Lethal Escherichia coli and Salmonella typhimurium endotoxemia is mediated through different pathways
Abstract
Despite the differences in the molecular structure between lipopolysaccharides (LPS) isolated from Escherichia coli, Klebsiella pneumoniae or Salmonella typhimurium, the potential differences in their biological effects in vivo have not been investigated. In the present study, TNF and LT double knock-out (TNF−/−LT−/−) mice were almost as susceptible as TNF+/+LT+/+ controls to S. typhimurium LPS, but they were significantly more resistant to lethal endotoxemia induced by E. coli or K. pneumoniae LPS. The effect was not due to endotoxin-associated proteins. In the knock-out mice, this difference in lethality was accompanied by decreased interleukin-1 (IL-1) and interferon-γ (IFN-γ) production after challenge with E. coli LPS, whereas after S. typhimurium LPS more IL-1 and IFN-γ were produced. In contrast, more IL-10 was produced after challenge of mice with E. coli LPS than with S. typhymurium LPS. The hypothesis that a combination of pro-inflammatory cytokines is responsible for the mortality after S. typhimurium LPS was suggested by experiments in mice deficient in IL-1β-converting enzyme (ICE−/− mice). ICE-/-mice, lacking mature IL-1β and IL-18, but also defective in IFN-γ and TNF production, were completely protected against both E. coli and S. typhimurium LPS. Experiments in Toll-like receptor (TLR)-4 defective mice suggested that the difference is not due to differential activation of TLR4. In conclusion, TNF and LT play a central role in the lethality due to E. coli LPS, whereas the lethal effects of S. typhimurium LPS are mediated through mechanisms also involving other cytokines such as IFN-γ, IL-1 and IL-18.
Abbreviations:
-
- LT:
-
lymphotoxin
-
- TLR:
-
Toll-like receptors
1 Introduction
Invasion of the host with Gram-negative bacteria may lead to a systemic inflammatory syndrome characterized by hypotension, disseminated intravascular coagulation, renal hepatic and cerebral damage. Most of these deleterious effects can be mimicked by infusion of endotoxin, the lipopolysaccharide (LPS) component of the cell wall of Gram-negative bacteria, and lethal endotoxaemia has beenextensively used as an experimental model of Gram-negative septic shock. Binding of LPS to various receptors on leukocytes triggers the production and release of proinflammatory cytokines such as TNF-α and IL-1β 1, 2. The importance of TNF as a central mediator of LPS effects has been supported by studies showing that neutralization of TNF with antibodies 3 – 6 or soluble receptors 7 protects against lethal endotoxemia or Gram-negative sepsis. Further support has come from measurements of elevated concentrations of TNF in patients with sepsis 8, 9, and from reports of a correlation between TNF activity and a deleterious outcome 10. These data strongly sustained the assertion that TNF plays a pivotal role in the events leading to shock and death during severe Gram-negative infections.
The development of mice deficient in TNF, lymphotoxin-α (LT-α) and TNF receptor (TNF−/−, LT−/− and TNFR−/−) has provided researchers with the possibility to study the role of these molecules in various pathological conditions, including lethal endotoxemia and Gram-negative sepsis. In contrast to earlier studies that suggested a key role of TNF as mediator during lethal endotoxemia, studies performed in TNF−/−, TNFR−/− or TNF−/−LT−/− mice surprisingly failed to demonstrate increased resistance of these animals against the lethal effects of high doses of LPS 11, 14. Only a limited degree of protection against LPS was reported by two recent studies 15, 16. These data have challenged the concept of TNF being the central mediator of Gram-negative septic shock.
The discrepancy between the earlier studies using anti-TNF antibodies that showed protection and the latter studies in TNF-deficient mice that were not protected against LPS, prompted us to investigate the possible mechanisms that explain the differences. It occurred to us that many of the studies investigating the effect of anti-TNF antibodies during lethal endotoxemia used LPS derived from Escherichia coli 3 – 6, whereas the studies performed with TNF−/− or TNFR−/− mice used LPS derived from Salmonella species 11 – 14. This led us to the hypothesis that the importance of TNF as proinflammatory mediator depends on the LPS source. LPS is a heterogenous molecule, with a great variation in the structure between various bacterial species 17. Thus, the aim of the present study was to assess the susceptibility of TNF−/−LT−/− mice to LPS derived from various microorganisms including E. coli, S. typhimurium and Klebsiella pneumoniae, and to compare the regulation of cytokine network during challenge with these different LPS types. The role of other pro-inflammatory cytokines such as IL-1α, IL-1β and IFN-γ for the lethality due to endotoxic shock was investigated by treating the mice with IL-1 receptor antagonist (IL-1Ra) and/or anti-IFN-γ antibodies, and by the challenge of IFN-γ−/− or IL-1β-converting enzyme (ICE−/− mice) mice with LPS.
2 Results
2.1 Survival of TNF−/−LT−/− mice during endotoxemia
To assess the role of TNF in mediating LPS toxicity, mice deficient in TNF and LT and wild-type controls were injected with LPS from various Gram-negative bacteria. Death occurred during the first 5 days after LPS challenge, without further deaths thereafter. TNF−/−LT−/− were slightly more resistant to low and moderate doses (0.5 – 1.5 mg/mouse) of S. typhimurium LPS than TNF+/+LT+/+ controls, whereas higher doses (2 mg/ mouse) lead to 100 % mortality in both control and deficient mice (Fig. 1 A). In contrast, TNF−/−LT−/− mice were completely resistant to challenge with LPS derived from E. coli, irrespective of the dose injected (Fig. 1 B). Similarly, injection of LPS from K. pneumoniae into TNF−/−LT−/− mice resulted in 100 % survival at all LPS doses tested, whereas only 20 % of the TNF+/+LT+/+ control mice survived when injected with 1 or 1.5 mg, and there was no survival when injected with 2.0 mg K. pneumoniae LPS (p < 0.01). Thus, differential susceptibility of TNF−/−LT−/− to various LPS types is not a phenomenon restricted to E. coli LPS.
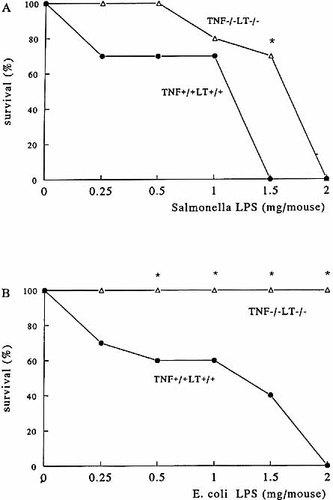
Survival of TNF−/−LT−/− mice to lethal endotoxemia. TNF+/+LT+/+ and TNF−/−LT−/− mice were injected i.p. with various amounts of S. typhimurium (A) or E. coli (B) LPS. The experiments were performed twice on two different occasions, with a total of 10 mice/group. *p < 0.05 compared with survival of TNF+/+LT+/+ mice.
2.2 Cytokine concentrations in vivo
In TNF+/+LT+/+ mice, the highest concentrations of TNF were found 90 min after challenge with either 0.25 mg of S. typhimurium or E. coli LPS, whereas TNF was not present in the TNF−/−LT−/− mice (Fig. 2 A and B). After injection of S. typhimurium LPS into TNF+/+LT+/+ mice, maximal IL-1α and IL-1β concentrations were found between 90 min and 6 h after injection of LPS (Fig. 2 A). In TNF−/−LT−/− mice challenged with S. typhimurium LPS, there was an ongoing production of both IL-1α and IL-1β at 3 to 6 h after injection of LPS, and the levels were significantly higher than those recorded in the control animals (p < 0.01) (Fig. 2 A). In contrast, after challenge with E. coli LPS, IL-1α and IL-1β kinetics were identical in TNF−/−LT−/− and TNF+/+LT+/+ mice, but the deficient mice produced significantly lower amounts of these two cytokines compared to controls (p < 0.05) (Fig. 2 B).
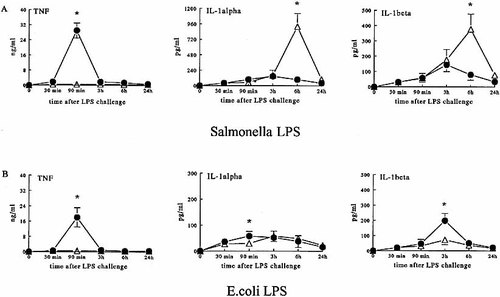
Circulating concentrations of pro-inflammatory cytokines. TNF+/+LT+/+ (closed circles) and TNF−/−LT−/− (open triangles) mice were injected i.p. with 0.25 mg/mouse of S. typhimurium LPS (A) or E. coli LPS (B). No TNF was detectable in the circulation of TNF−/−LT−/− mice after injection of either LPS. The experiments were performed twice on two different occasions, with a total of 10 mice/group. Cumulative data (means ± SEM) are presented. *p < 0.05 compared with TNF+/+LT+/+ mice.
The production of IFN-γ 6 h after challenge with LPS was significantly lower in the TNF−/−LT−/− mice than in TNF+/+ mice, independently of the LPS species (Fig. 3). It is, however, important to underscore that TNF−/−LT−/− mice produced 16-fold more IFN-γ after challenge with S. typhimurium LPS, then with E. coli LPS (23.4 ± 8.9 vs. 1.4 ± 0.5 pg/ml, p < 0.01) (Fig. 3). In contrast, the production of the antiinflammatory cytokine IL-10 was significantly lower in TNF−/−LT−/− mice challenged with both LPS species compared to wild-type mice (Fig. 3). However, mice stimulated with S. typhimurium LPS produced significantly less IL-10 then those challenged with E. coli LPS (Fig. 3). Similar data were obtained when mice were challenged with a lethal dose of LPS (1.5 mg/ mouse; Table 1).
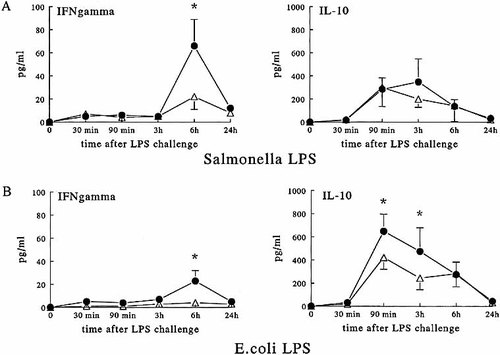
Circulating concentrations of T cell-derived cytokines. TNF+/+LT+/+ (closed circles) and TNF−/−LT−/− (open triangles) mice were injected i.p. with 0.25 mg/mouse of S. typhimurium LPS (A) or E. coli LPS (B), and IFN-γ and IL-10 were measured at the indicated time points. The experiments were performed twice on two different occasions, with a total of 10 mice/group. Cumulative data (means ± SEM) are presented. *p < 0.05 compared with TNF+/+LT+/+ mice.
|
|
E. coli LPS |
S. typhimurium LPS |
||
|---|---|---|---|---|
|
ng/ml |
TNF+/+LT+/+ |
TNF−/−LT−/− |
TNF+/+LT+/+ |
TNF−/−LT−/− |
|
IL-1α |
0.35 ± 0.10 |
0.49 ± 0.17 |
0.44 ± 0.11 |
1.27 ± 0.27* |
|
IL-1β |
0.19 ± 0.03 |
0.31 ± 0.17 |
0.17 ± 0.06 |
1.54 ± 0.74* |
|
IL-10 |
1.75 ± 0.71 |
0.22 ± 0.09* |
0.44 ± 0.29# |
0.23 ± 0.10* |
|
IFN-γ |
0.08 ± 0.07 |
0.04 ± 0.02 |
0.29 ± 0.08# |
0.40 ± 0.08 |
- Circulating concentrations of cytokines were measured 6 h after challenge of TNF+/+LT+/+ and TNF−/−LT−/− mice with 1.5 mg/mouse of S. typhimurium LPS or E. coli LPS. No TNF was detectable in the circulation of TNF−/−LT−/− mice after injection of either LPS. The experiments were performed with a total of five mice/group (means ± SEM). *p < 0.05 compared with TNF+/+LT+/+ mice, #p < 0.05 compared with E. coli LPS.
2.3 Effect of cytokine neutralization
To investigate whether the up-regulation of IL-1α and/or IL-1β production in the TNF−/−LT−/− after challenge with S. typhimurium LPS is responsible for the mortality in the absence of TNF and LT, groups of deficient animals were infused with either IL-1Ra or saline through osmotic minipumps, before and during challenge with endotoxin. Saturation levels of IL-1Ra did not protect TNF−/−LT−/− mice against the lethal effects of S. typhimurium LPS (Fig. 4 A). However, treatment of TNF−/−LT−/− mice with anti-IFN-γ antibodies tended to have a beneficial effect against the lethal effects of S. typhimurium LPS (mean survival time 91 ± 23 vs. 48 ± 18 h), although the difference did not reach statistical significance (p > 0.05 log rank test; Fig. 4 B). The protective effect of the anti-IFN-γ antibodies was not potentiated by the concomitant infusion of IL-1Ra (mean survival time 72 ± 25 vs. 38 ± 10 h in controls, p > 0.05). Thus, IL-1α and/or IL-1β do not seem to play a major role in the pathogenesis of Salmonella LPS-induced shock and death, but IFN-γ is suggested to be involved.
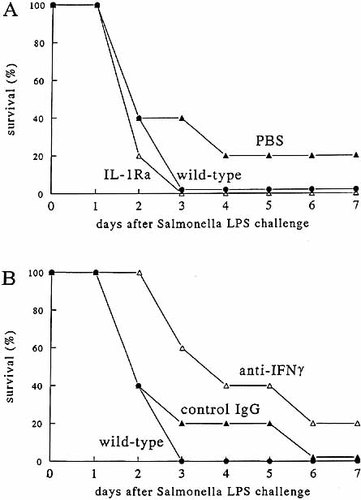
The effect of anti-cytokine treatment. TNF+/+LT+/+ and TNF−/−LT−/− mice were injected i.p. with 2 mg/mouse of S. typhimurium. Subgroups of five deficient mice received infusion with either PBS or IL-1Ra (A). In panel B, the TNF−/−LT−/− mice received either control rat IgG or anti-IFN-γ antibodies.
2.4 Cytokine redundancy in mortality induced by S. typhimurium LPS
To investigate the role of IFN-γ in the pathogenesis of endotoxic shock induced by S. typhimurium LPS, IFN-γ−/− mice were challenged with a low dose (0.25 mg/mouse) of Salmonella LPS. The survival was 67 % in IFN-γ−/− mice vs. 0 % in wild-type mice (p < 0.05; Fig. 5 A). However, when IFN-γ−/− mice were challenged with higher (1 mg/mouse) doses of S. typhimurium LPS, no protection against endotoxic shock was apparent (Fig. 5 B). This observation suggests that IFN-γ contributes, but is not solely responsible for the mortality after challenge with S. typhimurium LPS.
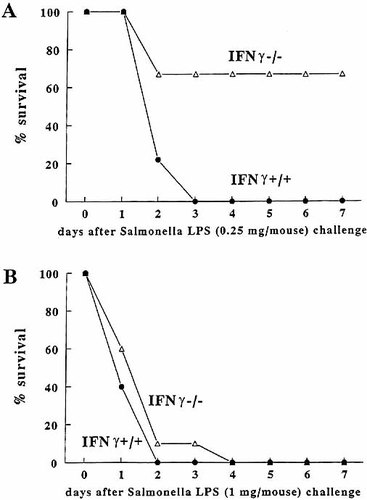
Survival of IFN-γ−/− mice to lethal endotoxemia. IFN-γ+/+ and IFN-γ−/− mice were injected i.p. with a 0.25 mg/mouse (A) or 1.0 mg/mouse (B) of S. typhimurium LPS. The experiments were performed twice on two different occasions, with a total of nine or ten mice/group. *p < 0.05 compared with survival of IFN-γ+/+ mice.
Because the data presented above suggest that Salmonella LPS is able to induce several cytokines with overlapping activities that contribute to lethality, we challenged ICE−/− mice with LPS. ICE−/− mice are not able to process immature precursors into mature IL-1β and IL-18, and as a consequence are also deficient in the production of IL-1α, IFN-γ and TNF 18. When ICE−/− mice were challenged with 1 mg of LPS, they were completely resistant to both E. coli and S. typhimurium LPS (100 % survival in ICE−/− mice, compared with 0 % survival in control animals, p < 0.01). There was no difference in the course of mortality between E. coli LPS and S. typhimurium LPS in control mice.
2.5 The role of endotoxin-associated proteins in the effects of E. coli and S. typhimurium LPS
In our experiments, we have used commercial LPS with a protein content of less than 10 %. However, because even these small amounts of endotoxin-associated proteins may influence cytokine induction by LPS, as previously suggested by some 19, although not all 20 authors, we tested whether the mortality induced in TNF−/−LT−/− mice by S. typhimurium LPS was due to qualitative or quantitative differences in endotoxin-associated proteins. While commercial LPS preparations induced small, but detectable amounts of TNF in peritoneal macrophages of LPS-resistant C3H/HeJ mice (190 ± 74 pg/ml after E. coli LPS, and 221 ± 59 pg/ml after S. typhimurium LPS), indicating the presence of endotoxin-associated proteins, the extra-purified LPS preparations (with undetectable protein contamination) from either E. coli or S. typhimurium were not able to induce any detectable TNF, IL-1α and IL-1β synthesis (all values below detection limit). The role of endotoxin-associated proteins for mortality was tested by injecting either commercial or purified S. typhimurium LPS into TNF−/−LT−/− and TNF+/+LT+/+ mice. Both commercial and extra-purified Salmonella LPS induced similar mortality patterns in the knock-out and control mice (Fig. 6). The remaining 1 % contamination of purified LPS with bacterial DNA is unlikely to be important, as C3H/HeJ macrophages did not respond with any cytokine production upon stimulation with purified E. coli or Salmonella LPS. Since bacterial DNA stimulates cytokine production through TLR9 whichis functional in C3H/HeJ mice, significant contamination with bacterial DNA would have led to induction of cytokines in these mice, which was not the case with our purified LPS preparations.
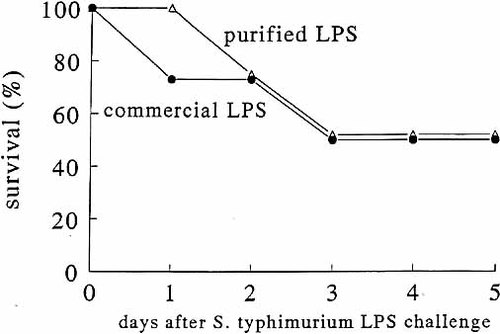
Survival of TNF−/−LT−/− to lethal endotoxemia induced by protein-free S. typhimurium LPS. TNF+/+LT+/+ and TNF−/−LT−/− mice were injected i.p. with 1 mg/mouse of either commercial S. typhimurium LPS (A) or repurified protein-free S. typhimurium LPS (B). The experiments were performed with a total of six mice/group.
In additional experiments, we investigated whether either LPS or endotoxin-associated proteins from the commercial preparations of E. coli or Salmonella LPS may differentially induce mortality in the absence of TLR4-dependent signals in the C3H/HeJ mice. While both preparations induced 80 to 100 % mortality in the susceptible C3H/HeN mice, no mortality was recorded inthe C3H/HeJ mice, independently of the LPS species injected in the mice (not shown).
3 Discussion
The present study shows that TNF−/−LT−/− mice are completely protected against E. coli LPS, whereas only a marginal protection of the double knock-out mice is present when they are injected with S. typhimurium LPS. Production of other pro-inflammatory cytokines such as IFN-γ after S. typhimurium LPS challenge, is likely to play a role in this phenomenon. These results demonstrate that endogenous TNF and LT are pivotal for the lethal effects of LPS, but this mechanism is dependent on the bacterial origin of the LPS.
The differential susceptibility of TNF−/−LT−/− mice to endotoxin of different bacteria provides the explanation for the unexplained discrepancy of reports showing the protective effects of anti-TNF antibodies in lethal endotoxemia suggesting a central role for TNF 3 – 6, and those demonstrating that TNF−/− 11, TNF−/−LT−/− 14 or TNFR−/− 12, 13 mice are susceptible to the lethal effects of LPS. Indeed, the former reports have used LPS derived from E. coli 3 – 6, whereas the latter used that from Salmonella species 11 – 14. Moreover, the only two reports showing a certain degree of protection of TNF−/− mice against lethal endotoxemia have employed E. coli LPS 15, 16, sustaining our results. As far as we know, this is the first studyto investigate the direct comparison of different LPS species in TNF−/− or TNF−/−LT−/− mice.
After sensitization of mice with galactosamine, the animals become particularly sensitive to TNF through blockade of the synthesis of protective acute-phase proteins, especially α1-acid glycoprotein 21. Due to this effect, the mice become sensitive to very low amounts of LPS. TNF−/− or TNF−/−LT−/− mice are fully protected in the galactosamine model, also when Salmonella LPS is used as a challenge 11, 12, 22, 23, indicating the crucial role of TNF in this model. The difference in the pathogenesis of this model compared with models using non-sensitized mice is also evident from reports showing that only TNFR p55 is involved in TNF-induced shock in galactosamine-sensitized mice,whereas both TNFR p55 and p75 are required for the lethal effects of TNF in non-sensitized mice 24. Thus, there are important differences in the pathogenesis of endotoxic shock in galactosamine-sensitized and non-sensitized mice. In addition, one should also not forget that mice deficient in TNF or TNFR are more susceptible to a wide variety of infections with live microorganisms, including with Gram-negative bacteria, underlining the differences between endotoxemia and infection, and the importance of a potent TNF response for host defense 22, 25, 26.
The different effects of high-dose E. coli and S. typhimurium LPS in TNF−/−LT−/− are accompanied by differential regulation of the cytokine network. Whereas stimulation with E. coli LPS results in low concentrations of IL-1α and IL-1β in TNF−/−LT−/− mice, S. typhimurium induced significantly higher IL-1α and IL-1β concentrations in the TNF−/−LT−/− mice compared to their wild-type controls. However, it is unlikely that IL-1α and/or IL-1β is responsible for the lethality after S. typhimurium LPS, since infusion of saturating levels of IL-1Ra were not able to protect the TNF−/−LT−/− mice. This is also supported by studies performed in mice deficient in IL-1β or IL-1R type I, which were also sensitive to thelethal effects of LPS 27, 28.
Another pro-inflammatory cytokine involved in the pathogenesis of septic shock is IFN-γ 29. In addition to the up-regulation of IL-1α and IL-1β, S. typhimurium also induced significantly more IFN-γ in TNF−/−LT−/− mice, compared with E. coli LPS. The possibility that IFN-γ may be responsible for some of the effects of S. typhimurium LPS is raised by the tendency of an increased survival of TNF−/−LT−/− mice when treated with anti-IFN-γ antibodies. The potential role for IFN-γ during Salmonella endotoxemia is further sustained by the increased resistance of IFN-γ−/− mice to the challenge with low doses of LPS. Interestingly, it has been reported that IFNR−/− mice are as susceptible as IFNR+/+ mice for Salmonella abortus equi LPS 30, while being resistant to lethal endotoxemia induced by E. coli LPS 31. However, the fact that the IFN-γ−/− mice are susceptible to high doses of Salmonella LPS, suggests that IFN-γ alone is not solely responsible for the differential susceptibility of TNF−/−LT−/− mice to E. coli andS. typhimurium LPS, and redundancy in the synthesis of pro-inflammatory cytokines induced by S. typhimurium LPS is likely involved in this phenomenon. This hypothesis is sustainedby the observation that ICE−/− mice, deficient not only in mature IL-1β and IL-18, but also with a 50 to 90 % lower production of IL-1α, IFN-γ and TNF, are completely protected against the lethal effects of S. typhimurium LPS. This observation suggests that mortality after challenge with Salmonella LPS is due to a network of several pro-inflammatory cytokines, including TNF, LT, IFN-γ and probably also IL-18. In a recent report we have shown that the differential cascade of cytokines induced by E. coli vs. S. typhimurium LPS is accompanied bya different profile of chemokine expression and neutrophil accumulation in the liver and lungs of the endotoxin-challenged animals 32.
The explanation for the differences in the induction of pro-inflammatory cytokines by the two LPS species investigated may in part be due to differential induction of IL-10. Indeed, IL-10 concentrations were significantly higher in mice stimulated with E. coli LPS, than in those challenged with S. typhimurium LPS, whereas IL-1 and IFN-γ were lower in the mice stimulated with E. coli LPS. However, the molecular mechanisms behind the differential regulation of the cytokine network endotoxaemia with E. coli or Salmonella LPS are not very well understood. One possible explanation for the differences observed with the various LPS preparations could be that quantitative and/or qualitative differences in the endotoxin-associated proteins present in commercial LPS preparations may be responsible for this effect. In the present study we have used commercial LPS chromatographically purified by gel filtration, with a protein contentless than 10 %. However, because even very small amounts of endotoxin-associated proteins may influence cytokine induction by LPS 19, we have tested this hypothesis by re-purifying the commercial LPS preparations. While the presence of contaminating endotoxin-associated proteins in the commercial LPS was demonstrated by the weak induction of TNF production in cells from LPS-resistant C3H/HeJ mice, a role of these proteins as an explanation of Salmonella LPS-induced death was excluded in experiments in which repurified protein-free LPS was still able to induce similar levels of mortality in TNF+/+LT+/+ and TNF−/−LT−/− mice. However, quantitative and/or qualitative differences in other possible trace contaminants of the LPS preparations (e.g. lipopeptides, peptidoglycans) cannot be totally excluded.
A second possible explanation for the differences between E. coli and S. typhimurium LPS may reside in differences in the actual structure of LPS itself. The structure of thetwo LPS species differs not only at the level of the polysaccharide chains, but also at the level of lipid A, the most active moiety of LPS. Lipid A from Salmonella contains an additional fatty acid 33, and different substituents of phosphate groups 34 compared with E. coli lipid A. As lipid A is considered to mediate binding to LPS-binding protein (LBP) and the CD14/TLR4 complex with subsequent stimulation of cytokine production 35, it is not surprising that differences in the structure of lipid A may lead to stimulation of different pathways of cytokine induction. Therefore, one may suggest that the differential cytokine response after E. coli or Salmonella LPS could be explained by aspects of stimulation at the level of cellular receptors, namely the recently described class of TLR 36, 37. However, this suggestion is negated by our data showing no mortality of the C3H/HeJ mice when challenged with either E. coli or Salmonella LPS, suggesting that both these two LPS species use TLR4 as a receptor. This is confirmed by the recent studies showing that LPS from both E. coli and Salmonella induce cytokines through TLR4 38, and the two types of LPS are equally potent in inducing intracellularsignals when bound to TLR4 39. Binding and signalling through other LPS receptors such as β2-integrins, macrophage scavenger-receptor and moesin, independently of TLR4, is unlikely to be relevant as β2-integrins act in concert with CD14/TLR4 to deliver an optimal signal after LPS stimulation 40, whereas the other receptors are mainly scavening receptors which do not induce an intracellular signal.
In conclusion, lethality due to E. coli and S. typhimurium is mediated through differential pathogenetic mechanisms, in which TNF and LT are central in lethal endotoxemia with E. coli LPS, whereas redundancy in the stimulation of multiple pro-inflammatory cytokines plays an important role during S. typhimurium endotoxemia. The molecular mechanisms responsible for the differential stimulatory pathways by the two LPS species are yet to be deciphered.
4 Materials and methods
4.1 Materials
LPS from E. coli (serotype O55 : B5), S. typhimurium and K. pneumoniae were obtained from Sigma Chemical Co (St. Louis, MO). Recombinant IL-1 receptor antagonist (IL-1Ra) was a kind gift from Amgen (Boulder, CO). Hybridoma cells producing rat anti-mouse IFN-γ monoclonal antibodies (clone B4-2A6) were obtained from the ATCC (Rockville, MD). Control rat IgG antibodies were obtained from Sigma.
4.2 Animals
TNF−/−/LT−/− mice were produced as previously described 23. IFN-γ−/− and the corresponding wild-type control mice were kindly provided by Organon (Oss, The Netherlands).The generation and background of ICE−/− mice were previously described 18, 41. C3H/HeJ and C3H/HeN mice were obtained from the Jackson Laboratory (Bar Harbour, ME). Specific pathogen-free knock-out mice and age- and weight-matched wild-type mice (20 – 25 g, 6 to 8 weeks old) were used. Mice were fed sterilized laboratory chow (Hope Farms, Woerden, The Netherlands) and water ad libitum.
4.3 Endotoxemia model
Groups of normal and deficient (TNF−/−LT−/−, IFN-γ−/−, or ICE−/−) mice were injected intraperitoneally (i.p.) with LPS in amounts varying from 0.25 to 2.0 mg/mouse. Survival was assessed during 1 week in groups of at least 10 mice. In addition, 30 min, 90 min, 3 h and 6 h after challenge with LPS, five animals per group injected with either 0.25 or 1.5 mg LPS/mouse were anesthetized with ether and bled from the retroorbital plexus for measurement of circulating cytokine concentrations.
In an additional experiment, the role of IL-1 and IFN-γ or the combination of both for the mortality induced by S. typhimurium LPS was assessed. IL-1Ra was administered to TNF−/−LT−/− mice using osmotic minipumps (model 1007D, Broekman Institute, Someren, The Netherlands). The pumps were loaded with 1.2 mg IL-1Ra and implanted 1 day before the LPS (2 mg/mouse) challenge. Thepumps give a sustained IL-1Ra release for 7 days, assuring an efficient neutralization of endogenous IL-1 42. A control group was implanted with minipumps loaded with sterile phosphate buffered saline. Anti-IFN-γ antibodies (0.5 mg/mouse) or control rat IgG were administered i.p. to the TNF−/−LT−/− mice, just before LPS (2 mg/mouse) administration. The survival was assessed for 1 week.
4.4 The role of endotoxin-associated proteins in the mediation of E. coli and S. typhimurium LPS efects
As "endotoxin associated proteins" have been shown to modulate LPS-induced cytokine production, we assessed whether protein contamination in commercial LPS preparations may be responsible for the possible differences between E. coli and S. typhimurium LPS. Commercial LPS preparations were purified as previously described by Manthey and colleagues 19. This method decreased protein contents of LPS preparations from 9 % (E. coli LPS) and 8 % (S. typhimurium LPS) to undetectable levels (as measured using the BCA assay), with a more than 90 % recovery of LPS 19. The DNA contamination (as detected using a Genequant spectrophotometer) decreased from 3.5 % and 3.1 % (E. coli and Salmonella LPS) to 1.3 % and 1.0 %, respectively. The purity of the newly extracted LPS preparations was tested by stimulation of cytokine production in peritoneal macropahges of LPS-resistant C3H/HeJ mice, as described 43. Briefly, resident peritoneal macrophages were harvested by injecting 4 ml of sterile PBS containing 0.38 % sodium citrate. After centrifugation and washing, thecells were resuspended in RPMI 1640 containing 1 mM pyruvate, 2 mM L-glutamine, 100 μg/ml gentamicin and 2 % fresh mouse plasma (culture medium). Cells were cultured in 96-well microtiter plates (Costar Corporation, Cambridge, MA) at 105 cells/well, in a final volume of 200 μl. The cells were stimulated with 10 ng/ml of either commercial or extra-purified LPS preparations. After 24 h of incubation at 37 °C, the plates were centrifuged (500 × g, 10 min), and the supernatant was collected and stored at − 80 °C until cytokine assays were performed.
The role of endotoxin-associated proteins for the mortality induced by S. typhimurium LPS was tested by injecting TNF+/+LT+/+ and TNF−/−LT−/− mice with 1 mg of either the commercial or the extra-purified LPS preparation. In addition, a possible difference between E. coli and Salmonella LPS in inducing mortality through TLR4-independent pathways in C3H/HeJ mice was tested by injecting the animals with either 1 or 2 mg of the two LPS species. Mortality was assessed during 1 week after the challenge, as described above.
4.5 Cytokine measurements
TNF-α, IL-1α and IL-1β concentrations were determined using specific radioimmunoassays (RIA) developed in our laboratory 42. Murine IFN-γ ELISA was purchasedfrom Biosource (Camarillo, CA), and used according to the guidelines of the manufacturer. Murine IL-10 ELISA was purchased from CLB (Amsterdam, The Netherlands), and used according to the guidelines of the manufacturer.
4.6 Statistical analysis
Differences in concentrations of cytokines were analysed using the Mann-Whitney test, and by Kruskal-Wallis test where appropriate. Survival data were analyzed using the Kaplan-Meyer log rank test. Differences were considered significant at p < 0.05. All experiments employed at least 5 mice per group at each time point for the plasma cytokine concentrations, and 10 mice pergroup for survival experiments, unless otherwise indicated. Data are presented as means ± standard deviation.
Acknowledgements
We thank Dr. Marcel van Deuren for his help with characterization of the LPS preparations, and for critically reading the manuscript.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




