A critical role for IL-12 in CCR5 induction on T cell receptor-triggered mouse CD4+ and CD8+ T cells
Abstract
Despite increasing evidence for the role of the chemokine system in leukocyte trafficking, the mechanism underlying the induction of chemokine receptors is poorly understood. Here, we investigated how CCR5, a chemokine receptor implicated in T cell migration to inflammatory sites, is induced in the T cell. CCR5 mRNA was hardly detected in resting T cells and marginally induced following T cell receptor (TCR) stimulation. However, TCR-triggered T cells expressed IL-12 receptor, and stimulation with recombinant IL-12 resulted in high levels of CCR5 expression on both CD4+ and CD8+ T cells. In contrast, IL-2 failed to up-regulate CCR5 expression. The effect of IL-12 was selective to CCR5 because IL-12 did not up-regulate CXCR3 expression. Surface expression of CCR5 was shown by staining with anti-CCR5 monoclonal antibody. Stimulation of these CCR5-positive T cells with the relevant chemokine MIP-1α elicited Ca2+ influx, showing that IL-12-induced CCR5 is functional. These results indicate a critical role for IL-12 in the induction of CCR5 on TCR-triggered T cells.
Abbreviation:
-
- IL-12R:
-
IL-12 receptor
1 Introduction
A considerable body of evidence highlights the role of chemokines/chemokine receptors in the regulation of leukocyte migration from the vascular compartment to inflammatory sites 1–3. This chemokine regulation rests on the ability of chemokines to induce affinity modulation of integrin adhesion molecules expressed on leukocytes 4, 5. Like other leukocyte subpopulations, T cells ap-pear to depend on chemokine-regulated mechanisms for recruitment to sites of inflammation 6–8.
T cells should express chemokine receptors to respond to chemokine stimulation. Intense effort has been made to determine which chemokine receptors are expressed on T cells and whether a givenchemokine receptor expression is restricted to particular subsets or states of T cells. The pattern of chemokine receptor expression appears to depend on the activation state of the T cell (reviewed in 9). Namely, expression of some chemokine receptors is up-regulated after mitogenic stimulation and/or prolonged treatment with IL-2 10, 11, and some chemokine receptors are restricted to activated T cells 12 or the memory subset 13. For example, CCR5 was reported to be up-regulated by long-term treatment of CD45RO+ human memory T cells with recombinant (r) IL-2 10. This chemokine receptor has been regarded as characteristic of CD4+ Th1 lymphocytes 14–16 and implicated in the recruitment of Th1 cells to inflammatory sites such as the synovial lesion in rheumatoid arthritis 15, 16, indicating its importance in T cell-mediated inflammatory responses. However, resting (TCR-un-triggered) T cells respond to neither IL-2 nor CCR5-reactive chemokines. Therefore, it remains to be determined how CCR5 expression and responsiveness to the relevant chemokines are induced in resting T cells following antigen sensitization. It is also unknown whether CD8+ T cells aswell as CD4+ T cells/Th1 cells express CCR5 following activation.
Recent studies have revealed that administration of IL-12 into tumor-bearing mice induces tumor regression by allowing T cells to infiltrate tumor masses 17–19. This suggested that IL-12 may have the capacity to influence the T cell adhesion mechanism that involves the coordination of adhesion molecules and chemokines/chemokine receptors. Based on this, the present study focused on the effect of IL-12 on the induction of CCR5 expression in the T cell. The results show that CCR5 is induced in both CD4+ and CD8+ T cells only when they are activated with anti-TCR plus anti-CD28 mAb and then stimulated with rIL-12. Because CCR5 expression was not induced in TCR-activated T cells by endogenous IL-2 and even by exogenous rIL-2, the results provide the first demonstration of the critical role for IL-12 in the induction of CCR5 on recently TCR-triggered T cells.
2 Results
2.1 CCR5 mRNA expression is induced in TCR-stimulated T cells depending on the exposure to IL-12
We investigated whether CCR5 is expressed on resting or TCR-stimulated T cells and if not, what type of stimulation is responsible for the induction of this chemokine receptor. Purified T cells were prepared from normal BALB/c lymph node cells and stimulated in vitro with immobilized anti-CD3 plus soluble anti-CD28 mAb for 48 h. Total RNA was isolated from these activated T cells as well as from unstimulated resting (freshly prepared) T cells and assayed for mRNA expression of CCR5 (Fig. 1A). The mRNA of CCR5 was hardly detected in resting T cells and only marginally expressed by anti-CD3/anti-CD28-activated T cells.
We next examined whether IL-12, the cytokine capable of promoting Th1 differentiation can up-regulate CCR5 mRNA induction. As shown in Fig. 1B, the expression of IL-12 receptor (IL-12R) β1 and β2 components was up-regulated in anti-CD3/anti-CD28-activated T cells. Stimulation of these activated T cells with IL-12 resulted in striking enhancement of CCR5 mRNA expression (Fig. 2A). This contrasted with the failure of exogenous rIL-2 to induce CCR5 up-regulation, i.e. the level of CCR5 mRNA after exposure to rIL-2 was comparable to that in a cytokine-unstimulated control group. Fig. 2B shows the time course of CCR5 mRNA induction. IL-12 induced the up-regulation of CCR5 mRNA expression in a time-dependent manner from 12 to 36 h after stimulation. IL-2 again failed to up-regulate throughout the entire culture period examined.
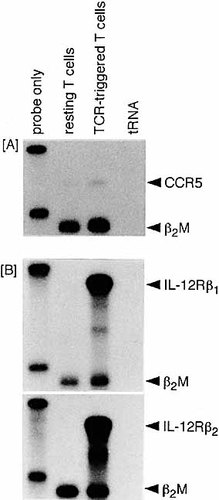
TCR triggering induces high and only marginal levels of IL-12R and CCR5 mRNA expression, respectively. Lymph node T cells were purified from normal BALB/c mice. The cells (1.5×106/well) were stimulated with immobilized anti-CD3 (5 μg/ml) and soluble anti-CD28 (2 μg/ml) for 48 h in 24-well culture plates. Total RNA was isolated from these stimulated T cells as well as unstimulated resting T cells and subjected to the RNase protection assay.
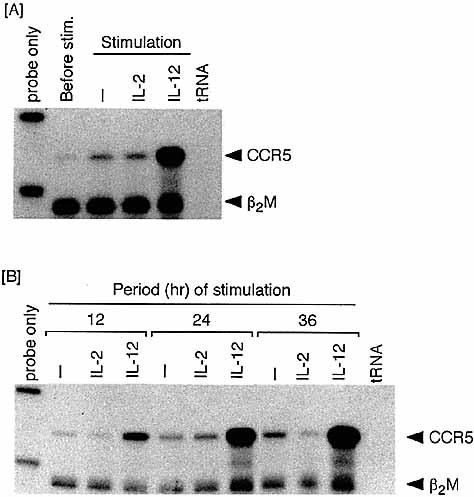
Induction of CCR5 mRNA in T cells exposed to IL-12 following TCR triggering. Purified lymph node T cells were similarly activated with anti-CD3 plus anti-CD28 for 48 h. (A) TCR-triggering T cells were harvested, washed and then stimulated with 100 U/ml rIL-2 or 250 pg/ml rIL-12 in the second culture for 24 h. Cells from the first TCR-triggering and second cytokine-stimulation cultures were examined for the expression of CCR5 mRNA. (B) Cells were harvested from the second cytokine stimulation cultures various times after stimulation.
To determine whether endogenous IL-12 derived from APC can induce CCR5 on TCR-stimulated T cells, a B cell-depleted splenic fraction (T+APC) instead of purified T cells was stimulated with anti-CD3 plus anti-CD28 in the presence of anti-IL-12 neutralizing mAb or control IgG. As shown in Fig. 3A, CCR5 expression in cultures containing anti-CD3/anti-CD28 was slightly up-regulated in the absence of anti-IL-12 mAb (in the presence of control rat Ig) when compared with a group of purified T cells alone. This CCR5 up-regulation was inhibited in the presence of anti-IL-12 mAb. The levels of CCR5 mRNA expression induced by a T+APC fraction in the absence of anti-IL-12 (Fig. 3A) were lower than those in a T+APC fraction stimulated in the presence of exogenous rIL-12 (Fig. 3B). The results suggest that a modest amount of IL-12 is produced by APC upon TCR stimulation of a T+APC population as previously shown 20 and induces detectable albeit slight levels of CCR5 expression on TCR-triggered T cells. Together with the results of Fig. 2, these observations show an important role for IL-12 in the induction of CCR5 expression on T cells.
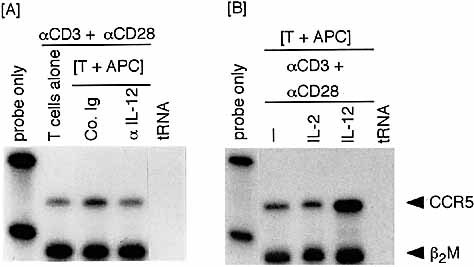
CCR5 expression in a TCR-stimulated [T+APC] fraction is down- or up-regulated by addition of anti-IL-12 mAb or rIL-12, respectively. A purified T cell population and a B cell-depleted splenic fraction (T cells + APC) were prepared. (A) A [T+APC] fraction was stimulated with anti-CD3 plus anti-CD28 for 72 h in the presence of control rat Ig or anti-IL-12 mAb (10 μg/ml) and as a control, a T cell population was also stimulated with anti-CD3/anti-CD28. (B) A [T+APC] fraction was stimulated with anti-CD3 plus anti-CD28 in the presence of rIL-2 (100 U/ml) or rIL-12 (250 pg/ml) for 72 h.
2.2 IL-12-induced CCR5 induction is IFN-γ independent
IFN-γ is produced following stimulation of TCR-triggered T cells with IL-12 21. We determined whether CCR5 expression is induced via the effect of IFN-γ produced as a result of IL-12 stimulation or directly via an IL-12 signal. TCR-triggered T cells were exposed to IL-12 in the presence of anti-IFN-γ mAb for the neutralization of IFN-γ produced in culture. Complete neutralization of IFN-γ in culture supernatant was confirmed by ELISA (data not shown). As shown in Fig. 4A, CCR5 induction was not influenced by neutralizing IFN-γ. Thus, IL-12-induced CCR5 expression is IFN-γ independent.
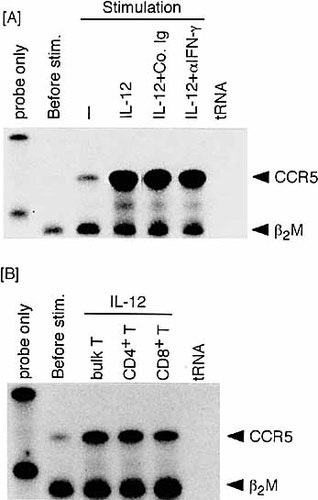
IL-12-induced CCR5 expression is IFN-γ-independent and observed in both CD4+ and CD8+ lymph node T cells. (A) TCR-triggered T cells were stimulated for 24 h with rIL-12 in the presence of anti-IFN-γ mAb (10 μg/ml) or control rat Ig. (B) T cells exposed to IL-12 following TCR triggering were separated to the CD4 or CD8 subset.
2.3 CCR5 is induced on both CD4+ and CD8+ T cell subsets
CCR5 has been shown to be induced on Th1 CD4+ T cells 15, 16, whereas only a few studies 22 have addressed the induction of CCR5 on CD8+ T cells. We examined whether exposure of TCR-triggered T cells to IL-12 induces CCR5 on both CD4+ and CD8+ T cells. Each CD4+ or CD8+ T cell population was separated from T cells exposed to IL-12 following TCR triggering. As shown in Fig. 4B, both CD4+ and CD8+ T cells expressed CCR5 mRNA. Thus, IL-12-induced CCR5 expression occurs in recently activated T cells irrespective of their phenotypes.
2.4 IL-12 induction of chemokine receptor is selective to CCR5
CXCR3 is expressed on Th1 T cells along with CCR5 expression 15, 16. We determined whether CXCR3 is also up-regulated by exposure to IL-12 (Fig. 5, left panel). This was examined in comparison with CCR5 and another control chemokine receptor CCR1. Anti-CD3/anti-CD28-stimulated T cells expressed negligible levels of CXCR3 mRNA. However, when these T cells were recultured free of stimulating reagents, high levels of CXCR3 expression were observed. While this CXCR3 expression was somewhat down-regulated in the presence of rIL-2, rIL-12 did not influence the levels of CXCR3 expression. Portions of the same RNA samples were examined for CCR5 and CCR1 expression. The pattern of CCR5 expression was essentially the same as shown in Fig. 2A. CCR1 was not induced in T cells following TCR/IL-12 stimulation, although unfractionated spleen cells including macrophages expressed CCR1 mRNA (data not shown). Thus, CCR5 is a chemokine receptor induced selectively in TCR-triggered T cells by exposure to IL-12.
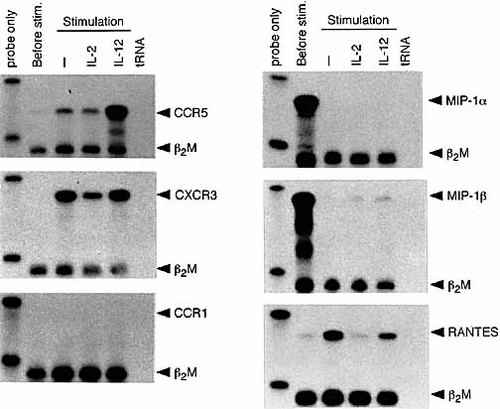
mRNA expression of various chemokine receptors and chemokines in T cells exposed to IL-12 following TCR triggering. RNA was isolated from TCR-triggered lymph node T cells or T cells stimulated with IL-2 or IL-12 following TCR triggering and examined for the expression of various chemokine receptors and CCR5-reactive chemokines.
2.5 The induction of CCR5-reactive chemokines in TCR-triggered T cells before and after IL-12 exposure
CCR5 is a chemokine receptor reactive to MIP-1α, MIP-1β and RANTES 9. We also examined whether these three chemokines are induced in TCR-triggered/IL-12-exposed T cells in parallel to CCR5 induction (Fig. 5, right panel). MIP-1α and MIP-1β mRNA were detected in TCR/CD28-triggered T cells and rapidly disappeared in the subsequent culture without stimulating antibody irrespective of whether these T cells were exposed to IL-12 or IL-2. RANTES mRNA expression was induced in TCR-triggered T cells when they were recultured, and was not enhanced by cytokine exposure but rather slightly or markedly down-regulated in the presence of IL-12 or IL-2, respectively. Thus, CCR5-reactive chemokine induction was achieved by TCR triggering but not by IL-12 exposure. It should be noted that the induction of the CCR5 chemokine receptor and CCR5-reactive chemokines do not synchronize in recently activated T cells.
2.6 Surface expression of CCR5 on TCR-triggered T cells after exposure to IL-12
We examined surface CCR5 expression by staining IL-12-stimulated T cells directly with anti-mouse CCR5 mAb. Stimulation of TCR-triggered T cells with IL-12 but not with IL-2 increased the incidence of CCR5-positive cells in the whole (CD4 plus CD8) T cell population (Fig. 6A). We further compared CCR5 expression between CD4 and CD8 T cell subsets and between naive and memory T cells. CCR5+ cells were induced in both CD4+ and CD8+ T cell subsets (Fig. 6B), but the incidence of CCR5+ cells was higher in the CD8 than the CD4 subsets. The results of Fig. 7 show that CCR5 induction occurred in both CD62L+ (naive) and CD62L– (memory) phenotypes of T cells, although the incidence in the latter is higher than that of the former.
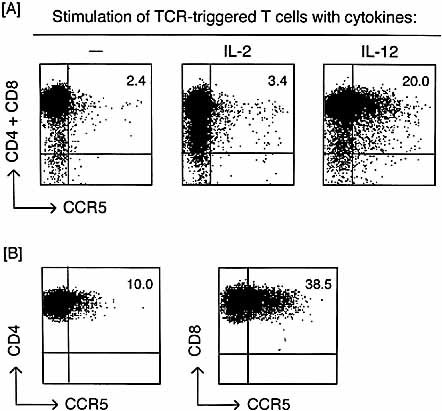
CCR5 expression on the surface of T cells exposed to IL-12 after TCR triggering. (A) TCR-triggered T cells were cultured in the presence of 100 U/ml rIL-2 or 250 pg/ml rIL-12 for 48 h. These cytokine-treated and untreated control cells were stained doubly with PE-conjugated anti-CCR5 mAb and a mixture of allophycocyanin-conjugated anti-CD4 plus anti-CD8 mAb. CCR5 expression was detected on both anti-CD4 and anti-CD8 stained cells. (B) TCR-triggered, IL-12-stimulated T cells were stained doubly with anti-CD4 plus anti-CCR5 or anti-CD8 plus anti-CCR5 mAb. CCR5 expression was detected by gating on each CD4 or CD8 population. The numbers in upper right corners on each figure are the percentages of CD4+ and/or CD8+ CCR5+ cells.
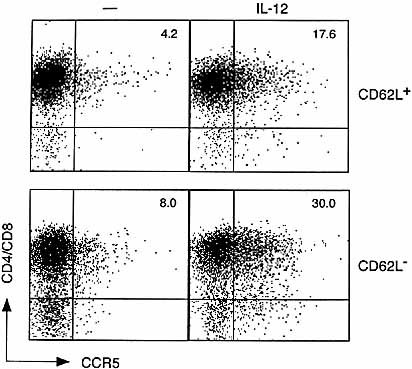
CCR5 induction on both naive and memory phenotypes of T cells. CD62L+ (naive) and CD62L– (memory) T cell-enriched populations were prepared as described. After TCR triggering, cells were stimulated with IL-12 and then stained with anti-PE-conjugated anti-CCR5 and a mixture of allophycocyanin-conjugated anti-CD4 plus anti-CD8 mAb.
2.7 Induction of Ca2+ mobilization by stimulation with a CCR5-reactive chemokine MIP-1α in IL-12-stimulated T cells following TCR triggering
To confirm that CCR5 expressed on the surface of IL-12-stimulated T cells is a functional receptor, we examined whether a CCR5-reactive chemokine, MIP-1α, induces Ca2+ mobilization as a result of surface CCR5 stimulation. Fig. 8 shows that stimulation with MIP-1α resulted in high levels of Ca2+ influx in IL-12-stimulated T cells following TCR triggering. This contrasted with low levels of Ca2+ influx in IL-2-stimulated T cells. Together, the results of Fig. 6 and 8 indicate that stimulation of TCR-triggered T cells with IL-12 results in the surface expression of CCR5 and that such CCR5 reacts with the relevant chemokines and works as a functional receptor.
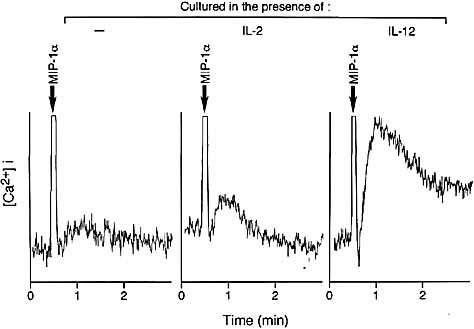
Chemokine-stimulated Ca2+ influx in T cells exposed to IL-12 following TCR triggering. TCR-triggered T cells exposed to IL-12 or IL-2 were stimulated with 1 nM rMIP-1α.
3 Discussion
The interaction of chemokines and the corresponding chemokine receptors is involved in the regulation of lymphocyte recruitment to inflammatory/infectious sites in many pathological situations 1–3. Accordingly, chemokine receptor expression on a given population of lymphocytes would allow only these cells to exhibit the migratory capacity. Within the T cell lineage, the expression of several chemokine receptors appears to be restricted to activated and memory (CD45RO+) types of cells 9, 23. Therefore, delineating which activation signals are required for the induction or up-regulation of chemokine receptor expression would be an area of recent intense study.
The present study demonstrates that CCR5 mRNA is marginally expressed in resting T cells, and the activation with anti-CD3/anti-CD28 induces only slight up-regulation of CCR5 mRNA expression. However, exposure of these TCR-triggered T cells to IL-12 resulted in striking levels of CCR5 expression. This was the case with both CD4+ and CD8+ T cells. Such an IL-12 capacity contrasted with the failure of IL-2 to up-regulate CCR5 expression. Consistently, TCR triggering in the presence of APC with the capacity to produce IL-12 during interactions with T cells 20 up-regulated CCR5 mRNA expression, although weakly, without requiring exogenous rIL-12, but this CCR5 up-regulation was inhibited by neutralizing IL-12 that would be produced from APC 20. The results also showed that IL-12-elicited CCR5 mRNA induction leads to cell surface expression of this chemokine receptor and that surface CCR5 mediates Ca2+ mobilization in response to the relevant chemokines.
The present results will be discussed from the following important aspects. First, CCR5 expression on human T cells, particularly of CD45RO+ T cells, was induced by selectively exposing these cells to IL-2 10, 24. IL-12 was shown to induce only slight levels of CCR5 expression in one study 10. In the other 24, IL-12 enhanced CCR5 mRNA expression acting on peripheral blood leukocytes. However, L-12 failed to induce surface CCR5 expression but rather down-regulated IL-2-mediated surface CCR5 induction.The discrepancy between CCR5 mRNA expression and surface protein expression is unknown. IL-12 stimulation simultaneously induced the production of CCR5-reactive chemokines. Surface CCR5 expression or detection might be influenced by the simultaneously produced ligands. In the present mouse model, IL-2 failed to up-regulate CCR5 expression on TCR-triggered T cells, and the expression of this chemokine receptor was inducible only by IL-12. Thus, the observations made in the present mouse model are discordant with the results obtained for human memory T cells.
The discordant results observed between human and mouse models may be explained by considering the following possibility. There is a critical difference in the nature of responding T cells used for cytokine exposure, particularly in terms of the activation state, between human and mouse models. Naive T cells express neither IL-12R nor IL-2R, and both of these receptors are induced only after TCR triggering 21, 25. Therefore, recently TCR-triggered T cells were used as responders in the present mouse model and exposed to IL-2 or IL-12. CCR5 mRNA expression was observed as early as 12 h after exposure to IL-12, and CCR5 surface expression and function were observed within 48 h after IL-12 stimulation. In contrast, memory T cells (previously TCR-triggered T cells) were exposed to IL-2 or IL-12 without restimulation with anti-CD3 in the human model. While IL-2 induced CCR5 expression on these T cells, considerably longer term (more than 8 days) exposure to IL-2 was required for its induction. Therefore, it is unclear whether this human model represents CCR5 expression on T cells (virgin or memory) that start to mobilize to inflammatory sites after antigen stimulation or restimulation.
Because previous studies 10, 24 did not examine whether memory (CD45RO+) T cells express sufficient levels of IL-12R for responding to IL-12 stimulation, the failure of IL-12 to induce CCR5 expression in human T cells may not necessarily be attributed to the inability of this cytokine itself. Namely, memory T cells may not express sufficient levels of IL-12R for promptly responding to IL-12 stimulation. If this is the case, it is possible that CCR5 is also induced on human T cells by IL-12 stimulation depending on IL-12R expression of responding T cells. In this context, our recent observations 26 showed that direct stimulation of peripheral blood lymphocytes (PBL) with IL-12 failed to induce CCR5 on the T cells,whereas IL-2 stimulation induced high levels of CCR5 after 8 days, which is consistent with previous studies 10, 24. However, when PBL or T cells separated from PBL were first stimulated with anti-CD3 plus anti-CD28 for 48 h to express detectable (enhanced) levels of IL-12R and then exposed to IL-12 for 24–48 h, CCR5 was induced on these T cells. This protocol was essentially the same as that used in the present mouse model. Thus, IL-12 has the capacity to induce CCR5 even in human T cells as long as their TCR have been recently triggered 26.
A second aspect that should be noted in this study concerns with the fact that IL-12-mediated CCR5 expression occurs in both CD4+ and CD8+ T cell subsets. Most of foregoing studies have dealt with the induction of chemokine receptor expression associated with differentiation of CD4+ T cells into Th1 and Th2. CCR5 and CXCR3 are preferentially expressed on Th1 cells, such as those recovered from the synovial fluid of rheumatoid joints, while Th2 cells preferentially express CCR4 14–16. Accordingly, it is likely that Th1 and Th2 cells differentially migrate to peripheral inflammatory sites in response to different chemokines 27. Although CD8+ T cells are also recruited to inflammatory sites such as tumor masses 17, 28, only a few studies 22 investigated the expression of CCR5 on CD8+ T cells recruiting into the site of inflammation. Even in such a study 22, how CCR5 is induced on CD8+ T cells was not clarified. Thus, the present study provides the first demonstration that IL-12 is capable of inducing CCR5 expression similarly on CD4+ and CD8+ T cells whose TCR have recently been triggered. These results are also compatible with the fact that both CD4+ and CD8+ T cells infiltrate tumor masses after IL-12 is given to tumor-bearing mice and stimulates a portion of IL-12R+ T cells 17, 28.
Third, differential induction of CCR5 and CXCR3 expression by IL-12 is also worth consideration. Whereas naive (cord blood) human T cells express neither CCR5 nor CXCR3, Th1-polarized cells express moderate levels of CCR5 and high levels of CXCR3 15. The expression of these receptors was also studied in T cells from rheumatoid joints, which acquire a Th1 phenotype in vivo 15, 16. They expressed high levels of CCR5 and CXCR3 9, 15, 16. In this study, CXCR3 was inducedin TCR-triggered T cells when they were allowed to rest. In contrast to the effect on CCR5 expression, IL-12 failed to up-regulate CXCR3 expression. Thus, while CXCR3 is expressed on Th1 cells infiltrating the inflammatory site, it appears that the expression of this chemokine receptor is regulated differently from that of CCR5, and IL-12 induces selective expression of CCR5 among chemokine receptors related to T cell recruitment to inflammatory sites.
In addition to the IL-12 effect on chemokine receptor induction, the present study examined whether chemokine expression of activated T cells is also modulated by IL-12. Previously, MIP-1αexpression was shown to be induced in T cells stimulated with anti-CD3 plus anti-CD28 mAb 29. Consistent with this, activation of purified T cells with anti-CD3 plus anti-CD28 resulted in high levels of MIP-1α and MIP-1β mRNA expression (Fig. 5). Interestingly, mRNA of another CCR5-reactive chemokine RANTES was only slightly induced after TCR triggering but considerably up-regulated when cells were re-cultured in conditions free of stimulating reagents. Thus, the induction of the ligands for CCR5 occurs in association with TCR triggering.Contrary to CCR5 induction, the expression of CCR5-reactive chemokines was not up-regulated in recently TCR-triggered T cells by IL-12 stimulation. Recent studies 24, 30, 31 investigating cytokine induction of chemokines showed that IL-12 has the capacity to enhance the production of CCR5-reactive chemokines 24. However, MIP-1α production was observed in longer term cultures (more than 8 day) of human memory T cells 24. Thus, it appears that IL-12 induction of CCR5-reactive chemokines differs dependingon the nature of responding T cells including of T cell subsets and the activation status.
Our present results illustrate that CCR5 is induced in TCR-triggered CD4+ and CD8+ T cells, and is dependent on the exposure to IL-12. These recently activated T cells that have expressed CCR5 in response to IL-12 do not produce chemokines reactive to CCR5. This contrasts with the observations that exposure of human memory T cells to IL-12 do not up-regulate CCR5 expression but produce its ligands 24. Despite such seemingly discordant observations, it should be noted that the capacities to express chemokine receptors and to produce chemokines donot simultaneously emerge at a given state of T cells. Differentially regulating these capacities could allow antigen-stimulated T cells in lymphoid organs to migrate to the site where they are needed to function.
4 Materials and methods
4.1 Mice
BALB/c mice were purchased from Shizuoka Laboratory Animal Center, Hamamatsu, Japan and used at 7–9 weeks of age.
4.2 Reagents
Mouse rIL-12 was provided by Genetics Institute, Inc. (Cambridge, MA). Mouse rIL-2 was kindly provided by Shionogi Co. (Osaka, Japan). Anti-CD3 (145–2C11) 32, anti-CD28 (Pv-1) 33, anti-I-Ad/b (34-5-3S) 34, anti-mouse IL-12 (C17.8) 35 and anti-IFN-γ (R4–6A2) (American Type Culture Collection, Bethesda, MD) mAb were purified from culture supernatants or ascites fluid of respective hybridomas. Mouse MIP-1α was purchased from R & D Systems (Minneapolis, MN). Allophycocyanin-conjugated anti-CD8 or anti-CD4 (PharMingen, San Diego, CA), and phycoerythrin (PE)-conjugated anti-mouse CCR5 mAb (PharMingen, Cat. no. 559923) were also obtained.
4.3 Preparation of various T cell populations
Lymph node or spleen cells were depleted of B cells and Ia+ APC by immunomagnetic negative selection as described 21. Cells were allowed to react with anti-I-Ad/b mAb and then incubated with advanced magnetic particles bound to goat anti-mouse Ig (Advanced Magnetic, Cambridge, MA). A T cell population depleted of anti-I-Ad/b-labeled and/or surface Ig+ cells was obtained by removing cell-bound magnetic particles with a rare earth magnet (Advanced Magnetic). CD4+ or CD8+ T cells were further depleted by incubating this population with anti-CD8 or anti-CD4 mAb followed by magnetic particles conjugated to goat anti-rat IgG (Advanced Magnetic). Purity of the resulting population was checked by flow cytometry.The purity was consistently >97% for the whole (CD4 plus CD8) T cell population and >95% for either CD4 or CD8 population. In some experiments, a population depleted of B cells alone was prepared and used as a T + APC population. To obtain CD62L+ and CD62L– cells, purified T cells were labeled with superparamagnetic microbeads conjugating anti-CD62L mAb (Miltenyi Biotec, Sunnyvale, CA). Labeled cells were separated from unlabeled cells by magnetic cell sorting using the MiniMACS (Miltenyi Biotec). The magnetically labeled cells were retained in a MiniMACS column inserted into a MiniMACS magnet, while the unlabeled cells passed through. Labeled cells were eluted after the column was removed from the magnet. The cells that passed though the column and those elutedfrom the column were used as CD62L– and CD62L+ T cell populations, respectively.
4.4 Stimulation of T cells with anti-CD3 plus anti-CD28 mAb
Anti-CD3 (5 μg/ml) was immobilized to individual wells of 24-well culture plates (Corning 25820, Corning Glass Works, Corning, NY) in a volume of 0.5 ml. After 3 h, solutions were discarded and plates were washed twice with PBS. Purified T cells were cultured in 2 ml RPMI 1640 medium supplemented with 10% fetal bovine serum and 2-mercaptoethanol (2-ME) at 1.5×106 cells/well in the presence of 2 μg/ml soluble anti-CD28 mAb for 48 h in a CO2 incubator.
4.5 Immunofluorescence and flow cytometry
CCR5 was stained using PE-conjugated anti-CCR5 mAb. Activated T cells were stained with anti-CCR5 mAb together with anti-CD4 and/or anti-CD8. CCR5 expression was detected on cells stained withanti-CD4 plus anti-CD8 or on cells stained with either anti-CD4 or anti-CD8 and then gated on a CD4+ or CD8+ T cell population. Stained cells were analyzed with a FACSCalibur (Becton Dickinson, Mountain View, CA).
4.6 cDNA probes
CCR5, CXCR3 and RANTES cDNA were cloned from purified mouse lymph node cells that were activated for 48 h with immobilized anti-CD3 (5 μg/ml) and soluble anti-CD28 (2 μg/ml) in the presence of 250 pg/ml mouse rIL-12. CCR1 cDNA was cloned from unfractionated spleen cells that were treated for 48 h with 5 μg/ml concanavalin A, harvested and then restimulated for 48 h with 100 U/ml mouse rIL-2 (Shionogi Pharmaceutical Co.). MIP-1α and MIP-1β cDNA were cloned from cells of the mouse macrophage cell line RAW 264 that were treated for 12 h with 100 pg/ml LPS (Escherichia coli 1027:B8; Difco Laboratories). RNA isolated from the above cells were used as templates for first-strand cDNA synthesis. The mouse CCR5, CCR1, CXCR3, MIP-1α, MIP-1β and RANTES coding sequences were cloned from these cDNA usingTaq DNA polymerase, standard PCR conditions and the following primers: CCR5, a 5′ sense oligonucleotide GGATTTTCAAGGGTCAGTTC and a 3prime; anti-sense oligonucleotide AACCTTCTTTCTGAGATCTGG based on sequences 77–96 and 580–600, respectively, from the sequence of CCR5 36; CCR1, a 5′ sense oligonucleotide ATGGAGATTTCAGATTTCACAG anda 3prime; anti-sense oligonucleotide TCAGAAGCCAGCAGAGAG based on sequences 1–22 and 1051–1068, respectively, from the sequence of CCR1 37; CXCR3, a 5′ sense oligonucleotide GCTAGATGCCTCGGACTTTG and a 3prime; anti-sense oligonucleotide GCTGATCGTAGTTGGCTGATA based on se-quences 30–49 and 566–589, respectively, from the sequence of CXCR3 38; MIP-1α, a 5′ sense oligonucleotide TGACACTCTGCAACCAAGTC and a 3prime; anti-sense oligonucleotide GAACGTGTCCTGAAGTCTTTC based on sequences 114–133 and 589–609, respectively, from the sequence of MIP-1α 39; MIP-1β, a 5′ sense oligonucleotide TGGATTACTATGAGACCAGCA and a 3prime; anti-sense oligonucleotide CAGTCATATCCACAATAGCAGA based on sequences 208–228 and 574–595, respectively, from the sequence of MIP-1β 40; and RANTES, a 5′ sense oligonucleotide AGCTGCCCTCACCATCATC and a 3prime; anti-sense oligonucleotide GTATTCTTGAACCCACTTCTTC based on sequences 54–72 and 270–291, respectively, from the sequence of RANTES 41. The PCR products were purified by agarose gel electrophoresis and ligated to the T-vector as described 42. Briefly, Bluescript (Stratagene, La Jolla, CA) plasmid was digested with EcoRV and incubated with Taq polymerase with the use of standard buffer conditions in the presence of 2 mM dTTP for 2 h at 70°C. After phenol extraction and precipitation, the T-vector was ready for cloning. PCR products were then ligated to the vector. cDNA for IL-12R β1 and β2 were cloned as previously described 43. cDNA for β2 microglobulin (β2M) was kindly provided by Dr. Takeshi Tokuhisa (Chiba University Medical School, Chiba, Japan).
4.7 Measurement of mRNA expression
Total cellular RNA was isolated by the acid guanidinium-thiocyanate-phenol-chloroform method and mRNA levels were determined using the RNase protection assay according to the procedure previously described 44. Briefly, 10 μg total cellular RNA was hybridized in solution to a 32P-labeled anti-sense riboprobe for overnight at 50°C in 80% formamide. The plasmid was linearized with Hinf I (CCR5), Hinc II (CCR1), Hpa I (CXCR3), Ava II (MIP-1α), Bgl II (MIP-1β), Fok I (RANTES), Hind III (IL-12R β1) or Nde I (IL-12R β2) and an in vitro transcription was performed in the presence of [α-32P]UTP. The protected fragment (172 bp for CCR5, 179 bp for CCR1, 279 bp for CXCR3, 216 bp for MIP-1α, 313 bp for MIP-1β , 232 bp for RANTES, 343 bp for IL-12R β1 or 235 bp for IL-12R β2) was separated on a denaturing sequencing gel. As an internal control for the amount of RNA loaded onto the gel, RNA was simultaneously hybridized to an anti-sense 32P-labeled probe for the β2M gene (127 bp).
4.8 Calcium mobilization assay
T cells were suspended at 1×107/ml in 2% FBS/PBS containing 3 μM fura-2AM (DOJINDO, Kumamoto, Japan) and incubated at 37°C for 30 min. Fura-2-loaded cells were pelleted and washed twice and then resuspended at 5×106/ml in PBS containing 0.5 mM CaCl2. The calcium response was initiated by addition of 1 nM MIP-1α. Cells were analyzed for free calcium ion by measurement of fura-2 fluorescence emission on fluorescence photometer HITACHI F-3000 (Tokyo, Japan).
Acknowledgements
The authors are grateful to Mrs. Mami Yasuda and Miss Mari Yoneyama for secretarial assistance. This work was supported by Special Project Research-Cancer Bioscience from the Ministry of Education, Science and Culture, Japan.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




