Complementarity and redundancy of the binding specificity of HLA-DRB1, -DRB3, -DRB4 and -DRB5 molecules
Abstract
The second HLA-DR molecules, which are encoded by loci different from HLA-DRB1 are weakly polymorphic. Predominant alleles such as HLA-DRB3*0101, HLA-DRB4*0101 and HLA-DRB5*0101 are therefore interesting targets to define antigenic peptides with major impact for the entire population. Strikingly, they have been poorly investigated. Thus we have characterized peptides from the major bee venom allergen that bind efficiently to these molecules and compared them to peptides specific for preponderant HLA-DRB1 molecules. Interestingly, DRB5*0101 and DRB1*0701 molecules share four bindingpeptides and use some identical anchor residues. Similarities are also found between DRB3*0101 and its haplotype-associated molecules DRB1*0301 and DRB1*1301. In sharp contrast, DRB4*0101 exhibits a unique binding specificity, which results from particular structural features of its peptide binding site. Yβ81 seems to alter the amino acid preferences of the P1 pocket, while Rβ71, Eβ74, Nβ26 and Cβ13 confer to the P4 pocket a unique topology. Our results show that the two HLA-DR molecules expressed in most haplotypes studied here have mostly complementary binding patterns. Only haplotype HLA-DR52 exhibits peptide binding redundancies. Finally our results document functional similarities among HLA-DR molecules and allow us to propose peptide sequences that might be useful for bee venom immunotherapy.
Abbreviation:
-
- Api m1:
-
Major bee venom allergen
1 Introduction
It is assumed that MHC class II molecules expressed by a given individual act in a complementary manner to select and activate T cells. In mice, I-E and I-A exhibit different binding specificities 1, 2 and each presents its own set of antigenic peptides. Similarly, in humans the homologous molecules, namely HLA-DR and HLA-DQ are characterized by distinct binding modes 3–5. However, the number of MHC II molecules differs from one species to another and from one individual to another in humans. Mice generally have only two MHC II molecules while humans possess three isotypes (HLA-DR, -DQ and -DP). Moreover HLA-DR molecules exist in different forms. The HLA-DRB1 gene encodes the beta chain of the first molecule and is expressed in all individuals. In contrast, HLA-DRB3, -DRB4 and -DRB5 loci are only present in particular individuals and provide them with a second HLA-DR product 6. These genes are in linkage disequilibrium with the first gene due to their close proximity and define three haplotype families: DR52, DR53 and DR51, respectively 6. Their products are thus associated with particular alleles of the first molecule. They are able to present antigenic peptides to T cells 7–10. They also have the particularity to be less polymorphic than DRB1 molecules and to possess alleles such as DRB3*0101, DRB4*0101 and DRB5*0101 that largely predominate in Caucasian populations 11. Peptides that efficiently bind to these alleles are therefore of potential interest in designing universal therapeutic peptides. However, the precise binding specificity of the second DR molecules and how they complement the first one to which they are associated remain unclear.
We have addressed these questions using peptides from the bee venom phospholipase A2 due to the recent interest for T cell epitopes of allergens 12. In fact, efficiency of conventional immunotherapy seems to result from the decline of proliferation of allergen-specific CD4+ T lymphocytes or from an alteration of their profile of cytokine secretion towards a TH1 response 13, 14. Production of the immunosuppressive interleukin-10 by these T cells has been also highlighted 14. Accordingly, short sequences of allergens that are recognized by T cells have been found to induce tolerance in allergic patients 15. Compared to crude allergenic extracts, such allergen fragments have the advantage of being weakly antigenic for specific IgE. Immunotherapy approaches that use engineered allergens or synthetic peptides have been previously proposed for the treatment of insect, pollen and animal allergies 15–18. They are of major interest for bee venom allergy since approximately fifteen percent of bee venom allergic patients suffer from serious side effects during conventional immunotherapy 19. The major allergen of bee venom is a phospholipase A2 (Api m1), which has been found to promote T cell proliferation in allergic patients 20–22. Most of the T cell epitopes appear to reside in the C-terminal half of Api m1 21, 22. In agreement with these data, we have previously reported that numerous binding determinants specific for the first HLA-DR molecules are located in this part of the allergen 23. These extensive binding data provide an interesting opportunity to compare the binding specificity of first and second HLA-DR molecules. In this study, we report peptide binding assays specific for DRB3*0101, DRB4*0101 and DRB5*0101 molecules, which allowed us to document their mode of recognition. We detail their complementarity with HLA-DR molecules from the same haplotype and highlight binding similarities between first and second HLA-DR gene products.
2 Results
2.1 Peptide-binding assays specific for the DRB3*0101, DRB4*0101 and DRB5*0101 molecules
We have established peptide-binding assays for the DRB3*0101, DRB4*0101 and DRB5*0101 molecules using as tracers, appropriate pairs of biotinylated peptides specific for each allele. Binding of the tracers was efficiently inhibited by their respective non-biotinylated counterpart but weakly by the other non-biotinylated peptide. Such patterns were previously described for the DR51 haplotype with the A3 152–166 and the HA 306–318 peptides 24. These peptides selectively bind to the DRB1*1501 and DRB5*0101 alleles, respectively (Table 1). We also defined such pairs of peptides for the DR52 and DR53 haplotypes. As shown in Table 1, the B1 21–36 25 and the LOL 191–210 26 peptides were remarkably specific for the DRB1*1301 and the DRB3*0101 alleles, respectively, while the YKL 27 and E2/E168 peptides allowed us to study the DRB1*0701 and DRB4*0101 alleles, respectively. These binding assays were sensitive since the IC50 values measured with the non-biotinylated peptides ranged from 2 to 275 nM (Table 1).
|
|
1st HLA-DR |
2nd HLA-DR |
||
|---|---|---|---|---|
|
Peptides |
Allele |
IC50 (nM) |
Allele |
IC50 (nM) |
|
A3 152 – 166 |
DRB1*1501 |
33 |
DRB5*0101 |
85,000 |
|
HA 306 – 318 |
DRB1*1501 |
2,500 |
DRB5*0101 |
6.5 |
|
B1 21 – 36 |
DRB1*1301 |
275 |
DRB3*0101 |
35,000 |
|
LOL 191 – 210 |
DRB1*1301 |
> 100,000 |
DRB3*0101 |
5 |
|
YKL |
DRB1*0701 |
35 |
DRB4*0101 |
950 |
|
E2 / E168 |
DRB1*0701 |
5,000 |
DRB4*0101 |
2 |
- a) Competitive binding assays were performed with biotinylated peptides as described in Sect. 4. For each peptide pair, the first IC50 column presents data obtained using the first peptide as biotinylated probe (bA3 152 – 166, bB1 21 – 36, bYKL) while the second IC50 column corresponds to the second peptide (bHA 306 – 318, bLOL 191 – 210, bE2 / E168).
2.2 Delineation of DRB3*0101-, DRB4*0101- and DRB5*0101-restricted determinants of Api m1
We then delineated the binding determinants of the major bee venom allergen (Api m1) according to the procedure previously described for HLA-DRB1 molecules 23. We evaluated the binding capacities of 18 amino acids (aa)-long peptides that encompassed the whole Api m1 sequence. The active and inactive peptides were distinguished on the basis of an upper inhibiting concentration threshold of 1,000 nM 23. As shown in Table 2, three regions in the N-terminal part of Api m1 were allele specific: the 13–38 and 41–66 regions towards the DRB5*0101 allele and the 53–74 sequence towards the DRB3*0101 molecule. In the C-terminal half of Api m1, two large regions (77–110 and 105–134) carried binding determinants for the three second DR molecules. We delineated more precisely the binding determinants using 13 aa-long peptides. The DRB5*0101 allele displayed active peptides all along the 73–108 sequence (Fig. 1). The peptide P85–97 was the most active and two other determinants (P77–89 and P94–106) of weaker activity were found. In contrast, less active peptides characterized the DRB3*0101 allele and were all concentrated in the 75–96 sequence (Fig. 1). Two distinct regions, represented by the P81–93 and P89–101 peptides, were observed for the DRB4*0101 allele. In the C-terminal part of Api m1 (Fig. 2), the three DR molecules displayed at least two distinct binding regions, which varied in the number of active peptides but systematically contained the P111–123 or P122–134 peptide. In conclusion, we observed a different number of determinants, restricted to each second DR gene product. The DRB5*0101 allele interacted with seven Api m1 regions, which spread over all the Api m1 sequence. Only five and four Api m1 regions bound to the DRB3*0101 and the DRB4*0101 allele, respectively, and were mainly located in the central and C-terminal parts of the allergen.
|
|
Alleles |
||
|---|---|---|---|
|
Peptides |
DRB5*0101 |
DRB3*0101 |
DRB4*0101 |
|
P1 – 18 |
9,000 ± 100 |
− |
46,000 ± 32,000 |
|
P5 – 22 |
25,000 ± 7,000 |
− |
70,000 ± 7,000 |
|
P9 – 26 |
65,000 ± 21,000 |
− |
24,000 ± 18,000 |
|
P13 – 30 |
600 ± 100 |
− |
15,000 ± 13,000 |
|
P17 – 34 |
175 ± 40 |
− |
26,000 ± 43,000 |
|
P21 – 38 |
35 ± 7 |
25,000 ± 7,000 |
65,000 ± 7,000 |
|
P25 – 42 |
− |
70,000 ± 30,000 |
75,000 ± 35,000 |
|
P29 – 46 |
95,000 ± 7,000 |
60,000 ± 14,000 |
75,000 ± 35,000 |
|
P33 – 50 |
28,000 ± 10,000 |
− |
90,000 ± 14,000 |
|
P37 – 54 |
− |
− |
− |
|
P41 – 58 |
770 ± 250 |
− |
− |
|
P45 – 62 |
40 ± 0 |
− |
70,000 ± 0 |
|
P49 – 66 |
430 ± 150 |
5,200 ± 3,400 |
− |
|
P53 – 70 |
− |
40 ± 15 |
32,000 ± 18,000 |
|
P57 – 74 |
20,000 ± 0 |
160 ± 70 |
60,000 ± 14,000 |
|
P61 – 78 |
23,000 ± 10,000 |
20,000 ± 5,000 |
43,000 ± 12,000 |
|
P65 – 82 |
80,000 ± 28,000 |
3,700 ± 1,000 |
14,000 ± 7,500 |
|
P69 – 86 |
− |
17,000 ± 8,000 |
− |
|
P73 – 90 |
− |
− |
− |
|
P77 – 94 |
300 ± 100 |
180 ± 30 |
400 ± 130 |
|
P81 – 98 |
4 ± 1 |
530 ± 330 |
− |
|
P85 – 102 |
3 ± 0 |
1,500 ± 500 |
58 ± 10 |
|
P89 – 106 |
320 ± 180 |
80,000 ± 0 |
600 ± 300 |
|
P93 – 110 |
100 ± 50 |
− |
− |
|
P97 – 114 |
− |
− |
− |
|
P101 – 118 |
− |
− |
77,000 ± 12,000 |
|
P105 – 122 |
170 ± 60 |
800 ± 350 |
400 ± 140 |
|
P109 – 126 |
25 ± 7 |
1,800 ± 800 |
350 ± 100 |
|
P113 – 130 |
40 ± 15 |
870 ± 550 |
500 ± 0 |
|
P117 – 134 |
73 ± 30 |
830 ± 300 |
580 ± 100 |
- a) IC50 are expressed in nM. Means and standards errors were measured from at least three independent experiments. IC50 lower than 1,000 nM are indicated in bold. "−" means activity higher than 100,000 nM.
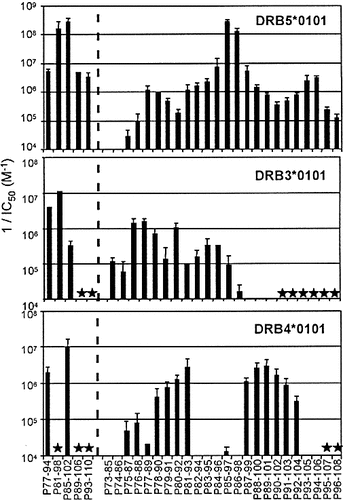
Binding capacities of 13-mer peptides covering the 73–108 sequence of Api m1 to 2nd HLA-DR molecules. Binding activities are expressed as inverse of mean IC50 (M-1). Means and standard errors were deduced from at least three independent experiments. Lack of histogram indicates that the peptide displayed no inhibitory activity at the maximal concentration tested (10–4 M). Stars mean "not defined" in these experiments.
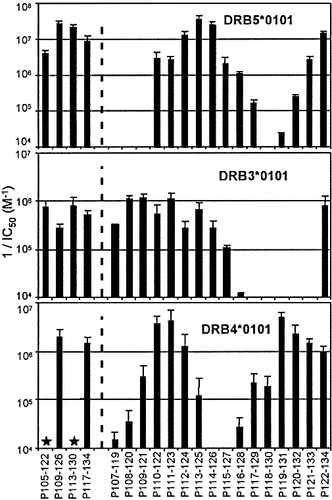
Binding capacities of 13-mer peptides from the C-terminal extremity of Api m1 to 2nd HLA-DR molecules. See legend Fig. 1.
2.3 The first and second DR molecules display overlapping Api m1 binding patterns
Peptides representative of the second HLA-DR molecules were compared with those previously described for the predominant HLA-DRB1 alleles 23. This revealed that the first and second DR molecules have binding peptides in common. For example, the P21–38 and P45–62 peptides were both common to the DRB5*0101 and DRB1*0701 molecules. The P53–70 peptide was restricted to the DRB1*0301 and DRB3*0101 alleles, while the P85–97 peptide bound to two second DR molecules and to six first molecules. This similarity between the first and second molecules was statistically documented by a factor analysis. We clustered the molecules based on the similarities of their binding pattern, using binding data from the 18-mer peptides that encompassed the whole Api m1 sequence. As shown in Fig. 3, the DRB5*0101 allele was very close to the DRB1*0701 and DRB1*1101 alleles while the DRB4*0101 allele was slightly more distant from these two DRB1 alleles. In contrast, the DRB3*0101 allele was rather close to the DRB1*0301 allele. Therefore, the DRB3*0101, DRB4*0101 and DRB5*0101 molecules do not constitute a functional group separate from the DRB1 gene products. On the contrary, first and second HLA-DR molecules clearly share functional similarities.
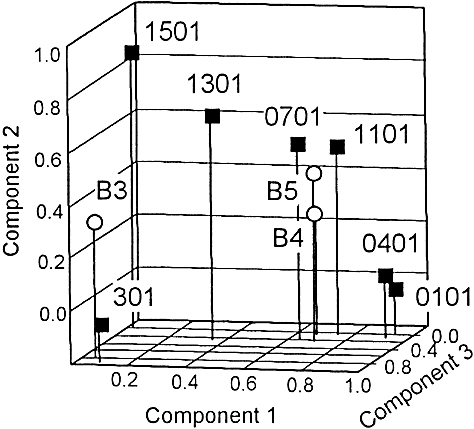
Factor analysis of Api m1 18-mer binding data to HLA-DR molecules. Each HLA-DR molecule was characterized by 30 coordinates corresponding to the IC50 values of the 18-mer peptides. As described previously 23, factor analysis projected the cloud of HLA-DR molecules into a space of smaller dimensions, while preserving as most as possible the information provided by the crude data. In our model, HLA-DR molecules are described by three components that account for 47%, 16% and 11% of the variance, respectively. The model explains 74% of the total variance. HLA-DRB1 gene product (black squares) second DR molecules (open circles). B3: DRB3*0101, B4: DRB4*0101, B5: DRB5*0101.
2.4 DRB3*0101, DRB5*0101 and DRB1 alleles bind Api m1 peptides in a similar way
We investigated whether the peptides common to first and second HLA-DR molecules shared similar anchor residues. Alanine and lysine scanning was performed on the P85–97A peptide, which bound to the DRB3*0101 and DRB5*0101 alleles as well as to six DRB1 molecules 23. The DRB3*0101 and DRB5*0101 alleles displayed a similar binding pattern (Fig. 4), as mainly illustrated by substitution of F88 by alanine or lysine, which resulted in a dramatic loss of binding. In agreement with computer modeling of the P85–97A peptide complexed to DRB3*0101 and DRB5*0101 alleles (Fig. 5A), we concluded that the P85–97A peptide bound to both DRB3*0101 and DRB5*0101 alleles using F88, I91, T93 and Y96 as P1, P4, P6 and P9 anchor residues, respectively. In these models, the global conformation of the peptide and the hydrogen bond network were similar to those observed in crystal structures of HLA-DR/peptide complexes 28–30. In particular, the predicted DRB3*0101 and the crystallized DRB1*0301 29 alleles shared the same P4 pocket sequence and adopted superimposable P4 structure (data notshown). Interestingly, we also noted that the P85–97A peptide interacted with some DRB1 molecules using the same binding mode as described above. As shown in Fig. 4, a strong negative effect at position F88 with both alanine and lysine scanning was observed on DRB1*1301 while negative effects also took place at positions F88, I91, T93 and Y96 for DRB1*0701. Therefore, the P85–97A peptide associates with HLA-DRB3*0101, DRB5*0101, DRB1*0701 and DRB1*1301 molecules by using the same binding frame.
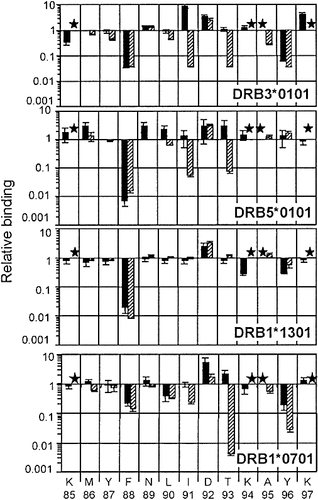
Relative binding capacities of single P85–97A analogues to DRB3*0101, DRB5*0101, DRB1*1301 and DRB1*0701 alleles. The P85–97A peptide contains an alanine at position 95 instead of a cysteine and binds to the HLA-DR alleles as well as the native P85–97 peptide (data not shown). Variants of the P85–97A peptide, substituted at each position with alanine (close bars) or lysine (hatched bars) were tested for their binding properties on first and second HLA-DR molecules as described in Sect. 4. Results are expressed as mean ratio between P85–97A IC50 relative to the substituted peptide value. They correspond to two to three independent experiments. Stars mean not tested.
2.5 The DRB4*0101 allele is characterized by a unique binding mode
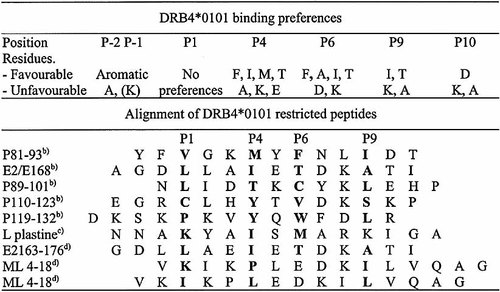
In sharp contrast to the DRB3*0101 and DRB5*0101 molecules, the DRB4*0101 molecule shares only two peptides with the other HLA-DR molecules. One of these, the P81–93 peptide also binds to the DRB1*1501 allele and therefore was submitted to alanine and lysine scanning. Binding pattern for the DRB1*1501 molecule (data not shown) dramatically differed from that of the DRB4*0101 molecule. For the latter, significant effects spread over a sequence of twelve residues, from Y81 to D92 (Fig. 6) and did not reveal any clear binding mode. Such effects generally affect amino acids located between the P-3 to P10 position of the peptide, as numbered by Stern et al. 28. By positioning the Y81, F82 and D92 residues in this range, only one possible combination may account for the data: V83 in P1, M86 in P4, F88 in P6 and I91 in P9. This binding mode is compatible with computer modeling of the P81–93/DRB4*0101 complex (Fig. 5). Global conformation of the model of the bound P81–93 peptide differs from X-ray HLA-DR/peptide complex structures only in the P-2 to P1 region. Its peptide backbone is pushed up and kinked towards the α chain of HLA-DR (Fig. 5B). Two hydrogen bonds stabilize the tyrosine in P-2: one with the side chain of βS88 and one with the backbone of βV85 (Fig. 5B). Interestingly, the hydrogen bond between the β81 MHC residue and the P81–93 peptide backbone is conserved despite that DRB4*0101 displays a tyrosine instead of a histidine in β81 (Fig. 5B). The side chains M86 and F88 appear to be deeply buried in the P4 and P6 pockets respectively. The top of the P4 pocket is in fact blocked by βR71 and βE74 while its bottom is enlarged by the presence of relatively small residues at positions β26 and β13 (Fig. 5C). In the P6 pocket, the alanine at position β11 creates a large cavity, which accommodates F88 (data not shown). Therefore, the DRB4*0101 molecule is characterized by structural particularities, which govern its binding specificity. Amino acid substitutions in the P81–93 peptide at the P1, P4, P6 and P9 positions revealed preferences for each position, except for pocket 1 (Fig. 6 and Table 3). These preferences allowed us to align the sequences of the peptides restricted to the DRB4*0101 allele reported in this work and in others 8, 31. Our study reveals that the DRB4*0101 allele possesses an original binding mode, different from the other HLA-DR molecules. Its main characteristics are the lack of preference at the P1 position and the accommodation of bulky side chains by the P4 and P6 pockets.
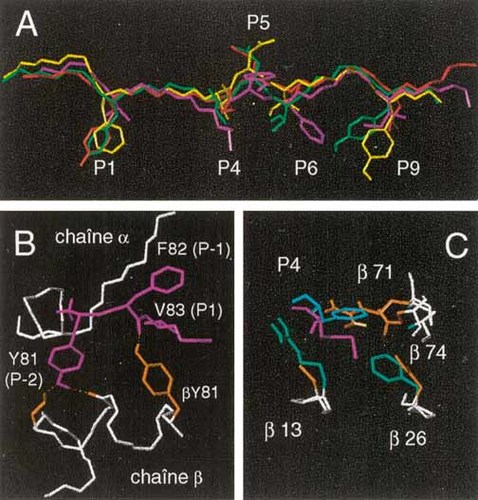
Computer modeling of HLA-DRB3*0101, -DRB4-*0101 and -DRB5*0101 molecules. Three complexes have been modeled using the cristallographic data of HLA-DR1 complex 28.(A) Comparison of peptide backbones. Only side-chains at positions P1, P4, P5, P6 and P9 are represented. (Red) HA 306–318 bound to DRB1*0101 28. (Green) P85–97 bound toDRB3*0101 and (yellow) to DRB5*0101. (Magenta) P81–93 bound to DRB4*0101. (B) Hydrogen bonds network in the N-terminal part of the P81–93 peptide. (White) DRB4*0101 backbone, (orange) DRB4*0101 side chains and (magenta) P81–93 peptide. (C) Topology of the DRB1*1501 and DRB4*0101 P4 pocket. Atomic coordinates of DRB1*1501 (green) complexed with the MBP peptide (blue) are fromSmith et al. 30. (Magenta) peptide P81–93. (Orange) DRB4*0101 side chains.
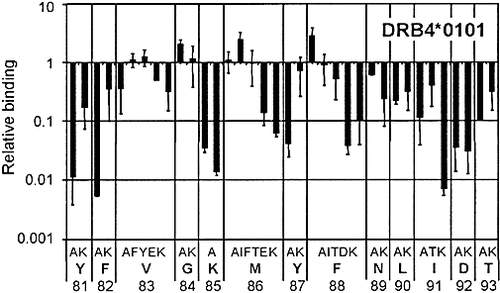
Relative binding capacities of P81–93 analogues toward DRB4*0101. Monosubstituted analogues of the P81–93 peptide were tested for their binding strength on the DRB4*0101 allele as described in Sect.4. Data are expressed as relative binding capacity (IC50 P81–93/IC50 analogue) and represent the mean of two to three independent experiments.
3 Discussion
HLA-DR genes have evolved from common ancestor genes and have been submitted to diversifying genetic mechanisms such as gene duplication, point mutation and gene conversion 32. The resulting molecules are thus expected to possess both their own functional characteristics and common properties inherited from ancestor genes or resulting from convergent events. Indeed, the occurrence of similarities and differences are documented by the data presented in this paper.
The HLA-DRB4*0101 molecule behaves in a singular manner as compared to other HLA II molecules. First, it does not show any amino acid preference in the P1 position, accepting for instance a lysine 31 or a tyrosine. Classically, identical P1 pockets, which contain a Val at position β86 prefer aliphatic amino acids 5, 24, 25. In the P81–93/DRB4*0101 complex, the tyrosine β81, which is exclusive of this HLA-DR molecule, may indirectly alter the specificity of pocket 1 by providing a higher flexibility to the peptide. Further amplifying the critical role of this position, it was observed that the simple disruption of the hydrogen bond between amino acid β81 and the peptide by introduction of an Asn both destabilized the I-Ad molecule 33, 34 and diminished its expression at the cell surface 35. Secondly, the P4 pocket accommodates aromatic and hydrophobic side chains as anchor residues. Such preference also occurs for the DRB1*1501 allele because an alanine is present at position β71 30. However, the DRB4*0101 P4 pocket is different and is shaped by small residues located at positions β26 and β13. Thirdly, aromatic and hydrophobic side chains are accepted by the P6 pocket. Fourthy, effects on binding strength were observed at the P-2 position and seem to result from interactions between the peptide and amino acids located outside the binding groove of DRB4*0101. Similar interactionshave been also proposed for the murine I-Ad molecule 36. Altogether, the DRB4*0101 molecule has a unique mode for accommodating peptides, which dramatically differs fromthat of other HLA II molecules.
On the contrary, several observations suggest that the DRB3*0101 and DRB5*0101 molecules frequently recognize antigenic peptides in the same manner as other HLA II molecules do. This was clearly demonstrated with the P85–97 peptide (Fig. 4). Based on HLA-DR peptide motifs 3, it seems to be also the case for other peptides. F24 and L50 may constitute P1 anchor residues in the P21–38 and P45–62 peptides for both the DRB5*0101 and DRB1*0701 molecules, while Y96 is a potential P1 anchor in the P94–106 peptide for the DRB5*0101, DRB1*0401, DRB1*0101 and DRB1*1101 molecules. L59 in P1 and D62 in P4 may participate to the binding of the P57–74 peptide to both the DRB3*0101 and DRB1*0301 alleles. In agreement with our data, the latter molecules were shown to be able to present a peptide from acetylcholine receptor to the same T cell clone and hence by the same binding mode 10. However, these similarities do not result from identical genetic mechanisms. DRB5*0101 and DRB1*0701 do not belong to the same haplotype. The observed similarities are therefore likely to result from an ancient common origin or from unexpected convergent events. In contrast, DRB1*1301 and DRB3*0101 seem to result from a recent gene duplication, while DRB1*0301 may have derived from a gene conversion between DRB3*0101 and DRB1*1301 genes 37. In particular, the entire P4 pocket of DRB1*0301 is identical to that of DRB3*0101. The P4 pocket is known to constitute a major and characteristic anchoring site for these molecules due to the presence of two lysines in position β71 and β74 29, 38. Their functional similarity may therefore result from a recent common lineage.
We have also noticed that the number of Api m1 binding determinants dramatically varies from one haplotype to another. Eleven determinants have been found for the DR51 haplotype which comprises the DRB5*0101 and DRB1*1501 molecules. In contrast, the DR1 haplotype, which contains only one molecule (DRB1*0101) gives rise to only two determinants 23. Very few peptides arecommon to the 1st and 2nd molecules of the DR51 and DR53 haplotypes, suggesting that these molecules have evolved towards complementary specificities. Redundancy between the 1st and 2nd molecules only occurs in the DR52 haplotype, as exemplified above with the P85–97 and P53–70 peptides. Such similarity may limit the number of peptides presented to T cells but may also enhance the density of the presented peptides, increasing specific T cell recruitment 10.
Finally, our data provide molecular basis for specific immunotherapy of bee venom allergic patients. In particular, we propose the P76–106 and P111–134 fragments, which encompass the majority of HLA-DR binding determinants as vaccine candidates for patients allergic to bee venom.
4 Materials and methods
4.1 Peptides
Peptides were synthesized as previously described 23, 39. HA 306–318 (PKYVKQNTLKLAT), YKL (AAYAAAKAAALAA), A3 152–166 (EAEQLRAYLDGTGVE), B1 21–36 (TER-VRLVTRHIYNREE), LOL 191–210 (ESWGAVWRIDTPDKLTGPFT) and E2/E168 (AGDLLAIETDKATI) were biotinylated with biotinyl-6-aminocaproic acid (Fluka Chimie, St Quentin Fallavier, France) on the N terminus. Peptides were cleaved from the resin by 95% trifluoroacetic acid. All peptides except those substituted by alanine and lysine were purified by reversed phase HPLC on a C18 Vydac column. Homogeneity of all preparations was assessed by HPLC and quality was controlled by electrospray mass spectroscopy.
4.2 Purification of HLA-DR molecules
EBV homozygous cell lines were used as sources of human HLA class II molecules. SCHU (DRB1*1501, DRB5*0101) and PITOUT (DRB1*0701, DRB4*0101) were from Dr. H. Grosse-Wilde (European Collectionfor Biomedical Research, Essen, Germany). 0206AD (DRB1*1301, DRB3*0101) was kindly provided by Dr. J. Dausset (Centre d'Etude du Polymorphisme Humain, Paris, France). HLA-DR molecules were purifiedby affinity chromatography using the monomorphic mAb L243 (American Type Culture Collection, Manassas, VA) coupled to protein A Sepharose CL 4B gel (Amersham Pharmacia Biotech, Orsay, France) 23.
4.3 HLA-DR peptide-binding assays
HLA-DR molecules were diluted in 10 mM phosphate, 150 mM NaCl, 1 mM DM, 10 mM citrate, 0.003% thimerosal buffer with an appropriate biotinylated peptide and serial dilutions of competitor peptides. Samples (100 μl/ well) were incubated in 96-well polypropylene plates (Nunc, Roskilde, Denmark) at 37°C for 24 h, except for the DRB1*1301 and DRB4*0101 alleles, which were incubated 72 h. After pH neutralization, samples were applied to 96-well Maxisorp ELISA plates (Nunc) previously coated with 10 μg/ml L243 mAb. They were incubated on the antibody-coated plates for 2 h atroom temperature. Bound biotinylated peptides were detected by using a streptavidin-alkaline phospha-tase conjugate (Amersham, Little Chalfont, GB) and 4-methylumbelliferyl phosphate as substrate (Sigma, St. Quentin Fallavier, France). Emitted fluorescence was measured at 450 nm upon excitation at 365 nm on a Fluorolite 1000 fluorimeter (Dynex, Issy les Moulineaux, France). Maximal binding was determined by incubating the biotinylated peptide with the MHC II molecule in the absence of competitor. Binding specificity was assessed by adding an excess of non-biotinylated peptide. Data were expressed as the concentration of peptide that prevented binding of 50% of the labeled peptide (IC50).
4.4 Statistical analysis
The factor analysis was done using the SPSS 8.0 software (SPSS France, Paris, France). The parameter estimates were calculated using the principal components extraction method as described previously 23. The varimax method of rotation was applied to facilitate the interpretation of the three-dimensional graph. Validity of the model was assessed by Kaiser-Mayer-Olkin (KMO) test (KMO = 0.71).
4.5 Molecular modeling
HLA-DR sequences were obtained from the ImMunoGeneTics Database (http://imgt.cnusc.fr:8104/). The models of the DRA*0101/DRB3*0101, DRA*0101/DRB4*0101 and DRA*0101/DRB5*0101 molecules in complex with Api m1 peptides were built on the crystallographic coordinates of the DRA*0101/DRB1*0101 (DR1) molecule complexed to the HA 306–318 peptide (PDB: 1dlh) 28. Amino acid replacement was performed using the BIOPOLYMER group of the SYBYL 6.5 software (Tripos Associates Inc, St. Louis, MO) run on an Octane station (Silicon Graphics Inc, Jouy en Josas, France). The models were then subjected to energy minimization in vacuo using the standard parameters fields of the X-PLOR 3.1 software 40 run on an R8000 station (Silicon Graphics). The charges of the lysine, arginine, glutamate and aspartate amino acids that were exposed to the solvent, were previously neutralized in order to lower the electrostatic effects on the surface of the complexes. Harmonic constraints were imposed on peptide bonds and chi 1 angles during a short mild temperature dynamics simulation and during the first steps of the energy minimization. After structure refinement, the root mean square (rms) deviations between the models and the DR1 molecule were calculated for the C-, N- and Cα-atoms involved in the secondary structures. They were 0.82 Å, 0.95 Å and0.90 Å for DRB3*0101, DRB4*0101 and DRB5*0101, respectively. As a comparison, the DRB1*0301/CLIP structure was calculated as described above, and presented a 0.84 Å rms value with both DR1/HA 28 and DR3/CLIP (PDB: 1a6a) 29 crystallographic structures.
Acknowledgements
We are grateful to Dr. B. Gilquin for useful advice in computer modeling and Dr. D. Gillet for critical reading of the manuscript. This work was supported in part by grant #99R013 from the Etablissement Français des Greffes.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH