CD4+CD8dim T lymphocytes exhibit enhanced cytokine expression, proliferation and cytotoxic activity in response to HCMV and HIV-1 antigens
Abstract
CD4+CD8dim T cells represent a minor subset of the total CD3+ T cell population in peripheral blood. Although transient and persistent expansions of these cells havebeen reported in both healthy and diseased individuals, the functional properties of the CD4+CD8dim population are largely unknown. In this study, we examined antigen-specific cytokine and proliferative responses of the CD4+CD8dim subset. In whole blood cultures stimulated with the viral antigens HCMV and HIV-1, a significant fraction of the CD4+CD8dim subset exhibited cytokine expression and proliferation in response to antigen activation. Typically, the CD4+CD8dim population contained two- to eightfold higher frequencies of antigen-specific cytokine producing cells than the CD4+CD8- population. Phenotypic analysis of the cytokine expressing CD4+CD8dim population indicated that these cells are memory T cells, with a high frequency of this population expressing the cytotoxic markers CD56 and perforin. Furthermore, the CD4+CD8dim cytokine responses to CMV were shown to be MHC class II dependent. Significantly, purified CD4+CD8dim T cells were found to possess higher CMV-specific cytotoxic activity than purified CD4+CD8– T cells in a standard 51Cr-release CTL assay. Thus, CD4+CD8dim T cells appear to be MHC class II dependent, are capable of cytolytic effector activity, and are highly enriched within the CD4+ cell populations specific for HCMV and HIV-1.
Abbreviations:
-
- APC:
-
Allophytocyanin
-
- HCMV:
-
Human cytomegalovirus
-
- PerCP:
-
Peridinin chlorophyl protein
-
- SEB:
-
Staphylococcal enterotoxin B
1 Introduction
Mature circulating human T lymphocytes typically ex-press high densities of the TCR complex in combination with either CD4 or CD8 surface antigens 1. In peripheral blood, CD4+CD8– T cells interact with MHC class II antigen presenting cells and exhibit helper/inducer functions, whereas CD4–CD8+ T cells experience antigen in the context of MHC class I, and are associated with cytotoxic/suppressor cell functions 2. In addition to these two major T cell compartments, several investigators have described the presence of a minor subset of circulating T cells exhibiting a double positive CD4+CD8dim phenotype. Generally, the CD4+CD8dim T cells occur at very low frequencies innormal blood (less than 2% of CD3+), however, transient and persistent expansions of this subset have been observed in both healthy individuals 3–6 and individuals with a wide range of unrelated diseases, including viral infections 7, various autoimmune diseases 8, 9 and T and B cell leukemias 10–12 and lymphomas 13, 14. These CD4+CD8dim T cells typically express CD8 α α homodimers, and are believed to be of extrathymic origin 10, 14–16.
Although the function of the CD4+CD8dim subset remains largely unclear, Watanabe et al. 14 have postulated that these cells may be cytotoxic T lymphocytes.Moreover, Bagot et al. 13 have demonstrated the presence of tumor-specific, MHC class I-restricted, cytotoxic CD4+CD8dim T cell clones inside cutaneous infiltrates from patients with cutaneous T cell lymphoma. In monkeys, swine and chicken, species that have markedly more abundant circulating CD4+CD8dim T cells than humans, the CD4+CD8dim subset appears to have both helper and cytotoxic functions 16–18.
In this study, utilizing a short-term, flow cytometry-based assay, we examined antigen-specific cytokine and proliferative responses of the CD4+CD8dim subset in whole-blood and PBMC cultures stimulated with HCMV or HIV-1 antigens. We observed enhanced frequencies of both cytokine-producing and proliferating memory T cells within the CD4+CD8dim subset in response to antigen stimulation. We further demonstrate that the CD4+CD8dim T cells exhibit enhanced CMV-specific cytotoxicity against autologous B and dendritic cell targets.
2 Results
2.1 CMV antigen activates two distinct subsets of CD4+ T cells
Staining of unstimulated peripheral blood from several healthy adult donors revealed variable frequencies of circulating CD4+CD8dim T lymphocytes. Among the 12 donors tested, the CD4+CD8dim population comprised between 1% and 13% of the CD3-positive population (Fig. 1A). These frequencies have remained relatively constant for several months.
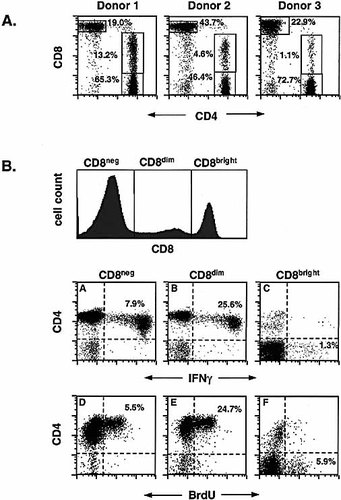
Frequencies of CD4+CD8dim T cells in whole blood, and CMV-induced IFN-γ expression and BrdU incorporation in CD4+CD8dim vs. CD4+CD8– and CD4-CD8bright T cells. (A) Unstimulated whole blood from three healthy donors was stained with CD4 FITC, CD8 PE and CD3 PerCP and 15,000 CD3+ events were analyzed for the co-expression of CD4 and CD8. The results shown are representative of 12 donors tested. (B) Whole blood (6 h stimulation) or PBMC (48 h stimulation) from a CMV-seropositive donor (Donor 1 in A) were stimulated with CMV lysate as described in Sect. 4. Activated cells were stained with CD3 PerCP, CD4 APC, CD8 FITC (cytokine assay) or CD8 PE (BrdU assay), and anti-IFN-γ PE or anti-BrdU FITC/DNase. A total of 60,000 CD3+ events were collected, and the co-expression of CD4 and IFN-γ (plots A–C) or BrdU (plots D–F) was assessed in the CD8–, CD8dim and CD8bright cell populations (see CD8 histogram). The results shown are representative of 6 donors tested.
Fig. 1B compares the CMV lysate-induced cytokine and proliferative responses of the CD4+CD8dim T cells versus the CD4+CD8– and the CD4–CD8bright T cells in one representative donor (Donor 1 in Fig. 1A). As illustrated by plot B, a relatively high percentage (nearly 26%) of the CD4+CD8dim cells expressed IFN-γ in response to 6-h CMV stimulation. Thus, although the CD4+CD8dim subset represents only 17% of the total resting CD4+ population in this donor, it contributed 39% of the total CMV-specific CD4+ T cell cytokine response. Similar results were observed for most donors tested. Generally, the CD4+CD8dim responder cells accounted for 10–40% of the total CD4+ IFN-γ response.
Within the CD4+CD8– T cell population, almost 8% of the cells were IFN-γ positive (Fig. 1B, plot A), while only about 1% of the CD8bright T cells produced IFN-γ to soluble CMV antigen.
To determine whether the CD4+CD8dim subset was also capable of proliferating in response to antigenic stimulation, DNA synthesis was measured by BrdU incorporation after a 48-h culture with CMV lysate. As illustrated by Fig. 1B, similar proportions of CD4 subsets undergoing DNA synthesis (48 h) and cytokine expression (6 h) were observed.
2.2 Both CD4+CD8– and CD4+CD8dim cytokine responses are MHC class II dependent
To determine whether the CD4+CD8dim antigen-specific responses are MHC class I or class II dependent, drugs and monoclonal antibodies (mAb) that inhibit either MHC class I- or class II-driven antigen processing and presentation were used. Fig. 2A depicts the effects of a mixture of three anti-MHC class II mAb (anti-HLA-DR,DP,DQ) on IFN-γ production in the CD4+CD8–, CD4+CD8dim and CD4–CD8bright T cell subsets. Consistent with our previous findings 19, the anti-MHC class II mAb cocktail suppressed the cytokine responses in the CD4+CD8– population by 77%, whereas an antibody directed against MHC class I determinants (anti-HLA-A,B,C) failed to suppress this response (Fig. 2B). Importantly, the inhibitory effect of the anti-class II cocktail was even more significant within the CD4+CD8dim T cell population (95% suppression), while only a minimal suppression (17%) of the cytokine response was observed due to the addition of the anti-class I mAb. In contrast, the CD4–CD8bright T cell cytokine responses, which have been shown to be MHC class I dependent 20, were not inhibited by the anti-class II mAb cocktail, but were suppressed by 70% in the cultures containing the anti-class I mAb (Fig. 2A and B). Additionally, an irrelevant mAb (CD45) showed no inhibitory effect on the cytokine responses for any of the T cell populations (Fig. 2A).
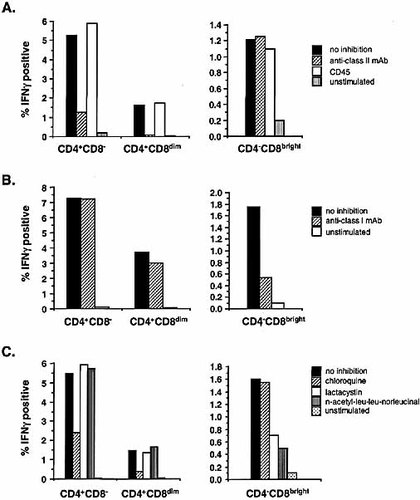
Inhibition of antigen presentation and subsequent cytokine expression by MHC class I and class II inhibitors. Whole blood was stimulated with CMV in the presence of anti-HLA-DR, DP, DQ or CD45 (A) or anti-HLA-A,B,C (B). The inhibitory mAb were added at 10 μg/ml, 20 min before addition of antigen and costimulus. The unstimulated control culture contained the costimulus but no antigen. Activated cells were stained with CD3 PerCP, CD8 FITC, CD4 APC and anti-IFN-γ PE. A total of 50,000 CD3+ events were collected and the frequencies of IFN-γ expressing cells were assessed in the CD4+CD8–, CD4+CD8dim and CD4–CD8bright populations. The calculated frequencies of IFN-γ-positive cells are expressed as % of the total CD4+ population for the CD8– and CD8dim cells, and as % of the total CD4- population for the CD8bright cells. (C) Chloroquine, lactacystin or n-acetyl-leu-leu-norleucinal was added to whole blood at 50 μg/ml, 20 min before addition of antigen and costimulus. Blood cultures were then activated with CMV for 6 h, as described in Sect. 4. Activated cells were stained with CD3 APC, CD8 PerCP, CD4 PE and anti-IFN-γ FITC and 40,000 CD3+ events were acquired. The results shown are representative of three experiments using different subjects.
A second set of inhibition studies was performed using two class I-specific proteasomal inhibitors, lactacystin and n-acetyl-leu-leu-norleucinal, and a class II-specific endosomal inhibitor, chloroquine 21–24. As expected, activation of whole blood in the presence of chloroquine resulted in a 56% and 75% suppression, respectively, of IFN-γ expression in the CD4+CD8– and CD4+CD8dim populations (Fig. 2C). These findings further confirm that the CD4+CD8dim response to CMV is strongly dependent on MHC class II antigen presentation. Note that chloroquine had no effect on the CD4–CD8bright cells (Fig. 2C). In the same experiments, the class I inhibitors, lactacystin and n-acetyl-leu-leu-norleucinal, had no inhibitory effect on IFN-γ production in the CD4+CD8– and CD4+CD8dim populations. These inhibitors did, however, suppress cytokine expression in the CD4-CD8bright cell population by 57% and 69%, respectively (Fig. 2C).
2.3 Antigen-specific CD4+CD8dim population exhibits a memory phenotype
Cell surface staining of unstimulated CD4+CD8– and CD4+CD8dim T cells for selected memory cell markers indicated that the CD4+CD8dim population generally contains a higher proportion of memory cells than the CD4+CD8- population (data not shown). This is reflected in the higher frequencies within the CD4+CD8dim subset of CD27–, CD45RO+, CD62L– and CD95+ cells 14, 25–28. Similarly, the frequencies of unstimulated cells expressing the cytotoxic markers CD56, CD57 and intracellular perforin 15, 29–31 are higher within the CD4+CD8dim subset. Cell surface expression of the coreceptor molecules CD28 and CD49d, which are associated with augmentation of antigen-specific cytokine responses 32, was also assessed. Interestingly, the CD4+CD8dim population in several donors contained significantly lower frequencies of CD28+ cells and slightly higher frequencies of CD49d+ cells. However, the magnitude of enhancement of the CMV-specific response by CD28 and CD49d co-stimulation was similar within both CD4+ subsets (data not shown).
Next we compared the phenotypes of the IFN-γ expressing CD4+CD8– and CD4+CD8dim T cells. Our previous studies have indicated that a large majority of the CMV-specific responder cells within the single positive CD4 population express the memory cell phenotype CD27–, CD45RO+, CD95+ (33 and unpublished observations). As expected, the majority of the cytokine-producing cells within the CD4+CD8dim subset were also CD27–, CD45 RO+ and CD95+ (Fig. 3A). It should be noted that in most donors, we observed activation-induced down-modulation of the CD95 antigen after a 6-h culture with CMV. At 48 h, however, all proliferating cells (BrdU+) were brightly CD95+, consistent with the memory phenotype (data not shown). These data suggest that CD95 is only transiently down-modulated during the early period of activation.

Phenotype of the CMV-specific response in the CD4+CD8– versus the CD4+CD8dim cells. Whole blood was stimulated with CMV for 6 h, as described in Sect. 4. Activated cells were stained with CD4 PerCP/Cy5.5, CD8 APC and anti-IFN-γ PE vs. CD27 FITC or CD45RO FITC (A), CD56 FITC, or CD57 FITC (B) or anti-IFN-γ FITC vs. CD95 PE (A) or anti-perforin PE (B). Gates were drawn to include CD4+CD8– or CD4+CD8dim lymphocytes and the co-expression of IFN-γ and the cell surface markers or intracellular perforin was assessed. The percentages displayed in the upper left and right quadrants represent % of the total responding (IFN-γ+) cells. A total of 11,000 events are shown in the CD4+CD8– gate and 4,000 events are shown in the CD4+CD8dim gate. Plots A–C depict the IFN-γ response within the CD4+CD8– and plots D–F within the CD4+CD8dim subset. The results shown are representative of five experiments using different donors.
Expression of CD56 has been shown to correlate strongly with CTL effector function in CD8+ T cells 30. In our study, CD56 expression defined a phenotypic difference between the two CD4 subsets. Namely, while the bulk of the CD4+CD8– responder cells lacked CD56 antigen expression, the majority of the CD4+CD8dim responder cells were dimly CD56+ (Fig. 3B, plots A, D). Lastly, the cytokine producing cells in both subsets exhibited similar staining patterns for CD57 and intracellular perforin (Fig. 3B, plots B, E, C, F).
With the exception of the transient down-modulation of the CD95 antigen, the phenotype of the cytokine producing cells at 6 h was maintained in the proliferating cells observed at 48 h (data not shown).
2.4 Enhanced CMV-specific cytotoxicity was observed within the CD4+CD8dim T cell subset
The increased frequencies of CD56, CD57 and intracellular perforin expressing cells within the CD4+CD8dim subset suggest that these cells may have a cytotoxic function in vivo. Consequently, chromium-release CTL assays were performed to compare the cytolytic activity of purified CD4+CD8– versus CD4+CD8dim T cells against CMV lysate-pulsed autologous B or dendritic cell targets. The purified T cell populations were obtained by sorting from fresh, unstimulated PBMC.
As seen in Fig. 4 (Donor 1), at E:T ratio of 40:1, incubation of CMV-pulsed EBV-transformed B cells (B-LCL) with CD4+CD8dim effector cells resulted in 23% specific lysis, while the CD4+CD8– subset induced 9% lysis of the target cells. The control target cells, autologous B cells without CMV lysate, were lysed at levels ranging from 8% (CD4+CD8–) to 15% (CD4+CD8dim). This elevated "nonspecific" killing of the control targets by the CD4+CD8dim T cells was observed in all donors tested, and may reflect a higher inherent cytolytic potential of the CD4+CD8dim subset against some other constitutively expressed determinants, perhaps EBV antigens, expressed on the B cell targets.
In an attempt to reduce the CMV-unrelated killing observed with B-LCL, autologous monocyte-derived dendritic cells (DC) were used as targets for Donor 2 in Fig. 4. For this donor, the most significant difference in cytolytic activity between the two CD4 effector cell populations was observed at E:T ratio of 10:1. At this ratio the CD4+CD8dim subset induced 18% lysis, while the CD4+CD8– subset induced 6% lysis of the CMV-pulsed dendritic cell targets. Importantly, the incubation of either effector cell population with the control target cells, autologous dendritic cells without CMV lysate, resulted in 0% specific lysis.
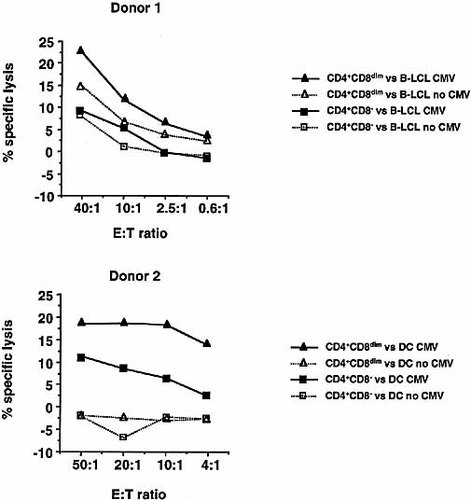
51Cr-release CTL assay of sorted CD4+CD8– versus CD4+CD8dim T cells against an autologous, EBV-transformed B cell line (B-LCL) pulsed with CMV lysate (Donor 1) or autologous monocyte-derived dendritic cells (DC) pulsed with CMV lysate (Donor 2). Purified CD4+CD8– and CD4+CD8dim T cells were obtained by sorting from fresh, unstimulated PBMC. The cytotoxic activity is presented as % specific lysis of the CMV-pulsed target cells and control target cells, by CD4+CD8– cells (closed square and open square, respectively), and by CD4+CD8dim cells (closed triangle and open triangle, respectively). The results shown are representative of five separate experiments using three different donors.
2.5 Enhanced frequencies of antigen-specific IFN-γ expressing cells were observed within the CD4+CD8dim subset in HIV-1 antigen-activated blood cultures
A persistent expansion of the CD4+CD8dim subset in an HIV-infected individual has been observed previously 7. As a part of a recent HIV-1 vaccine study 34, in which we measured CD4+ T cell cytokine responses to a gp120-depleted native HIV-1 immunogen (REMUNETM, Immune Response Corporation, Carlsbad, CA), we also compared HIV-1-specific IFN-γ expression within the CD4+CD8– and CD4+CD8dim subsets in whole blood from 13 HIV seropositive subjects enrolled in this study. Prior to immunization, the subjects generally demonstrated low or unmeasurable CD4+CD8– and CD4+CD8dim T cell responses to HIV-1 antigen, whereas one month after immunization, in vitro HIV-1-specific responses were observed in 12/13 subjects. As illustrated by Fig. 5, following a single immunization with REMUNETM, significantly higher frequencies of cells responding to in vitro stimulation with HIV-1 antigen were observed within both CD4+ subsets. However, the mean frequency of HIV-1-specific IFN-γ+ cells was proportionally much higher, both pre- and post-vaccine therapy, within the CD4+CD8dim-gated population. The mean frequency of IFN-γ+ cells within the CD4+CD8dim subset was 0.5% pre-, and 3.7% post-immunization (range 0–2.1% and 0–17.4%, respectively). Within the CD4+CD8– population, the mean frequency of IFN-γ+ cells before vaccination was 0.1% (range 0–0.5%) and post vaccination 0.5% (range 0–0.9%).
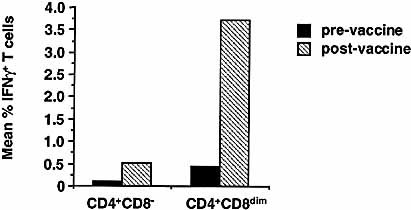
Mean frequencies (n=13) of IFN-γ expressing cells within the CD4+CD8dim subset vs. CD4+CD8– subset in HIV seropositive donor blood activated with HIV-1 antigen. Whole blood from chronically infected HIV seropositive subjects was stimulated with purified gp120-depleted HIV-1 antigen as described in Sect. 4. The activated samples were stained with anti-IFN-γ FITC, CD69 PE (an early activation antigen), CD3 PerCP or CD4 PerCP and CD8 APC. A total of 100,000–200,000 live-gated (FSC vs. SSC) events were collected. Cytograms of CD4 vs. CD8 and CD3 vs. CD8 were used to select subsets for display and analysis of the co-expression of IFN-γ and CD69.
3 Discussion
In this study, we compared functional responses of single positive CD4 T cells and CD4+CD8dim T cells to two purified viral antigens, CMV and HIV-1. CMV-specific cytokine and proliferative responses as well as CMV-specific killing of target cells were measured with cells from healthy CMV seropositive adult subjects. HIV-1-specific cytokine responses were measured in whole blood from HIV seropositive adult subjects.
In most donors tested, the CD4+CD8dim subset comprised 1–2% of the CD3+ lymphocytes. However, three subjects showed CD4+CD8dim T cell frequencies between 2 and 5%, and in one subject, about 13% of the T lymphocytes were CD4+CD8dim (Fig. 1A). After stimulation with CMV, an enriched fraction of responding cells was generally observed within the CD4+CD8dim population (Fig. 1B). Typically, the CD4+CD8dim subset contained proportionally two-to eightfold higher frequencies of CMV-specific IFN-γ+ cells than the CD4+CD8– subset. Similar proportions of the T cell subsets were observed undergoing proliferation at 48 h (Fig. 1B). The enhanced frequencies of cytokine-expressing and proliferating cells within the CD4+CD8dim subset observed in response to CMV antigen, suggest that these cells may represent a significant component of T cell immunity to infection.
Weiss et al. 7 have described persistent expansions, in an HIV-infected person, of Vβ-restricted CD4+CD8dim T lymphocytes that express cytotoxicity-associated molecules and are committed to produce IFN-γ and TNF-α. To determine whether the observed functional responses of the CD4+CD8dim T cells were unique to CMV lysate activation, we also examined cytokine responses to HIV-1 antigen. We demonstrated enhanced frequencies of HIV-1-specific cytokine producing cells within the CD4+CD8dim subset in chronically HIV-1-infected subjects receiving immunizations with an HIV-1 immunogen, REMUNETM. Proportionally higher frequencies of CD4+CD8dim than CD4+CD8–T cells expressed IFN-γ in response to in vitro stimulation with HIV-1 both before and after vaccine treatment (Fig. 5). Similar in vitro response patterns were observed to HIV antigens from other sources (purified HIV-1 gag p24, rp17 and rp55), although the frequencies were generally lower and more variable between donors (data not shown).
In contrast, when blood from selected CMV-seropositive subjects was stimulated with antigens not associated with chronic infection, such as mumps or measles virus, or the bacterial superantigen SEB, we observed a slightly smaller fraction of cytokine expressing cells within the CD4+CD8dim than the CD4+CD8– subset (data not shown). These findings imply that the CD4+CD8dim subset may play a unique role in the control of chronic viral infections such as CMV and HIV.
Previous studies in other species, including monkeys and swine, suggest that after activation, single positive CD4+ memory T cells could acquire the ability to express the CD8 α chain and subsequently permanently maintain the CD4+CD8dim double positive phenotype 16, 18. In our study, a number of observations indicated that while being distinct from the CD4+CD8– subset, the CD4+CD8dim subset shares major attributes with the single positive CD4 population. Single positive CD4 memoryT cells express IL-2, IFN-γ and TNF-α upon stimulation by viral antigens, whereas single positive CD8 memory T cells typically lack IL-2 expression 19. Among our CMV-seropositive subjects, IL-2 as well as IFN-γ and TNF-α expression was generally observed within the CD4+CD8dim subset, hence resembling the cytokine profile of the CD4+CD8– population (data not shown). Incidentally, neither CD4 population expressed the T helper type 2 cytokine IL-4 in response to CMV activation. The CD4+CD8dim cytokine responses were also shown to be MHC class II-restricted, as illustrated by the suppression of these responses by the class II-specific inhibitor, chloroquine, and a cocktail of anti-class II mAb (Fig. 2). And lastly, activation of blood from several CMV-seropositive donors with an HLA-A2-matched CMV pp65 nonapeptide resulted in a complete lack of cytokine expression within the CD4+CD8– and CD4+CD8dim subset, whereas robust IFN-γ responses were observed within the CD4–CD8bright subset (unpublished observations).
CMV-specific T cells within the CD4+CD8– and CD4+CD8dim subsets expressed memory T cell markers (Fig. 3A). Additionally, high frequencies of both IFN-γ+CD4+CD8– and IFN-γ+CD4+CD8dim T cells from CMV lysate-stimulated cultures expressed CD57 and perforin, whereas, CD56 was predominantly expressed on the cytokine producing cells within the CD4+CD8dim population (Fig. 3B).
The expression of cytotoxicity-associated markers suggested that the CD4+CD8dim population might possess enhanced cytolytic effector function. To investigate this possibility, we compared purified populations of CD4+CD8dim and CD4+CD8– T cells for their ability to kill CMV lysate-loaded autologous targets in a chromium-release CTL assay. On a per cell basis, we observed enhanced CMV-specific cytolytic activity within the CD4+CD8dim subset against autologous B or dendritic cell targets when compared with the CD4+CD8– subset (Fig. 4). In this regard, it is of interest that both CMV and HIV-1 infect APC (monocyte/macrophages and/or dendritic cells), and have the capacity to down-modulate class I MHC 35, 36, thus making class II-restricted CTL of potential importance in host defense against these agents.
The data presented in this report demonstrates that CD4+CD8dim and CD4+CD8– T cells are for the most part phenotypically and functionally similar. The observation that a high proportion of CD4+CD8dim cells are capable of responding to chronic viral antigens and the increased cytolytic activity associated with this T cell subset suggest that CD4+CD8dim cells may represent a cytotoxic CD4+ effector population involved in control of chronic viral infections such as HIV and CMV.
4 Materials and methods
4.1 Study subjects
For the CMV study, blood from 12 CMV-seropositive adult subjects (30–50 years old, selected from BDIS blood donor program) was used. For the HIV-1 study, blood from 13 HIV-1-seropositive adultsubjects was used. Median baseline CD4 count for the HIV-1 seropositive subjects was 659/mm3 (range 426–1,969/mm3). Median baseline plasma HIV-1 RNA copy number was 216 (range 1384,967). Each HIV-1-seropositive subject received one intramuscular injection of HIV-1 immunogen (REMUNETM) in incomplete Freund's adjuvant on day 1. Blood samples for the study were drawn on day 1 (prior to immunization) and at 4 weeks post immunization.
4.2 Antigen stimulation for cytokine assays
Antigen stimulation of whole blood has been described elsewhere 19, 37. Briefly, 0.5–1 ml aliquots of heparinized venous blood were stimulated with 5 μg/ml of purified CMV lysate (Advanced Biotechnologies, Columbia, MD) or 10 μg/ml of gp120-depleted HIV-1 antigen (REMUNETM) in 15-ml conical polypropylene tubes. CD28 and CD49d mAb (1 μg/ml of each) were included in all cultures as costimuli 31. The blood cultures were incubated in a humidified 37oC incubator for a total of 6 h, with the final 4 h inthe presence of the secretion inhibitor Brefeldin A (10 μg/ml, Sigma, St. Louis, MO). For inhibition studies, anti-MHC class I or class II mAb (anti-HLA-A,B,C or anti-HLA-DR, DP, DQ, 10 μg/ml final concentration), or an irrelevant control antibody (CD45, 10 μg/ml), as well as inhibitors of proteasome function (lactacystin and n-acetyl-leu-leu-norleucinal, 50 μg/ml, Sigma) or endosome function (chloroquine, 50 μg/ml, Sigma) were used. These inhibitors were added to the whole-blood cultures 20 min prior to the addition of antigen.
At 6 h, a final concentration of 2 mM EDTA was added to each tube for 15 min at room temperature, after which the culture tubes were vortexed vigorously. Blood samples were subsequently aliquoted into staining tubes at 200 μl per tube. Alternatively, the activated blood cultures were frozen for later use. For frozen storage, the EDTA-treated samples were lysed/fixed for 10 min at room temperature using 1X FACSTM Lysing Solution (BD Immunocytometry Systems, BDIS, San Jose, CA; 1:10 dilution of blood in the lysing solution). After the 10-min incubation, the sample tubes were placed in a –80oC freezer.
4.3 Antigen stimulation for BrdU incorporation assays
Flow cytometric assessment of proliferating T cells was performed as previously described 38. Briefly, venous blood was collected into cell preparation tubes (CPT) containing sodium heparin anticoagulant (BD Vacutainer Systems, Franklin Lakes, NJ). PBMC were isolated by centrifugation, resuspended to the original volume in autologous plasma, and aliquoted into 15-ml conical polypropylene tubes at 0.5–1 ml per tube. These cultures were stimulated with 5 μg/ml of CMV lysate and CD28 plus CD49d mAb, and incubated at a 5-degree slant in a 37oC, 5% CO2 incubator for 42 h. Brefeldin A and BrdU (60 μM, Sigma) were then added, and the cultures were incubated an additional 6 h. At 48 h, the cells were treated with EDTA as described above and aliquoted into staining tubes at 200 μl per tube.
4.4 Immunofluorescent staining
Fresh whole-blood and PBMC samples were first lysed/fixed for 10 min at room temperature with 2 ml of 1X FACS Lysing Solution. Cells were then washed in wash buffer (PBS, 0.5% BSA, 0.1% NaN3) and resuspended in 500 μl of 1X FACS Permeabilizing Solution (BDIS) for 10 min at room temperature. Previously frozen cells were thawed rapidly in a 37oC water bath, aliquoted into staining tubes, washed in wash buffer and permeabilized as described above. Permeabilized cells were washed once and staining was performed for 30 min at room temperature, as previously described 37. Isotype-matched control antibodies were included in all experiments. Staining of some cell surface antigens can be compromised by pre-fixation of cells. Therefore, in the phenotyping experiments (Fig. 3), a 30-min surface stain was carried out first, using mAb against various cell surface antigens. Next, the samples were lysed/fixed and permeabilized, followed by the intracellular staining step for the detection of IFN-γ and perforin. After staining, samples were washed once, fixed in 1% paraformaldehyde in PBS, and stored at 4oC until FACS analysis.
4.5 Flow cytometric analysis
Four-color flow cytometric analysis was performed on a FACSCaliburTM flow cytometer (BDIS). Data were acquired and analyzed using CELLQuestTM software (BDIS). Typically 50,000–60,000 gated CD3+ events were collected using FL3 or FL4 (CD3 PerCP or APC) as a fluorescent trigger. In some experiments (Fig. 1B), a second set of gates was drawn to include CD8–, CD8dim or CD8bright T cells, and IFN-γ expression or BrdU incorporation vs. CD4 expression was assessed. Alternatively, a second gate was drawn around the CD4+ T cells and the percentage of cytokine producing cells in the CD8– vs. CD8dim populations was expressed as the fraction of the total CD4+ cells (Fig. 2). The CD8bright cytokine responses were similarly assessed using gates that included CD4-CD8bright T cells. In the phenotyping experiments (Fig. 3), gates were drawn to include either CD4+CD8– or CD4+CD8dim lymphocytes to determine the co-expression of IFN-γ and various cellsurface markers.
4.6 Cell sorting and CTL assays
For use as effector cells in the CTL assays, CD4+CD8– and CD4+CD8dim T cells were sorted from fresh, unstimulated PBMC using a FACSVantageTM cell sorter (BDIS). PBMC were isolated by centrifugation in CPT tubes, washed twice in PBS + 2% fetal bovine serum (FBS) and stained for 30 min at room temperature with CD3 FITC, CD8 PE and CD4 APC (Donor1 in Fig. 4). Alternatively (Donor 2 in Fig. 4), washed PBMC were first stained for 30 min with streptavidin IMag Particles (BD PharMingen, San Diego, CA) labeled with biotinylated CD4. Isolation of CD4+ cells was subsequently performed using an autoMACS magnetic cell sorter (Miltenyi Biotec Inc., Auburn, CA). For further sorting on a FACSVantage, the CD4-enriched cell population was stained with CD8 PE and CD4 APC for 30 min. After staining, cells were washed twice and resuspended at 50×106 cells/ml in PBS + 2% FBS. Thetwo T cell subsets were sorted into glass tubes containing 0.5 ml of ice-cold FBS, using gates to include either CD3+CD4+CD8– or CD3+CD4+CD8dim T cells (Donor 1) or CD4+CD8– or CD4+CD8dim T cells (Donor 2). The purity of the sorted populations was analyzed on a FACSCalibur flow cytometer and was found to be >90%.
Target cells in the CTL assays were either autologous EBV-transformed B cells (B-LCL, Donor 1) or autologous monocyte-derived dendritic cells (DC, Donor 2). The B cells were incubated overnight in cRPMI (RPMI-1640 + 10% heat-inactivated FBS) containing 50 μCi 51Cr (NEN Life Science Products, Boston, MA) with or without CMV lysate (5 μg/ml).
Monocyte-derived dendritic cells were generated in vitro using a modification of the protocol by Romani et al. 39. Briefly, dendritic cells were derived from adherent monocytes by culture in AIM-V medium (GibcoBRL, Grand Island, NY) with 100 ng/ml IL-4 and 250 ng/ml GM-CSF (R & D Systems, Minneapolis, MN). On day 5, the loosely adherent cells were harvested and cultured in AIM-V and monocyte conditioned medium with GM-CSF, IL-4, and TNF-α (30 ng/ml, R & D Systems) for 2 more days in the presence or absence of CMV lysate (5 μg/ml). The cells were harvested on day 7 and pure dendritic cell populations were obtained by immunomagnetic depletion of any remaining T cells (CD3+), monocytes (CD11b+) and NK cells (CD16+) using a MACS dendritic cell isolation kit and an autoMACS cell sorter (Miltenyi Biotec Inc.). The purity of the dendritic cells obtained this way was >90% based on surface phenotype (HLA-DR+/lineage–). Both the CMV-pulsed dendritic cells and the control dendritic cells were incubated for 3 h in cRPMI containing 50 μCi 51Cr. All target cells were washed twice in RPMI-1640 before use in the CTL assay.
Sorted CD4+CD8– and CD4+CD8dim T cells were resuspended in cRPMI at the desired cell concentrations and seeded in 96-well round bottom plates (Costar, Cambridge, MA) containing 51Cr-labeled target cells (5,000 cells/well). Each E:T ratio was set up in triplicate wells. After a 4 h incubation at 37oC, 5% CO2, the supernatants were harvested using a supernatant harvester (Molecular Devices Corporation, Sunnyvale, CA) and radioactivity was counted with a 1272 Clinigamma counter (LKB Wallac, Turku, Finland). The percentage of specific lysis was calculated as follows: % specific lysis = (experimental lysis – spontaneous lysis) / (maximum lysis – spontaneous lysis) × 100. Spontaneous lysis was obtained by incubating the target cells in culture medium alone. Maximum lysis was obtained by exposure of the target cells to 1% Triton x-100.
4.7 Monoclonal antibodies
Monoclonal antibodies CD28, CD49d, anti-HLA-DR (clone L203), anti-HLA-DP, anti-HLA-DQ, CD45, CD3 PerCP and APC, CD4 FITC, PE, PerCP, PerCP/Cy5.5 and APC, CD8 FITC, PE, PerCP and APC, CD27 FITC, CD45RO FITC, CD56 FITC, CD57 FITC, CD69 PE, CD95 PE, anti-IFN-γ FITC and PE, anti-BrdU FITC/DNase, and IgG1 and IgG2a FITC and PE were obtained from BDIS. Anti-HLA-A,B,C (clone G46–2.6) and anti-Perforin PE (clone δG9) were obtained from BD PharMingen, San Diego, CA. All antibodies were used at the manufacturer's recommended concentrations.
Acknowledgements
The authors wish to thank Dr. Ken Davis for providing the CD4-labeled IMag Particles and Drs. Bruce Koppelman and Steve Anderson for helpful discussions.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH



 CD57
CD57 T lymphocytes are enriched in antigen-specific T cells capable of down-modulating cytotoxic activity.
T lymphocytes are enriched in antigen-specific T cells capable of down-modulating cytotoxic activity.
