function as homo- or heterodimers thus further increasing the potential repertoire of specificities 11. TLR activate NFκB and induce the expression of a variety of immune defense genes 3, 12. Recent studies have reported the expression of TLR in murine and human myeloid DC 13–18.
In human peripheral blood two DC subsets can be identified 19. The first is represented by myeloid DC (mDC), which are CD11c+, CD45RO+, CD123lo, ILT1+, CD13+ and CCR5+. These cells mature in response to a variety of stimuli, but produce IL-12 primarily in response to LPS or CD40L stimulation 20. The second subset is represented by plasmacytoid DC (pDC) 21. These cells are CD11c-, CD45RA+, CD123high, ILT1-, CD13- and CXCR3+ and, upon exposure to viruses produce very high levels of type I interferon (IFN-I) 22, 23 and are therefore identical to the previously identified interferon-producing cells 24. In humans IFN-I is a Th1-polarizing cytokine and indeed, after exposure to viruses, pDC have been shown to induce potent Th1 responses 25, 26. Previous studies, however, showed that upon stimulation with CD40L the same cells mature and acquire the capacity to prime Th2 responses 27.
We have compared mDC and pDC for their capacity to modulate chemokine receptor expression and synthesize cytokines in response to different PAMP. We show that mDC and pDC express complementary sets of TLR and respond to different PAMP by up-regulating CCR7 expression and by producing distinct cytokine combinations.
2 Results and discussion
2.1 pDC and mDC respond to different PAMP by up-regulating CCR7 and down-regulating CCR5 or CXCR3
pDC and mDC were purified from peripheral blood by a combination of magnetic and FACS sorting. The cells were stimulated in vitro with different PAMP or CD40L and chemokine receptor expression was measured after 24 h (Fig. 1a). mDC responded to LPS and PG by up-regulating CCR7 and down-regulating CCR5 expression, a switch which is characteristic of the maturation process of in vitro generated monocyte-derived DC (moDC). In striking contrast, pDC did not show any change in chemokine receptor expression in response to LPS or PG; however, they up-regulated CCR7 and down-regulated CXCR3 expression in response to live influenza virus and to the CpG oligonucleotides 2006 (Fig. 1) and 2216 (not shown). In contrast, mDC were unresponsive to both CpG and died in response to influenza virus infection. Finally, both DC types responded to CD40L. In all cases switch in chemokine receptor expression was accompanied by up-regulation of B7.1, B7.2, CD83 and HLA-DR (data not shown). Interestingly, the extent of CCR7 up-regulation was consistently much higher in pDC than in mDC (Fig. 1b). The high level of CCR7 expression characteristic of mature pDC would be consistent with their extravasation at the level of high endothelial venules (HEV) around which they have been shown to localize 28. Indeed, it has been shown that extravasation requires higher levels of chemokine receptor expression than migration within tissues 29. It remains to be established whether pDC can mature in the blood and migrate directly to the lymph nodes or whether they first enter as immature cells into inflamed peripheral tissues and, upon maturation, migrate to the lymph nodes following the same route of myeloid lineage DC.

pDC and mDC modulate chemokine receptor expression in response to different stimuli. (A) Freshly isolated pDC and mDC were stained with antibodies to CCR7, CCR5 or CXCR3 20 h following exposure to the indicated stimuli (filled histograms) or to medium alone (solid lined open histograms). The control antibody staining is shown in broken lined open histograms. (B) Extent of CCR7 up-regulation in mDC, moDC and pDC in response to different stimuli. RMFI represents the ratio of CCR7 staining and isotype control. Data are from two experiments out of five.
2.2 pDC and mDC express reciprocal sets of TLR
To explain the differential responsiveness of mDC and pDC to PAMP we investigated the expression of TLR (TLR 1–9) using RT-PCR on freshly isolated highly purified (>98%) DC populations (Fig. 2a). pDC express only TLR7 and high levels of TLR9 mRNA, consistent with their responsiveness to CpG, which has been shown to require TLR9 10. In contrast, TLR7 and TLR9 are not expressed by mDC, which express a complementary set of TLR, namely TLR2, TLR3, TLR4, TLR5, TLR6, and TLR8.
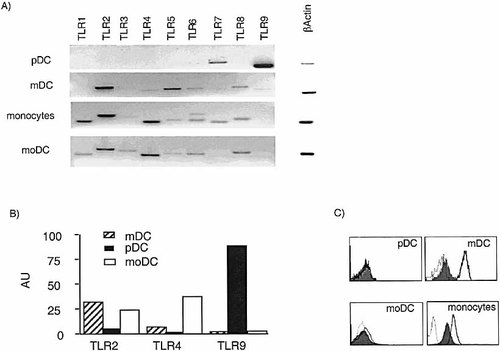
Reciprocal expression of TLR on DC subsets. TLR expression was determined by: (A) RT-PCR using primers specific for TLR1–9; (B) real time RT-PCR using primers and probes specific for TLR2, TLR4, TLR9 (AU, arbitrary units normalized to beta actin); and (C) surface staining for TLR2 (solid lined open histograms) and TLR4 (filled histograms). Isotype control staining is shown as broken line open histograms.
The expression of TLR2 and TLR4 on mDC correlates with their capacity to respond to PG and LPS, respectively. Peripheral blood monocytes and moDC expressed the same spectrum of TLR as mDC with, in addition, TLR1. TLR1 and TLR6 have been shown to form heterodimers with TLR2 having different signaling capacity 11. Real time PCR experiments confirmed the differential expression of TLR2 and TLR4 on mDC and TLR9 on pDC (Fig. 2b). Consistent with the mRNA expression data, antibodies to TLR2 and TLR4 stained monocytes and myeloid lineage DC. Altogether both phenotypic and functional studies indicate that pDC and myeloid lineage DC express complementary sets of TLR, which endow them with the capacity to respond to different PAMP.
2.3 Specialization, flexibility and exhaustion of cytokine production by pDC and mDC
The pattern of cytokines produced by pDC and mDC following exposure to different maturation stimuli was analyzed by measuring cytokine accumulation in the culture supernatant (Table 1). Consistent with previous observations 22, 23, 30, 31, pDC produced high levels of IFN-α in response to viral infection and to CpG 2216, but not in response to CpG 2006 or to CD40L, although both stimuli induced CCR7 up-regulation and production of other cytokines, such as IL-6. IFN-α was produced rapidly in response to influenza virus peaking at approximately 8 h (Fig. 3A). This production, however, was transient since it fell dramatically at later time points, although the cells were still alive (Fig. 3B). Interestingly, IFN-α and IL-12 were produced by pDC in a reciprocal fashion: CpG 2006 induced IL-12, but not IFN-α production; conversely CpG 2216 induced strong production of IFN-α with no concomitant induction of IL-12. It is tempting to speculate that the different stimulatory capacity of the two CpG tested may be mediated through TLR9 homo or heterodimers. Finally, pDC produced IL-6 and TNF-α, but never IL-10 and, in most cases, did not produce cytokines in response to CD40L stimulation. Under the stimulatory conditions tested, mDC did not produce IFN-α, but produced IL-12 in response to LPS and PG. In some experiments low levels of IL-12 were produced by mDC in response to CD40L. The above results show that the two main Th1-polarizing cytokines are very tightly regulated both in terms of elicitation in response to different stimuli and kinetics of production. Accordingly, both mDC and pDC can be considered either DC1 or DC2 (Th1 or Th2 inducers), depending on the nature of the maturation stimulus and the kinetics of activation 32, 33.
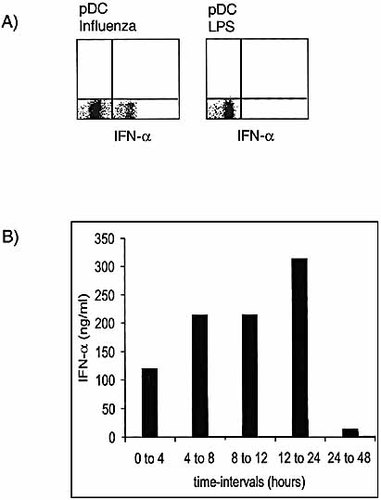
Kinetics of IFN-α production by pDC. (A) pDC were challenged with influenza virus or LPS for 8 h, fixed, permeabilized and stained for intracellular IFN-α. Monensin and brefeldin A were added for the last 4 h of incubation. The quadrants were set according to the staining with irrelevant antibodies. (B) Supernatants of pDC challenged with influenza virus were collected at different time points. After each collection, cells were washed and replated with influenza virus to provide continuous stimulation.
|
|
IL-6 |
IL-10 |
TNF-α |
IL-12 |
IFN-α |
|
|---|---|---|---|---|---|---|
|
|
- |
<6 |
<6 |
<6 |
<3 |
<5 |
|
|
CpG 2216 |
472 |
<6 |
516 |
<3 |
>80,000 |
|
pDC |
CpG 2006 |
2,050 |
<6 |
162 |
176 |
<5 |
|
|
Influenza |
950 |
<6 |
256 |
<3 |
>80,000 |
|
|
CD40L |
35 |
<6 |
<6 |
<3 |
<5 |
|
|
- |
<6 |
<6 |
<6 |
<3 |
<5 |
|
|
LPS |
137 |
<6 |
635 |
6 |
<5 |
|
mDC |
PG |
2,200 |
1,223 |
51 |
10 |
<5 |
|
|
CD40L |
28 |
<6 |
<6 |
<3 |
<5 |
|
|
- |
<6 |
<6 |
<6 |
<3 |
<5 |
|
|
LPS |
11,200 |
3,640 |
2,350 |
574 |
<5 |
|
moDC |
PG |
9,800 |
5,300 |
2,500 |
35 |
<5 |
|
|
CD40L |
6,200 |
116 |
1,125 |
8 |
<5 |
- a) Supernatants were collected 40 h after addition of the indicated stimuli. Cytokines were measured by ELISA and the results (pg/ml) were normalized to 105 cells. Results are from one representative experiments out of five.
Altogether the direct comparison of pDC and mDC reveals common as well as divergent themes. The first common theme is the maturation-induced CCR7 up-regulation that drives the migration of maturing DC towards T cell areas. The second common theme is the flexibility of cytokine production in response to various stimuli and its rapid exhaustion following induction of maturation. pDC and mDC, however, differ in a novel important aspect, namely the expression of TLR. It is tempting to speculate that the different capacity to respond to pathogens may result in a concerted action of pDC and mDC in the initiation of the immune response. By producing high levels of IFN-α in response to viruses or bacteria, pDC may protect myeloid lineage DC from the cytopatic effect of the virus 34 and may exert an adjuvant effect on antibody responses 35.
3 Materials and methods
3.1 Isolation and stimulation of DC
mDC and pDC were isolated from PBMC of healthy donors using a combination of magnetic and FACS sorting. Briefly, PBMC were incubated with CD1c-FITC and BDCA4-PE mAb followed by anti-FITC and anti-PE magnetic beads (Miltenyi, Bergisch Gladbach, Germany). The labeled cells were separated using Automacs (Miltenyi) and were further stained with CD19-APC (Becton Dickinson, San Jose, CA) and CD14-APC (Immunotech, Marseille, France) to detect B cells and monocytes, respectively. mDC (CD1c+, BDCA4-, CD19- and CD14-) and pDC (BDCA4+, CD1c-, CD19- and CD14-) were then sorted on a FACSVantage SE (Becton Dickinson). moDC were generated as previously described 36. All DC preparations analyzed were >98% pure. Cells were cultured in RPMI1640 supplemented with 10% FCS (Hyclone, Logan, UT) and either 1,000 U/ml IL-4 and 50 ng/ml of GM-CSF (for mDC and moDC) or 10 ng/ml of IL-3 (R&D Systems, Minneapolis, MN) for pDC. DC were challenged with one of the following maturationstimuli: 0.1 μg/ml LPS, 5 μg/ml of PG ( both from Sigma Chemicals Co., St. Louis, MO); 5 μg/ml CpG oligodeoxynucleotide 2006 (5′-GGGGGACGATCGTCGGGGGG-3′) 37 or 2216 (5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′) 31; 40 HAU/ml influenza virus (strain A/Beijing/353/89; kind gift from I. Julkunen) or rCD40L (Alexis Biochemicals, San Diego, CA).
3.2 Surface markers and cytokine production
Cell surface staining was performed using either directly conjugated mAb or purified mAb followed by PE-conjugated goat anti-mouse antibodies (Southern Biotechnology Associates, Inc., Birmingham, AL). The following antibodies were used: mouse CD80, CD86, CD83, HLA-DR (Immunotech, Marseille, France), rat CCR7 (kind gift of M. Lipp), mouse CXCR3 and CCR5 (Becton Dickinson). Production of type-I IFN was evaluated after 8 h of activation with influenza virus by intracellular staining with anti-IFN-α mAb (Research Diagnostics, Inc., Flanders, NJ) followed by FITC-conjugated goat anti-mouse Ig. In the last 4 h, 2 μg/ml of monensin and brefeldin A were added. Cells were analyzed on a FACSCalibur cytometer (Becton Dickinson). Cytokines were measured in culture supernatants after 40 h of activation with the various stimuli using commercially available ELISA kits for IL-6, IL-10, IL12, TNF-α (R&D Systems) and IFN-α (PBL Biomedical Laboratories).
3.3 TLR expression
Total RNA was isolated from pDC, mDC and moDC (3×105–10×105) using the RNeasy kit (Qiagen, Valencia, CA). cDNA was synthesized using random hexamers and the TaqMan Reverse Transcription kit (Perkin-Elmer Applied Biosystems, Foster City, CA). Semiquantitative PCR on the cDNA was performed with the following primers: TLR1 (F:CGCATGGTCCACATGCTTT, R:GCCACATCCAGGAAGGTCAGT), TLR2 (F: CCCTGGGCAGTCTTGAACATT, R:GCCTCCGGATTGTTAACGTTT), TLR3 (F:GGGTCCCAGCCTTACAGAGAA, R: CTAGGTGGCCCAACCAAGAG), TLR4 (F:TGGTGTCCCAGCACTTCATC, R: CTGCATATCTAGTGCACCATGG), TLR5 (F:TCCACGGAAGGTTGTGATGA, R:GACCCAACCACCACCATGA), TLR6 (F: CCTCATGCACCAAGCACATT, R: TCTGGCAGCTCTGGAAGAAA), TLR7 (F: CGAACCTCACCCTCACCATTA, R: GGGACGGCTGTGACATTGTTA), TLR8 (F: GCCAGCGAGTCTCACTGAACT, R: GCCAGGGCAGCCAACATA), TLR9 (F:TGGACACTCCCAGCTCTGAAG, R:TGGGACACTTGGCTGTGGATG). All reagents were from Stratagene. Real-time quantitative RT-PCR was performed as described 38. A relative quantification of TLR2, 4 and 9 mRNA was done using an ABI PRISM 7700 Sequence Detector (Perkin-Elmer) and TaqMan probes provided by Applied Biosystems. The sequences of probes and oligos used were:TLR2 (5′-3′:CAGCACTGGTGTCTGGCATG, Probe: CTGTGCTCTGTTCCTGCTGATCCTGC, 3′-5′: GAGCC AGGCCCACATCATT), TLR4 (5′-3′: GTTTCCTGCAATGGATCAAGGA, Probe: TCGTTCAACTTCCACCAAGAGCTGCCT, 3′-5′TGCTTATCTGAA GGTGTTGCACAT), TLR9 (5′-3′: TGAAGACTTCAGGCCCAACTG, Probe: AGCACCCTCAACTTCACCTTGGATCTGTC, 3′-5′: TGCACGGTCACCAGGTTGT). Amplification of beta actin was done for each experimental sample as endogenous controlto account for differences in the amount and quality of total RNA added to each reaction.
Acknowledgements
We thank Gioacchino Natoli for critical reading and comments. This study is supported by the Swiss National Science Foundation (contract no. 31-63885.00). The Basel Institute was founded and supported by Hoffmann-La Roche. AL is supported by the Helmut Horten Foundation.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




