Failure of MHC class II expression in neonatal alveolar macrophages: potential role of class II transactivator
Abstract
Neonatal peritoneal and blood macrophages are known to be ineffective in antigen-presentation functions, and this manifests as inefficient MHC class II expression in response to IFN-γ. Theunderlying mechanisms responsible for this maturational deficiency have not been elucidated. We show here that MHC class II expression in alveolar macrophages (AM) from neonates is also refractory to IFN-γ stimulation. Furthermore, by examining the intracellular pathway leading to MHC class II expression, we demonstrate that the site of the impairment is at the level of transcription. Thus, expression of mRNA encoding the class II transactivator (CIITA), MHC class II (RT1.B) and invariant chain (Ii) was low or undetectable in neonatal AM stimulated with concentrations of IFN-γ that induced adult AM to up-regulate MHC class II expression. The failure of AM from young animals to express MHC class II was not due simply to deficient IFN-γ receptor function since IFN-γ-responsive genes such as IRF-1, IRF-2 and IP-10 were up-regulated in a dose-dependent manner from animals of all ages investigated. Importantly, the responsiveness of neonatal AM to IFN-γ, as determined by MHC class II expression, could be modulated to adult levels when pre-cultured in vitro. This suggests that microenvironmental factors operative in vivo may play a role in suppressing the expression of MHC class II in AM from young animals. We have investigated the role of type I interferons but did not find them to be responsible for the inability of AM from young animals to induce MHC class II in response to IFN-γ.
Abbreviations:
-
- AM:
-
Alveolar macrophage
-
- CIITA:
-
Class II transactivator
-
- Ii:
-
Invariant chain
1 Introduction
Both antigen presentation 1 and MHC class II expression 2, 3 by neonatal macrophages have previously been shown to be defective when compared to macrophages from adult animals. However, this functional immaturity has not been demonstrated in alveolar macrophages (AM) and, furthermore, the mechanism of this dysregulation in neonatal macrophages is still unknown. Thus, we wished to determine if AM from neonates were similarly inefficient in expressing MHC class II in response to a defined stimulus such as IFN-γ. In addition, we wished to elucidate the nature of this maturational "defect" in neonatal macrophages in some detail by attempting to identify specific point(s) of dysregulation within the intracellular MHC classII processing pathway.
The IFN-γ-inducible MHC class II pathway is initiated via the ligation of IFN-γ to its receptor, resulting in Stat 1a phosphorylation, dimerization and the subsequent translocation into the nucleus, where it is bound to defined DNA sequences (GAS for gamma-activated site) (reviewed in 4). This leads to the transcription of several genes including IRF-1 5 and IRF-2 6. IRF-1 together with Stat 1 and a ubiquitous factor known as USF-1 cooperatively bind to their respective cis-acting element, resulting in the activation of the class II transactivator (CIITA) promoter 7. The resultant transcription of CIITA is critical in regulating the expression of MHC class II genes 8 and has been shown to be controlled and induced by IFN-γ 9. In addition, CIITA has also been shown to regulate at least two other genes involved in antigen presentation, namely those coding for the invariant chain (Ii) 10 and HLA-DM 11.
The MHC class II molecule is a heterodimer composed of non-covalently-linked α and β subunits 12. Three MHC class II α β dimers are assembled onto a set of invariant chain (Ii) trimers to form a nine-subunit complex 13, a process facilitated by the endoplasmic reticulum (ER)-resident protein, calnexin 14. One of the major roles of Ii is the targeting of MHC class II α β complex to appropriate compartments, thus directing the trafficking of MHC class II complexes from ER to Golgi and endosomes 15, 16.
Before MHC class II molecules can bind antigen-derived peptides for presentation to T cells at the cell surface, the α β heterodimers must first be released from their interaction with Ii. This is achieved through stepwise removal of Ii fragments generated by proteolytic cleavage by the cysteine protease cathepsin S 17, which leaves a peptide associated with the MHC class II molecule known as CLIP (class II-associated Ii peptide). The final removal of CLIP from the class II α β complex is facilitated by HLA-DM 18, a molecule encoded within the class II region of the MHC 19. This "mature" MHC class II molecule is now loaded with peptide and ready for export to the cell surface for presentation to T cells in conjunction with appropriate costimulatory molecules.
In this study, we demonstrate that induction of MHC class II by IFN-γ in AM from young animals is reduced when compared to those from adult animals. This is not due to defective IFN-γ responses, as shown by the dose-dependent induction of IFN-γ responsive genes IRF-1, IRF-2 and IP-10. By investigating expression of genes that are critical in the MHC class II pathway, we have shown that AM from young animals have reduced ability to express CIITA in response to IFN-γ, suggesting that transcription of CIITA may be inhibited in AM from young animals. Importantly, 7 days in vitro pre-culture resulted in equivalent up-regulation of CIITA and MHC class II (mRNA and protein) by neonatal AM when compared to adults, suggesting suppression by microenvironmental factors. Factors that may play a role in the suppression of MHC class II expression in neonates are also discussed.
2 Results
2.1 Induction of MHC class II by IFN-γ is reduced in AM from young animals
AM from newborn and adult animals obtained by bronchoalveolar lavage (BAL) were cultured in the presence of varying doses of IFN-γ for 24 h, and the cells were then fixed in ethanol and immunostained with mAb specific for rat MHC class II (OX6).
As shown in Fig. 1, AM from newborn animals showed a reduced ability to up-regulate MHC class II expression in response to IFN-γ when compared to those derived from adult animals. Determination of cells staining positively for OX6 mAb showed significantly greater numbers of adult AM up-regulated MHC class II surface expression at all concentrations of IFN-γ stimulation (p<0.0001 at 10, 100 and 1,000 U/ml; n=4) compared to AM from newborn animals. Note that whereas AM from adult animals significantly up-regulated their MHC class II expression in response to 10 U/ml IFN-γ, newborn AM required 1,000 U/ml IFN-γ for significant expression of MHC class II as detected by immunostaining.
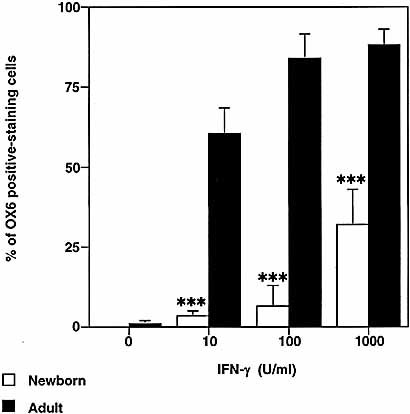
Induction of MHC class II by IFN-γ is significantly reduced in AM from newborn when compared to adults. AM from newborn (1-day-old) and adult (9–11-week-old) animals were cultured for 24 h in the presence of different doses of IFN-γ (0–1,000 U/ml). AM were then fixed and stained with OX6 mAb for the detection of MHC class II expression. AM that were positively stained for OX6 mAb were scored under a light microscope. Results are expressed as mean of percentages of OX6-positive staining AM ± SD (n=4). Significant differences to adult AM at each concentration are indicated as *** p<0.0001
To further elucidate the regulation of MHC class II expression in AM, reverse transcription (RT)-PCR was used to detect the expression of MHC class II (RT1.B) mRNA. As shown in Fig. 2, the pattern of mRNA expression was found to reflect closely that of surface expression as shown in Fig. 1. Thus, significant up-regulation of RT1.B mRNA expression was observed after exposure to 10 U/ml IFN-γ in the adults, whereas AM from newborn animals were unresponsive to IFN-γ stimulation until relatively high concentrations (1,000 U/ml) were used. Similarly, AM from 7-day-old animals were also unresponsive to IFN-γ, with significant RT1.B expression only occurring after exposure to 1,000 U/ml IFN-γ.
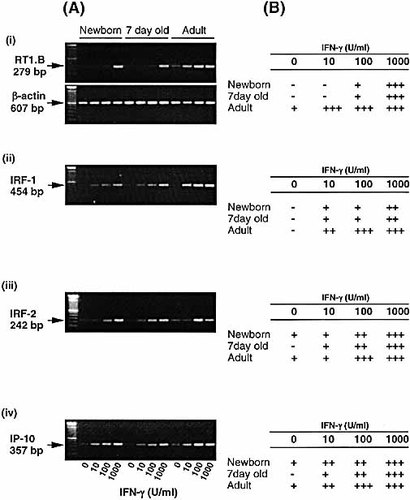
AM from newborn and young animals showed reduced RT1.B (MHC class II) mRNA induction but not expression of IFN-γ-responsive genes in response to IFN-γ. AM from newborn (1-day-old), 7-day-old and adult (9–11-week-old) animals were cultured in the presence of IFN-γ (0–1,000 U/ml) for 24 h. RNA was then extracted from the AM and reverse-transcribed. (A) PCR determination of (i) RT1.B, (ii) IRF-1, (iii) IRF-2 and (iv) IP-10 mRNA induction is shown in the photographs of ethidium bromide-stained gels. (B) Levels of mRNA were semiquantitated by expressing mRNA levels as a ratio to their respective β-actin and presented as negative and positive scores: –, <0.1; +, 0.1–0.5; ++, 0.5–0.75; and +++, >0.75. Representative data from a series of four separate experiments are shown.
2.2 Responses of other IFN-γ-sensitive genes are not defective in AM from newborn animals
The expression of two IFN-γ-regulated transcription factors (IRF-1, IRF-2) and an IFN-γ responsive protein (IP-10) in animals of different ages was examined in response to various doses of IFN-γ.
As shown in Fig. 2A(ii), AM from newborn, 7-day-old and adult animals all up-regulated the expression of IRF-1 in response to IFN-γ in a dose-dependent manner. However, as shown in Fig. 2B(ii), when the expression of IRF-1 was normalized as a ratio to its corresponding β-actin and presented as a scoring system as described, IRF-1 induction from adult animals appeared more sensitive to IFN-γ stimulation, up-regulating to a greater extent when compared to young animals. In comparison, IRF-2 expression by AM from the three different age groups all up-regulated in a dose-dependent manner and to a similar extent [Fig. 2(iii)]. The responses of AM to IFN-γ were further investigated with IP-10, its induction being exquisitely sensitive to IFN-γ 20. As shown in Fig. 2 B (iv), IFN-γ induced the expression of IP-10 mRNA in a dose-dependent manner with no significant differences between AM from animals of different age groups.
2.3 Diminished expression of CIITA in newborn AM compared to adults in response to IFN-γ
Since immunohistochemistry as well as PCR showed that AM from neonatal animals are inefficient in expressing MHC class II [Fig. 1, 2(i)] despite normal (adult-equivalent) responses to IFN-γ [Fig. 2(ii–iv)], the MHC class II pathway was investigated in detail to identify possible points of dysregulation in the AM from young animals. The importance of CIITA in regulating the transcription of MHC class II-associated molecules has been previously shown 8. Thus, the expression of CIITA in AM from animals of different age groups was investigated. As shown in Fig. 3, CIITA mRNA displayed similar patterns of induction by IFN-γ as RT1.B [Fig. 2(i)]. Thus, expression of CIITA by newborn and 7-day-old animals was absent or significantly lower than that of the adults when exposed to relatively low concentrations of IFN-γ (10–100 U/ml). Significant detectable expression of CIITA by AM from young animals required up to 1,000 U/ml IFN-γ stimulation. On the other hand, expression of CIITA by AM from adult animals was apparent after exposure to 10 U/ml IFN-γ, and was further enhanced with increasing concentrations of IFN-γ.
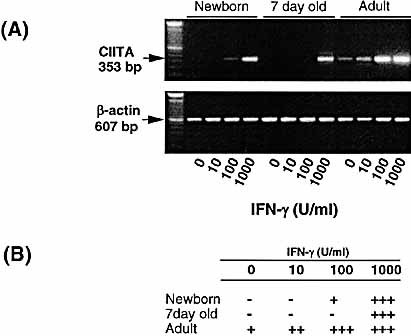
AM from newborn and young animals show reduced CIITA mRNA induction in response to IFN-γ. AM from newborn, 7-day-old and adult animals were cultured in the presence of IFN-γ (0–1,000 U/ml) for 24 h. RNA was then extracted from the AM and reverse transcribed. (A) PCR determination of CIITA mRNA induction is shown in the photograph of ethidium bromide-stained gel. (B) Levels of mRNA were semiquantitated and expressed as in Fig. 2. Representative data from a series of four separate experiments are shown.
2.4 Induction of Ii by IFN-γ is significantly reduced in AM from newborn and young animals when compared to adults
Since CIITA is a transcription factor that not only controls MHC class II expression but also the expression of Ii 10, 11, experiments were performed to determine whether expression of Ii is "defective" in the AM of young animals. In an identical experiment to that for the investigation of MHC class II expression (Fig. 1), AM from newborn and adult animals were investigated for their Ii expression in response to IFN-γ by immunohistochemistry using rat Ii-specific mAb, RG11. As illustrated in Fig. 4, AM from newborn animals showed diminished ability to express Ii, with little expression even at 1,000 U/ml IFN-γ stimulation. In contrast, increased expression of Ii in adult AM was induced with 10 U/ml IFN-γ, which continued to respond in a dose-responsive manner. Thus, whereas adult AM up-regulated their expression for Ii following IFN-γ stimulation, significantly fewer AM from newborn animals were RG11 positive at the corresponding doses of IFN-γ (p<0.05 at 10 U/ml, p<0.005 at 100 U/ml and at 1,000 U/ml).
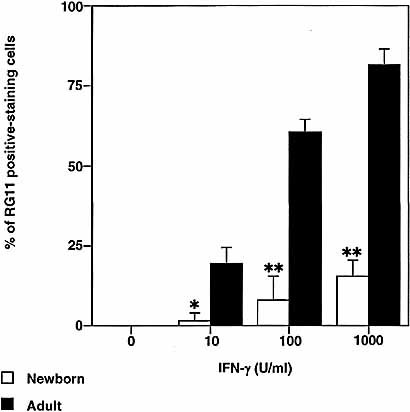
Induction of Ii by IFN-γ is significantly reduced in AM from newborn animals when compared to adults. AM from newborn and adult animals were cultured in the presence of IFN-γ (0–1,000 U/ml) for 24 h. AM were then fixed and stained with RG11 mAb for the detection of Ii expression. AM that were positively stained for RG11 mAb were scored under a light microscope. Results are expressed as mean of percentages of RG11-positive staining AM ± SD (n=4). Significant differences to adult AM at each concentration are indicated as * p<0.05, ** p<0.005.
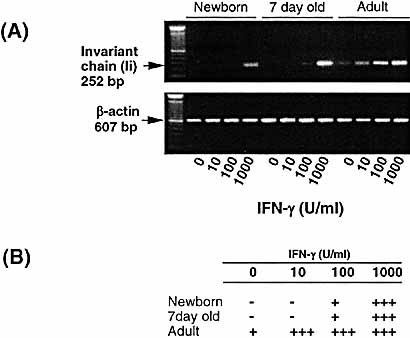
Expression of Ii by AM from young animals are inefficient when compared to the AM from adult animals. AM from newborn, 7-day-old and adult animals were cultured in the presence of IFN-γ (0–1,000 U/ml) for 24 h. RNA was then extracted from the AM and reverse transcribed. (A) PCR determination of Ii mRNA induction is shown in the photograph of ethidium bromide-stained gel. (B) Levels of mRNA were semiquantitated and expressed as in Fig. 2. Representative data from a series of four separate experiments are shown.
When the induction of Ii mRNA expression was investigated using RT-PCR, the pattern of expression reflected that seen by immunohistochemistry and was similar in pattern to RT1.B and CIITA mRNA expression. Thus, AM from newborn and 7-day-old animals showed significantly reduced ability to express Ii mRNA in response to lower doses of IFN-γ (10–100 U/ml), and 1,000 U/ml was required before adult-equivalent levels of Ii mRNA expression were observed (Fig. 5). In contrast, adult AM displayed some constitutive expression of Ii mRNA, which was further up-regulated in a dose-dependent manner in response to IFN-γ. Significantly, despite comparable to adult levels of mRNA expression in AM from newborn animals when stimulated with 1,000 U/ml of IFN-γ (Fig. 5), the level of Ii expression as detected by RG11 mAb (Fig. 4) remained significantly lower than adults with similar stimulation.
Another important component of the class II antigen-presentation pathway that has been reported to be regulated by CIITA is HLA-DM (RT1.DM in the rat) 11. When the expression of RT1.DM was investigated, no apparent defect in expression of both the alpha and the beta chain was found when AM from young animals were compared to AM from older animals as shown in Fig. 6(i) and 6(ii), respectively. Importantly, significant constitutive expression of both chains of RT1.DM was observed in AM from animals of all ages. While the alpha chain (RT1.DMa) appeared to be induced only by high doses of (1,000 U/ml) IFN-γ [Fig. 6(i)], the beta chain [Fig. 6(ii)] did not appear to respond to IFN-γ significantly.
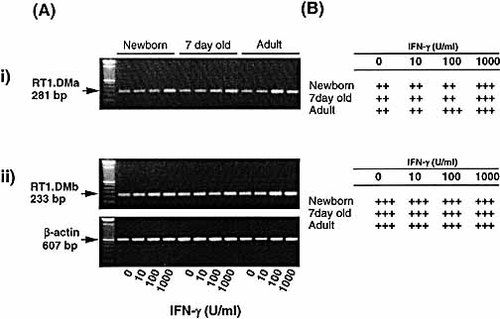
Constitutive expression of RT1.DM by AM from animals of all ages. AM from newborn, 7-day-old and adult animals were cultured in the presence of IFN-γ (0–1,000 U/ml) for 24 h. RNA was then extracted from the AM and reverse transcribed. (A) PCR determination of RT1.DM alpha chain (i) and beta chain (ii) mRNA induction is shown by the photographs of ethidium bromide-stained gel. (B) Levels of mRNA were semiquantitated and expressed as in Fig. 2. Representative data from a series of four separate experiments are shown.
2.5 Prolonged in vitro culture of neonatal AM results in up-regulation of their IFN-γ responsiveness to adult levels
It is apparent from the data described above that AM from young animals are able to express MHC class II in response to IFN-γ stimulation. However, it is also clear that AM from young animals are much less responsive to this IFN-γ stimulus. Several previous reports have indicated that MHC class II expression is subject to regulation by microenvironmental factors 2, 21, 22. The possibility that such factors may play a role in the reduced ability of neonatal macrophages to express MHC class II was accordingly investigated. Thus, AM from 1-day-old animals were cultured in vitro for 7 days prior to exposure to increasing doses of IFN-γ. As shown in Fig. 7 A, this prolonged culture resulted in strong up-regulation of IRF-1, RT1.B, CIITA and Ii mRNA expression. When the levels of these genes were normalized against the expression of their corresponding house-keeping gene, β-actin, AM from newborn animals that had been pre-cultured in vitro for 7 days showed similar levels of induction to those from adult AM, which were both clearly different to those from AM that were freshly isolated from newborn animals (Fig. 7 B).
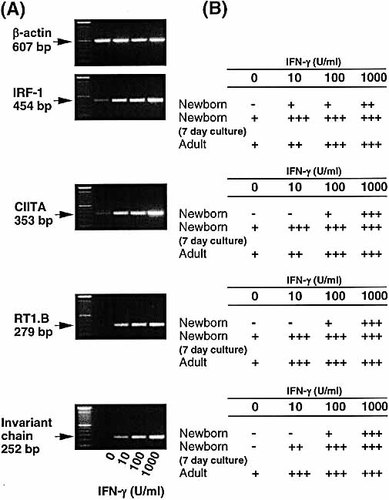
In vitro culture of newborn AM enhances their ability to up-regulate MHC class II and associated genes in response to IFN-γ stimulation. AM from newborn animals were pre-cultured for 7 days prior to stimulation with different concentrations of IFN-γ (0–1,000 U/ml) for 24 h. (A) RNA was then extracted and reverse-transcribed. PCR determination of IRF-1, CIITA, RT1.B and Ii expression is shown by the photographs of ethidium bromide-stained gel. (B) Levels of mRNA from 7-day-old newborn AM culture were compared to freshly isolated AM from newborn and freshly isolated AM from adult animals, and results are shown as in Fig. 2. Representative data from a series of four separate experiments are shown.
3 Discussion
Deficiencies in MHC class II expression by neonatal peritoneal macrophages has been reported by several groups 1, 2 but this has not been investigated in macrophages from the lung nor has there been any attempt to examine this phenomenon at the molecular level. Data reported here show that induction of MHC class II (RT1.B) expression by neonatal AM is almost non-existent when compared to AM from adult animals in response to 24-h IFN-γ exposure. We have also examined the induction of MHC class II after 48 and 72 h and have shown that there was a minor improvement in the responses of AM from newborn and young animals to IFN-γ (data not shown); however, expression levels in AM from the young animals remained well below those of adults. This may reflect the fact that the AM were "in vitro" for this extra 24–48 hours, i.e. see 7-day pre-culture experiment. To investigate the mechanisms involved in the reduced ability of newborn animals to express MHC class II in response to IFN-γ, the 24-h time point was chosen because this represents a time when the difference (in the induction of MHC class II by IFN-γ) between adult and young animals was maximal and is closer to in situ responses.
The induction of MHC class II mRNA by neonatal AM was shown to be refractory to IFN-γ as determined by RT-PCR, suggesting that the "functional immaturity" occurs at the transcriptional level. Since the major regulatory molecules involved in IFN-γ stimulation of MHC class II expression are now known, the various components of this pathway, as well as a range of other IFN-γ-sensitive genes, were investigated in an attempt to identify anomalies that may be responsible for the "functional immaturity". Due to limited rat-specific reagents to identify many of the components of the MHC class II pathway, we have used RT-PCR to study specific mRNA expression under steady-state conditions and in response to IFN-γ stimulation. However, where possible (such as experiments involving MHC class II and Ii), mAb were used to identify protein expression of these factors.
An important initial question was whether neonatal AM express functional IFN-γ receptors. As these have not been cloned from the rat, this was addressed by investigating the expression of three separate genes that are induced by IFN-γ. We reasoned that by showing positive induction of these genes, we could assume a functional IFN-γ receptor. AM from animals of all ages were found to respond to IFN-γ by up-regulating the expression of IRF-1 and IRF-2 in a dose-dependent manner. Interestingly, while acknowledging the limitations in RT-PCR semiquantitation, adult AM appear to express higher levels of IRF-1 in response to IFN-γ. Since IRF-1 is an integral factor in activating the CIITA promoter 7, the possibility exists that higher levels of IRF-1 may play a significant role in the initiation of CIITA transcription. Indeed, the role of IRF-1 in activating promoters of CIITA has been shown 23. It is plausible that specific intracellular events (including MHC class II expression) post-IFN-γ receptor engagement and signaling may be different in adults compared to young animals. Thus, additional studies such as more precise quantitation of IRF-1 are required to draw further conclusions regarding the involvement of the IFN-γ pathway in the differential response of AM from different age groups. Notwithstanding, the failure of neonatal AM to up-regulate MHC class II expression in response to IFN-γ does not appear to be a result of defective IFN-γ receptor expression or signaling since AM from both newborn and 7-day-old animals were capable of responding to IFN-γ effectively as shown by their significant and dose-dependent increase in IRF-1 and IRF-2 expression in response to IFN-γ stimulation. In addition, dose-dependent increases in other IFN-γ responsive genes such as IP-10, cathepsin S (data not shown) and B7.1 (data not shown) strengthened the conjecture of normal interferon responsiveness in newborn AM. Importantly, there appeared to be no difference in the level of expression of these factors (IRF-2, IP-10, cathepsin S and B7.1; data not shown) amongst AM from animals of different age groups. Thus, events responsible for the failure of neonatal AM to up-regulate MHC class II in response to relatively low (10–100 U/ml) levels of IFN-γ are more likely to be downstream from IFN-γ receptor engagement and signaling in the MHC class II pathway.
The expression of mRNA for MHC class II (RT1.B, Fig. 2) was found to correspond closely to that of protein expression as detected by immunohistochemistry (Fig. 1). Significantly, this pattern of mRNA expression was almost identical for both CIITA and Ii. Since CIITA regulates the expression of both MHC class II and Ii, it appears that inefficient responses of AM from young animals may occur as early on the MHC class II processing pathway as CIITA transcription. However, another factor that has been reported to be controlled by CIITA, namely HLA-DM, did not seem to be as tightly regulated by CIITA in the model used here. In this context, significant constitutive expression of both alpha and beta-chains of HLA-DM was observed in the present study, and their expression was also relatively refractory to IFN-γ (Fig. 6), suggesting that expression of HLA-DM, at least in the rat, may not be strictly controlled by CIITA. Of particular interest are the recent papers showing that deficiency in MHC class II and CIITA expression in embryonic trophoblast cell lines are controlled by epigenetic mechanisms via the methylation of the IFN-γ inducible promoter of CIITA 24, 25. This is a mechanism that may be functioning in neonatal AM as indicated by their inability to express CIITA despite apparent normal IFN-γ pathways. This will be an area of further research in our laboratory.
The presence of microenvironmental factors that alter during the course of maturation has been reported to modulate MHC class II responses of macrophages in neonates 21, 22. In addition, a wide range of mediator molecules 26–28 are produced locally at high levels during the rapid growth and remodelling phase of the lungs in the newborn period, but decrease with maturation of the animals. To determine if the presence of "age-specific" microenvironmental factors may affect MHC class II expression, AM were cultured in vitro for a period of 7 days prior to IFN-γ exposure. The rationale here was to isolate AM from any influence from factors present in the local milieu. Indeed, by removing neonatal (1-day-old) AM from their microenvironment, the sensitivity of these cells to IFN-γ was shown to increase to that of adult levels. Hence, MHC class II, Ii and CIITA mRNA expression were readily inducible with as little as 10 U/ml IFN-γ. This did not appear explicable simply on the basis of time-dependent maturation since AM freshly prepared from 7-day-old animals were shown to be relatively refractory to IFN-γ in terms of their MHC class II induction. Thus, this suggests that factors in the neonatal lung microenvironment may suppress the ability of macrophages to up-regulate MHC class II expression in response to IFN-γ stimulation.
To investigate the microenvironmental factors that may cause the inhibition of MHC class II expression in neonatal AM further, the effects of IFN-α and IFN-β were examined (data not shown). Specifically, these two type I interferons have been reported to contribute to the diminished IFN-γ responsiveness of MHC class II expression in neonatal macrophages 2, 3. However, our findings suggest that type I interferons may not be the critical factors involved in this system, as IFN-α and IFN-β effectively down-regulated surface expression of MHC class II, but did not diminish expression of mRNA specific for MHC class II (RT1.B; data not shown), suggesting that the antagonistic effects of α and β interferons occur at the post-transcriptional level. Attempts to block potential endogenous IFN-α or IFN-β in neonatal AM using a commercial rat interferon polyclonal antibody were unsuccessful due to a lack in specificity of the antibodies towards IFN-α and IFN-β. Notwithstanding, since the data presented here with neonatal AM suggests that the inhibition of MHC class II expression occurs early in the MHC class II processing pathway at CIITA and thus the transcription of MHC class II mRNA, the inhibition of MHC class II expression by endogenous IFN-α or IFN-β as proposed by Inaba et al. 2 seems unlikely as these were unable to block MHC class II at the transcriptional level. Additionally, CIITA is known to control not only the expression of MHC class II but also MHC class I expression 29. Thus, the previously reported ability of IFN-β to block MHC class II but not MHC class I expression 30 suggests that if the defect in newborn AM responsible for MHC class II dysregulation occurs at the level of CIITA expression, it is unlikely that type I IFN plays a significant role in the process.
In conclusion, results presented here have shown that microenvironmental factors are involved in suppressing the ability of newborn AM to express MHC class II in response to IFN-γ, and that these act at the level of CIITA transcription. However, further investigations are re-quired to identify these endogenous factors. A possible explanation for this diminished capacity in MHC class II expression in response to an inflammatory cytokine may be the requirement for an intrinsic mechanism to down-regulate excessive activation of adaptive immune responses during the fragile period of infant life when the lungs are still undergoing rapid remodelling. Host defense during this period in early life may instead rely on passive transfer of maternal antibodies (especially via the milk 31, 32), and also on enhanced expression of certain innate immune functions 33. Indeed, studies have shown that the recruitment and persistence of airway inflammatory cells and the formation of airway wall fibrosis during respiratory diseases in early life leads to long-term airway dysfunction 34. Thus, by keeping the immune-activation potential of MHC class II antigen presentation to a minimum, the relatively immunosuppressed environment of the newborn lungs may be advantageous to proper development.
4 Materials and methods
4.1 Animals
PVG rats were used throughout all experiments and were specific pathogen-free derived and barrier maintained. Animals were housed on low-dust bedding to minimize background airway inflammation, and were serologically free of Sendai infection and other known pathogens. Animals were fed ad libitum on autoclaved rat chow. All animal experimentation was conducted with prior approval of the TVW Telethon Institute for Child Health Animal Ethics Committee, which operates under guidelines set down by the Australian National Health and Medical Research Council.
4.2 Bronchoalveolar lavage
Rat AM were lavaged from animals within 24 h of birth (newborn, 1-day-old), and from 7-day-old, 21-day-old (weanling), and adult (9–11-week-old) animals. Rats were anesthetized with ether before administration of a lethal dose (0.5 ml/ 100 g body weight) of Lethobarb (pentobarbitone sodium; Virbac Pty, NSW, Australia), delivered intraperitoneally via a 21-gauge needle. Tracheas were exposed and catheterized. For adult animals, lungs were lavaged with 10 ml ice-cold phosphate buffered saline pH 7.4 (PBS), which was instilled gently into the lungs and withdrawn immediately. This procedure was repeated five to six times to obtain a total volume of 50 ml of lavage fluid. In newborn animals, 200 μl PBS was instilled in the lungs in a similar fashion. In this case, extreme care was taken to avoid damage to the lungs due to pressure generated by instillation of the lavage fluid. A total of 1–2 ml of lavage fluid was collected from each newborn animal. Lavage fluid from several animals was pooled and centrifuged at 4°C for 7 min at 600×g. Cells were resuspended in macrophage-serum free medium (MSFM; Gibco-BRL, Life Technologies, NY) containing antibiotic and antimycotic (100 μg/ml penicillin, 100 μg/ml streptomycin and 250 ng/ml amphotericin B; Sigma-Aldrich Pty, NSW, Australia).
4.3 Immunohistochemistry
BAL cells were cultured in tissue-culture grade chamber slides (Becton Dickinson, NJ). Immunostaining was performed with primary antibodies (OX6, IgG1, anti-rat MHC class II molecule 35, and RG11, IgG2a, anti-rat Ii 36) for 1 h at room temperature. Slides were then washed three times (10 min each) with PBS, and incubated with biotinylated sheep anti-mouse Ig (Amersham Australia) containing 10% (v/v) normal rat serum (NRS) and streptavidin-conjugated horseradish peroxidase (Amersham Australia). The reaction was visualized with 3,3prime;-diaminobenzidene and 0.015% (v/v) hydrogen peroxide in PBS. Slides were counterstained with hematoxylin, dehydrated, mounted and analyzed. A minimum of 500 cells were counted in each experiment.
4.4 Analysis of gene expression by RT-PCR
AM, suspended in MSFM with antibiotic and antimycotic were cultured in a 96-well plate (Falcon 3072, Becton Dickinson Labware, NJ). For experiments investigating the expression of MHC class II-associated genes, AM were exposed in vitro, for 24 h to recombinant rat IFN-γ (Biosource, Belgium) or in combination with IFN-α or IFN-β (Serotec, GB) or GM-CSF (Biosource, Belgium). Total RNA was prepared by lysis in RNAzol B (BIOTECX laboratories, TX) according to the manufacturer's instructions. The recovered RNA was resuspended in 10 μl of sterile water, followed by cDNA synthesis with the total recovered RNA primed with 250 ng oligo(dT) (Biotech International, Australia). Reverse transcription was performed with the total oligo(dT) primed RNA in a final volume of 50 μl containing 7.5 mM MgCl2, 0.4 mM each dNTP, 10 U RNase inhibitor (all from Biotech International, Australia) and 5 U reverse transcriptase (Promega, WI) with reaction buffer consisting of 50 mM Tris-HCl, pH 8.3 (42°C), 50 mM KCl, 10 mM MgCl2, 10 mM DTT and 0.5 mM spermidine. This mixture was incubated at 42°C for 60 min. The cDNA mixture was then inactivated at 95°C for 10 min in a heating block prior to storage at 4°C.
PCR reactions were performed with 1 μl cDNA in a total volume of 12.5 μl containing 1.5 mM MgCl2; 0.2 mM each dNTP; 2.5 pmol each of 3prime; and 5′ primers; sterile H2O; 0.25 U platinum Taq DNA polymerase (Gibco, Life Technologies) in storage buffer consisting of 20 mM Tris-HCl pH 8.0, 40 mM NaCl, 2 mM Na3PO4, 0.1 mM EDTA, 1 mM DTT, stabilizers, 50% (v/v) glycerol and reaction buffer consisting of 200 mM Tris-HCl pH 8.4, 500 mM KCl. The mixture was capped with 50 μl of DNA grade mineral oil (Sigma-Aldrich, NSW, Australia). PCR was performed for 22–38 cycles depending on primers in a solid block thermal cycler (Perkin Elmer DNA Thermal Cycler 480). The number of PCR cycles used was first determined by amplifying cDNA through 15–40 cycles to obtain a standard curve. The cycle number chosen satisfies easy visualization and amplification along the linear part of the standard curve. The sequences of PCR primers pairs used are listed in Table 1.
|
|
Primer sequence (5′-3prime;) |
|
|
|---|---|---|---|
|
Target mRNA |
Sense |
Antisense |
Product size (bp) |
|
IRF-1 |
ACA CCT TAT CTG ACG GAC TG |
GTG AAG ACA CGC TGT ATG C |
454 |
|
IRF-2 |
TGA GTA TGC GGT CCT GAC |
CAC TGT CGG TAG TTT CGC |
242 |
|
IP-10 |
AAC TGT CCC TGT TTC TCC TG |
GGA AGG TGG TGG TAA GTT TG |
357 |
|
RT1.B |
CAG TCA CAG AAG GCG TTT ATG |
GAT CGC AGG CCT TGA ATG ATG |
279 |
|
CIITA |
CAG ACC CAG AGG CTG AGA AAC |
GTA CAAA GCT CAG CCT TAG GAG |
353 |
|
Ii |
TGA AGA ATG TTA CCA AGT ATG G |
TGG TCA ATA CTT TAG GTG GAG |
252 |
|
RT1.DMa |
AAC ATA GGG CTC TCC GAG |
ATG AAA CAG ACC AGC GTG |
281 |
|
RT1.DMb |
GTC CAA GTA GCC CAA ACC |
ACC ACG CAG GTG TAG ATG |
233 |
PCR products were electrophoresed on an ethidium bromide stained 1.5% (w/v) agarose gel (Progen Industries, Queensland, Australia). Gel photographs were scanned with a UMAX Vista S-6 scanner using PhotoShop software (Adobe System) on a Macintosh computer followed by densitometry using Scan Analysis software (Biosoft, Ferguson, MO). The data obtained was expressed as a ratio relative to the density of the β-actin band for each sample and presented as "-" or "+" according to the scale described with each figure.
4.5 Statistical analysis
Statistical analyses were performed using standard InStat software package (GraphPad Software).
Acknowledgements
This work was supported by grants from the SIDS foundation of Western Australia and Glaxo Wellcome Pty Ltd. P.G.H. is supported by the National Health and Medical Research Council of Australia.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




