Ligand motif of the autoimmune disease-associated mouse MHC class II molecule H2-As
Abstract
The MHC class II molecule H2-As, expressed in the SJL mouse strain, is the principle restriction element of autoreactive CD4+ T cells mediating experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. We deduced the H2-As ligand motif from the analysis of naturally processed self peptides and from peptide binding studies. Major anchor residues were identified using various sets of substituted and truncated peptides, derived from natural peptide ligands and known H2-As binders like myelin basic protein 81 – 99. The nine-residue H2-As core binding motif comprises an arrangement of anchors in relative positions P1, P4, P6, P7, and P9. The P1 pocket is relatively unspecific and the P6 pocket favors hydrophobic-aliphatic side chains. The P1 pocket contributes little to peptide binding. Primary anchors were identified in P4, P7, and in particular in P9. The preferred anchor residues are Lys (P4), His / Arg (P7), and Pro (P9), respectively. Ala-polysubstituted peptides containing only one of these dominant anchor residues still retain the capacity to bind to H2-As. Thus, the presence of only one suitable anchor side chain in P4, P7, or P9 is sufficient for high-affinity peptide binding, at least in the absence of negatively charged side chains nearby. The identified ligand motif facilitates the analysis of immunogenic peptides interacting with H2-As and will allow a better prediction of pathogenetically relevant peptide antigens in the autoimmune mouse model.
Abbreviations:
-
- Abu:
-
α-Aminobutyric acid
-
- AMCA:
-
7-Amino-4-methylcoumarin-3-acetic acid
-
- CNS:
-
Central nervous system
-
- HPSEC:
-
High-performance size-exclusion chromatography
-
- IC50:
-
Peptide concentration required for half-maximal competition
-
- MBP:
-
Myelin basic protein
-
- MS:
-
Multiple sclerosis
-
- mTFR:
-
Mouse transferrin receptor
-
- PLP:
-
Proteolipid protein
-
- PX:
-
Relative agretope core / anchor position X
-
- Rel. pos.:
-
Relative position
-
- TFA:
-
Trifluoroacetic acid
1 Introduction
Multiple sclerosis (MS) is a chronic disabling inflammatory-demyelinating disease of the central nervous system (CNS). Despite its unknown pathogenesis, an autoimmune response is considered to be important, caused at least in part by a dysregulated T cell response (for reviews see 1 – 4). In addition, certain HLA class II alleles are significantly associated with an increased risk of developing MS 3.
In the animal model of MS, experimental autoimmune encephalomyelitis (EAE), similar clinical and histopathological features can be induced in susceptible strains by immunization with myelin components or transfer of myelin-reactive T cells 5. EAE is mediated by MHC class II-restricted CD4+ T cells of the Th1 phenotype (reviewed in 3, 6). The two most abundant myelin proteins, proteolipid protein (PLP) and myelin basic protein (MBP), as well as other CNS proteins, such as myelin oligodendrocyte glycoprotein (MOG) and S-100β, have been demonstrated to be encephalitogenic 3, 7, 8. Like in MS, EAE susceptibility depends on the MHC background 9 and different peptides are immunogenic and encephalitogenic in different mouse strains 5, 10, 11.
The SJL mouse (H-2s haplotype) is increasingly being used for EAE, because several clinical, immunological and histopathological features resemble human MS more closely than in other rodent strains 12. Due to a deletion in the gene coding the H2-E α-chain, H2-As represents the only functional MHC class II molecule expressed by SJL mice. In the SJL EAE model a high correlation between binding affinity to H2-As, immunogenicity and encephalitogenicity of peptides has been observed for the major myelin antigens, PLP and MBP, with mouse MBP(81 – 100) and PLP(139 – 151) containing major encephalitogenic epitopes 10, 11, 13. H2-A and its homolog HLA-DQ are less well characterized than H2-E and HLA-DR. Rudensky and coworkers 14 first reported an H2-As binding motif, derived from sequence alignment of five different eluted self peptides, and suggested peptide anchor residues in relative position (rel. pos.) P1 (hydrophobic), P2 (Thr, Ser, Ala) and P7 (His, Arg). However, this motif could not explain the observed binding affinities of a variety of peptides, including those immunogenic in the SJL mouse, and is unsuitable for epitope predictions. In subsequent detailed studies focussing on T cell recognition of PLP(139 – 151), main anchor side chains contacting H2-As were identified in the middle and C-terminal part of the peptide at a spacing of three residues (Leu145, Pro148) 15. The recent identification of the crystal structures of H2-Ad and H2-Ak peptide complexes, as well as peptide binding studies at these and other H2-A molecules, revealed an MHC class II-characteristic P1-P4-P6-P9 binding motif mediated by allele-specific pockets in the MHC-binding groove 16, 17, with an anchor usage partly divergent from the kind of peptide binding commonly observed for HLA-DR and H2-E molecules (see http://134.2.96.221 at Tübingen University or http://wehih.wehi.edu.au / mhcpep for a summary of MHC ligand motifs). Such a P1-P4-P6-P9 motif has also been suggested for H2-As by Reizis et al. 18. Here, we describe a markedly improved binding motif for H2-As, deduced from analyses of bound self peptides as well as from binding studies with various sets of synthetic peptides.
2 Results
2.1 Binding of MBP- and PLP-epitopes to purified H2-As molecules
Overlapping 11-mer MBP-peptides (81 – 91) to (95 – 105), spanning the encephalitogenic region of MBP with highest binding affinity to H2-As 13, were tested for their binding capacity in competition assays against the fluorescence-labeled standard binder PLP(139 – 153). Fig. 1 shows that two different agretope regions can be distinguished within MBP(81 – 105). The determination of competitor IC50 values for MBP(83 – 93) (IC50 = 52 μM) and MBP(86 – 96) (IC50 = 25 μM) revealed that these overlapping agretopes combine within MBP(81 – 99) to give a high-affinity H2-As binding peptide (IC50 = 5.8 μM).
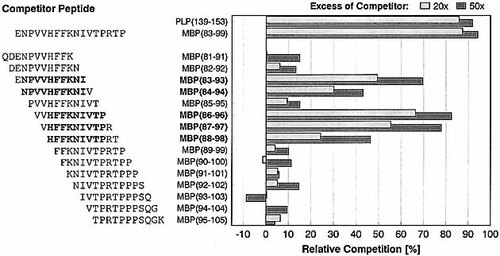
Determination of core agretopes within encephalitogenic peptide MBP(81 – 105) using overlapping 11-mer peptides (H2-As competition assay). The relative competition of test peptides is shown at a 20- and 50-fold molar excess, respectively, against AMCA-labeled PLP(139 – 153) for binding to H2-As. At least two agretope regions are embedded within MBP(81 – 105), comprising the dominant agretope core (88 – 96) present in MBP(86 – 96) / (87 – 97) /(88 – 98) and an additional agretope core (84 – 93) contained in MBP(83 – 93) / (84 – 94) (suggested agretope cores within peptide sequences emphasized in bold).
L-Ala scans were then performed with monosubstituted derivatives of MBP(86 – 96), MBP(83 – 93), and PLP (139 – 151), and the differences in competition of substituted peptides relative to the unsubstituted peptide were calculated. Difference value < 10 % are judged to be within the error limit of the assay. Fig. 2 A shows the results of an L-Ala scan for MBP(86 – 96). Three Ala-sensitive peptide anchor positions are clearly found. They correspond to Lys91, Ile93 and Pro96 in rel. pos. P4, P6 and P9, respectively, within a probable nonamer agretope (88 – 96), with Lys91 in P4 and in particular Pro96 in P9 being the preferred anchor residues. The substitution Pro96 → Ala96 results in an almost complete abolishment of H2-As binding. Strikingly, an N-terminal anchor residue (rel. pos. P1) cannot be observed in this assay.
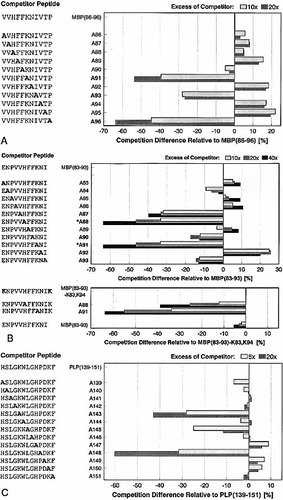
L-Ala-scans of MBP(86 – 96) (A), MBP(83 – 93) (B) and PLP(139 – 151) (C) to determine peptide side chains influencing H2-As binding (competition assay). The competition differences of Ala-monosubstituted peptides relative to the corresponding unsubstituted peptides competing against AMCA-PLP(139 – 153) (A, B) and AMCA-mTFR(203 – 218) (C), respectively, are shown at different competitor concentrations. Reference competition values come up to 46.5 % and 64 % at a 10- and 20-fold molar excess, respectively, of MBP(86 – 96) (A), 33 %, 49 % and 63.5 % at a 10-, 20- and 40-fold molar excess, respectively, of MBP(83 – 93) (B), and 63 % and 80.5 % at a 5- and 20-fold molar excess, respectively, of PLP(139 – 151) (C). The Cys140 → Ser140-substituted derivatives of PLP-peptides are used (C). Due to poor peptide solubility (*, uncertain data), additional derivatives of MBP(83 – 93) supplemented by terminal Lys residues (MBP(83 – 93)-K83,K94-peptides) were synthesized and tested for competition, with reference competition of 36 %, 55 % and 64 % at a 10-, 20- and 40-fold molar excess, respectively, of MBP(83 – 93)-K83,K94 (B, lower panels). MBP(86 – 96) (A) and PLP(139 – 151) (C) use similar anchor side chain combinations, weaker H2-As binding MBP(83 – 93) (B) reveals a diverged more complex anchor usage. The results correspond to a (P1)-P4-P6-P7-P9 binding motif.
Fig. 2 B shows the results of the L-Ala scan for MBP(83 – 93). Here, the identification of anchor residues is more difficult because of partly reduced peptide solubility and because of data that do not match directly with the (P1)-P4-P6-P9 binding motif as deduced for MBP(86 – 96). Substitution of the more soluble peptide MBP(83 – 93)-K83,K94 at His88 and Lys91, respectively, clearly leads to a reduced competition capacity, and substitution of Lys91 even completely abolishes H2-As binding. Thus, His88 and Lys91 have to be judged as major anchor residues. Significant binding effects are also observed for Val87, Phe90, Ile93 (reduced binding) and Asn92 (increased binding). This can be explained by at least two overlapping agretopes spanning residues (84 – 92) and (85 – 93). The first uses Val87 in rel. pos. P4 and may be inhibited from strong H2-As binding by an unfavorable Asn92 in P9. The second preferred agretope probably uses His88 in P4 and Lys91 in P7 as primary anchors and Phe90 in P6 as well as Ile93 in P9 as potential weak auxiliary anchors. Distinct P1 anchor residues are not detectable by the Ala scan.
Fig. 2 C shows the results of the L-Ala scan for PLP(139 – 151), which represents the encephalitogenic PLP peptide with the highest binding affinity to H2-As 11. As for MBP(86 – 96), three anchor positions can be identified, Lys143, Leu145 and Pro148. Provided that MBP(86 – 96) and PLP(139 – 151) use the same anchor arrangement within a nonamer agretope, a P4-P6-P9 anchor combination within the agretope (140 – 148) mediates the strong H2-As binding of PLP(139 – 151), with Lys143 in P4 and in particular Pro148 in P9 as primary anchor residues. Again, a P1 anchor residue cannot be identified.
2.2 Identification of naturally processed peptide ligands of H2-As and characterization of their binding to purified H2-As molecules
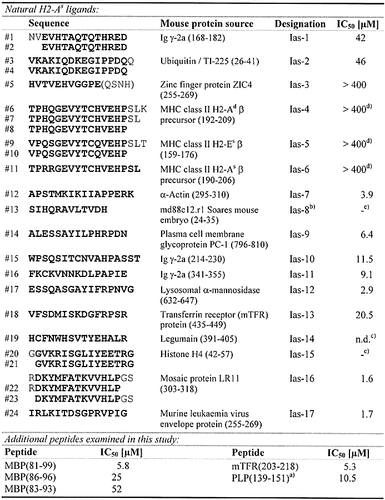
Naturally processed peptide ligands were identified by acidic elution from immunoaffinity-purified H2-As : peptide complexes with subsequent peptide separation and characterization. The sequence of 24 distinct peptides was determined, distributed among 15 different HPLC fractions (Table 1), and could finally be summarized into 17 different self-peptide sequences derived from 15 different mouse protein sources. These were mainly membrane-bound or vesicular / secretory proteins characteristic of the MHC class II-associated antigen presentation pathway. The peptides were synthesized and tested for binding to H2-As by determination of IC50 values (Table 1) where ten peptides were identified as moderate (IC50 < 400 μM) to high-affinity (IC50 < 40 μM) binders. Of the remaining seven peptides (IC50 > 400 μM), four clearly showed H2-As binding at a higher peptide concentration (Ias-3, -4, -5, -6; IC50 400 – 800 μM). In contrast, no significant competition capacity was observed for Ias-8, -14 and -15. In the case of Ias-14 this is mainly due to its poor solubility. The Cys → Ser-substituted soluble derivative of Ias-14, however, also reveals only weak binding (IC50 > 400 μM).
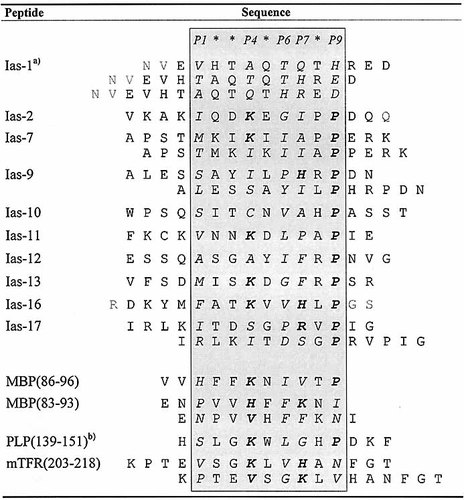
Table 2 shows the alignment of all identified self-peptide sequences with established H2-As binding (IC50 < 400 μM) listed according to a putative (P1)-P4-P6-P7-P9 binding motif (in P4 and P7 basic residues, in P9 Pro highly favored). This is compared with anchor patterns deduced for the MBP, PLP and mouse transferrin receptor (mTFR) peptides also examined in this report. The presence of basic residues in P4 and P7, and of hydrophobic residues in P6, respectively, but in particular the accumulation of Pro in P9 is generally noticed. Moreover, all H2-As ligands containing anchors matching this motif turn out to be high-affinity binders.
- Shown in the upper part are the 24 identified primary sequences (#1 – #24), which partly comprise N- and C-terminal truncation variants of the same peptide. Uncertain sequence sections are shown in parentheses. For each of the resulting 17 different core peptides (emphasized in bold), one longer peptide covering the whole sequence (designated Ias-1 to Ias-17), respectively, was synthesized and tested in H2-As competition assays. For comparison, IC50 values of some additional peptides are added below. IC50, competitor concentration leading to 50 % inhibition of binding of AMCA-PLP(139 – 153) to purified H2-As molecules. a) Cys140 → Ser140-substituted derivative, competing against AMCA-mTFR (203 – 218); b) sequence uncertain at the C-terminus; c) n. d., not reliably detectable because of peptide precipitation during the assay; d) binding reduced by peptide oxidation; e) no binding observed at peptide concentrations up to 400 μM.
- Amino acid residues in assumed anchor positions P4, P6, P7, P9 as well as in P1 written in italic, potential main anchor residues in P4, P7 and P9 emphasized in bold. The alignment was also performed in consideration of the results obtained by the various substitution analyses presented herein. The frequency of Pro near the C-terminus of naturally processed H2-As ligands, in assumed anchor position P9, is striking, facilitating the alignment of most peptide sequences. Some ligands may contain several or alternative agretope patterns, which are also listed. For comparison, probable agretope patterns of MBP-, PLP- and mTFR-peptides deduced herein are added below. a) Uncertain peptide alignment; b) Cys → Ser-exchange in peptide position 140.
The high-affinity binding of selected Ias-peptides was analyzed further by use of derivatives containing single and multiple L-Ala substitutions in the putative agretope anchor positions. For Ias-12, substitution of Ile in P6 has little effect, but substitution of Pro in P9 decreases peptide binding to a third of the starting competition value (data not shown). Obviously, the Pro-residue serves as primary anchor as suggested above.
Ias-16 and Ias-17 are among the best H2-As binding peptides that we have found. For Ias-16, introduction of single Ala residues at P1, P4, P6 and P7 has little effect (Fig. 3). Only substitution of Pro in P9 leads to a significant reduction in competition (Ias-16-A9), with comparable results also obtained for the double- and triple-substituted peptides. Complete loss of competition ability is observed for Ias-16-A4 / 6 / 7 / 9, derived from the triple-substituted peptide by further substitution of His in P7. We concluded that four anchor residues within one agretope, located in rel. pos. P4 (Lys), P6 (Val), P7 (His) and P9 (Pro), together mediate the peptide's strong binding. Again, Pro in P9 is the primary anchor, but His in P7 as well as the combination Lys in P4 / Val in P6 also contribute to this. Ias-16 appears to match the agretope requirements of H2-As so closely that only multiple anchor substitutions can diminish its binding ability substantially. Moreover, the presence of only one suitable anchor residue is sufficient to maintain peptide binding to H2-As.
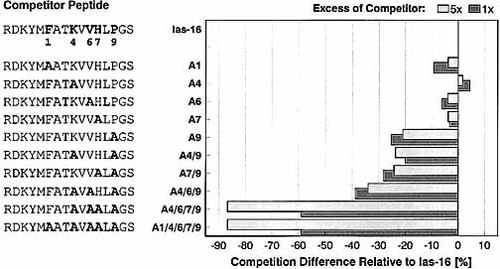
L-Ala-substitution analysis of self-peptide Ias-16 (H2-As competition assay). The competition capacity of Ias-16 and derivatives substituted at P1, P4, P6, P7 and P9 is tested at one- and fivefold molar amount of competitor against AMCA-PLP(139 – 153) at H2-As. The difference in competition relative to Ias-16 is calculated for the substituted peptides. Reference competition of Ias-16 is 59 % at onefold and 87 % at fivefold molar amount, respectively. Ias-16 is characterized by a multiple anchor usage in P4, P6, P7 and P9, with Pro (P9) and at least His (P7) as important H2-As interacting residues.
For Ias-17, single or multiple Ala-substitutions at putative anchor positions P1 (Ile), P6 (Pro), P7 (Arg), P9 (Pro), but not at P4 (Ser), are accompanied by a clear decrease in competition capacity (data not shown), pointing to an alternative agretope pattern compared with Ias-16. Besides favored amino acid residues in suited anchor positions, Ias-17 contains two overlapping agretopes each C-terminally anchored by a different Pro-residue, which combine to mediate a high-affinity binding at H2-As in the same order of magnitude as does the single agretope / multiple anchor combination within Ias-16. The presence of these agretopes within Ias-17 could be demonstrated by analysis of two truncated peptides, Ias-17-L (IRLKITDSGPR) and Ias-17-R (KITDSGPRVPI): IC50 (Ias-17-L) = 300 μM, IC50 (Ias-17-R) = 43 μM, compared with IC50 (Ias-17) = 1.7 μM. Thus, two overlapping suboptimal agretopes (RLKITDSGP and ITDSGPRVP; putative main anchor residues in bold) combine to result in one high-affinity binding peptide, as observed more clearly for peptide MBP(81 – 99), covering MBP(83 – 93) and MBP(86 – 96) (see Fig. 1 and Table 1). In a similar analysis, we conclude that mTFR(203 – 218), an additional natural H2-As ligand identified previously by Rudensky et al. 14, may also use two agretopes (listed in the lower part of Table 2).
2.3 Additional studies on anchor positions P4 and P7
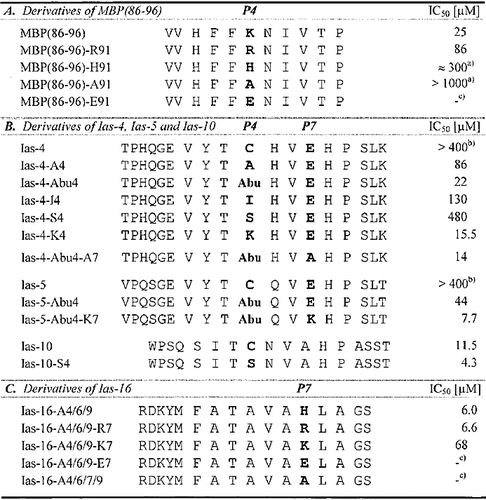
MBP(86 – 96) is suitable for studying the anchor usage in rel. pos. P4 because of the clear effects upon Ala-substitution within this peptide (Fig. 2 A). Substitution of Lys in P4 by basic or acidic residues other than Ala allows the ranking of anchor preferences in P4 in the order Lys > Arg > His > Ala > Glu, the latter completely abolishing H2-As binding (Table 3 A). Thus, positively charged side chains are favored but negatively charged ones excluded.
Ias-4, Ias-5 and Ias-6 represent H2-As ligands with a strikingly high sequence homology and weak binding (Table 1). All three peptides are probably anchored by Pro in P9 and contain a Cys residue at rel. pos. P4, suggesting oxidation-sensitive Cys as a possible anchor. To evaluate the role of Cys, we determined IC50 values for Ias-4 derivatives containing the following substitutions in P4: aliphatic side chains of different length (Ala, Abu, Ile), polar (Ser), or charged (Lys) groups. α-Aminobutyric acid (Abu) was used because of its structural and physicochemical similarity to Cys. As shown in Table 3 B, clear differences in IC50 values are observed. The residue in P4 serves as anchor mediating H2-As binding in the order Lys > Abu >> Ala > Ile >> Ser > Cys. However, in the case of Cys (Ias-4) peptide binding is strongly dependent on the oxidation state of its sulfhydryl group, with binding in its reduced form coming up to values observed for Ias-4-Abu4, but successively dropping upon oxidation (not shown). Comparable results are also obtained for Ias-5: a Cys → Abu substitution clearly improves its H2-As binding (Table 3 B, Ias-5-Abu4). Substitution of (oxidized) Cys by Ser applied to Ias-10 has a less pronounced effect (Table 3 B, Ias-10-S4). We conclude that most of the binding affinity in P4 is mediated by electrostatic interactions but that small aliphatic residues are similarly favored. In accordance with these results we have already judged Lys and Val as important P4-anchor residues (Fig. 2). Ser (e. g. in Ias-17) has to be considered as a non-anchor residue but Cys (in Ias-4, Ias-5, Ias-6 and Ias-10) in its reduced more hydrophobic sulfhydryl form as a favored residue in P4. Interestingly, eliminating Glu in P7 by insertion of Ala leads to a further increase of binding (compare Ias-4-Abu4 and Ias-4-Abu4-A7 in Table 3 B) due to the preference for basic residues at this position. Insertion of Lys in P7 of Ias-5-Abu4 caused its IC50 value to drop to an even lower level and converts this peptide to a high-affinity binder (Table 3 B, Ias-5-Abu4-K7).
- Anchor residues in P4 (A, B) and P7 (B, C) emphasized in bold; IC50-values determined against AMCA-PLP(139 – 153). a) inexact data due to partial peptide precipitation at elevated concentration; b) IC50-values at least coming up to 400 – 800 μM but inexact data significantly lowered due to peptide oxidation; c) no binding detectable.
The triple Ala-substituted derivative of Ias-16, Ias-16-A4 / 6 / 9, still shows high-affinity H2-As binding but is considered to contain only one major anchor residue (His) remained in rel. pos. P7 (Fig. 3), therefore being well suited for a further characterization of P7-anchor usage (Table 3 C). Exchange of His by Arg (Ias-16-A4 / 6 / 9-R7) has only a minor effect, but exchange by Lys (Ias-16-A4 / 6 / 9-K7) leads to a clear decrease of binding. No competition ability remains upon substitution of His for Glu (Ias-16-A4 / 6 / 9-E7) as similarly observed upon Ala-substitution (Ias-16-A4 / 6 / 7 / 9). We conclude that acidic residues are unfavorable but basic residues highly preferred in P7 following the order His ≈ Arg > Lys, and that a positive charge contributes to the main part but not all of the binding strength mediated by this anchor.
2.4 Binding of L-Ala-polysubstituted minimal anchor peptides
The minimal requirements for peptide binding to H2-As were determined by Ala-polysubstituted peptides containing the most favorable residues in main anchor positions P4 (Lys), P7 (His), and P9 (Pro), respectively. Peptides containing only one such anchor residue are able to compete with high affinity at H2-As (Fig. 4). Pro in P9 (poly-AK-P) and His in P7 (poly-AK-H) are equally important anchor residues both superior to Lys favored in P4 (poly-AK-K). A further increase of binding is observed for the peptides containing two anchor residues (poly-AK-KP, poly-AK-HP), whereas insertion of a third anchor leads to only a minor additional increase (poly-AK-KHP). The IC50 values of poly-AK peptides embedding two or three dominant anchor residues approach those determined for Ias-16 and Ias-17.
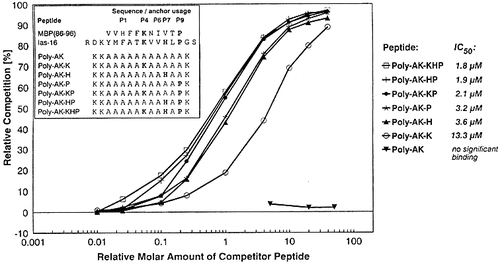
H2-As binding of L-Ala-polysubstituted minimal anchor peptides (competition assay). Poly-AK-peptides, derived from MBP(86 – 96) and Ias-16, are based on an 11-mer L-Ala core embedding the most favourable residues in main anchor positions P4, P7 and P9 (bold), supplemented by N- and C-terminal Lys for maintenance of peptide solubility. IC50 values are determined by testing competition at 0.01- to 40-fold molar amount (0.03 – 100 μM) relative to the binding of AMCA-PLP(139 – 153) to H2-As. Poly-AK without any anchor residue shows no significant binding, even at elevated peptide concentrations (100 – 400 μM), enabling an evaluation of agretope structure by successive introduction of suited anchor residues.
2.5 H2-As peptide binding motif
The H2-As peptide binding motif deduced by these experiments is summarized in Fig. 5. In general, acidic residues are rather unfavorable but hydrophobic and basic residues are well tolerated or even preferentially used as anchor side chains at different agretope positions. Pro in P9 serves as conserved primary anchor of many H2-As ligands (Table 2), but comparable contributions to H2-As binding are also mediated by electrostatic MHC : peptide interactions in rel. pos. P4 and P7. The agretope pattern is characterized by several dominant anchor positions corresponding to marked pockets within the MHC binding groove, which can be alternatively employed for high-affinity binding.
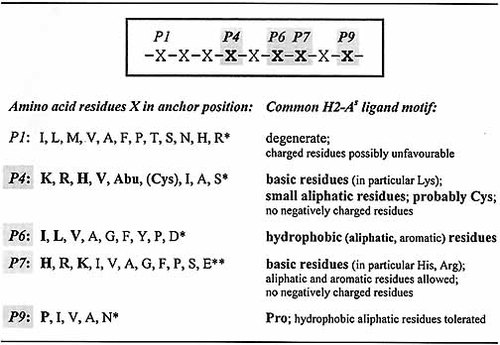
Summary of (tolerated and anchor-) amino acid residues found in probable anchor positions and deduction of a common H2-As ligand motif. Major anchor residues proven to contribute to H2-As binding are emphasized in bold; *, may lead to reduced binding; **, reduced binding proven. MHC : peptide interactions are preferentially electrostatic / hydrophobic in rel. pos. P4, hydrophobic in P6, electrostatic in P7 and hydrophobic / sterical in P9. A strong influence on peptide binding is mediated by the interactions concerning three primary anchor positions P4, P7 and in particular P9, with one favoured anchor enabling the presence of less favoured residues at other positions. Side chains located in P6 are classified as secondary anchors; side chains in P1 are considered to contribute only insignificantly to peptide binding.
3 Discussion
We have deduced an H2-As peptide binding motif (Fig. 5) by analysis of naturally processed peptide ligands and peptide substitution assays. Natural H2-As ligands were investigated similarly to the method employed by Rudensky et al. 14. Three of five different H2-As self peptides described by them were also recovered by us (Table 1; Ias-1, Ias-10, Ias-17). A fourth peptide shows striking homology to Ias-16, differing by three residues. The fifth peptide, mTFR(203 – 218), was not found by us but has high affinity to H2-As (Table 1). Nevertheless, our binding motif clearly differs from that deduced by Rudensky et al. 14 in the absence of binding studies, but we can confirm His as a dominant anchor in rel. pos. P7.
Further studies of peptide binding to H2-As have been rather scarce. Some aspects of our motif were anticipated theoretically by Reizis et al. 18. A significant difference is that instead of the Pro-anchored agretope 91 – 99 within MBP (KNIVTPRTP) predicted by them, we found MBP(83 – 93) to harbour other agretope(s) in addition to MBP(86 – 96) (Fig. 1). The identification of at least two agretopes and the corresponding anchor usage within MBP(81 – 99) (Figs. 1, 2) should facilitate the analysis of this peptide's T cell recognition. It is striking but probably accidental that the immunodominant region 81 – 105 of human MBP also contains various agretopes capable of binding to a set of different HLA-DR molecules 19 – 21, even though the anchor recruitment of HLA-DR and H2-As molecules is substantially different.
For PLP(139 – 151) (Fig. 2 C), Leu145 and Pro148, but not reliably Lys143, have been described as H2-As anchor residues 15. According to this report, positively charged side chains appear unfavorable in P6 and P9. PLP (100 – 119) (FGDYKTTICGKGLSATVTGG), PLP(178 – 191) (NTWTTCQSIAFPSK) and PLP(260 – 276) (ATYNFAVLKLMGRGTKF) represent further encephalitogenic or immunogenic determinants of PLP for which high-affinity H2-As binding was demonstrated 11. Applying the present motif we predict for PLP(100 – 119) Lys110 (P4) and Leu112 (P6), for PLP(178 – 191) Ile186 (P6) and Pro189 (P9), and for PLP(260 – 276) Lys268 (P4) and Met270 (P6), respectively, as major anchors. For αB-crystallin, another important myelin antigen, we predict H2-As binding of its dominant T cell epitope 43 – 52 (SLSPFYLRPP) via Leu49 (P6), Arg50 (P7) and Pro52 (P9) 22. It may be possible to explain the MHC : agretope interaction of other known H2-As-binding peptides in a similar manner, but experimental verification would be appropriate in cases of poorly recognizable anchor recruitment.
The simple structural demands for H2-As binding particularly revealed by the Ala-polysubstituted peptides (Fig. 4) – one proper anchor residue in rel. pos. P4, P7 or P9 necessary and sufficient to convert a non-binder into a high-affinity binder – are characteristic of MHC class II molecules, as most of the MHC : peptide binding energy is mediated rather unspecifically by the peptide backbone 16, 17, 23. Therefore, a major criterion determining H2-As binding specificity is also the absence of inhibitory residues disturbing MHC : peptide interaction. The structural basis for the peptide-binding specificity of homologous mouse / rat H2-A / RT.1B and HLA-DQ molecules, compared with well-characterized HLA-DR / H2-E motifs, was discussed in detail recently 18. A principle P1-P4-P6-P7-P9 anchor pattern is characteristic of MHC class II molecules including HLA-DR and H2-E 23 – 25 as well as various HLA-DQ 26 – 28 and H2-A molecules 16 – 18. However, peptide binding to most HLA-DQ / H2-A vs. HLA-DR / H2-E molecules is characterized by a different hierarchy of anchor positions and specificities, e. g. pockets P4 and P9 are marked and pocket P1 of H2-A in general is more polymorphic than that of H2-E molecules. The pronounced role of an additional P7 anchor residue possibly distinguishes H2-As from other H2-A alleles. Computer modelling of H2-As indicated that the central region of its binding groove is dominated by two acidic residues, β28 (Asp) and β74 (Glu), which favor binding of peptides with basic side chains at P4 and / or P7 22.
Another special aspect of peptide binding by H2-As is the unusual spcificity for Pro as dominant C-terminal anchor, although not mandatory for high-affinity binding (Fig. 4). The accumulation of Pro-residues at or near the C terminus of many natural H2-As ligands is striking (Tables 1, 2). Apart from being primary anchors they may also be stop signals for antigen processing. Due to the latter function some accumulation of Pro (both at the N and C termini) was also found among other natural MHC class II ligands 29, however, a role of Pro as a primary anchor residue has not been confirmed as clearly as in the case of H2-As. This strong preference for Pro may be due to an abrogated peptide backbone hydrogen bond formation at P9 because of a rare α69 Asn → Thr substitution in H2-As 18 along with a β61 Trp → Tyr substitution. Conserved residues at P9 rather than at P1 likewise appear to be important for peptide binding to the H2-A homologous, EAE-associated RT1.Bl molecule of the Lewis rat 30, 31. The relatively degenerate nature of P1 is in good agreement with some HLA-DQ binding characteristics 26 – 28, 32, suggesting a common organization of H2-A / DQ motifs. Residues in P9 critical for selective peptide binding may be particularly involved in H2-A / DQ-associated susceptibility to autoimmune diseases 18, 33.
The deduced H2-As motif allows a more precise prediction of high-affinity binding peptides, e. g. facilitating the identification of epitopes in novel target proteins relevant in the pathogenesis of EAE. The Ala-polysubstituted minimal-anchor peptides may be further employed as tool for (a) the identification of additional anchor or inhibitory residues to improve the present H2-As motif, (b) the design of derivatives of encephalitogenic peptides like MBP(81 – 99) or PLP(139 – 151) allowing the analysis of potential TCR-contact residues, and (c) they may act as lead structures for the design of altered peptide ligands and peptide analogs. Such compounds could be used to modulate T cell responses in the SJL mouse model and may advance therapeutic approaches in autoimmune diseases.
4 Materials and methods
4.1 Cell lines and isolation of MHC molecules
The hybrid B lymphoma cell line LS102.9 (coexpressing H2-As and H2-Ed) 34 was maintained in RPMI 1640 medium (Gibco, Grand Island, NY) plus 10 % heat-inactivated fetal calf serum, antibiotics, and 2-ME. The mouse hybridoma cell line Y-3P was used for isolation of the anti H2-As mAb Y-3P 35. H2-As molecules were purified from cell lysates by immunoaffinity chromatography, using Y-3P 14 covalently coupled to cyanbromide-activated Sepharose CL-4B (Pharmacia Biotech, Freiburg, Germany), essentially as performed for isolation of HLA-DR molecules 20 with Zwittergent-12 (Calbiochem, San Diego, CA) as detergent. Purity was checked by SDS-PAGE, high-performance size-exclusion chromatography (HPSEC) and Western blotting.
4.2 Release and analysis of H2-As bound self-peptides
Peptides were eluted from H2-As complexes by treatment with diluted trifluoroacetic acid (TFA) at pH 2 and 4 °C, essentially as described 20, using Y-3P column eluates of highest purity (4.5 mg effective protein amount). The low-molecular-weight fraction was separated by ultrafiltration through a 20-kDa cut-off membrane and concentrated to < 1 ml by speed vac. The concentrated peptide pool was fractionated by capillary liquid chromatography on a reversed phase-C18-column (300 μm × 250 mm; LC-Packings, Amsterdam, The Netherlands) equilibrated with 95 % system A (0.025 % TFA / H2O), 5 % system B (84 % acetonitrile in 0.025 % TFA / H2O). Peptides were eluted under monitoring at 214 / 295 nm applying a gradient of 5 % – 50 % system B in 90 min followed by 50 % – 100 % system B in 15 min at a flow rate reduced to 4 μl / min by a pre-column split. Column eluates were collected in 3 – 6 μl-fractions according to the UV absorbance. HPLC fractions with the highest UV absorbance (20 fractions) were analyzed by mass spectrometry and Edman degradation. One fourth of each fraction was used for low flow electrospray ionization mass spectrometry (ESI-MS; TSQ 7000, Finnigan, Bremen, Germany) and matrix-assisted laser desorption ionization mass spectrometry (REFLEX III, Bruker Daltonik, Bremen, Germany), recording peptide masses as well as peptide fragment spectra (ESI-MS / MS). The remaining three fourths were applied to Edman-sequencing on an ABI 494CLC gas-phase sequenator (Applied Biosystems / Perkin-Elmer, Weiterstadt, Germany). Peptides of heterogeneous fractions were identified by combination of Edman degradation data, the MS / MS technique, accurate peptide mass measurements and database searches.
4.3 Peptide synthesis and fluorescence labeling
Peptides were prepared by solid-phase synthesis on a multiple automatic peptide synthesizer system (Syro, MultiSynTech, Bochum, Germany) as described 36. Identity of the peptide amides was confirmed by ESI-MS and purity (> 80 %) was determined by reversed phase-HPLC. N-terminal labeling of peptides was performed using the fluorescent dye 7-amino-4-methylcoumarin-3-acetic acid (AMCA; Calbiochem), as described 20. Peptide stock solutions were prepared at 1 – 5 mg / ml in 0.02 % TFA or 5 % DMSO. For PLP peptides the Cys140 → Ser140-substituted derivatives are used. The MBP-peptide nomenclature is related to the sequence of the 170 amino acid residue isoform of human MBP.
4.4 H2-As peptide binding / competition assay
MHC peptide binding / competition assays were performed by HPSEC 20. Purified H2-As molecules (0.3 μM) were coincubated with standard binding peptides AMCA-PLP(139 – 153) or AMCA-mTFR(203 – 218) (2.6 μM) and competitor peptides in HPSEC buffer (150 mM sodium phosphate pH 6.0, 15 % acetonitrile, 0.1 % Zwittergent-12), supplemented with protease inhibitors. Competitor peptides were added in various molar amounts relative to the AMCA-labeled peptide (40 μl final assay volume). After incubation for 48 h at 37 °C, 15-μl aliquots were analyzed on a Superdex 75 PC 3.2 / 30 high-performance gel filtration column (Pharmacia), using a Merck-Hitachi HPLC-system (Merck, Darmstadt, Germany). Fluorescence signals of bound AMCA-labeled peptides (350 / 450 nm) were normalized to corresponding UV signals of eluting H2-As molecules (214 nm); relative competition values were calculated from normalized fluorescence signals in the absence (Fo) and presence (Fc) of competitor peptide: % competition = 100 × (Fo – Fc)/Fo. The error limit of the assay was ≤ 5 %.
Acknowledgements
We thank Dr. P. Marrack (Howard Hughes Medical Institute, National Jewish Center for Immunology and Respiratory Medicine, Denver, Colorado) and Dr. K. Reske (Institute of Immunology, University of Mainz) for supplying cell lines LS102.9 and 14-4-4S, Dr. A. Bittner (Department of Neurology, Medical School, University of Tübingen) for help with cell culture, and A. Torun (Institute of Organic Chemistry, University of Tübingen) for synthesis of peptides. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 510) and the Gemeinnützige Hertie-Stiftung.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH