CD4+CD8+TCRlow thymocytes express low levels of glucocorticoid receptors while being sensitive to glucocorticoid-induced apoptosis
Abstract
While signaling by either the TCR or glucocorticoid receptor (GR) can induce apoptosis in thymocytes, recent studies have shown that combining these signals results in survival of CD4+CD8+ thymocytes. Although glucocorticoids (GC) in this way may directly affect T cell selection, no data are available addressing GR expression in thymocyte subsets and in individual cellswithin subsets. We studied GR expression by combining immunofluorescence cell surface staining for CD4, CD8 and TCR with intracellular staining of GR in four-color cytometry. Significant differences of GR expression were observed in various thymocyte subsets, although a homogeneous distribution of GR expression in individual thymocyte subsets emerged. The highest GR expression was found in CD4–CD8–TCR– thymocytes, and decreased during development via the CD4–CD8+TCR– subpopulation into the CD4+CD8+TCRlow subset. Interestingly, the latter population, although expressing less than half the GR density of CD4–CD8–TCR– cells, is the most sensitive subset to GC-in-duced apoptosis. Up-regulation of TCR expression by the CD4+CD8+TCRlow subset to CD4+CD8+TCRhigh cells was accompanied by a parallel increase in GR expression. The latter finding and the presence of a homogeneous distribution of GR in each thymocyte subset provides an experimental basis for the concept that GR can antagonize TCR-mediated signals at a constant rate relative to TCR expression.
Abbreviations:
-
- CORT:
-
Corticosterone
-
- DAPI:
-
4′,6-Diami-dino-2-phenylindol
-
- FTOC:
-
Fetal thymic organ culture
-
- GC:
-
Glucocorticoid(s)
-
- GR:
-
Glucocorticoid receptor
1 Introduction
The successful treatment of inflammation and autoimmune diseases by synthetic glucocorticoid (GC) hormones suggests that the natural counterparts have an inhibitory function on in vivo immune function. A recent revival in GC research, however, shows that these hormones may also have immunostimulatory properties. Thus, GC up-regulate the expression of a number of cytokine receptors. To date, it has been shown that receptors for IL-1, IL-2, IL-4, IL-6, IFN-γ, GM-CSF and CSF-1, as well as the common signal transducer gp130, are induced by GC on several cell types (reviewed in 1). In addition, GC in various experimental settings have been shown to act synergistically with exogenously added cytokines, such as IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, IFN-γ, G-CSF, GM-CSF and oncostatin M 1. These permissive actions of GC contrast with their well-known inhibitory effects on various immune and inflammatory responses, which are generally thought to be due to the inhibition of cytokine production by GC 2. Although, in general, the functional relevance of these contradictory effects of GC is not known, at least one experimental system has shown that the net effect of restricting the synthesis of a given cytokine and simultaneously inducing its receptor may lead to a faster development of the biological response, which is thereafter rapidly terminated 3. Recently, it has been shown that in vivo GC can also act as immunostimulatory compounds. DTH responses were shown to be enhanced by preceding acute stress, and both GC and epinephrine were shown to mediate this effect 4.
In the thymus, GC have long been known to induce cell death (apoptosis) 5–8. While most studies have been performed by injecting pharmacological doses of (synthetic) GC, it is important to note that physiological levels of endogenous GC can also induce thymocyte apoptosis 9. Interestingly, we and others have recently shown that the thymus itself can synthesize GC 10, 11, although conflicting data have been reported with respect to the localization within the thymus, i.e. thymic epithelial cells 10, 11 or a thymocyte subset 12. A potential function of these intrathymically produced hormones has been suggested by the so-called `mutual antagonism' hypothesis, which states that the combination of signals delivered by GC and the TCR results in survival of immature CD4+CD8+ thymocytes, i.e. these signals, both of which induce apoptosis when present alone, antagonize each other 13, 14. Thus, GC may directly affect T cell development and selection. However, this concept is partially based on the assumption that the signal mediated by GC receptors (GR) must be relatively constant, while the TCR-mediated signal is variable. In addition, TCR expression increases during maturation of immature CD4+CD8+TCRlow thymocytes so that TCR signaling is also quantitatively variable. Presently, no data are available addressing GR expression in individual thymocyte subsets, and in the CD4+CD8+TCRlow subset in particular. Ligand-binding assays have the disadvantage of requiring that the endogenous ligand be removed by adrenalectomy, a procedure that in itself modulates GR expression 15. Moreover, this type of assay cannot determine whether GR expression is homogeneously distributed within each thymocyte subset. We, therefore, studied GR expression in different thymocyte subsets by combining immunofluorescence cell surface staining for CD4, CD8 and TCR with intracellular staining of GR in four-color cytometry. The highest GR expression was observed in CD4–CD8–TCR– thymocytes, whereas the lowest expression was found in the CD4+CD8+TCRlow subset. In each thymocyte subset, GR was homogeneously distributed. Interestingly, the CD4+CD8+TCRlow subset, although expressing less than half the GR density of CD4–CD8–TCR– cells, is the most sensitive to GC-induced apoptosis, as revealed by in vitro thymocyte suspension cultures as well as in fetal thymic organ culture (FTOC). Up-regulation of TCR expression by the CD4+CD8+TCRlow subset to CD4+CD8+TCRhigh cells was accompanied by a parallel increase of GR expression in this latter subset. Since the GR appeared to be homogeneously distributed among CD4+CD8+TCRlow cells, and its expression increased along with the TCR in the CD4+CD8+TCRhigh subset, our data support the view that the GR can, at least on basis of its concentration, produce a constant signal relative to that of the TCR.
2 Results
2.1 Detection of GR in thymocyte subsets
Previous studies have measured GR expression mainly by radioligand-binding assays (cytosol binding as well as whole cell binding), which have the disadvantage of endogenous ligand binding to an unknown fraction of all GR available. While removal of endogenous ligand by adrenalectomy has been employed in several studies 15–17, this procedure alone can alter expression of GR 15. In addition, ligand-binding studies estimate the level of GR in a given cell type as the average concentration of the entire cell population, since expression of receptors by individual cells cannot be assessed. Consequently, such assays are not appropriate to answer the question of whether GR is homogeneously expressed within a given thymocyte subset. We, therefore, measured GR expression by FCM with an mAb (BuGR2) that binds GR even in the presence of hormone 18 and does not cross-react with a number of other steroid hormone receptors, such as the mineralocorticoid, progesterone, androgen or estrogen receptors 19, 20. Surface reactivity for CD4, CD8 and TCR (Fig. 1A) was combined with intracellular staining for GR. Fig. 1B shows that the GR is homogeneously distributed among CD4+CD8+TCRlow (DP TCRlow), CD4+CD8+TCRhigh (DP TCRhigh), CD4+TCRhigh and CD4–CD8–TCR– thymocytes. CD8+ cells can be subdivided into mature CD8+TCRhigh and immature CD8+TCR– subsets 21. Although the proportion of the CD8+TCR– subset is variable between mouse strains, thymi of BALB/c mice contain significant numbers of this population 22. Fig. 1B shows that CD8+TCR– cells express more GR than the CD8+TCRhigh subset. The highest levels of GR are expressed by CD4–CD8–TCR– cells, which decreased during development via the CD4–CD8+TCR– subpopulation into the CD4+CD8+TCRlow subset (Fig. 2). The latter subset expressed less than half the GR density of CD4–CD8–TCR– cells. The mature CD4+TCRhigh and CD8+TCRhigh subsets express equal levels of GR that are intermediate between CD4–CD8–TCR– and CD4+CD8+TCRlow cells (Fig. 2).
To independently confirm the determination of GR levels by mAb BuGR2, as performed above, we used a second anti-GR mAb (clone 5E4). Although both mAb bind a different epitope of the GR, it is clear that GR staining in various thymocyte subsets is very similar for both mAb (Fig. 3).
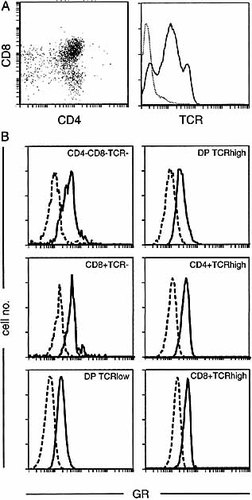
Flow cytometric analysis of GR in thymocytes. Fresh BALB/c thymocytes were preincubated with anti-CD16/32, stained with CD4, CD8 and TCR mAb, fixed in 0.5% PFA and permeabilized in 0.05% Triton X-100. The GR was stained with anti-GR mAb BuGR2 (or an isotype control), followed by FITC-conjugated anti-mouse IgG F(ab')2-fragment. Cells were analyzed in a FACS-Vantage. Thymocyte staining for CD4, CD8 and TCR is shown in (A) and the analysis of GR in various thymocyte subsets is presented in (B); DP: double-positive (CD4+CD8+). Results shown are representative of 11 independent experiments.
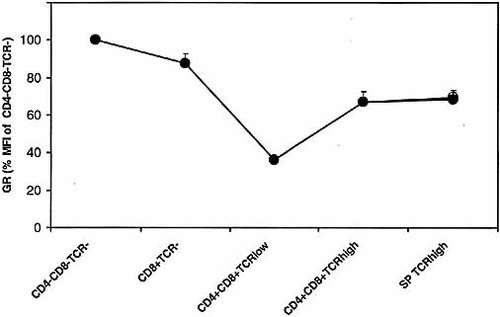
GR expression in various thymocyte subsets. Thymocytes were stained and analyzed as described in the legend of Fig. 1. GR staining is expressed as % median fluorescence intensity (MFI) of CD4–CD8–TCR– cells. Results (expressed as mean MFI ± SEM) are derived from 11 independent experiments. Single-positive (SP) TCRhigh cells: CD4+, filled circles; CD8+, empty circles.
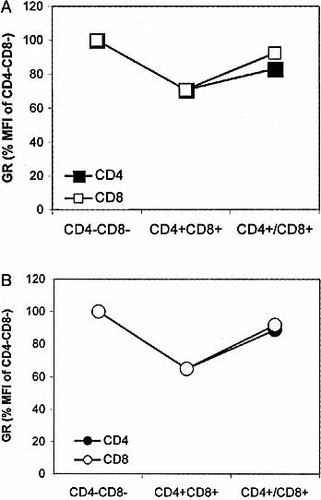
GR expression in thymocytes as determined with two different mAb. GR expression was assessed by either mAb 5E4, known to bind a conserved sequence (150–176 amino acids) of the regulatory part of the GR (A), or mAb BuGR2, which binds a single epitope in the DNA-binding domain of the GR (B). Thymocytes were stained and analyzed as described in the legend of Fig. 1, except that mAb 5E4 was directly FITC conjugated. Results shown are representative of three independent experiments.
|
GR assay |
CEM C7H2 |
CEM C1 |
Ratio |
|---|---|---|---|
|
FCM (MFI)a) |
1,129 |
402 |
2.8 |
|
Ligand binding (sites/cell)b) |
17,671 |
6,928 |
2.6 |
- a) Data are representative of three independent experiments.
- b) Data from 23.
To evaluate whether different levels of GR, as measured by FCM, were related to comparable GR concentrations measured by conventional ligand binding assays, we tested two T cell lines with a known amount of GR 23. Table 1 shows the results of stainings of these cell lines with mAb 5E4 (which also binds human GR 24). The ratio of the estimation of GR by ligand binding of both cell lines appears to be very similar to the ratio of GR of these cell lines as assessed by FCM.
2.2 GR expression during the cell cycle in thymocyte subsets
It has been reported that expression of GR varies during the cell cycle 25, which may cause bias as to the determination of GR within thymocyte subsets. We, therefore, stained cells for CD4, CD8 and GR, as described in Sect. 4, incubated with 4′,6-diamidino-2-phenylindol (DAPI; 1 μg/ml) and analyzed the cells by FCM. Fig. 4A shows CD4+/CD8+ gating of the cells depicted in Fig. 4B. Gating on either G0/1 (R1) or G2/M (R2) of the cell cycle revealed that cells in G2/M (Fig. 4C, shaded histogram) expressed about twice as much GR than cells in G0/1 (Fig. 4C, open histogram). Despite this difference, evaluation of GR expression in G0/1 of various thymocyte subsets did not substantially alter the median fluorescence intensity (MFI) obtained with cell cycle independent analysis (data not shown).
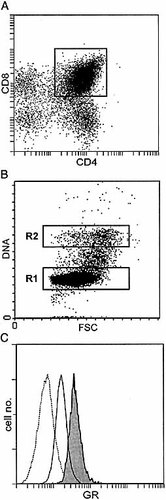
Cell cycle-dependent expression of GR. Thymocytes were stained for CD4, CD8 and GR, as described in the legend of Fig. 1, subsequently fixed in 75% ethanol and incubated with DAPI. Thymocytes were gated on CD4, CD8 (A) and DNA (B) and analyzed for GR expression in (C). (C) The open histogram shows cells in G0/1 (gate R1) and the shaded histogram shows cells in G2/M (gate R2, ±5% of cells) of the cell cycle. Open histogram with broken line, isotype control. Results shown are representative of three independent experiments.
2.3 Confocal laser microscopy analysis of GR in thymocyte subsets
To further confirm the expression of GR as assessed by FCM, aliquots of thymocytes were stained for CD4, CD8, GR and DNA and analyzed by confocal laser microscopy. Fig. 5A shows an example of a mixture (1:1) of both isotype control and anti-GR-stained cells expressing CD8 and/or CD4. In Fig. 5B single stainings for CD4, CD8, GR and DNA of a representative CD4+CD8+ thymocyte are shown separately.
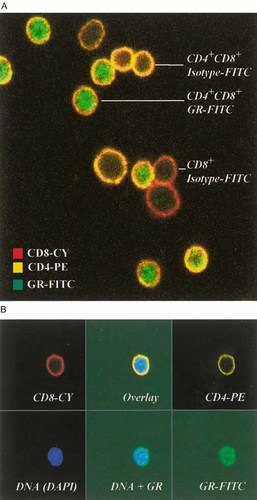
Confocal laser microscopical analysis of GR in thymocytes. Thymocytes were stained for CD4, CD8 and GR and DNA, as described in the legend of Fig. 4, washed and mounted with Mowiol. In (A), isotype control and GR stained thymocytes were mixed 1:1; (B) shows single stainings for GR and DNA, or combined together, of a CD4+CD8+ thymocyte. Results shown are representative of three independent experiments.
2.4 GC-induced apoptosis in thymocyte subsets
Having established the presence and distribution of GR in various thymocyte subsets, we wondered why the CD4+CD8+TCRlow subset, which has been shown to be most sensitive to GC-induced apoptosis 26, 27, expressed less than half the GR of the relatively GC-resistant CD4–CD8–TCR– subset. We determined sensitivity to GC of the various subpopulations by incubating thymocytes with corticosterone (CORT; 10–7 M) for 15 h followed by staining with anti-CD4, anti-CD8, DAPI and Annexin-V. FCM showed that CD4+CD8+ cells, indeed, were very sensitive to GC-induced apoptosis, whereas the CD4–CD8– subset appeared to be resistant (Fig. 6).

GC-induced apoptosis in different thymocyte subsets. Thymocytes were cultured in RPMI 1640 medium in 24-well plates (5×106 cells/well) for 15 h in the presence or absence of 10–7 M CORT. After 15 h, cells were counted, and stained with mAb for CD4 and CD8, DAPI for DNA and Annexin-V for apoptotic cells. Results shown are representative of four independent experiments.
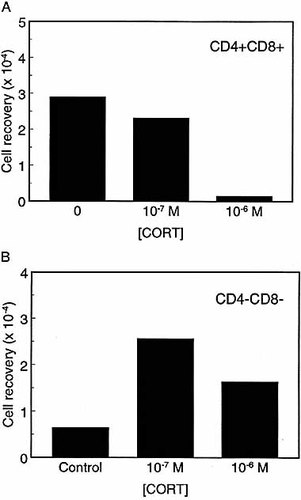
GC-induced apoptosis in FTOC. Embryonic day 15 thymic lobes were cultured in a 0.4-μm culture plate insert in serum-free X-vivo 20 medium for 3 days in the presence or absence of various concentrations of CORT (10–7–10–6 M). Thymocytes were harvested from the lobi, counted and stained for CD4 and CD8. (A) Recovery of CD4+CD8+ cells, (B) recovery of CD4–CD8– cells. Results shown are representative of four independent experiments.
Since single-cell suspensions of thymocytes display a certain degree of 'spontaneous' apoptosis, we tested whether the effects of CORT would be comparable in FTOC. Embryonic day 15 thymic lobes were cultured in serum-free medium for 3 days in the presence or absence of various concentrations (10–7–10–6 M) of CORT. At the end of the culture period, thymocytes were counted, stained for CD4 and CD8 and analyzed by FCM. The number of CD4+CD8+ cells was decreased by CORT in a dose-dependent manner (see Fig. 7A). In contrast, the number of cells of the CD4–CD8– subset appeared to be increased in the presence of CORT (Fig. 7B).
2.5 bcl-2 expression in thymocyte subsets
It has been previously reported that expression of bcl-2, a protein with important anti-apoptotic properties, is very low in CD4+CD8+ cells 28, 29. Since CD4–CD8–TCR– cells down-regulate both bcl-2 28, 29 and GR (Fig. 2), when differentiating into CD4+CD8+ cells, we tested how the reduction of both proteins are related to one another. Thymocytes were stained for CD4, CD8, TCR and bcl-2, or with an isotype control. CD4–CD8–TCR– immature thymocytes and mature CD4+TCRhigh and CD8+TCRhigh cells appear to express bcl-2 in the same amounts (Fig. 8), in agreement with previous studies 28, 29. In contrast, more than 90% reduction rendered bcl-2 expression in CD4+CD8+TCRlow hardly detectable. Thus, these cells appear to down-regulate bcl-2 more strongly than GR, which is reduced by approximately 60% (Fig. 2).
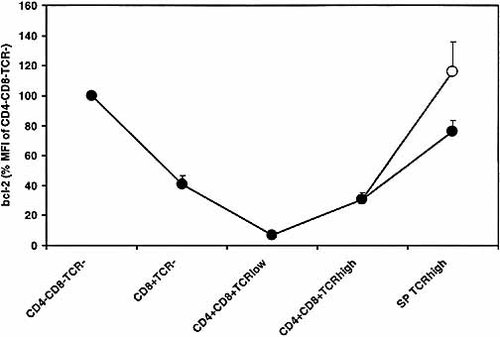
Bcl-2 expression in various thymocyte subsets. Thymocytes were stained with CD4, CD8 and TCR mAb, fixed in 0.5% PFA and permeabilized in 0.05% Triton X-100. Bcl-2 was stained directly with an anti-bcl-2-FITC mAb or an FITC-conjugated isotype control. Bcl-2 expression in CD4+CD8+TCRlow cells was hardly detectable. Bcl-2 staining is expressed as % MFI of CD4–CD8–TCR– cells. Results (expressed as mean MFI ± SEM) are derived from four independent experiments. Single-positive (SP) TCRhigh cells: CD4+, filled circles; CD8+, empty circles.
3 Discussion
The present study demonstrates that GR expression, as assessed by four-color FCM, is uniformly distributed within each thymocyte subset. The CD4+CD8+TCRlow subset expresses less than half the GR level of CD4–CD8–TCR– cells, although the former subpopulation is sensitive to GC-induced apoptosis and the latter is not. Interestingly, GR expression is up-regulated along with the TCR as CD4+CD8+TCRlow cells mature to CD4+CD8+TCRhigh cells. These findings, on one hand, provide the basis for constant GR signaling in each thymocyte subset, and on the other hand suggest a mutual antagonism of TCR and GR signaling at a quantitatively constant rate, since CD4+CD8+TCRlow cells up-regulate both receptors.
It has been shown that signals produced by GC and TCR ligands antagonize each other's capacity to induce apoptosis in immature CD4+CD8+ thymocytes 13, 30. Based on these and other data, a hypothesis was put forward by the group of J. D. Ashwell 13, 14 that GC can directly influence the generation of the T cell repertoire by setting the TCR avidity window for thymocyte selection. Indeed, a reduction of the GC concentration in FTOC of TCR β-transgenic mice results in apoptosis of CD4+CD8+ thymocytes, whose TCR recognize self-Ag/MHC with an avidity that would normally result in positive selection 31. In addition, mice whose thymocytes express low levels of GR due to the transgenic expression of GR antisense transcripts responded normally to the complex Ag-purified protein derivative (PPD), but were nonresponders to pigeon cytochrome c 81–104 (PCC), indicating the presence of an Ag-specific 'hole' in the T cell repertoire 32. The concept of mutual antagonism between GR- and TCR-mediated signals, however, is based on the assumption that the signal mediated by GR is relatively constant, while the TCR-mediated signal is variable. In addition, TCR signaling is not only qualitatively variable, but also quantitatively, since TCR expression increases during maturation of immature CD4+CD8+TCRlow thymocytes to CD4+CD8+TCRhigh cells. Thus, GR expression during the maturation of individual CD4+CD8+ thymocytes is an important factor that needs to be addressed. Previous studies estimating GR concentrations in various cell types and tissues did not address GR expression by individual thymocytes 16, 17, 33, 34. The measurement of GR by FCM has a number of advantages over ligand-binding studies. First, it allows determination of GR in individual cells rather than an average number of receptors per cell. Second, assessment of GR can be performed in the presence of endogenous ligand, which allows more accurate estimation of the total number of available GR. Third, the assay can be carried out on fresh, heterogeneous thymocyte populations without any prior experimental manipulations, such as adrenalectomy or purifications of various thymocyte subsets. In addition, very few cells are required in this assay compared to ligand-binding analysis.
CD4–CD8–TCR– thymocytes expressed the highest concentration of GR. The functional significance of this observation is presently not clear, although it is of interest that transgenic mice whose thymocytes express reduced levels of GR have small thymi, a phenome-non partially explained by inefficient progression of CD4–CD8– to CD4+CD8+ cells 35. While the mature CD4+TCRhigh and CD8+TCRhigh subsets express equal levels of GR intermediate between CD4–CD8–TCR– and CD4+CD8+TCRlow subsets, the most interesting observation is the low level of GR in CD4+CD8+TCRlow cells. Since CD4+CD8+ cells are highly sensitive to GC-induced apoptosis (26, 27 and the present study), such a result is unexpected. Differential sensitivity of thymocyte subsets to GC-induced apoptosis has been ascribed to up-regulation of bcl-2 in mature thymocytes 36. Since CD4–CD8–TCR– cells down-regulate both bcl-2 and GR, we were interested in determining how the reduction of both proteins were related to one another. While bcl-2 is hardlydetectable in CD4+CD8+TCRlow cells, GR is clearly present, although at reduced levels. The substantial down-regulation of bcl-2 (or other, apoptosis-related, proteins) inCD4+CD8+TCRlow cells may have a greater impact on their high sensitivity to GC-induced apoptosis than down-regulation of the GR would have on the survival of these cells.Why the GR is down-regulated at all remains unclear.
An interesting observation was that GR is up-regulated in CD4+CD8+ cells concurrently with TCR expression. If GC set the TCR avidity window for thymocyte selection by antagonizing TCR-mediated signals, then one would expect that GC-induced signals would not vary in relation to signals produced by the TCR. The finding that the GR is homogeneously expressed in CD4+CD8+TCRlow thymocytes is in agreement with that prediction. As soon as TCR expression increases during maturation of a given CD4+CD8+TCRlow thymocyte to the CD4+CD8+TCRhigh stage, the TCR-mediated signal is expected to change quantitatively. If the degree of antagonism of the GR relative to TCR is constant, then the GR should also increase in number which indeed was observed. Alternatively, other factors that influ-ence the transcriptional activity of the GR may also change during the transition of CD4+CD8+TCRlow to CD4+CD8+TCRhigh cells.
The results of the present study support the theoretical basis of a model in which the balance of the GC- and TCR-mediated signals regulates thymocyte selection. This model, however, has recently been challenged by the finding that thymocyte recovery, thymocyte subset analysis, and staphylococcal enterotoxin B (SEB)- and anti-CD3/CD28-induced apoptosis were normal in GR-knockout mice 37. In addition, conflicting data exist with respect to thymocyte recovery and thymocyte subset analysis in two transgenic mouse strains bearing an antisense construct of the GR 35, 38. A detailed characterization of the TCR repertoire in both GR knockout and GR antisense transgenic mice is still lacking and will help to clarify a potential role of GR in T cell development and selection. Finally, it will be of great interest to study the consequences of aberrant regulation of GC action in the thymus, either at the level of thymocyte responsiveness to GC (GR expression), or of GC production, which the thymus itself can synthesize 10, 11. At present, we are performing comparative analyses of GRexpression in thymocytes and thymic GC production in normal vs. autoimmune-prone mice.
4 Materials and methods
4.1 Mice
BALB/c mice were bred in the Central Laboratory Animal Facilities of the University of Innsbruck. They were housed under standard light cycles and temperatures. Food and tap water were available ad libitum. Timed (12 h) pregnant BALB/c mice, mated in the Central Laboratory Animal Facilities, were used for FTOC. The presence of a vaginal plug was designated as day 0.
4.2 Antibodies, conjugates and reagents
For flow cytometric stainings, the following Ab were used: anti-CD4-PE (clone GK1.5), anti-CD8-Cy-ChromeTM (CY) (clone 53–6.7), anti-TCR-β-biotin (clone H57–597), anti-CD16/anti-CD32 (Fc-Block; clone 2.4G2), anti-bcl-2-FITC, FITC-conjugated isotype control, IgG2a isotype control (clone MPOC-173), IgG1 isotype control (clone MOPC-21; all Ab from PharMingen, San Diego, CA), anti-GR (clone BuGR2; Affinity BioReagents, Golden, CO). An additional anti-GR-FITC (clone 5E4) was kindly provided by A. Falus 24. FITC-conjugated donkey anti-mouse IgG (H+L) F(ab')2 fragment was obtained from Jackson Immunoresearch (West Grove, PA). Annexin-V-FITC, and streptavidin-FITC were obtained from PharMingen (San Diego, CA). Streptavidin-Alexa350 and DAPI were purchased from Molecular Probes (Leiden, The Netherlands). CORT was purchased from Sigma (Vienna, Austria).
4.3 Cell lines
The GC-sensitive cell line CEM-C7H2, and the GC-resistant cell line CEM-C1 23, kindly provided by R. Kofler (Innsbruck, Austria), were maintained in RPMI 1640 medium supplemented with 100 μg/ml streptomycin, 100 U/ml penicillin and 10% heat-inactivated FCS (Life Technologies, Paisley, GB).
4.4 FCM
Fresh BALB/c thymocytes, prepared as described in Sect. 4.6, were analyzed for cell surface markers (TCR, CD4, CD8) as well as intracellular determinants (GR, bcl-2, DNA) by four-color immunofluorescence. Before staining, Fc II/III receptors on cells were blocked by preincubation with anti-CD16/32 (Fc-Block). Cell surface markers were then stained either directly (CD4-PE, CD8-CY) or indirectly (TCRβ-biotin) in PBS/1%BSA for 30 min at 4°C. Cells were washed twice and the indirectly-stained cells incubated with streptavidin-FITC or streptavidin-Alexa350 for 30 min at 4°C and washed three times. For intracellular staining of GR or bcl-2, cells (surface-stained as described above) were fixed in 0.5% PFA for 30 min at room temperature, washed and permeabilized with 0.05% Triton X-100 in 0.1% sodium citrate for 5 min at 4°C. After three additional washing steps, cells were stained either directly (anti-bcl-2-FITC or a FITC-conjugated isotype control) or indirectly (GR or an isotype control) for 70 min at room temperature. Cells were washed three times and the indirectly stained cells were incubated with a FITC-conjugated anti-mouse IgG(H+L) F(ab')2 fragment (which was shown to have no cross-reactivity with bovine, chicken, goat, guinea pig, Syrian hamster, horse, human, rabbit, rat and sheep serum proteins) for 30 min at room temperature. After three final washing steps, cells were analyzed in a FACS-Vantage (Becton Dickinson, Sunnyvale, CA).
For control purposes in some experiments, cells (either fresh thymocytes or the cell lines CEM-C7H2 and CEM-C1) were stained with an FITC-conjugated anti-GR mAb (or an iso-type control). This mAb was raised against a conserved sequence (150–176 amino acids) of the regulatory part of the GR 24.
To analyze GR expression during the cell cycle, cells were stained for CD4, CD8 and GR, as described above, and subsequently fixed in 75% ethanol for 5 min. DAPI (1 μg/ml) was added and incubated for 15 min at 4°C. After three washing steps, cells were analyzed by FCM.
Apoptosis of cultured thymocytes (see below) in the presence or absence of CORT was determined by staining with both Annexin-V and DAPI. Cells were stained for CD4 and CD8 and Annexin-V-FITC, as described above, except that 'binding buffer' (10 mM Hepes, 140 mM NaCl, 2.5 mM CaCL2) was used for this, and subsequent, incubations. DAPI (1 μg/ml) was added 15 min before analysisto discriminate between intact and dead cells, i.e. cells that excluded DAPI but stained for Annexin-V were considered apoptotic.
4.5 Confocal laser microscopy
Morphological analysis of GR staining was performed by confocal laser microscopy. Briefly, thymocytes were stained for CD4, CD8 and GR and DAPI, as described above, washed and mounted with Mowiol (Sigma, Vienna, Austria). Samples were analyzed with a LSM 510 confocal laser scanning microscope (Zeiss, Göttingen, Germany).
4.6 In vitro apoptosis of thymocytes
Thymi from BALB/c mice were aseptically removed and gently disrupted through a cell strainer (pore size 100 μm, Becton Dickinson) to obtain single-cell suspensions. Cells were then washed (400×g, 8 min) and cultured in RPMI 1640 medium, supplemented with 2 mM L-glutamine, 50 μM 2-ME, 100 U/ml streptomycin, 100 μg/ml penicillin and 10% heat-inactivated FCS (Life Technologies, Paisley, GB) in 24-well plates (5×106 cells/well) for 15 h in the presence or absence of 10–7 M CORT. After 15 h, cells were counted, stained with anti-CD4-PE, anti-CD8-CY, DAPI and Annexin-V-FITC, and analyzed for apoptosis by FCM.
4.7 FTOC
Embryonic day 15 thymic lobes were aseptically removed and placed in a 0.4 μm culture plate insert (4 lobes/insert; Millipore-CM; Millipore, Bedford, MA). Cultures were carried out in serum-free X-vivo 20 medium (BioWhittaker, Walkersville, MD) supplemented with 2 mM L-glutamine, 100 mM nonessential amino acids, 1 mM sodium pyruvate, 50 μ M 2-ME, 100 μg/ml streptomycin and 100 U/ml penicillin in 12-well plates for 3 days. Various concentrations of CORT (10–7–10–6 M) were added at the start of culture. Thymocytes were harvested by gently disrupting the lobes in a 1-ml syringe and subsequent pressing through a screen cloth (pore size 40 μm). After counting, cells were stained with anti-CD4-PE, anti-CD8-CY, and analyzed by FCM.
Acknowledgements
We are grateful to Dr. H. Reul for critically reading the manuscript and Dr. Melanie Vacchio for helpful suggestions. This study was supported by the Austrian Ministry of Education, Science and Culture as part of the Austrian-French Scientific Cooperation program, the Jubiläumsfonds of the Austrian Nationalbank (Grant no. 8131), and the Austrian Science Fund (project 14466).
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




