A novel Fc receptor for IgA and IgM is expressed on both hematopoietic and non-hematopoietic tissues
Abstract
By contrast to well-defined Fcγ and Fcϵ receptors, the structural and functional characteristics of Fcμ receptor are unclear. We have recently described a novel mouse Fc receptor, designated Fcα/μ receptor, and its human homologue, which bind both IgM and IgA. Here we show that the Fcα/μ receptor is expressed on mature, but not immature, B lymphocytes and acquires the ability to bind IgM and IgA antibodies after stimulation of B lymphocytes. Moreover, stimulation with phorbol 12-myristate 13-acetate increased endocytosis of IgM-coated microparticles mediated by the Fcα/μ receptor expressed on pro-B cell line Ba/F3 cells. We also show that the Fcα/μ receptor is expressed in secondary lymphoid organs, such as lymph node and appendix, kidney and intestine, suggesting an important role of the receptor for immunity in these organs.
Abbreviations:
-
- mFcα/μR:
-
Mouse Fcα/μ receptor
-
- hFcα/μR:
-
Human Fcα/μ receptor
1 Introduction
Receptors for the Fc portions of immunoglobulin (FcR) play important roles in a wide array of immune responses. The binding of the Fc portions of antibodies or immune complexes to cell surface FcR on hematopoietic cells can trigger or inhibit immunological functions, including antibody-dependent cellular cytotoxicity, mast cell degranulation, phagocytosis, cell proliferation, antibody secretion and enhancement of antigen presentation 1–3. Considerable progress has been made in elucidating the structural and functional diversity of the FcR for IgG and IgE 4–11. Functional FcR for IgM have been reported on subpopulations of lymphocytes 12–21. However, because a Fcμ receptor gene has not been cloned, the structural and functional characteristics of FcμR remain unclear.
We have recently identified a novel mouse FcR, designated Fcα/μ receptor (Fcα/μR), and its human homologue which bind both IgM and IgA 22. The Fcα/μR is a type 1 transmembrane protein and a member of immunoglobulin superfamily. The Fcα/μR is preferentially expressed on the majority of B lymphocytes and macrophages, but not on granulocytes and T and NK cells 22. Furthermore, we have reported that the Fcα/μR mediates endocytosis of immune complex composed of Staphylococcus aureus and IgM anti-S. aureus antibody by primary B lymphocytes, suggesting that the Fcα/μR may play an important role for the primary stage of the immune response against microbes 22. However, the mechanism of endocytosis mediated by the Fcα/μR on primary B lymphocytes has not been elucidated. Furthermore, distribution and function of the Fcα/μR expressed on non-hematopoietic tissues remains unclear.
Here we show that the Fcα/μR is expressed on mature, but not immature, B lymphocytes and acquires the ability to bind IgM and IgA antibodies after stimulation of B lymphocytes. The Fcα/μR is also abundantly expressed in secondary lymphoid organs, kidney and intestine, suggesting an important role of the Fcα/μR for the immune defense in these organs.
2 Results and discussion
2.1 Fcα/μR is expressed on mature, but not immature, B lymphocytes
The Fcα/μR expression is restricted on B220+ B lymphocytes and macrophages, but not on the other lymphohematopoietic cells and progenitors 22. We further examined the expression of the Fcα/μR during the developmental stage of B lymphocytes. Mouse spleen or bone marrow cells were simultaneously stained with anti-Fcα/μR, anti-IgM, and anti-IgD antibodies and the Fcα/μR expression was compared between IgD–, IgM+ immature and IgD+, IgM+ mature B lymphocytes. Fig. 1 shows that IgD+ mature B lymphocytes from spleen expressed significant amount of the Fcα/μR. By contrast, the Fcα/μR was not expressed on the majority of IgD– immature B lymphocytes. Similar pattern of the Fcα/μR expression was observed in bone marrow B lymphocytes (Fig. 1). We also found that pre-B cell tumor line Ba/F3 did not express the Fcα/μR 22. These results suggest that the expression of the Fcα/μR is developmentally regulated and that the Fcα/μR may have functional role in some aspect of B cell maturation and activation.
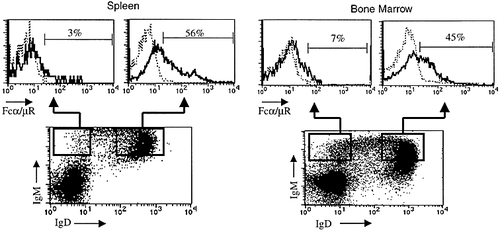
Mouse spleen or bone marrow cells were simultaneously stained with anti-Fcα/μR, anti-IgM, and anti-IgD antibodies and analyzed by flow cytometry. IgD+, IgM+ mature B lymphocytes expressed significant amount of the Fcα/μR. By contrast, the Fcα/μR is not expressed on the majority of IgD–, IgM+ immature B lymphocytes.
2.2 Binding of the Fcα/μR on primary B lymphocytes to IgM and IgA
To examine whether the Fcα/μR expressed on primary B lymphocytes can bind mouse IgM and IgA, we incubated mouse spleen cells with FITC-conjugated mouse IgM or IgA antibodies. While B220+ B lymphocytes in spleen express sufficient amount of the Fcα/μR (Fig. 2A), binding of IgM and IgA to B lymphocytes were barely detectable, as determined by flow cytometry (Fig. 2B, C). Although the Fcα/μR expressed on Ba/F3 transfectant bind IgM and IgA with intermediate or high affinity 22, these results suggest that the affinity of the Fcα/μR expressed on resting B lymphocytes for IgM and IgA is low.
We examined whether an activation of B lymphocytes increases binding ability of the Fcα/μR with IgM or IgA. Spleen cells were cultured for 24 h either in medium supplemented with 10% FBS or in the medium containing LPS and plate-coated anti-IgM and anti-CD40 mAb. While the Fcα/μR expression on B lymphocytes remained constant (Fig. 2A, D, G), binding of both IgM and IgA to B220+ B lymphocytes was significantly increased after culture in the medium supplemented with FBS (Fig. 2E, F). We did not observe any further additive effect of the stimulation with LPS and anti-IgM and anti-CD40 mAb on the binding ability of the Fcα/μR to IgM and IgA (Fig. 2H, I). The pretreatment of the cultured spleen cells with anti-Fcα/μR mAb totally inhibited the binding of IgM and IgA to the cultured B lymphocytes (Fig. 2E, F, H, I), indicating that the interactions between B lymphocytes and IgM or IgA antibodies were mediated by the Fcα/μR. These results suggest that the affinity of the Fcα/μR on primary B lymphocytes for IgM and IgA was increased after culture, in which cellular interaction between B lymphocytes and the other spleen cells, cytokines produced from these cells, and/or exogenous factors in FBS might have been involved.
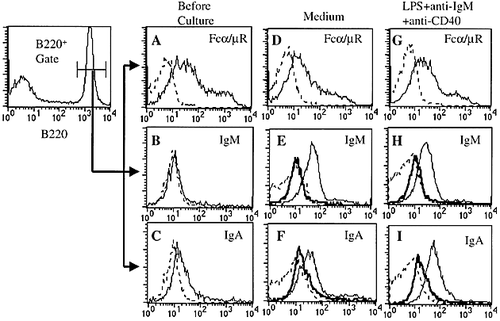
Fresh or cultured mouse spleen cells were stained with anti-B220 mAb and either anti-Fcα/μR, or IgM or IgA antibodies. While fresh resting B lymphocytes express comparable amount of the Fcα/μR with cultured B lymphocyte (A, D, G), the binding of fresh B lymphocytes to IgM and IgA was hardly detected (B, C; thin lines). By contrast, the B lymphocytes after culture significantly bound IgM and IgA (E, F, H, I; thin lines). These bindings were totally inhibited by pretreatment of B lymphocytes with anti-Fcα/μR (E, F, H, I; thick lines). Dotted lines indicate the staining of cells using rat or mouse IgG control antibodies.
We have reported that the Fcα/μR mediates endocytosis of the immune complex composed of S. aureus and an IgM anti-S. aureus antibody by a subset of B lymphocytes after 24 h of culture of spleen cells 22. However, we did not observe the endocytosis by B lymphocytes within a few hours of culture of spleen cells (data not shown), consistent with the present result that resting B lymphocytes bind undetectable level of IgM. Taken together, these results suggest that the Fcα/μR requires stimulation of B lymphocytes from the microenvironment in lymphoid tissues to mediate its function.
2.3 Stimulation with PMA increases endocytosis of IgM-coated microparticles mediated by the Fcα/μR
We have described that the Fcα/μR expressed on a mouse pre-B cell Ba/F3 mediated endocytosis of IgM-coated microparticles, for which the di-leucine motif in the cytoplasmic portion of the mFcα/μR is essentially required (22 and Fig. 3, upper panel). Because the culture condition increased the IgM binding of the Fcα/μR expressed on primary B lymphocytes, we examined whether stimulation of the Ba/F3 transfectant results in increase in endocytosis of IgM-coated microparticles. As demonstrated in Fig. 3, stimulation of the transfectant expressing the wild-type Fcα/μR with PKC activator PMA significantly increased endocytosis of the IgM-coated microparticles, although the expression of the Fcα/μR remained constant after stimulation (data not shown). It has not yet been determined whether the Fcα/μR contains a substrate for activated PKC. However, PKC activation seemed either to increase affinity of the Fcα/μR with IgM-coated microparticles, as observed in primary B lymphocytes which were nonspecifically stimulated after culture, or to drive the machinery of Fcα/μR-mediated endocytosis.
We further examined whether PKC activation restores the ability for endocytosis, which was lost by the Fcα/μR mutated at residue 519 (L-A), 520 (L-A) or both (LL-AA) (22 and Fig. 3, upper panel). The Ba/F3 transfectants expressing any of the mutant Fcα/μR did not show endocytosis, even when these cells were stimulated with PMA (Fig. 3). Thus, PKC activation by PMA increases endocytosis of the IgM-coated microparticles mediated by the Fcα/μR, but still can not restore the ability of the mutant Fcα/μR to perform endocytosis. These results suggest that the di-leucine motif is primarily important for Fcα/μR-mediated endocytosis.
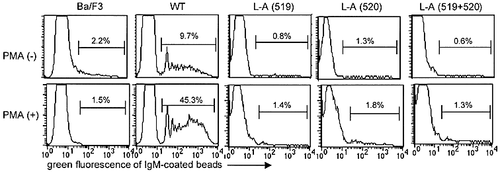
The Ba/F3 transfectants expressing wild-type (WT) or site-directed mutant Fcα/μR were stimulated or not with PMA for 2 h. The cells were then incubated overnight at 37°C with mouse IgM (DX18 mAb)-coated green fluorescent beads, treated with 0.1% trypsin and analyzed by flow cytometry. The IgM-coated green fluorescent beads were only detected in the cytoplasm of transfectants expressing the wild type (22 and upper panel). The stimulation of the transfectants with PMA significantly increased the endocytosis of IgM-coated beads in the transfectant expressing the wild-type, but not mutant, Fcα/μR. The expression of the wild-type and mutant Fcα/μR were comparable one another both before 22 and after (not shown) stimulation.
A di-leucine motif is also involved in endocytosis mediated by two major alternative splicing isoforms of Fcγ receptor-IIB1 and IIB2 23,24, which are expressed on B lymphocytes and macrophages, respectively. While the Fcγ receptor-IIB2 rapidly internalizes upon cross-linking, Fcγ receptor-IIB1 requires a longer time for internalization, in which detergent-insoluble cytoskeleton associated with Fcγ receptor-IIB1 seems to be involved 25. Because endocytosis of IgM-coated microparticles by Fcα/μR occurred after 24 h of culture and was accelerated by PMA, undefined Fcα/μR-associated molecules or detergent-insoluble cytoskeleton that are affected by activated PKC might be involved in endocytosis mediated by Fcα/μR. Several other FcR, including FcγRI, FcγRIIIA, FcϵRI, and FcαRI, also mediate endocytosis 26–29 that is dependent on the associated subunit FcR γ chain 30. However, we did not observe an association of the Fcα/μR with the γ chain 22. Further studies are required to clarify the signaling mechanism which regulates the affinity and endocytosis of the Fcα/μR.
2.4 Fcα/μR transcript expression in hematopoietic and non-hematopoietic organs
We have reported that the mFcα/μR transcript is expressed not only on hematopoietic cells but also in non-hematopoietic tissues, as determined by reverse transcription (RT)-PCR 22. To compare relative amount of the mFcα/μR expression, we performed quantitative RT-PCR using mRNA from various mouse hematopoietic and non-hematopoietic tissues. As demonstrated in Fig. 4, the expression of the mFcα/μR transcript was relatively higher in kidney and intestine than in the other tissues examined. Lower but significant level of the mFcα/μR transcript was also detected in liver, lung and heart.
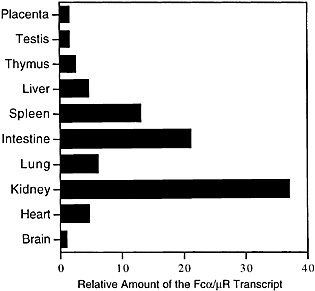
Expression of mFcα/μR transcript was analyzed by quantitative RT-PCR in various mouse tissues. The expression of the mFcα/μR transcript was relatively higher in kidney and small intestine than the others. Lower but significant amount of the Fcα/μR transcript was also detected in spleen, heart, lung and liver. Data are shown as relative number when the amount of the transcript in brain is taken as one. Data are representative in several independent experiments.
To confirm the amount of the mFcα/μR transcript expression, we further examined the transcript by Northern blot analysis using a 32P-labeled mFcα/μR cDNA as a probe. Consistent with the results of quantitative RT-PCR, the mFcα/μR transcript was most abundantly expressed in kidney (Fig. 5A). Less amount of the mFcα/μR transcript was also detected in spleen, heart, liver, and lung. It was of note that two sizes of the mFcα/μR transcripts (7 and 2.3 kb) were detected in these tissues, which may be due to the different usage of polyadenylation sites. However, they were hardly detected in brain, skeletal muscle, and testis. We also examined the expression of hFcα/μR receptor transcript in various tissues using a 32P-labeled hFcα/μR cDNA as probe. Similarly, the hFcα/μR transcript was also detected in kidney and small intestine (Fig. 5B). Moreover, secondary lymphoid organs such as lymph node and appendix were found to express significant amount of the transcript.
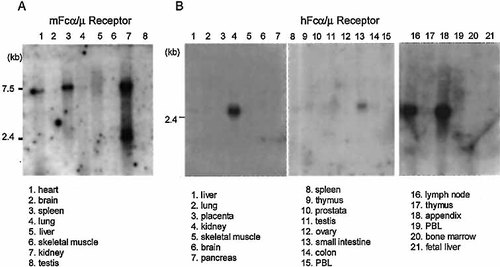
Expression of mouse (A) and human (B) Fcα/μR transcript was analyzed by Northern blot analysis. The mFcα/μR transcript was most abundantly expressed in kidney. It was also detected in spleen and heart, and faintly in lung and liver. Similarly, hFcα/μR transcript was detected in kidney and small intestine. Moreover, it was also abundantly detected in lymph node and appendix. The comparable amount of β-actin transcript expression in each lane using human β-actin cDNA was confirmed (data not shown).
2.5 Concluding remarks
To clarify the physiological and pathophysiological significance of the Fcα/μR, it will necessary to determine the type of cells that specifically express the Fcα/μR in these hematopoietic and non-hematopoietic tissues. Nonetheless, because the Fcα/μR mediates the endocytosis of IgM-coated bacteria by B lymphocytes, the Fcα/μR may plays an important role in immunity in these organs. Recent reports have demonstrated that mice deficient in the secretory form of IgM exhibit delayed development of specific IgG antibodies to T cell-dependent foreign antigens 31 and dissemination of micropathogens in peripheral organs but not in secondary lymphoid organs 32. The results described here, together with these reports, suggest that the Fcα/μR may play an essential role in the priming of helper T lymphocytes in secondary lymphoid organs and in immune defense against bacteria in peripheral organs.
3 Materials and methods
3.1 Antibodies
For binding assays and analyses of Fcα/μR expression on B lymphocytes, fluorescein isothiocyanate (FITC)-conjugated anti-human CD3 mAb (UCTH1, mouse IgG1), FITC-conjugated DX18 mAb (mouse IgM), FITC-conjugated M18–254 mAb (mouse IgA), phycoerythrin (PE)-conjugated anti-mouse CD45R/B220 (rat IgG), biotin-conjugated anti-mouse IgM (R6–60.2, rat IgG2a), FITC-conjugated anti-mouse IgD (11–26c.2a, rat IgG2a) and anti-mouse Fcα/μ receptor (TX-6 mAb, rat IgG) were used. Anti-human CD3, M18–254, anti-mouse CD45R/B220, anti-mouse IgM, and anti-mouse IgD mAb were purchased from PharMingen (San Diego, CA). DX18 mAb was a gift from DNAX Research Institute (Palo Alto, CA). Anti-mFcα/μR was generated in our laboratory, as described previously 22. As a second-step antibody and a reagent, allophycocyanin (APC)-conjugated anti-rat IgG antibody (Caltag Laboratories, Burlingame, CA) and PE-conjugated streptavidin (PharMingen) were used.
3.2 Flow cytometry
For analyses of the Fcα/μR expression on B lymphocytes, spleen cells from 6–10-week-old C57BL/6 mice were stained with anti-mFcα/μR, followed by APC-conjugated anti-rat IgG secondary antibody. Cells were then treated with excess amount of rat IgG to block the binding of the anti-rat IgG secondary antibody to rat IgG used for two- or three-color analysis. For two-color analysis, cells were then stained with PE-conjugated anti-mouse CD45R/B220. For three-color analysis, cells were stained with biotin-conjugated anti-mouse IgM, followed by PE-conjugated streptavidin and FITC-conjugated anti-mouse IgD. For binding assay of mouse IgM or IgA to the Fcα/μ receptor on B lymphocytes, spleen cells were pretreated or not with anti-Fcα/μR mAb and then incubated with PE-conjugated anti-mouse CD45R/B220 and either FITC-conjugated mouse IgG (UCTH1) as a negative control, FITC-conjugated mouse IgM (DX18) or FITC-conjugated mouse IgA (M18–254). Cells were analyzed by flow cytometry (FACSCalibur, Becton Dickinson, San Jose, CA), as described previously 33, 34.
3.3 Stimulation of B lymphocytes
Spleen cells from 6–10-week-old C57BL/6 mice were cultured for 24 h in DMEM medium (Gibco BRL, Rockville, MD) supplemented either with 10% FBS (Life Technologies, Grand Island, NY) alone or with the combination of LPS (Sigma, St. Louis, MO) at a concentration of 100 μg/ml and plate-precoated anti-IgM (PharMingen) and anti-CD40 mAb (PharMingen) in addition to 10% FBS.
3.4 Endocytosis of IgM-coated fluorescent beads by Ba/F3 transfectant
Ba/F3 transfectants expressing wild-type or site-specific mutant mFcα/μR (with mutations at residues 519, 520, or both) hae been described previously 22. Ba/F3 transfectants were incubated for 24 h in DMEM medium supplemented either with 10% FBS alone or with PMA (Sigma) at a concentration of 100 ng/ml in addition to 10% FBS. The assay for endocytosis of IgM-coated fluorescent beads (FluoSpheres Fluorescent Microspheres, 0.5 μm in diameter, Molecular Probes, Eugene, OR) was performed as described previously 22.
3.5 Quantitative RT-PCR
Total RNA were extracted from various mouse tissues using ISOGEN (Nippon Gene, Tokyo, Japan) and mRNA were isolated using Quick Prep Micro mRNA Purification Kit (Amersham Pharmacia, Little Chalfont, GB). First strand cDNA were synthesized from mRNA isolated using SuperScript II (Gibco-BRL) and the amount of cDNA were quantified by quantitative PCR using ABI PRISM7700 system (Applied Biosystems, Foster City, CA). Primers used for amplification of mFcα/μR transcripts for a TaqMan analysis were sense, 5′-CTCCCTTTCAGGTACAAATGCA-3prime;; antisense, 5′-TCTTTGATGCCTGTTGACTGAG-3prime;, and the Taq Man probe was 5′-ATCCATTGCCATTACGCCCCCT-3prime;. The data were collected and analyzed by ABI PRISM7700 system.
3.6 Northern blot analysis
Mouse and human Fcα/μR and human β-actin cDNA were labeled with [32P]dCTP using the Ready to Go DNA Labelling Beads (Amersham Pharmacia). Membranes containing approximately 2 μg poly(A+) RNA in each lane from different mouse or human tissues (Mouse and human MTN Blots, Clontech, Palo Alto, CA) were hybridized with the [32P]-dCTP-labeled cDNA probes at 68°C in modified Church's hybridization buffer (0.5 M Church's phosphate buffer, 7% SDS, 1% BSA). The membranes were washed at 68°C for 1 h in Church's washing buffer (40 mM Church's phosphate buffer, 1% SDS) and developed by autoradiography.
Acknowledgements
We thank Lewis Lanier for critical reading of this manuscript, Satoshi Yamazaki and Michie Ito for technical and secretarial assistances, respectively. This research was supported in part by the grants provided by the Ministry of Education, Science and Culture of Japan, and Special Coordination Funds of the Science and Technology Agency of the Japanese Government (to A.S.).
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




