Somatic diversity of the immunoglobulin repertoire is controlled in an isotype-specific manner
Abstract
We have studied two aspects of the IgE immune response. First, we have compared the kinetics of the IgE response to the T cell-dependent antigen ph-Ox coupled to ovalbumin with that of the IgG1 response and we have assessed the quality of the IgE response. Second, we have studied the generation of somatic diversity, understood as the combined effect of somatic mutation and the selection of D(iversity) and J(oining) elements, in germinal center B cells at the molecular level, using the germ-line sequence of the prototype anti-ph-Ox heavy chain variable element VHOx1 as reference. We evaluated sequences derived from μ-, γ1- and ϵ-variable elements and showed that somatic diversification was different for all isotypes studied. We further compared the IgE responses of wild-type mice with those of mice expressing a truncated cytoplasmic IgE tail (IgEKVKΔtail). IgEKVKΔtail mice showed a more diverse sequence pattern. We corroborated previous results suggesting that short CDR3 regions are indicative for high-affinity antibodies by measuring relative affinities of phage-expressed Fab fragments with prototype long and short CDR3 regions. Therefore, the composition of the antigen-receptor is responsible for the selection process and the expansion of antigen-specific cells, leading to an isotype-specific antibody repertoire.
Abbreviations:
-
- AFC:
-
Antibody-forming cell
-
- ph-Ox:
-
4-Eth-oxymethylene-2-phenyl-2-oxazolin-5-one
-
- IgEKVKΔtail:
-
Truncated cytoplasmic IgE tail
-
- WT:
-
Wild type
1 Introduction
The humoral immune response plays a critical role in the containment and elimination of many pathogenic organisms. It does so by first generating a broadly reactive, not very specific, but often effective, IgM-dominated primary response 1. Later, the response becomes more focused, reflected in a switch 2, 3 from the IgM-based to an IgG-, IgA- or IgE-mediated response and an increased affinity of the antibodies. Class switch and affinity maturation are exquisitely T cell dependent, and take place in a specialized microenvironment, the germinal center 4–7. During these processes, two other important events take place: the generation of short- and long-lived antibody-forming cells (AFC, also known as plasma cells) 8, ensuring an immediate and a longer-lasting specific antibody level in the serum, and the generation of immunological memory 9, which allows a rapid, high-affinity response to a secondary encounter with the pathogen. In principle, immune memory has a T and a B cell component. B cell memory is thought to rely on the selection and persistence of a B cell subset that has acquired affinity-enhancing somatic mutations. Previously, we showed for murine IgE 10 that the generation of a class-switched, affinity-maturated repertoire is dependent on the expression of the membrane-bound isotype during the immune response. Similar results were reported for murine IgG1 11 and later for human IgG2a 12. However, the nature and effects of the signals generated by the antigen receptor are mostly unknown. We 10 and others 11 showed that the 28-amino acid-long cytoplasmic tail of murine IgE and IgG1, respectively, strongly influenced both the number of cells that were recruited in the IgE- or IgG1-AFC pool and the quality of the response.
We have now studied the influence of the cytoplasmic domain of Ig receptors on the somatic diversity of the antibody repertoire. The immune response was monitored over a 49-day period after immunization with the T cell-dependent hapten 4-ethoxymethylene-2-phenyl-2-oxazolin-5-one (ph-Ox) with particular attention for the quality and quantity of serum antibodies, and the generation of diversity at the molecular level in germinal center B cells. We studied these aspects of the immune response also in mutant mice with a truncation of the cytoplasmic tail of IgE (IgEKVKΔtail), rendering this tail similar to that of IgM and IgD, and compared them to wild-type (WT) IgE and IgG1 and IgM responses.
2 Results
2.1 IgE immune response in answer to immunization with ph-Ox
We first analyzed the IgG1- and IgE-mediated immune response in WT BALB/c mice. Mice were immunized with the T cell-dependent hapten ph-Ox coupled to ovalbumin 13 over a 49-day period. The immunization protocol is schematically depicted in Fig. 1. IgG1 antibodies were readily found a few days after immunization (Fig. 2a), rose further after a first booster immunization and remained on a high level. Strong support exists in the literature that during this phase-affinity maturation takes place 4–7, 14 and long-lived plasma cells are generated 8. Specific IgE antibodies were only detectable after the first booster immunization, peaked at day 21, and then declined rapidly, until the next encounter with antigen (Fig. 2b). These results clearly show that IgE responses are short-lived. This may be partly due to the very short half-life time of IgE 15, 16 but it also is reflective of a poor recruitment of long-lived plasma cells in the IgE immune response (Fig. 2b, and unpublished observations). The responses therefore reflect the efficiency of the recruitment of short-lived IgE AFC. These AFC have not necessarily undergone extensive somatic mutations 8. IgE responses do show "memory" in the sense of a swift response to secondary and tertiary immunization 8. In mice with a deletion of the 25 C-terminal amino acids of the IgE cytoplasmic tail (IgEKVKΔtail) 10 specific IgG1 titers were similar to those in WT mice, as expected; however, IgE titers were 70% lower than in WT mice after immunization with ph-Ox. Titers only increased slowly, indicative of a poor recruitment of AFC in the immune response.
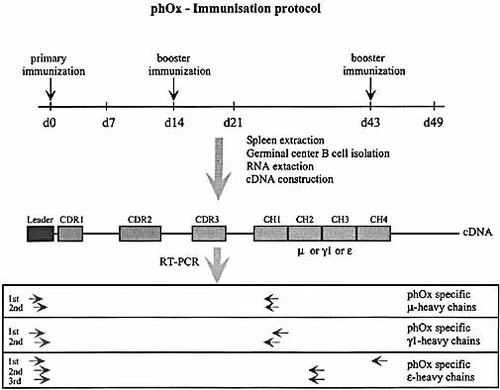
Scheme of the ph-Ox specific amplification of variable heavy chains of different isotypes. Primer positions correspond to the original location. μ- and γ1-heavy chains were amplified by a standard nested PCR protocol. ϵ-heavy chains could only be amplified after a triple nested protocol.
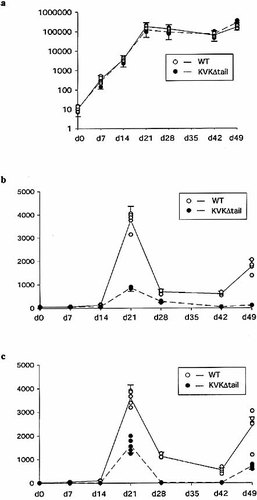
ph-Ox-specific IgG1 and IgE serum levels. Mice (five animals per group) were immunized with ph-Ox11-OVA and received booster immunizations 2 and 6 weeks later. Mice were bled at the indicated time points and analyzed individually. Results are expressed as the serum dilution where half-maximum absorbance was obtained for IgG1 (a), IgE (b) and IgE after depletion of the serum with protein G-Sepharose (c).
2.2 Determination of the relative affinity of ph-Ox-specific IgE antibodies
Next, we assessed the relative affinity of anti-ph-Ox specific IgE antibodies in two ways. Because IgE antibody levels are measured in about a 1,000-fold excess of IgG1 antibodies we first depleted the serum antibodies with protein G-Sepharose for competing excess IgG antibodies (a reversed "classical" affinity measurement) 16. Depletion of the IgG did not markedly increase the titers in WT mice, indicating that IgE antibodies of sufficient affinity that were not influenced by competing IgG1 antibodies, were produced (Fig. 2c). In IgEKVKΔtail mice specific IgE responses were sensitive to IgG depletion by protein G (Fig. 2c). The latter result implies that the affinity of the IgE antibodies was sufficiently low to be influenced by competing IgG1 antibodies. These results were confirmed by a second approach where the relative affinity 17 of anti-ph-Ox-specific IgE and IgG1 antibodies was measured using "low-haptenated" and "high-haptenated" ELISA plates 17. In WT mice we could not detect differential binding to the differently haptenated ELISA plates. In IgEKVKΔtail mice, a twofold difference in titer between the highest-(ph-Ox35-BSA) and the lowest haptenated ELISA-plate (ph-Ox11-BSA) was seen at each time point at which specific IgE was detected (Fig. 3b), but was most impressive at day 21. These results and dilution experiments (data not shown) proved that these results were independent of the titer of IgE antibodies. Using the paired t-test, statistical significance at the level of 0.1>p>0.001 was calculated. The absence of the cytoplasmic tail of IgE causes a low-titered response of a poorer quality. The cytoplasmic tail of Ig receptors could thus influence both the efficiency of the somatic mutation process in germinal centers and the efficiency of the recruitment of (high-affinity) B cell clones into the AFC pool.
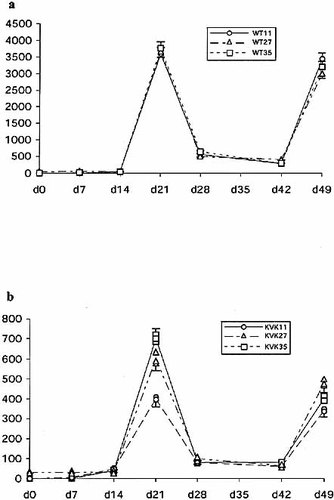
ph-Ox-specific IgE serum levels in dependence of hapten density. WT (a) and IgEKVKΔtail mice (b) were bled at the indicated time points and the sera of each group were pooled. ELISA plates were coated with BSA coupled with 11, 27 or 35 2-phenyloxazol-5-one groups per BSA molecule (ph-Ox11-, ph-Ox27-, ph-Ox35-BSA). Results are expressed as described in Fig. 2.
Therefore, we studied the pattern and extent of somatic diversification in germinal center B cells early and late in the immune response. We compared the occurrence of somatic mutations and the use of DJ elements in the prototype anti-ph-Ox heavy chain variable region, VHOx1 14, 18, in IgM, IgG1 and IgE antibodies, both in WT and IgEKVKΔtail mice. A schematic representation of the experimental protocol is given in Fig. 1. Spleen cells of three BALB/c and three IgEKVKΔtail mice that were immunized at days 0, 14 and 43 13 were isolated on days 7, 14, 21, 43 and 49, and pooled. Germinal center B cells were isolated based on the expression of the surface marker CD45R and the binding of peanut agglutinin (PNA). This procedure excludes plasma cells that do not bind PNA 19. Germinal center B cells were found with an average frequency of 5%. RNA was prepared from 106 cells, followed by cDNA synthesis. For PCR, primers complementary to Cμ, C γ1 or Cϵ constant regions were combined with a specific primer (Fig. 1) for the leader region of VHOx1 4, 20. A proofreading polymerase was used throughout these experiments to minimize experiment-based mutations.
2.3 Evaluation of somatic diversity of IgM antibodies
To get an impression about the pool of somatic variants used, we first sequenced 36 μ-heavy chain clones from day 7 to day 49 of immunization (Fig. 4) and aligned the sequences with the ph-Ox-specific hybridoma H26 20, expressing the VHOx1 germ-line sequence. As expected, VHOx1 was the preferentially amplified VH prototype. However, in 20–40% of the primary sequences of all isotypes a repeated pattern of substitutions was found. These VH regions resembled the MC101 sequence 21, 22 (marked with one asterisk) and most likely represent a VH subset that is closely related to VHOx1. As observed before, the CDR3 region displayed a high degree of diversity, generated by VDJ rearrangement. The length differed from 7 to 17 amino acids. All four J elements were used, with a preference for J3 7 and J4. Interestingly, during the whole period of immunization not a single identical sequence was found twice (Table 1, Fig. 4). Table 1–4 also summarize the frequency and type of mutations. In these tables, the MC101-like sequences are not represented, because the germ-line sequence of this VH member has not been established. The number of mutations per sequence did not vary significantly during the course of immunization and replacement mutations were more frequent than silent mutations. In summary, a highly diverse repertoire of VHOx1-based antibodies accumulated in the IgM pool, with no indication of one preferred type during the course of immunization.
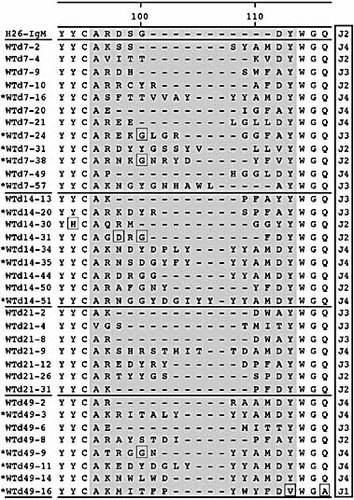
Sequence alignments, showing the CDR3 region of ph-Ox specific WT μ-heavy chains. Sequence files were analyzed using the DNASTAR package. Amino acid positions are according to Kaartinen et al. 18. CDR3 starts with amino acid position 96 and varies in length from 7 to 17 amino acids. The J element used is indicated at the end of the sequence. MC101-like sequences are marked with one asterisk. The full sequence alignments can be downloaded on request.
|
WT (Fig. 4) μ-heavy chains |
μ-VH total no. of sequences |
% CDR3 diversity |
μ-MC101 total no. of sequences |
μ-VHOx1 no. of sequences |
Total mutations/ sequence |
Silent mutations / sequence |
AA exhanges / sequence |
R / S values |
|---|---|---|---|---|---|---|---|---|
|
Day 7 |
12 |
100 |
5 |
7 |
10 |
3.1 |
4.7 |
2.1 |
|
Day 14 |
9 |
100 |
4 |
5 |
9.6 |
3 |
4.8 |
2.2 |
|
Day 21 |
7 |
100 |
0 |
7 |
12.1 |
3.8 |
6.1 |
2.1 |
|
Day 43 |
– |
– |
– |
– |
– |
– |
– |
– |
|
Day 49 |
8 |
100 |
5 |
3 |
13.3 |
6 |
5.3 |
1.2 |
- a) In Tables 1 – 4, the number of mutations and the type of mutations were counted and analyzed with the hybridoma H2620 as reference. The ratio of replacement to silent mutations (R/S values) were calculated only for VHOx1 representatives. MC101-like sequences (sequences marked with one asterisk in Figs. 4 – 7), as well as sequences arising more than once per day (sequences marked with two asterisk in Figs. 4 – 7) were not considered. For calculation of CDR3 diversity VHOx1 and MC101-like sequences were used (AA: amino acid)
|
WT (Fig. 5) γ1-heavy chains |
γ1-VH total no. of sequences |
% CDR3 diversity |
γl-MC101 total no. of sequences |
γ1-VHOx1 no. of sequences |
Total mutations / sequence |
Silent mutations / sequence |
AA exhanges / sequence |
R / S values |
|---|---|---|---|---|---|---|---|---|
|
Day 7 |
13 |
61 |
4 |
9 |
9.1 |
3.2 |
4.8 |
1.8 |
|
Day 14 |
15 |
80 |
6 |
9 |
15.7 |
5.4 |
8.3 |
1.9 |
|
Day 21 |
17 |
52 |
0 |
17 |
4.6 |
1.1 |
3.1 |
3.1 |
|
Day 43 |
10 |
60 |
5 |
5 |
4.2 |
1.2 |
2.8 |
2.5 |
|
Day 49 |
15 |
53 |
1 |
14 |
11.2 |
3.3 |
6.8 |
2.3 |
|
WT (Fig. 6) ϵ-heavy chains |
ϵ-VH total no. of sequences |
% CDR3 diversity |
ϵ-MC101 total no. of sequences |
ϵ-VHOx1 no. of sequences |
Total mutations/ sequence |
Silent mutations / sequence |
AA exhanges / sequence |
R / S values |
|---|---|---|---|---|---|---|---|---|
|
Day 7 |
13 |
23 |
4 |
9 |
16.4 |
4.2 |
9.8 |
2.9 |
|
Day 14 |
10 |
20 |
0 |
10 |
18.3 |
8.1 |
8.1 |
1.2 |
|
Day 21 |
13 |
38 |
0 |
13 |
13.4 |
5.9 |
6.6 |
1.2 |
|
Day 43 |
13 |
38 |
3 |
10 |
16.4 |
4.4 |
9.3 |
2.7 |
|
Day 49 |
11 |
36 |
2 |
9 |
7.3 |
1.8 |
5.3 |
3 |
|
KVK (Fig. 7) ϵ-heavy chains |
ϵ-VH total no. of sequences |
% CDR3 diversity |
ϵ-MC101 total no. of sequences |
ϵ-VHOx1 no. of sequences |
Total mutations / sequence |
Silent mutations / sequence |
AA exhanges / sequence |
R / S values |
|---|---|---|---|---|---|---|---|---|
|
Day 7 |
6 |
83 |
3 |
3 |
43 |
11.3 |
18.6 |
2.8 |
|
Day 14 |
10 |
60 |
0 |
10 |
19.2 |
6.9 |
10 |
1.7 |
|
Day 21 |
6 |
50 |
0 |
6 |
1.8 |
1.1 |
0.6 |
0.5 |
|
Day 43 |
18 |
55 |
6 |
12 |
7.5 |
2.6 |
4.5 |
1.8 |
|
Day 49 |
11 |
91 |
3 |
8 |
9.7 |
3.8 |
4.3 |
1.5 |
2.4 Molecular analysis of the somatic diversity following an IgG1 response
A rather different situation was found in γ1-heavy chains. We analyzed 81 γ1-specific heavy chain sequences from day 7 up to day 49 of immunization (Fig. 5). In the primary and late (day 43) response, MC101-like sequences as well as the prototype VHOx1 sequence were observed. The CDR1 region at position 31–35 showed a higher rate of amino acid exchanges than in μ-heavy chains, particularly late in the immune response (e.g. Ser-31, Tyr-32 and Gly-33). In the CDR2 region, fidelity for amino acid substitutions is comparable to μ-heavy chains, with a few new amino acid substitutions (e.g. Ser at position 56). Highly different sequence motifs were detected in the CDR3 region. During the primary immunization period (days 7 and 14) the sequence motifs are very similar to those in the μ-heavy chains. The length of the CDR3 region varies from 7 to 15 amino acids; however, the total number of different DJ elements was lower (Table 2, Fig. 5). The length of the CDR3 region decreased during the secondary response. Clones with a short CDR3 most likely represent high-affinity anti-ph-Ox antibodies 21. Nine different sequence motifs, which varied only by single amino acid substitutions, dominated CDR3 at day 21. Still, no sequence was identical over the entire VH region, and an immediate interdependence of the sequenced clones is not visible. Late in the secondary response (day 43) a new pool of anti-ph-Ox heavy chains accumulated. The CDR3 region of these resembled the primary set found at days 7 and 14. Also, the MC101-like sequence is found again (marked with one asterisk). At 7 days after the secondary booster immunization, the immune response is as restricted as at day 21, with a short CDR3 region. However, additional sequence motifs occurred, neither identical nor related. The number of mutations and the ratio between replacement and silent mutations was not significantly different from those found in μ-heavy chain sequences (Table 1). Summarizing, the pool of anti-ph-Ox γ1-heavy chains is more restricted than that of μ-heavy chains, in particular as to the length and composition of CDR3 early in the secondary and tertiary response.
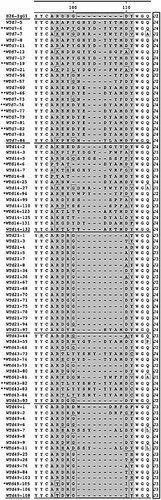
Sequence alignments, showing the CDR3 region of ph-Ox-specific WT γ1-heavy chains. CDR3 starts with amino acid position 96 and varies in length from 7 to 15 amino acids. Nearly 50% of the analyzed sequences use the J4 element. MC101-like sequences are marked with one asterisk. Sequences that were identical at the nucleotide level are marked with two asterisks.
2.5 Evaluation of somatic diversity following an IgE response
We next asked, whether the IgE response follows a maturation pattern similar to the IgG1 response. We analyzed 65 specific ϵ-VH gene segments (Fig. 6). During the whole course of immunization the IgE response was rather restricted. Again, MC101-like sequences were found early in the primary and late secondary response. Late in the primary (day 14), early in the secondary (day 21) and in the tertiary response (day 49) a very restricted repertoire was found, with a clear relationship between the sequences. With "a clear relationship" we mean their similarity, in the sense of for instance CDR3 composition, but without a direct "hereditary" relationship. This may reflect the low titer found in the IgE response, with a low number of IgE-specific germinal centers. The reduction in length of the CDR3 region was less pronounced than for γ1 and a few dominant motifs characterized CDR3. Diversity returned in the sequences obtained from day 43. At day 49 new CDR3 motifs showed up. None of these variants were found in the sequence motifs at earlier time points. The ratio of replacement to silent mutations was not significantly changed (Table 3).
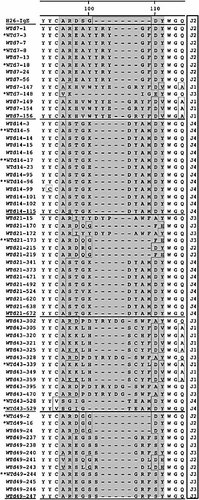
Sequence alignments, showing the CDR3 region of ph-Ox-specific WT ϵ-heavy chains. Comparable to γ1, CDR3 starts with amino acid position 96 and varies in length from 7 to 15 amino acids. In contrast to μ- and γ1-heavy chains, the four J elements are distributed equally. Nine MC101-like sequences were identified (marked with one asterisk). Sequences that were identical at the nucleotide level are marked with two asterisks.
Summarizing, the IgE response is more restricted than the IgG1 response. The length of the CDR3 region is longer than that found in IgG1 antibodies, suggesting a lower affinity. It differs strongly from the sequence motifs found in the CDR3 regions of IgG1. However, there is no doubt that the IgE response matures. A set of antigen receptors with a distinct accumulation of mutations is selected and preferentially expanded.
2.6 Comparison of somatic diversity of IgE antibodies in IgEKVKΔtail mice with those of IgM antibodies in WT mice
We finally asked whether the poor affinity maturation seen in IgEKVKΔtail mice was due to an insufficient somatic mutation process or a poor recruitment of high-affinity B cells. Analysis of IgM-and IgG1-derived VHOx1 sequences in IgEKVKΔtail mice revealed a pattern as found in WT mice (data not shown). Fifty-seven IgE-derived sequences were analyzed (Fig. 7). Again, in the primary and late secondary response MC101-like sequences were found. The primary response was rather different from that in WT mice, particular in the CDR3 region. Only late in the primary response (day 14) did one clone type resemble a dominant clone type seen in WT mice. The secondary response is characterized by a CDR3 pattern that is strongly represented in γ1 antibodies. As for the other isotypes, the response was more diverse at day 43, with longer CDR3 regions. A most significant difference to WT BALB/c mice was detected at day 49. Sequences were more diverse than in WT mice, with long CDR3 regions, resembling the pattern seen in μ-heavy chains. The ratio of replacement to silent mutations was not significantly changed (Table 4). These results indicate that the mechanism of somatic mutation in IgE antibodies is not interfered with in IgEKVKΔtail mice, but that the selection of B cells with high-affinity receptors is impaired.
2.7 Expression of anti-ph-Ox-specific IgE Fab fragments on the surface of phages
Our premise that a short CDR3 region is indicative of a higher affinity anti-ph-Ox antibody is based on the work of Griffiths et al. 21. To support this interpretation, we expressed anti-ph-Ox-specific IgE Fab fragments on the surface of M13 phages 23 and measured their affinity for ph-Ox with surface plasmon resonance analysis. We first cloned two different ph-Ox-specific light chains, using primer combinations as described by Berek et al. 4. Light chain variant I comprised VKOx1 and the JK5 element. The histidine at position 34 identifies this light chain as a low-affinity anti-ph-Ox variant 4. This light chain was cloned into phagemid pComb3 23, resulting in plasmid pEL3. Light chain variant II showed an exchange of histidine to asparagine, resulting in a high-affinity anti-ph-Ox light chain variant. Cloning of this variant into pComb3 resulted in pEL6. Two VH regions were chosen: WT IgE d7–1 and WT IgE d22–170 as representatives of a long and a short CDR3 region, respectively. They were then re-amplified and subcloned in pComb3, generating plasmids pEL15 and pEL16, respectively, in pEL3, generating plasmids pEL8 and pEL12, respectively, and in pEL6, generating plasmids pEL10 and pEL14. The four plasmids that functionally expressed anti-ph-Ox-specific Fab fragments (pEL8 and pEL12 as well as pEL 10 and pEL14) and the four control plasmids (pEL3, pEL6, pEL15 and pEL16) were analyzed for their binding affinity using surface plasmon resonance technology. ph-Ox OVA and OVA as reference were immobilized to an appropriate sensorchip. Fab fragments, expressed on the surface of M13 phage, were directly injected onto the sensorchip. The relative response units, expressed as differences (Fc1–Fc2) of ph-Ox OVA (Fc1) and OVA (Fc2) are proportional to binding affinities and are summarized in Table 5. Injection of pEL3 and pEL6, which express only the (low- and high-affinity) light chain does not show binding to ph-Ox. Similarly, injection of the control plasmids pEL15, which expresses the heavy chain segment WT IgE d7–1, and pEL16 which expresses the heavy chain segment WT IgE d22–170 do not show a measurable response. Injection of pEL8, which expresses both the light chain and the WT IgE d7–1 heavy chain segment representing a long CDR3, shows a marginal response. In contrast, injection of pEL12, which expresses the light chain segment together with the WT IgE d22–170 (pEL12) heavy chain segment representing a short CDR3, results in a remarkable increase in binding affinity. Measurements of pEL10 and pEL14 (heavy chains as described before in combination with a high-affinity light chain variant) showed an additional increase in binding affinities if compared to pEL8 and pEL12. This result fully supports our evaluation of the sequence motifs presented in Fig. 4–7, where we interpret short CDR3 regions as representative of high-affinity and long CDR3 regions as representative of low-affinity antibody variants.
|
|
pEL 3 |
pEL 15 |
pEL 16 |
pEL 8 |
pEL 12 |
pEL 6 |
pEL 10 |
pEL 14 |
|---|---|---|---|---|---|---|---|---|
|
|
Fcl-Fc2 RelResp |
Fc1-Fc2 RelResp |
Fc1-Fc2 RelResp |
Fc1-Fc2 RelResp |
Fc1-Fc2 RelResp |
Fc1-Fc2 RelResp |
Fc1-Fc2 RelResp |
Fc1-Fc2 RelResp |
|
Start of injection |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
End of injection |
38 |
2 |
1 |
23 |
125 |
57 |
103 |
181 |
|
End of elution |
3.9 |
− 2 |
− 4 |
14 |
122.6 |
10 |
80 |
171 |
- a) Two anti-ph-Ox-specific light chains were subcloned into pComb 3 (pEL3 low-affinity variant; pEL6 high-affinity variant) and combined with heavy chain WT IgE d7 – 1 (long CDR3 region; plasmid pEL8 and pEL10), and heavy chain WT IgE d22 – 170 (short CDR3 region; plasmid pEL12 and pEL14). WT IgE d7 – 1 and WT IgE d22 – 170 heavy chains, subcloned in pComb3 without a light chain (pEL15 and pEL16, respectively) serve as controls. The relative response units, expressed as differences (Fc1 – Fc2) of ph-Ox OVA and OVA are proportional to binding affinities. "End of injection" expressed the final onrate value. "End of elution" expresses the final off-rate value.
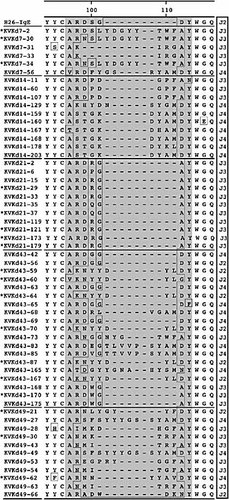
Sequence alignments, showing the CDR3 region of ph-Ox-specific IgEKVKΔtail ϵ-heavy chains. Comparable to WT ϵ-heavy chains, CDR3 starts with amino acid position 96 and varies in length from 7 to 15 amino acids. Like in μ-heavy chains, J3 and J4 elements dominate the immune response. Twelve MC101-like sequences were identified (marked with one asterisk). Sequences that were identical at the nucleotide level are marked with two asterisks.
3 Discussion
The B cell antigen receptor plays a central role in almost all processes along the developmental pathway of B cells, i.e. the generation, maturation, survival and activation of B cells. The cytoplasmic domains of B cell antigen receptors are short and range from only three amino acid residues in the case of mIgM and mIgD (Lys-Val-Lys-COOH in each case) to 28 residues for the other mIg subclasses. B cell antigen receptors exist as a complex between mIg and at least two other transmembrane polypeptides, called Ig-α and Ig-β, which connect the antigen receptor to the signal transduction pathways in the cell. All isotypes of mIg can form a complex with Ig-α and Ig-β 24. It is now understood that the B cell antigen receptor regulates three central features of the immune system function 25. First, it acts as a checkpoint regulator in B cell development, controlling both allelic exclusion and early cellular transitions. Second, as predicted by the clonal selection theory, cross-linking the B cell receptor with antigen can lead to clonal expansion and the production of specific, high-affinity antibodies. The third function of the membrane-bound immunoglobulin is as a receptor for antigen capture and presentation.
Upon activation by antigen, B cells follow one of two differentiation pathways 26: they may differentiate directly into IgM-secreting plasma cells, or they participate in the development of germinal centers. In these specialized structures within lymphoid organs class switch from IgM to other isotypes (IgG, IgA or IgE) takes place together with successive rounds of mutation of the immunoglobulin V region genes. This is followed by expression of the gene products on the cell surface and selection of the cells on the basis of the affinity of the mutated antigen receptors. Selected cells can now expand clonally and form the precursor pool for B memory cells and for short- and long-lived antibody-forming cells.
We have studied the influence of the cytoplasmic domain of Ig receptors on the somatic diversity of the antibody repertoire. The immune response was monitored over a 49-day period after immunization with the T cell-dependent hapten ph-Ox with particular attention for the quality and quantity of serum antibodies, and the generation of diversity at the molecular level in germinal center B cells. We studied these aspects of the immune response also in mutant mice with a truncation of the cytoplasmic tail of IgE (IgEKVKΔtail), rendering this tail similar to that of IgM andIgD, and compared them to WT IgE and IgG1 and IgM responses.
Analysis and comparison of the types of mutations (silent or replacement) and the number of mutations per analyzed sequence in IgM, IgG1 and IgE in WT and IgEKVKΔtail mice showed a mostly comparable mutation type for the different isotypes (Table 1–4), with a slightly higher number of mutations per analyzed sequence for IgE antibodiesfrom WT mice during the whole immunization period. In WT mice sequence motifs found in IgE antibodies differed from those found in IgG1 antibodies. This argues against the notion that consecutive switch recombination via γ1 is a major operational mechanism in the generation of IgE-expressing B cells in the mouse in vivo. This contrasts with certain data obtained after in vitro restimulation of IgG1-expressing B cells with antigen and sufficient Th2 cells, where proof for a consecutive switch was found 27, 28. The only exception is found in the CDR3-derived sequences revealed in the secondary response (day 21) of IgEKVKΔtail mice; they are strongly related to those found in IgG1 antibodies. This could well reflect a rare event of consecutive switching, revealed through the low number of IgE-expressing cells generated the "normal" way, and the low affinity of IgE antibodies in IgEKVKΔtail mice. However, mutations found in CDR1- and CDR2-derived sequences of IgG1 antibodies at day 21 in IgEKVKΔtail mice were not reflected in those of IgE antibodies, suggesting either an abrupt stop inaffinity maturation of IgE antibodies in IgEKVKΔtail mice, or a serendipitous result. Remarkably, the short CDR3 found in IgE antibodies of IgEKVKΔtail mice at day 21 did not correlate with an improved affinity (Fig. 2 and 3, see also below).
Both IgG1 and IgE showed affinity maturation, as judged by a decrease in the diversity of DJ elements used in CDR3 and the length of CDR3. In contrast, IgM in WT mice and IgE in IgEKVKΔtail mice lacked these signs of affinity maturation, which was substantiated by the measurement of the relative affinities of IgE antibodies in WT and IgEKVKΔtail mice. We corroborated the notion that a short CDR3 correlates with a higher affinity 21 in experiments in which we measured relative affinities of phage-expressed anti-ph-Ox Fab fragments with a short or a long CDR3. Surface plasmon resonance analysis showed a tenfold higher response for Fab fragments with a short CDR3 than for those with a long CDR3. Apparently the truncated cytoplasmic tail precluded an efficient selection of high-affinity AFC.
Serum antibodies are produced by short- and long-lived AFC. The number of short-lived AFC for IgE in spleen after a tertiary booster is 500–1,000-fold lower than for IgG1 10. Remarkably, long-lived plasma cells, which are easily found in the bone marrow, are very rare for IgE (unpublished observations). This of course fits well with the observed rapidly waning antigen-specific serum titers of IgE. The decay of IgE antibodies, which have an extremely short half-life, is not compensated by de novo synthesis.
Somatic mutations and diversification caused by DJ recombination as found in germinal center B cells are not always representative of those present in AFC 8, but they are clearly indicative for the effectiveness of the mechanism of diversification itself. IgEKVKΔtail mice show a normal type and frequency of somatic mutation of IgE antibodies, but show a defective recruitment of high-affinity IgE-AFC. A similar phenomenon is seen in IgM antibodies of all mice, leading to the conclusion that the truncated tail of mIgM in WT mice and mIgE in IgEKVKΔtail mice is the cause for the poor selection. These results seem to be in disagreement with recently published results of White and Gray 29 who find evidence for affinity maturation in IgM-derived VHOx1 sequences. White and Gray 29 based their conclusions on expression profiles of mRNA derived from total spleen, bone marrow or blood but did not specify the B cell type that made mRNA. Plasma cells for instance can severely bias expression profiles. Our studies concentrated on VHOx1-specific expression profiles in germinal center B cells. Here we did not find evidence for extensive somatic diversification in IgM-derived sequences, which does not exclude the presence of a few affinity matured IgM-producing plasma cells. The mechanisms leading to selection of high-affinity B cell clones remain unknown. In view of the growing knowledge in the field of the physiology of the B cell receptor, and the low number of IgE-producing cells, we assume that an altered signal transduction in B cells with an Ig receptor with a short cytoplasmic domain (KVK) leads to a decreased survival and, therefore, a poor selection of high-affinity B cell clones.
Our experiments show very clearly that signals generated by the antigen receptor are needed for the selection process and the expansion of antigen-specific cells, and that isotype-specific patterns arise. Therefore, the signals generated by the cytoplasmic tail of IgE are an important target for intervention in allergic patients, in whom the titer and the affinity of the IgE antibodies for the allergen are directly related to disease activity.
4 Materials and methods
4.1 Immunizations
Mice were immunized subcutaneously (s.c.) with 20 μg ph-Ox11-OVA, precipitated in alum with Bordetella pertussis as adjuvant. At days 14 and 43, mice received booster immunizations with 20 μg ph-Ox11-OVA s.c. and 20 μg ph-Ox11-OVA intraperitoneally (i.p.). ph-Ox was conjugated to OVA and BSA according to Mäkelä et al. 13.
4.2 ELISA assays
Serum was taken at different times (days 0, 7, 14, 21, 28, 43 and 49) after immunization. Sera were depleted for IgG as described by Yu et al. 10, 16. For measuring relative affinities of ph-Ox-specific IgE and IgG1, ELISA plates were coated with BSA coupled to varying densities of the hapten ph-Ox (ph-Ox11-, ph-Ox27- or ph-Ox35)-BSA. Specific IgG1 and IgE antibodies to ph-Ox were detected with alkaline phosphatase-conjugated isotype-specific goat anti-mouse antibodies (Southern Biotechnology Associates) or rat anti-mouse IgE monoclonal antibody (EM-95–3). Titers are expressed as the reciprocal serial dilution, at which a half-maximal absorbance value was measured.
Because of the low titer of specific IgE in the serum, sera of three mice per data point were pooled. Measurements of individual mice were made to validate the use of pooled sera. The measurements in individual sera were in full agreement with those obtained in pooled sera.
4.3 Flow cytometry
Mouse spleens were homogenized and erythrocytes were lysed isotonically. Subsequently, the obtained single-cell suspension was prepared for fluorescence activated cell sorting using a FACS-Vantage flow cytometer (Becton Dickinson). Phycoerythrin-labeled antibody to CD45R (clone 6B2), a B cell marker and the lectin PNA (peanut agglutinin) coupled with FITC were used to stain the germinal center B cells 4. Double-positive cells were sorted. For further RNA extraction, 106 double-positive B cells were used.
4.4 RNA extraction
B cells (106) were homogenized with QIAshredder according to manufacturer's instructions. The cell lysate was used for further total RNA extraction using RNeasy (Qiagen). About 20 μ total RNA per 106 B cells was isolated; 3 μg total RNA was used for further cDNA construction.
4.5 Reverse transcription-PCR
cDNA was synthesized using oligo(dT) priming. After cDNA synthesis, antigen specific μ-, γ1- and ϵ-heavy chain variable regions were amplified by nested PCR 30. The primers bind specifically to the leader sequence of the VHOx1 heavy chain variable region and to the constant region (CH1 or CH2 or CH4) of μ-, γ1- and ϵ-heavy chains, respectively. PCR products were subcloned into the pCR-Script cloning vector (Stratagene) and sequenced. Cloning of an anti-ph-Ox-specific light chain was achieved with a semi-nested approach: nested 5′ primers (leader VLOXA and leader VLOXB), complementary to the leader region of the ph-Ox-specific V element VKOx1 were used; for cloning purposes, VLOXB contains an internal Sac 1 restriction site. As 3prime; primer Lκ-reverse2, complementary to the 3prime; end of the constant region of the κ-locus with an internal Xba 1 restriction site was used. PCR products were subcloned into the pCR-Script cloning vector (Stratagene) and sequenced. For all amplifications a proof-reading DNA polymerase was used (Pfu polymerase, Stratagene).
Nested PCR conditions were: 2 min at 95°C (primary denaturation); 45 s at 95°C; 45 s at 59°C; 1 min at 72°C; (35 cycles); 3 min 72°C (final extension).
4.6 Primers
The following primers were used: oligo(dT): 5′-GAGAGAGAGAGAGAGAGAGAACTAGTCTCGAGTTTTTTTTTTTTTTTTT-3prime;; leader VH OXA: 5′-TCCTCTCTAGAGCCCCCATCAGAGCATGGC-3prime;; leader VH OXB: 5′-GCCTGGTTGAATTCCAAGCTGTGTC-3prime;; CH4ϵ-reverse: 5′-GATATTGTTTTCTCCAGTTTC-3prime;; CH2ϵ-reverse: 5′-CAGTTTGTGCAAGTGTATCAG-3prime;; CM-reverse: 5′-CAGGGGGCTCCTGCAGGAGACGAGG-3prime;; CG1-reverse: 5′-CAGCAGCTGCAGGGGCCAGTGGATAG-3prime;; CG2-reverse: 5′-GGGAAATAGCTCTAGACCAGGC-3prime;; leader VLOXA: 5′-CCTGCTAATCAGTGCCTCAGTCATAATA-3prime;; leader VLOXB: 5′-TCCAGAGGAGAGCTCCAAATTGTTCTC-3prime;; Lκ-reverse2: 5′-ACCTTTGTCTTCTAGACTAACACTCATTC-3prime;.
4.7 pComb3 cloning
The anti-ph-Ox-specific light chain was subcloned in pComb3 23 by digesting the PCR product with Sac 1 and Xba 1 followed by direct insertion into the pComb3 plasmid. Anti-ph-Ox specific heavy chains were re-amplified with a 5′ primer (leaderVH OXC), with an internal Xho 1 restriction site and a 3prime; primer (CH1Comb) with an internal Spe 1 restriction site. Internal restriction sites were designed to guarantee in frame fusion of the light chain with the pelB-leader, and the heavy chain with the pelB-leader and the gIII phage surface protein, respectively;leader VH OXC: 5′-GTGTCCTGTCCCTCGAGCAGGTGCAGC-3prime;; CH1Comb: 5′-GACAGGTCGAACACTAGTTAGGATAGTCC-3prime;. PCR-con-ditions were as described before.
4.8 Generation of phages
pComb3 plasmids, containing in frame fusions with anti-ph-Ox-specific light and heavy chains were transformed to E. coli strain XL1-blue. E. coli were grown to an optical density of OD600≥0.5. Helper phage VCS-M13 (1011/ml) was added. After incubation (1 h at 37°C) kanamycin was added (final concentration 35 μg/ml) and incubated overnight at 30°C. After centrifugation (20 min, 4,000×g), phage were precipitated with 4% PEG 8000 and 3% NaCl for 2 h on ice. After centrifugation (20 min, 11,000×g) the phage pellet was dissolved in 1 ml TBS (10 mM Tris-HCl pH 7.5, 150 mM NaCl). Titer was determined by diluting the phage from 10–9 to 10–11 and re-infection of 100 μl E. coli XL1-blue (OD600>0.5) (10 min, room temperature). Infected XL1-blue were plated on LB-amp plates. The generated phage were diluted to a titer of 1011 plaque-forming units. The phages were directly used for surface plasmon resonance analysis.
4.9 Surface plasmon resonance analysis
Using Biacore-J, the sensorchip CM5 (Biacore) was activated and coupled with ph-Ox-OVA and OVA as reference, respectively, according to the manufacturers specifications. 9,000 resonance units (RU) were coupled for each. Analysis was performed on the Biacore J. Phage (1011) were injected for 3 min. Phages were eluted for 2 min. Binding curves were calculated using the BIA-viewer software program. Relative response units after the elution step are indicative for binding affinities.
Acknowledgements
We thank Thomas Boehm and Fátima Ferreira for supportive critique and Claudia Berek for critically reading the manuscript and stimulating discussions. Animal experiments were conducted in accordance with guidelines provided by the Austrian law on experimentation with live animals. The scientific work was supported by the Austrian Science Foundation (FWF Project: P12274-MED and S8809-MED), the Austrian National Bank (OENB Project: 7362) and the University of Salzburg for the acquisition of the FACS-Vantage.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




