Impaired development of HIV-1 gp160-specific CD8+ cytotoxic T cells by a delayed switch from Th1 to Th2 cytokine phenotype in mice with Helicobacter pylori infection
Abstract
Th1 and Th2 cells play a central role in immunoregulation during infection. We show that Helicobacter pylori induces Th1 cytokine responses early (2 weeks) but predominantly Th2 responses later (6 weeks) in infection. The switch is principally mediated by urease-specific CD4+ T cells, and correlates with a loss of urease-specific high-avidity JNK+ Th1 and gain of low-avidity JNK– (possibly Th2) cells at the later stage of infection, concomitant with a 100-fold higher colonization level of H. pylori at 6 weeks than at 2 weeks that might tolerize high-avidity Th1 cells. Furthermore, differentiation of HIV gp160-specific CD4+ Th and CD8+ cytotoxic T lymphocytes (CTL) into effector cells is impaired in 6-week H. pylori-infected mice immunized with vaccinia expressing gp160, and serum IL-12 stimulated by vaccinia infection is barely detectable. Adoptive transfer of urease-specific Th2 cells to mice infected only with gp160-expressing vaccinia abrogates Th1 polarization of the gp120 response, down-modulates virus-specific CTL responses, and delays virus clearance. Therefore, the H. pylori urease-mediated immunoregulation in the switch from JNK+ Th1 to JNK– Th2 phenotype, and the preceding low IL-12 response, are likely critical steps in the impairment of antiviral immunity.
Abbreviations:
-
- GST:
-
Glutathione S-transferase
-
- JNK:
-
c-jun NH2-terminal kinase
-
- moi:
-
Multiplicity of infection
1 Introduction
Helicobacter pylori is a gram-negative spiral bacterium 1 responsible for a large portion of chronic gastritis, duodenal and gastric ulcers and probably an increased risk of gastric adenocarcinoma 2 – 4. It infects over half the world adult population 5, and is also common in HIV-1-infected patients 6. Cellular immunity in the H. pylori-associated diseases, including superinfection by viruses such as HIV-1, has not been characterized well. There has been some uncertainty in the literature as to whether a Th1 or Th2 type of immune response predominates in Helicobacter infection 7 – 13, depending on the system studied, such as host genotype and Helicobacter species (H. pylori vs. H. felis). For example, studies of H. felis infection in C57BL / 6 mice found that a Th1 response dominated, but a Th2 response could be revealed if IFN-γ was neutralized in vivo 12, and that Th1 cells enhance gastritis whereas Th2 reduce bacterial load 13. Also, in BALB / c mice, recombinant urease induced a Th2 response and cured H. felis infection 10. In a study of human blood T cells, live H. pylori stimulated a Th1 response whereas urease stimulated IL-10 production 11. Here, using H. pylori in BALB / c mice, we provide a new perspective by showing a switch from Th1 to Th2 response over the course of infection, concomitant with a switch from urease high-avidity T cells to low-avidity T cells, possibly caused by high urease concentration-mediated tolerization of high-avidity T cells.
Th1 or Th2 effector cells mediate inflammatory or humoral responses, respectively 14, 15. IL-12 activates the differentiation of Th1 cells and induces IFN-γ production by these cells 16 – 18, whereas IL-4-mediated signals favor a Th2 cell response 19. IL-4 can down-regulate IL-12 receptor expression on Th2 cells, making them insensitive to IL-12 signals that would normally invoke a Th1 cell response 20. In certain infectious diseases in mice and humans, the Th1 pattern of cytokines is associated with resistance to infection, whereas the Th2 pattern is associated with disease progression 21 – 24. It also has been reported that a switch from Th1 to Th2 cytokine phenotype is a critical step in the progression of HIV-1 disease 24.
T cell anergy is a state of functional unresponsiveness characterized by the inability to produce IL-2 upon TCR stimulation 25, 26. c-Jun NH2-terminal kinase (JNK) is a mitogen-activated protein kinase thought to play a key role in transmission of signals from the cell membrane to AP-1-binding DNA sequences inside the nucleus. Therefore, a defect in JNK function could be responsible for the poor AP-1-dependent transcription observed in anergic T cells 27. Further, there is an impairment in Th1 differentiation in the absence of JNK2 28. The attenuation of Th1 responses is primarily due to reduced IL-12-stimulated IFN-γ secretion by CD4+ T cells at early stages of the differentiation process. Thus, the JNK signaling pathway may play a critical role in regulating the balance of Th1 and Th2 cells. Induction of anergy or apoptosis in CD4+ T cell clones occurs following exposure to supraoptimal antigen doses 29 – 31. The urease of H. pylori, an essential and highly immunogenic protein 10, 32, is a major component of the bacterium (between 5 and 10 % of the total protein) and localizes very densely at the bacterial membrane on the gastric mucosa 33, 34. We therefore asked whether supraoptimal concentrations of urease could induce functional loss of urease-specific T cells.
Previously, we reported that during persistent H. pylori infection of mice, there is down-modulation of Th1 cytokine production and CD8+ CTL response toward HIV-gp120 (a subunit of gp160), with concomitant delay of virus clearance 35. However, the mechanisms of these effects remain unclear. To address the role of H. pylori urease, we generated a urease-negative mutant of H. pylori strain KS612. We now report that a urease-dependent persistence of H. pylori infection and urease-mediated immunoregulation causes a delayed switch from Th1 to Th2 cytokine phenotype associated with a switch from high to low avidity of urease-specific T cells, reduced JNK activity, and low serum IL-12, leading to reduction in both Th1 cytokine and CTL responses toward HIV-1 gp120 and impairment of immune protection against natural viral infection.
2 Results
2.1 Th1 to Th2 switch in cytokine pattern during the course of H. pylori infection
H. pylori was detected in the stomach of all mice infected with a fresh clinical isolate (KS612) or the ure-mutant (KS612ure–) at 3 days after the infection, whereas at 2, 3 and 6 weeks the colonization was detected only in the mice inoculated with the wild-type strain. Vaccinia infection had no effect on the colonization of H. pylori. To compare the effect of the wild-type strain KS612 and the ure– mutant on immune responses to H. pylori itself and to a virus, 3 weeks after the H. pylori infection, BALB / c mice were inoculated i. p. with vPE16, a recombinant vaccinia virus expressing the HIV-1 gp160 envelope protein 36. We measured the cytokine production by spleen cells in response to urease at 0, 2, 3 and 6 weeks after the H. pylori infection (Fig. 1). H. pylori infection induced Th1 cytokine (IFN-γ, IL-2) responses specific for urease at 2 weeks, while Th2 cytokine (IL-4) responses predominated at 6 weeks after the infection (Fig. 1). Mixed cytokine phenotypes were observed at 3 weeks. The cytokine responses to H. pylori lysate showed a pattern similar to that of responses to purified urease in wild-type H. pylori infection, suggesting that urease dominated or was representative of the overall response, but the transient infection of ure– mutant induced no Th2 cytokine response to any antigen contained in the H. pylori lysate at any time tested (data not shown). All these cytokine responses were found to be produced by CD4+ T cells (not shown). Thus, the Th1 to Th2 cytokine phenotype switch correlates with the urease-specific CD4+ T cell response during persistent H. pylori infection.
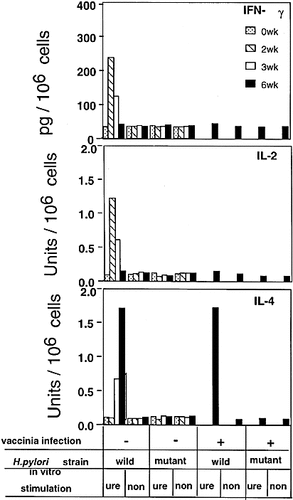
Cytokine production in vitro in response to urease in mice infected with recombinant vaccinia vPE16 expressing gp160, vPE16 plus H. pylori KS612, vPE16 plus H. pylori ure– mutant (KS612ure–), KS612 alone or KS612ure– alone. Mice 3 weeks after infection with H. pylori and uninfected control mice were infected with vPE16. Three weeks later, spleen cells at 5 × 105 cells / ml were incubated with 0.1 μM urease. Supernatants were assayed for IFN-γ, IL-2 and IL-4 as described, and expressed as amounts of cytokine per 106 cells present. Data represent mean responses of five mice per time point, with SE < 10 % of the mean. Thus, the error bars were not shown for simplicity of presentation. The experiment shown is representative of two repeat experiments which gave comparable results. The differences in cytokine production remained qualitatively similar when normalized for percentage of spleen cells that were CD4+ and CD8+. Ure; urease, non; no antigen.
2.2 Prolonged infection with H. pylori diminishes CTL and Th1 cytokine responses to gp160 and vaccnia
Mice infected with vPE16 alone generated strong CTL activity against gp160 and P18-I10, an immunodominant CTL epitope of gp160 (RGPGRAFVTI) 37, whereas the mice infected with H. pylori KS612 3 weeks prior to the vPE16-infection had much lower responses at 6 weeks of H. pylori infection (Fig. 2 a). Mice infected with H. pylori KS612 1 week after or at the time of the vaccinia infection (killed and tested at 2 or 3 weeks of H. pylori infection, respectively) and mice infected with the mutant strain KS612ure– prior to vaccinia infection mounted strong CTL responses against the gp160-expressing transfectant, 15-12, or P18-I10 (Fig. 2 a), at all E / T ratios tested, superimposable on those of the mice infected with vPE16 alone. Since the transfectant 15-12 expresses MHC class I molecules but not class II, CTL must recognize the epitope in the context of class I molecules. Thus, colonization of H. pylori KS612 for 6 weeks resulted in reduced CTL generation against gp160. The vaccinia-specific CTL activity was also reduced (three- to ninefold in lytic units, not shown) in the mice doubly infected with vaccinia plus KS612 for 6 weeks compared to the mice infected with vaccinia alone or vaccinia plus KS612ure– or with KS612 for only 2 or 3 weeks (Fig. 2 a). The CTL activity was predominantly CD8 dependent (not shown).
The mechanism of inhibition of CTL induction by H. pylori infection might be related to a shift in cytokine production from a Th1-like to Th2-like pattern. Therefore, we assessed whether cytokine production in response to HIV-1 gp120 was affected by H. pylori infection. Mice were immunized with vPE16, and their cells stimulated in vitro with recombinant gp120, the major subunit of gp160 available as soluble protein. Three weeks after virus infection, spleen cells from the mice infected with vPE16 alone or with vPE16 and the non-colonizing strain KS612ure–, or after only 2 or 3 weeks infection with KS612, produced high levels of IFN-γ and IL-2 in response to in vitro stimulation with recombinant gp120 (Fig. 2 b and c), but little IL-4 (Fig. 2 d). Conversely, spleen cells from KS612 (6 weeks)-vPE16 doubly infected mice showed reduced IFN-γ and IL-2 (Fig. 2 b and c), and modest IL-4 production (Fig. 2 d) in response to gp120. Production of IFN-γ, IL-2 and IL-4 was found to be CD4+ cell dependent in experiments in which CD4+ or CD8+ T cells were selectively depleted (not shown).
Because IL-12 activates Th1 cells 16, we investigated whether endogenous IL-12 plays a role in the up-regulation of CTL activity against virus. When naive mice were injected with vaccinia, serum IL-12 was strongly induced, whereas such induction was almost fourfold lower in 6-week H. pylori-infected mice, but only slightly reduced at 2 weeks (Fig. 2 e). These results indicate that the impaired development of anti-gp160 and anti-vaccinia CTL responses may be related to decreased production of IL-12 in response to vaccinia infection.
Virus clearance from tissues of mice infected with vPE16 after 6 weeks of infection with the colonizing strain KS612 of H. pylori was delayed 4 days in the spleen and ≥ 14 days in the liver as described previously 35, whereas no significant delay was observed when vPE16 was inoculated 2 weeks after infection with KS612 or 6 weeks after infection with the ure– strain (Fig. 2 f).
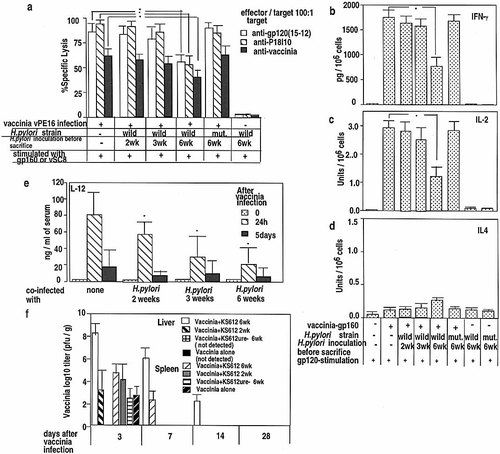
Anti-gp160 CTL and cytokine response to gp120 or H. pylori antigens in mice infected with wild-type or ure– mutant strains of H. pylori. H. pylori-infected or uninfected mice were co-infected with vPE16 1 week prior to, at the time of, or 3 weeks after H. pylori infection and killed 3 weeks later in (a – d). (a) Spleen cells were stimulated with gp160-expressing 15-12 cells or syngeneic spleen cells infected with control vaccinia vSC8. Six days later, CTL activity was tested against 51Cr-labeled 15-12 cells, P18 I10-pulsed or vSC8-infected (moi of 10) or control fibroblasts at E : T = 100 : 1. Lysis of control 3T3 fibroblasts was < 5 % in all cases (not shown). No CTL were detected in non-vaccinia-infected mice (< 5 %; not shown). (b, c, d) IFN-γ (b), IL-2 (c) and IL-4 (d) from spleens cells (5 × 105 cells / ml) stimulated with gp120 (2 μg / ml). Results remained similar when normalized for percentage of CD4+ and CD8+ cells. (e) Serum IL-12p70 after vPE16 in 2-, 3-, or 6-week-H. pylori-infected mice. Mice were challenged i. p. with vPE16 after the indicated times of infection with H. pylori KS612 and sera taken 0, 2 and 5 days after challenge (means of five mice per time point ± SEM). * p < 0.01 by Wilcoxon′s rank sum test vs. values in mice not infected with H. pylori. (f) Wild-type H. pylori infection delays clearance of vaccinia. H. pylori-uninfected mice 2 or 6 weeks after H. pylori infection were infected with vPE16, and at the times indicated the liver and spleen were titered for vaccinia. Three experiments showed comparable results. mut.; urease-negative mutant strain.
2.3 Adoptive transfer of urease- or gp120-specific CD4+ T cells: effects on CTL, cytokines and virus clearance
To assess whether the H. pylori urease-specific CD4+ T cells might affect the induction of the virus-specific CTL in vivo in vaccinia-infected mice, we transferred CD4+ T cells isolated from a urease-specific T cell line to the mice after vPE16 infection and examined the CTL and cytokine response against gp120. The urease-specific CD4+ cells produced predominantly IL-4 (2.8 U / 106 cells), but not IL-2 and IFN-γ, in response to 0.1 μM urease (data not shown). As a control, a T cell line specific for the T1 peptide of gp120, producing IFN-γ (452 pg / 106 cells) and IL-2 (3.2 U / 106 cells), but not IL-4, in the presence of 0.1 μM T1, was transferred to the mice. APC pulsed with the relevant antigen (urease or T1) were also transferred to stimulate the transferred T cells in vivo. Mice were killed at 3 weeks after the vaccina challenge. The vPE16-infected mice that received four injections of the urease-specific T cell line showed down-modulation of CD8+ CTL responses and down-regulation in Th1 cytokine (IFN-γ, IL-2) production toward HIV-gp120 similar to that observed in the mice infected with vPE16 plus H. pylori (Fig. 3 a – d), with concomitant delay of virus elimination (Fig. 3 e). Conversely, transfer of the T1-specific T cell line resulted in even higher Th1 cytokine responses. Transfer of naive spleen CD4+ T cells also showed no significant effect on the cytokine and CTL responses to gp120 (not shown). The mice receiving urease- and T1-specific T cells showed Th2 and Th1 cytokine responses to the corresponding antigen stimulation in vitro, respectively (not shown).
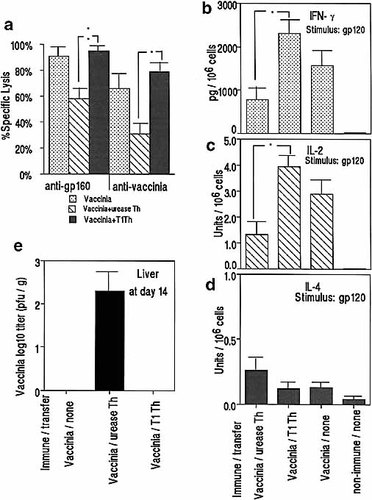
The CD4+ T cells purified from a H. pylori urease-specific CD4+ T cell line that predominantly produces IL-4, and a T1-specific CD4+ T cell line that produces IL-2, were injected i. v. (1 × 107 / mouse / injection) to vPE16 gp160-recombinant vaccinia-infected H-2d mice 4 times on days 1, 5, 8, and 12 after vaccinia infection, along with 1 × 107 APC pulsed with the specific antigen (urease or T1) at 1 μM for 2 h. CTL responses (a) and production of IFN-γ (b), IL-2 (c) and IL-4 (d), against gp120 were measured 3 weeks after vaccinia infection whereas virus in the liver was measured after 2 weeks (e). Lysis of control fibroblasts was < 5 % in all cases. Data represent mean responses of five mice per group ± SEM. * p < 0.01 by Wilcoxon′s rank sum test. The experiment is representative of two repeat experiments, with comparable results.
2.4 Changes in JNK activity and avidity of T cells early and late in H. pylori infection in parallel with cytokine shift
To assess whether high density of urease protein induces anergy in CD4+ T cells, T cell responses (proliferation and IL-2 and IFN-γ production) were generated from spleen cells of mice infected with H. pylori alone at 2 or 6 weeks after infection, or mice infected with H. pylori plus vPE16 (inoculated 3 weeks after H. pylori infection) at 6 weeks after H. pylori infection, by in vitro stimulation with various concentrations of urease protein (Fig. 4 a, b). T cell responses generated at 2 or 6 weeks of infection are distinct in the antigen requirement for proliferation (Fig. 4 a). At 2 weeks, proliferation and IL-2 production were stimulated by 0.0001 μM urease but not by 30 μM urease, whereas at 6 weeks only the high dose urease protein (30 μM) was able to stimulate either activity. The concentration of protein antigen required for optimal proliferation varied by nearly 100-fold betwen the two T cell populations, high- and low-avidity T cells (data not shown). In the presence of 30 μM urease protein, the Th1 cytokine-producing high-avidity T cells present at 2 weeks of the infection (shown in Fig. 1), generated on autologous APC as stimulators, were completely inhibited in their proliferative response. In contrast, the low-avidity T cells generated at 6 weeks were not susceptible to inhibition by the same high antigen dose. In fact, concentrations of protein antigen that were inhibitory for the high-avidity T cells promoted optimal proliferation of the urease-specific low-avidity T cells.
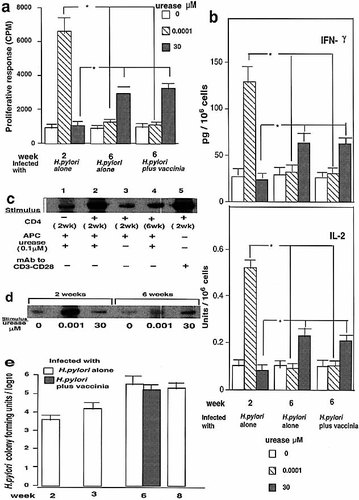
Urease-dependent activation of JNK kinase and anergy of urease-specific CD4+ T cells. Purified CD4+ T cells from mice infected as indicated with H. pylori and / or vaccinia (inoculated 3 weeks after H. pylori) were stimulated with urease and APC, and proliferation (a) and IL-2 and IFN-γ production (b) measured. (c) JNK activities of purified CD4+ T cells treated with urease. Without the effector cells, APC alone with antigen did not show any JNK activity. (d) JNK activity in the high- and low-avidity purified CD4+ T cells after stimulation with urease. (e) The colonization levels of H. pylori in the stomach at times indicated. Data represent means of five mice per time point ± SEM. * p < 0.01 Wilcoxon′s rank sum test. The experiments shown are representative of two repeat experiments, with comparable results.
Because Yang et al. 28 had shown a requirement for JNK2 activity in the differentiation of Th1 but not Th2 cells, we investigated the role of JNK in Th1 cell activation by urease during the switch from Th1 to Th2 cytokine phenotype, measuring the levels of JNK activity in the T cells after infection with H. pylori plus vPE16. Purified splenic CD4+ T cells from the infected mice, at 2 or 6 weeks, were stimulated with urease in the presence of APC. At day 5, the cells were extensively washed and counted and equal numbers of cells were restimulated with urease in the presence of APC or with antibodies to CD3 and CD28, and the levels of JNK activity were determined by kinase assay with glutathione S-transferase (GST)-cJun as substrate. Urease-stimulated T cells exhibited a marked increase in JNK activity at 2 weeks but only a modest increase at 6 weeks of infection; moreover, anti-CD3 and anti-CD28 could greatly enhance kinase activity in Th cells at 2 weeks of infection (Fig. 4 c). Thus, these data suggest that JNK may play a role in the activation of urease-specific effector Th1 cells in the early stage of H. pylori infection but may not be required for urease-specific effector Th2 cells in the later stage of the infection, consistent with the difference in requirement for JNK2 for Th1 vs. Th2 differentiation observed by Yang et al. 28.
To examine the relationship between high- and low-avidity T cells and JNK activity, we measured the levels of JNK activity in high- and low-avidity T cells from the spleen cells at 2 weeks (mice infected with H. pylori alone) and 6 weeks (mice infected with H. pylori plus vPE16) of infection, respectively, after stimulation with high or low concentrations of urease (Fig. 4 d). At 2 weeks of infection the high-avidity T cells present showed a marked increase of JNK activity induced by low dose urease but a reduction in activity by high dose urease, in parallel with the Th1 cytokine responses and proliferation by urease-specific T cells (Fig. 4 a, b). Thus, high dose urease inhibited JNK activity. In contrast, at 6 weeks the low dose was only marginally effective and the high dose was only slightly more effective at inducing JNK activity. The medium alone did not induce a measurable change in the levels of the activity of JNK. H. pylori urease-specific T cell activation by stimulatory signals and JNK-dependent transactivation are critical steps in the immune regulation of the Th1 response toward urease.
To assess whether the colonization levels of H. pylori in the stomach reflect the determinant density inducing Th1 cell anergy, we measured the colonization levels of H. pylori at 2 weeks and 6 weeks (mice infected with H. pylori alone or mice infected with H. pylori plus vaccinia) after the infection, when the cytokine production and JNK activity were measured using the samples from the same mice. The colonization levels were about 100 times higher at 6 weeks than at 2 weeks (Fig. 4 e), suggesting that they may be causally related to Th1 cell anergy induced by high determinant density of the specific antigen, urease.
3 Discussion
Th1 type CD4+ cells producing IL-2 and IFN-γ and Th2 type CD4 cells producing IL-4 and IL-5 have been identified in mice and humans 21, 38 – 40. In certain chronic infectious diseases, the Th1 pattern of cytokines is associated with resistance to infection, whereas the Th2 pattern is associated with progressive infection 21 – 24. It is therefore essential to understand the cytokine environment and specific intracellular signals that control the differentiation of precursor CD4+ T cells into effector Th1 and Th2 cells and the activation of these effector cells.
In the current study, we focus on the effect of Helicobacter infection on CTL and cytokine responses to HIV-1 gp160 expressed in vaccinia. We demonstrate a delayed switch from Th1 to Th2, associated with increased density of H. pylori urease antigen and involving a JNK signaling pathway that controls the initiation of Th1 responses, as well as a decrease in CTL responses to recombinant vaccinia-expressed HIV gp160 and delayed clearance of the virus. We also observe a shift from high-to low-avidity urease-specific CD4+ T cells consistent with deletion or anergy of high-avidity T cells caused by high levels of urease antigen. The enhanced Th2 phenotype at 6 weeks after H. pylori infection in our system is consistent with the observation 13 of striking enhancement in the levels of Helicobacter-specific IgG1 (Th2 dependent) in contrast to low levels of IgG2 (Th1 dependent) at 5 weeks of murine H. felis infection. However, in the case of different Helicobacter species, different results have been reported for the predominant cytokine response to Helicobacter antigens in mice, depending on the system used 12, 41, but cytokine responses to non-Helicobacter antigens were not examined. In our case, BALB / c mice, which have more of a predilection for T2 responses, were used. The sequential shift from Th1 to Th2 is reminiscent of that seen in murine malaria, where both responses may contribute to protection 42, 43, showing another parallel with parasitic disease.
We observed several potentially related changes in H. pylori-infected mice, each of which could contribute to decreased virus-specific CTL production and delayed clearance of virus: decreased production of IL-12, decreased activity of JNK in CD4+ T cells, a shift from Th1 to Th2 phenotype of cytokine production over the course of infection, and a loss of urease-specific high-avidity CD4+ T cells and gain of low-avidity T cells coincident with the cytokine switch and with increased H. pylori load. The exact causal relationship between these events cannot be determined with certainty from the existing data. However, the urease-specific CD4+ T cells can clearly mediate the cytokine shift in the gp120-specific response, the reduction in HIV- and vaccinia-specific CTL, and the delay in virus clearance, as shown by adoptive transfer of urease-specific CD4+ T cells (Fig. 3). Thus, during persistent infection by colonizable H. pylori and co-infection with recombinant vaccinia, the reduction in IFN-γ and IL-2 expression by CD4+ T cells may be relevant to the decrease observed in CTL against gp160 and vaccinia itself.
These findings prompted us to investigate whether H. pylori affected endogenous IL-12 production, which is required for up-regulating the activity of CTL against the virus 44, 45. Surprisingly, serum IL-12 was barely detectable in H. pylori-infected mice. Further, the cytokine phenotype switch from Th1 to Th2 was preceded by a reduced level of IL-12. These results suggested a mechanism in which H. pylori infection impaired IL-12 production needed for IFN-γ-producing Th1 differentiation and development of anti-gp160 and anti-vaccinia CTL.
JNK2 is required for the production of IFN-γ in Th1 cells but not for IL-4 production in Th2 cells 28. We have shown here that JNK activity is rapidly induced in Th1 effector cells but not in Th2 cells. JNK phosphorylates jun, and jun and Fos form AP-1 which is necessary for IL-2 production 27. Thus, it is possible that the selective activation of JNK in Th1 cells functions to regulate the transcriptional machinery that leads to the expression of Th1-specific cytokines. We demonstrated that the JNK signaling pathway correlates with the early initiation of the differentiation of precursor CD4+ T cells into effector Th1 cells, and the loss of JNK activity correlates with the switch to Th2 dominance.
The data also suggest a role for the density of protein determinants presented by the APC. High-avidity T cells, capable of being activated by a low number of antigen / MHC complexes, are deleted by exposure to APC with a very high density of antigen / MHC complexes, while low-avidity T cells are maximally stimulated at this same high determinant density. There is evidence that this high-dose antigen induction of anergy or apoptosis in CD4+ T cells occurs primarily in the Th1 subset 31. There is also independent evidence that antigen density can influence the balance of Th1 and Th2 response 40, 46 – 48. Thus, high level of urease in late H. pylori infection may contribute to the cytokine imbalance not only by deleting high-avidity Th1 cells, but also by favoring the outgrowth of lower avidity cells, which may correspond to the Th2 cells. The defect in Th1 cytokine production and selective block in JNK-dependent activation after stimulation with high dose urease was profound in persistently H. pylori-infected mice. The colonization levels of H. pylori were about 100 times higher at 6 weeks than at 2 weeks of infection. Therefore, the high colonization level is likely to be related to the anergy of Th1 cells caused by high levels of antigen (Fig. 4 d).
In summary, we hypothesize that the initial response to H. pylori is dominated by high-avidity urease-specific Th1 cells expressing JNK2, but as the urease density increases, the high-avidity cells are anergized or deleted, and low-avidity Th2 cells with low JNK2 activity are selectively stimulated to become the major population. The early decrease in IL-12 production, possibly contributing to the decrease in Th1 cells and CTL, may be related to some effect of urease-producing H. pylori on APC, but the mechanism is unclear. Further, since IFN-γ is necessary for up-regulation of the IL-12 receptor β2 chain 20, decreased IFN-γ production could further compound the problem. Once generated, the urease-specific Th2 cells are sufficient to cause the switch in cytokine phenotype for the HIV antigens, as well as inhibit the CTL production, with resultant delayed viral clearance, as shown by the adoptive transfer experiments, but we cannot exclude a role for other cells making IL-10 or TGF-β.
A number of organisms have been considered as possible cofactors influencing the rate of progression of HIV-mediated disease, and our results suggest that H. pylori, like schistosomiasis 22, may be an important candidate for this list. Our results bring the first direct evidence that a sequential cytokine phenotype switch from Th1 to Th2, preceded by low IL-12 response, is generated during the course of H. pylori infection. Therefore, the change in T cell avidity and switch from Th1 to Th2 phenotype are critical steps in the loss of immune protection against viral infection.
4 Materials and methods
4.1 Recombinant vaccinia viruses, H. pylori ure– mutant and antigens
vSC8 (recombinant vaccinia virus containing the Escherichia coli lacZ gene), and vPE16 (recombinant vaccina virus expressing the HIV-1 IIIB gp160 envelope glycoprotein), have been described 36, 49. A H. pylori ure– mutant, which had no detectable urease activity by the conventional sensitive urease test, was developed by allelic exchange mutagenesis of the urease B subunit, ureB, gene of H. pylori KS612, as described 32. The mutant lacks urease subunit A and B protein by immunoblot (not shown). Preparation of H. pylori lysate was as described 35. Urease was purified as described 50. The purified urease (purity > 98 %) was composed of equivalent amounts of the 31-kDa (UreA) and 62-kDa (UreB) subunits, and contained < 3.0 ng / mg endotoxin by Limulus amebocyte assay, a level insufficient to affect T cell proliferation significantly.
4.2 Mice and H. pylori
Eight-week-old SPF female BALB / c mice (Japan Charles River Labs, Tokyo), shown to be free of Helicobacter, were orogastrically administered 3 times at 2-day intervals an aliquot of 2 × 108 CFU of H. pylori (a clinical isolate, KS612 35; urease+, cagA+, vacA+) or the isogenic urease mutant (KS612 ure–). H. pylori strains were cultivated at 37 °C in Brucella broth (Difco) containing 5 % heat-inactivated horse serum under microaerobic conditions with shaking 35. H. pylori colonization was assessed by the urease test, growth in culture and morphology as described 32, 51. A week prior to or 3 weeks after H. pylori inoculation, or as indicated, H. pylori-infected and uninfected control mice were inoculated i. p. with 2 × 106 PFU of vPE16 or vSC8.
4.3 Preparation of CD4 T cells and APC and T cell lines and adoptive transfer
CD4+ T cells were isolated from spleen by negative selection using anti-CD8 (Pharmingen) and anti-MHC class II Ab to deplete CD8+ cells and B cells, respectively 52, followed by positive selection by cell sorting for the CD4+ cell fraction using en EPICS ELITE flow cytometer (Coulter Electronics, Hialeah, FL) as described 53 (purity > 95 %). APC were prepared from spleen by complement-mediated lysis of Thy1+ T cells (purity > 95 %). H. pylori urease-and HIV-gp120 T1 peptide (421 – 436, KQIINMWQEVGKAMYA)-specific T cell lines were established from spleen cells of BALB / c mice infected with H. pylori for 6 weeks or immunized with T1 peptide (20 μg peptide in CFA), respectively, as described 54. T cell lines were maintained by the same repeated stimulation with urease or T1 peptide at 0.1 μM biweekly with mitomycin C (MMC)-treated syngeneic spleen cells for 8 weeks. T cell lines were used for in vitro assays 5 days after antigen stimulation. Vaccinia-infected mice were inoculated i. v. via the tail vein with 1 × 107 cells of the 8 week-cultured urease- or T1-specific T cell lines (at 2 days after the antigen stimulation) in 100 μl PBS on days 1, 5, 8, and 12 after vaccinia infection, along with 1 × 107 APC pulsed with 1 μM antigen (urease or T1) for 2 h. Tissues were harvested 3 weeks after vaccinia infection.
4.4 T cell proliferative and cytokine responses
Spleen cells (2 × 105) or CD4+ T cells as indicated were cultured with 2 × 105 MMC-treated (50 μg / ml for 30 min) syngeneic APC in 200 μl complete T cell medium 35 in triplicate wells of a 96-well flat-bottom microtiter plate (Costar) under 5 % CO2, at 37 °C in the presence or absence of antigens 35. After 48 h, 1 μCi [3H] thymidine / well (Amersham) was added and 3H incorporation into DNA was measured 16 h later. For cytokine responses, spleen cells or CD4+ T cells were stimulated with H. pylori, urease or gp120 as described 35. IL-2, IL-4 and IFN-γ were determined as described 22. Levels of IL-12 p70 molecules after vaccinia challenge were determined using ELISA kits (Genzyme). To determine the phenotype of T cells producing cytokines, the spleen cells were also stimulated in vitro after treatment with either with anti-CD8 mAb (3.155; rat IgM) 55 or anti-CD4 mAb (RL.174; rat IgM) 56 plus complement, or complement alone.
4.5 CTL generation and assay
Three weeks after vaccinia virus infection, immune spleen cells (5 × 106 / ml in 24-well culture plates) in complete T cell medium 35 were restimulated for 6 days in vitro with either 2 × 105 gp160 gene-transfected H-2d fibroblasts (15-12 cells) per ml (MMC treated) or 2.5 × 106 syngeneic spleen cells infected with vSC-8 per ml [1 h at 37 °C; multiplicity of infection (moi) of 10] and 10 % Con A supernatant-containing medium (rat T-Stim; Collaborative Research, Inc., Bedford, MA) as a source of IL-2. Cytolytic activity after 6 days of in vitro stimulation was measured as previously described 37 by a 6-h assay with 5000 51Cr-labeled targets per well: 15-12 transfectant, P18I10 (RGPGRAFVTI)-pulsed (1 μM) BALB / c 3T3 fibroblasts, or unpulsed 3T3 fibroblasts as a negative control at the E / T cell ratio indicated. Lysis of control 3T3 fibroblasts was < 5 % in all cases.
4.6 Vaccinia virus elimination study
At the indicated days post infection, mice from each group were killed, and tissues were aseptically removed, weighed, and frozen at − 80 °C before analysis of virus titer as described 22. The lower limit of detection was 70 PFU / g, corresponding to a log10 of 1.85.
4.7 Induction and assay of JNK activity
Purified immune CD4 T cells (5 × 105 cells / ml) were stimulated with urease and syngeneic irradiated APC (5 × 105 cells / ml). At day 5, the cells were extensively washed, counted, and restimulated at 106 cells / ml with urease in the presence of APC (106 cells / ml) or with plate-bound anti-CD3 and anti-CD28 (plates precoated with antibody at 10 μg / well) for 30 min. Protein kinase asays were performed as described 57. T cell lysates were incubated with GST-c-Jun immobilized on GSH-agarose beads. After 12 h at 4 °C, the beads were washed extensively and the activity of the bound JNK was detected by the addition of [32P] ATP for 30 min at 30 °C. The reaction products were resolved by SDS-PAGE and the incorporation of [32P] phosphate was quantitated by Phosphorimager analysis.
Acknowledgements
We thank Dr. Roger J. Davis (Howard Hughes Medical Institute and Program in Molecular Medicine, Department of Biochemistry and Molecular Biology, University of Massachusetts Medical School, 373 Plantation Street, Worcester, MA 01605, USA) for a gift of the c-jun plasmid, Dr. Teruko Nakazawa for for technical advice, and Drs. Patricia Earl and Bernard Moss (NIAID) for a gift of vPE16 and vSC8 recombinant vaccinia viruses. We thank Drs. Marika Kullberg, Warren Leonard, and Alan Sher for critical reading of the manuscript and helpful suggestions. This work was supported in part by a grant-in-aid from the Ministry of Education, Science and Culture of Japan (11470133).
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




