Axolotl MHC class II β chain: predominance of one allele and alternative splicing of the β1 domain
Abstract
The axolotl MHC is composed of multiple polymorphic class I loci linked to class II B loci. In this report, evidence of the existence of one class II B locus (Amme-DAB) that codes for two different transcripts is given. A 2.1-kb transcript is translated to a complete β chain and a shorter transcript of 1.8 kb encodes a molecule lacking the β1 domain. For two complete class II B mRNA synthesized, up to one mRNA devoid of the β1 domain is synthesized. Alternative splicing involving a peptide binding domain at a class II B locus evidenced in axolotl (Ambystoma mexicanum) is also observed for A. trigrinum, the tiger salamander. Very little variability is found among various axolotl MHC class II B cDNA sequences, and the same allele is obtained from inbred and wild axolotls. The transcription of one MHC class B locus in two class II B isoforms in thymic cells and in splenic lymphocytes may shed new light on the well-known deficient immune responder state of the axolotl.
Abbreviations:
-
- gDNA:
-
Genomic DNA
-
- UTR:
-
Untranslated region
-
- BAm:
-
Basel stock A. mexicanum
-
- WAm:
-
Wild A. mexicanum
-
- WAt:
-
Wild A. tigrinum
-
- RT:
-
Reverse transcription
-
- RFLP:
-
Restriction fragment length polymorphism
-
- aa:
-
Amino acid
-
- PBD:
-
Peptide-binding domain
-
- RACE:
-
Rapid amplification of cDNA ends
1 Introduction
The axolotl (Ambystoma mexicanum) is the most studied urodele amphibian species used for investigating the immune system. As such, the axolotl represents a key model for phylogenetic studies of the MHC genes and molecules and is very different from the other well-known amphibian, the anuran Xenopus 1, 2. cDNA encoding axolotl antigen receptors, Ig, TCR as well as MHC class Ia and class Ib have been previously characterized. The axolotl antigen receptor sequences display a mammalian type pattern with a large diversity of Ig and TCR repertoires 3 – 5. Important MHC class I polylocism and variability have also been described in this species 6, 7.
Despite this mammalian pattern at the molecular level, the axolotl, as all urodele amphibians, displays a weak experimental immune response 8. The paucity of the humoral response could be due to a deficient antigenic presentation by MHC class II molecules. To gain a better insight into this question, we decided to characterize axolotl MHC class II B genes and molecules. The class II molecule consists of one α and one β chain encoded by MHC DXA and DXB genes, respectively, where D designates class II and X specifies the class II family 9. The membrane-distal α1 and β1 domains constitute the peptide-binding domain (PBD).
Previous data suggested the presence of oligomorphic class II molecules in axolotl based on immunoprecipitation techniques with mammalian cross-reactive MHC class II polyclonal and monoclonal antibodies 10 – 12. However, these data have not been confirmed by protein or cDNA sequence characterization of class II α or β chains.
We undertook molecular cloning of axolotl MHC class II B cDNA using a degenerated primer-based reverse transcription (RT)-PCR strategy 13, 14. This approach allowed the isolation of a specific probe used to screen an axolotl cDNa splenic library and to design oligonucleotides for RT-PCR. Partial overlapping splenic cDNA clones were analyzed and β1 and β2 probes were derived from the longer splenic Amme-DB.010 clone 15. Thanks to these specific probes we investigated the architectural pattern of the axolotl MHC by Southern blotting and cosegregation studies of class Ia, class Ib and class II B genes 7. The present study extends the previous analysis by describing full-length axolotl class II B cDNA sequences and allelic polymorphism of the β1 domain. The presence of alternatively spliced class II B transcripts lacking the β1 domain is reported in the axolotl population as well as in the tiger salamander ( A. tigrinum), another ambystoma species.
2 Results
2.1 MHC class II B loci defined by Southern blotting with the β2 and β1 probes
Genomic DNA (gDNA) of blood cells from axolotls of different origins were digested, Southern blotted, and hybridized with the Amme-DB.010 β2 probe. Whatever the restriction enzyme used, BamHI, EcoRV, EcoRI or NcoI, this probe revealed a single hybridizing band (Fig. 1). With BamHI, the single band revealed was polymorphic: 3 kb (lane 1) or 10 kb (lane 2). With EcoRV, a single 16-kb fragment was evidenced whatever the sample origin: wild or Basel stock for A. mexicanum (WAm and BAm, lane 3), or for a wild A. tigrinum (WAt, lane 4). A restriction fragment length polymorphism (RFLP) was observed with EcoRI when the two ambystoma species were compared: a unique 9.5-kb EcoRI fragment was observed for sibs derived from the three BAm families (26 animals tested; lane 5), a unique but different 11-kb fragment was evidenced in A. tigrinum (lane 6). With NcoI, a new RFLP was evidenced between the two species, an 8-kb fragment was in BAm stock individuals or in WAm (lane 7), and a 12-kb fragment in WAt (lane 8).

Southern Blotting with the class II β2 probe on different endonuclease digests. Hybridization obtained with Amme-DB.010 β2 domain probe on BamHI (lanes 1,2), EcoRV (lanes 3,4), Eco RI (lanes 5,6), and NcoI (lanes 7,8) digests of gDNA from axolotls of different origins. Each lane represents one pattern shared by several individuals. Shown are patterns of BAm (lanes 1,2,3,5,7) or WAm (lanes 3,5,7). Lanes 4,6 and 8 represent the patterns obtained from two WAt individuals. Calculated lengths of the detected restriction fragments are indicated on the right. Whatever the restriction enzyme used, the class II β2 probe revealed a single hybridizing band.
Then, Southern blots of Eco RI digests were hybridized with the Amme-DB.010 β1 and β2 probes (Fig. 2). Again, even under non-stringent washing conditions, a single 9.5-kb EcoRI fragment was detected with the β2 probe in all BAm (Fig. 2, B2 lane 1), and in wild axolotls (lane 4). More complex restriction profiles were obtained with the β1 probe (Fig. 2, B1). Indeed, under the same experimental conditions, different profiles were obtained, each composed of three to four bands. This feature was shared by the BAm stock (Fig. 2 lanes 2,3 = ten sibs of the A family as an example) and the wild animals (Fig. 2, lanes 5,6 = nine sibs of a WAm family). In all cases, the β1 probe revealed a 9.5-kb band for all BAm and for all WAm axolotls.
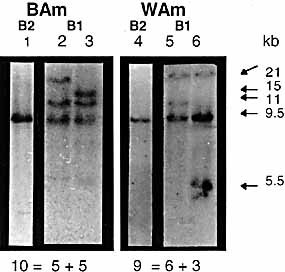
Southern Blotting with the β1 and β2 probes on EcoRI digests from various individual gDNA samples. As an example, one family of A. mexicanum derived from the Basel stock BAmA (lanes 1 – 3), and one family derived from two wild A. mexicanum (WAm, lanes 4 – 6) are shown. EcoRI digests were performed on gDNA of ten individuals (BAmA) and nine individuals (WAm). The hybridization profiles with the β2 probe are shown in lanes 1 and 4, and with the β1 probe in lanes 2 – 3, 5 – 6. The number of individuals showing the same profiles is indicated below the lanes for each group of animals. A single β2 EcoRI fragment is detected in all individuals. More complex restriction profiles were obtained with the β1 probe.
The fact that the β1 and β2 probes revealed both a 9.5-kb fragment in all analyzed samples may indicate the presence of β1 and β2 exons on the same 9.5-kb fragment. The additional β1 bands may correspond to MHC class II B loci, unrevealed by our β2 probe.
2.2 Thymic cDNA library screening with both β1 and β2 probes and analysis of full-length class II B clones
In order to look for putative β2 divergent sequences associated to β1 sequences, broad screening of the thymic axolotl cDNA library was carried out with a mix of β1 and β2 probes. Using low stringency hybridization conditions, this analysis allowed isolation of 14 clones. Among these, (1) eight clones hybridized with both β1 and β2 probes: four clones were 5′ truncated (starting at different sites in the β1 exon) and four started in the 5′ untranslated region (UTR). The longer ones (clones 020 and 021) were completely sequenced and are shown in Fig. 3. (2) six clones hybridized with the β2 probe only: one clone started in the β2 exon (5′ truncated) and five clones lacked the β1 exon. Among them, two started in the leader peptide sequence and three in the 5′UTR sequence. Two clones (022 and 023) were entirely sequenced (Fig. 3). (3) No clone hybridized with the β1 probe only.
Clones 020 and 021 were independent since their 5′ and 3prime; extremities were different (Fig. 3). Their sequences overlapped on 1928 bp. Length inequality was due in part to different starts in the 5′UTR (23 to 45 bp long, respectively) and to the presence of two polyadenylation signals separated by 140 bp. Clone 021 (1968 bp) used the first polyadenylation signal, and encoded a mature MHC class II β chain of 240 amino acids (aa), with a 26-aa long putative leader peptide, identical to the coding sequence of the Amme-DB.010 partial splenic cDNA clone already described 15. Both β1 and β2 domains were 93 residues long, the connecting peptide and transmembrane region included 34 residues and the cytoplasmic tail was composed of 20 residues. The coding sequences of the six other clones which hybridized with both β1 and β2 probes were identical to clone 021.
This result pinpoints the fact that no other functional class II B locus can presently be evidenced in thymus, which would hybridize with the β1 probe but not with the β2 probe. These eight β1 and β2 positive clones, and the previously described partial splenic clones 15, were then renamed Amme-DAB.00x to assign the same locus origin.
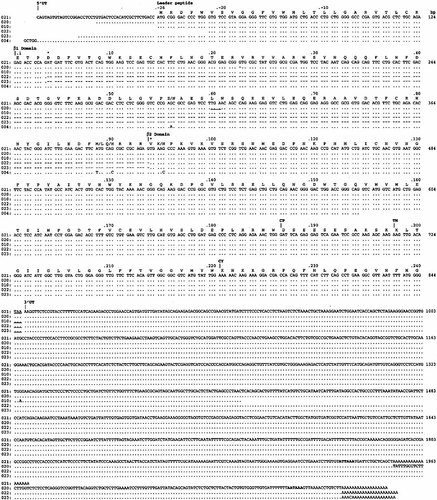
Axolotl MHC class II B cDNA nucleotide sequences. Comparison of nucleotide sequences of Amme-DAB.020 to Amme-DAB.023 clones with the partial splenic cDNA Amme-DAB.010 and Amme-DAB.004 clones previously described. Amme-DAB.021 is a full-length transcript encoding a classical β chain, Amme-DAB.020 presents a nucleotide deletion at position 167 in a GC-rich context (CGGTG for CGGGTG); for the remaining part, it overlaps with Amme-DAB.021. This deletion could be due to cloning artefact. Amme-DAB.022 and Amme-DAB.023 are two independent full-length transcripts encoding a smaller isoform devoid of the β1 domain. Identical nucleotides are denoted by dots, gaps by dashes, and domain borders by bars. The stop codons are underlined, and polyadenylation signals are in bold. Stars indicated the putative intron 1 and intron 2 positions. GenBank accession numbers (Amme-DAB. 004, AF213378), (Amme-DAB.010, AF209117), (Amme-DAB. 020, AF209116), (Amme-DAB.021, AF209115), (Amme-DABmB1.022, AF209118) and (Amme-DABmB1.023, AF209119).
2.3 Evaluation of PBD sequence variability and allelic polymorphism
The β1 domain sequence is expected to be polymorphic as part of the PBD of the MHC class II molecule. However, all the cDNA clones obtained from the thymus cDNA library encoded identical β1 sequences common with the 5′ truncated sequence previously obtained from a spleen library 15. Thus, we turned to RT-PCR strategy on spleen cDNA to ascertain these unexpected results and to test more thoroughly Amme-DAB variability. RT-PCR were performed using specific primers flanking the β1 domain, and cDNA of three axolotls corresponding to different β1 RFLP profiles obtained from EcoR I digests (from BAm A, C and WAm samples). The amplified products were cloned and six clones were sequenced from each of the initial cDNA samples. All 18 sequences were identical to Amme-DAB.021, thus demonstrating that β1 restriction polymorphism was not linked to an allelic polymorphism. We also performed 5′ rapid amplification of cDNA ends (RACE)-PCR experiments with an antisense primer located in the NGDW conserved region of the β2 domain and a sense primer corresponding to the universal adapter primer.
Finally, out of 52 β1 sequences, obtained from different batches of cDNA (80 animals from Ax 6 stock for the thymic library, three different animals from Ax 6 stock for the splenic library, six others from Ax 6 stock and two wild axolotls WAm for RT-PCR), 51 were identical and only one presented three non-synonymous mutations. This Amme-DAB.004 clone was found once by PCR but was never isolated by library screening 15. It may be a result of PCR artefact or represent a variant, sharing 98.9 % identity with the dominant allele at the DAB locus.
2.4 Analysis of cDNA lacking the β1 sequence
The sequences of two β2-positive and β1-negative clones obtained by thymic library screening were analyzed. Clones 022 and 023 were 1825 bp and 1832 bp long and overlapped on 1825 bp. Length difference between the two clones was due to different starts in the 5′UTR. While lacking 270 bp of the β1 sequence, these two cDNA clones were identical for all the other domains with Amme-DAB.021 clone and included a 5′UTR stretch, the complete presumptive leader sequence, the first three codons, and the first nucleotide of the fourth codon (G) of the β1 domain which is spliced onto the second nucleotide (T) of the first codon of the β2 domain. The β2 domain was followed by the connecting peptide, the transmembrane domain, the cytoplasmic tail, and the 3prime;UTR sequences. The two clones thus encoded the same truncated β chain of 150 aa preceded by a 26-aa putative leader peptide and may share the same common pre-messenger RNA with clone Amme-DAB.021. They were then renamed Amme-DABmB1.022 and Amme-DABmB1.023 (mB1 for minus β1-encoding domain), as coding for a shorter MHC class II B isoform.
2.5 Detection of the two different class II B transcripts
Northern Blot analysis of spleen and thymus has already shown two different transcripts of approximately 2.2 kb and 1.9 kb revealed with the β2 probe, the β1 probe revealing only the 2.2-kb band 15. The size of the longer band is compatible with a full-length Amme-DAB cDNA. The shorter one is compatible with an Amme-DABmB1 cDNA lacking the 270-bp sequence encoding the β1 domain. We carried out a quantitative study on Northern blots performed with splenic mRNA extracted from Ax 6 axolotls (Fig. 4). Autoradiography density and PhosphorImager analyses were made on hybridization profiles obtained with the β2 probe. Using the two techniques, the estimated ratio DAB versus DABmB1 isoforms was 2 / 1 in spleen mRNA. A similar ratio was obtained from mRNA from thymic epithelial cells.
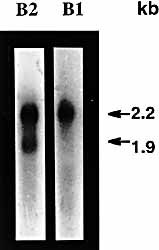
Detection of MHC class II B isoforms by Nothern Blot. Northern blot hybridization of Amme-DAB.010 β1 domain (B1) and β2 domain (B2) probes with 0.3 μg poly A+ RNA extracted from axolotl spleen cells. Calculated mRNA lengths are indicated on the right. Amme-DAB class II transcripts at 2.2 kb were abundantly revealed with the two probes. The β2 probe revealed a shorter isoform transcript at 1.9 kb, Amme-DABmB1.
To estimate the presence of the two Amme-DAB transcripts in different wild individuals, their transcription was assessed by RT-PCR using a sense primer located in the leader sequence and an antisense primer located in the β2 domain (Fig. 5). Similar PCR profiles, two DNA bands, were obtained with different splenic cDNA derived from stock A. mexicanum, from wild A. mexicanum and from a wild A. tigrinum. The hybridization profiles obtained from these PCR products confirmed the presence of the β2 sequence in both bands, but no hybridization signal was detected on the shorter band when using the β1 probe (Fig. 5).
These results suggest the natural co-expression in ambystomidae thymic and splenic cells of two MHC class II B cDNA isoforms encoding a complete β chain and a shorter chain lacking the β1 domain.

Detection of MHC class II B isoforms by PCR. PCR products were obtained with a sense primer (located in the leader sequence) and an antisense primer (located in the middle of β2 domain) on A. mexicanum DNA (BAmA, lane 1), or on different splenic cDNA samples derived from Basel stock A. mexicanum (BAmA, lane 2, and BAmC, lane 3), wild A. mexicanum (WAm, lanes 4, 5, 7, and 8), or from a wild A. tigrinum (WAt, lane 6). These PCR products were electrophoresed and stained with ethydium bromide (EtB) and blotted and hybridized (Hyb) with the Amme-DAB.010 β1 (β1) and β2 probes (β2). The hybridization profiles confirmed the detection of two class II B isoforms in all A. mexicanum and A. tigrinum samples tested. Absence of amplification on gDNA sample testified to the absence of cDNA contamination of the PCR reaction mix (lane 1).
2.6 Phylogenetic analysis of vertebrate class II β chain
β1 and β2 aa sequences from axolotl, Xiphophorus, Xenopus, pheasant and human were aligned (Fig. 6). Compared to Amme-DAB, the highest identities were obtained with Xenopus Xela-DAB and human HLA-DRB1 (52 % and 55 % for β1 and 48 % and 46 % for β2, respectively). These various vertebrate class II B sequences were also used to build phylogenetic dendrograms for β1 and β2 domains (Fig. 7). The branching order observed for the β2 tree matches the known phylogenetic relationships of major vertebrate classes, with a specific amphibian cluster located between fishes and birds. A particular node was observed with birds (chicken and pheasant) but not with Xenopus β1 sequences. The inconsistent feature for the β1 domain phylogenetic tree may relate to a different evolution timing for each exon: in contrast to the β1 domain subjected to positive selection as part of the PBD, the β2 domain could have evolved at a more constant and slower rate.
Despite the ability of the Amme-DAB locus to perform an alternative splicing, this phylogenetic analysis shows that the axolotl DAB locus is not divergent and belongs to the vertebrate classical class II B family.

Alignment of vertebrate MHC class II B aa sequences. Comparison of the aa sequence encoded by the axolotl Amme-DAB.021 clone was carried out with aa sequences of representative MHC class II β chains of selected vertebrates. Numbering of the aa residues is based on the HLA-DRB1 sequence. Accession numbers: Xiphophorus fish Xima-DAB (AF040760) and Xima-DXB (AF040761), Xenopus Xela-DAB (D13688), Xela-DBB (D13684) and Xela-DCB (D13685), pheasant Phco-DAB (AJ224344) and human HLA-DRB1 (X03069). Residues identical to Amme-DAB.021 are denoted by dots and gaps by dashes. Invariant refers to residues shared by all (underlined characters) or all but one (regular characters) sequences in the alignment. Calculated percentages of aa identities with the Amme-DAB.021 β1 or β2 sequence are indicated on the right of each sequence alignment.
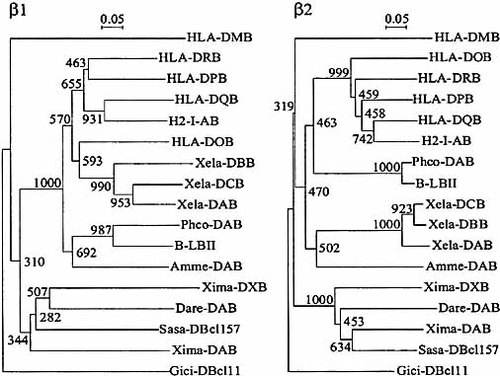
Phylogenetic relationships among the MHC class II β1 and β2 domain sequences across vertebrate classes. Species origin and accession numbers of the deduced MHC class II β amino-acid sequences are: axolotl Amme-DAB.021 (AF209115); human (HLA-DRB, X03069; HLA-DPB, PO4440; HLA-DQB, X96420; HLA-DMB, HSU15085; HLA-DOB, P13765), mouse (H2-I-AB, V01527), pheasant (Phco-DAB, AJ224344), fowl (Gaga-B-LBII, M29763), Xenopus (Xela-DAB, D13688; Xela-DBB, D13684; Xela-DCB, D13685), Xiphophorus fish (Xima-DAB, AF040760; Xima-DXB, AF040761), zebrafish (Darre-DAB, U08870); salmon (Sasa-DBcl157, X70166), and shark (Gici-DBcl11, L20274). The dendrograms were constructed by the neighbor-joining method. Scale shows genetic distances expressed as proportion of non identical aa. Numbering on nodes shows the number of times a particular branch is recovered per 1000 bootstrap replications.
3 Discussion
Our Southern blotting experiments using an MHC class II β2 domain probe with four different restriction enzymes on more than 40 wild and inbred individuals from the two species A. mexicanum and A. tigrinum revealed only one class II B locus. For the axolotl, A. mexicanum, this unique class II B locus is revealed on a 9.5-kb Eco RI fragment in laboratory strains as well as in wild animals. However, several bands were detected by Southern blotting using the MHC class II β1 domain probe with all restriction enzymes tested, BamHI, EcoRV, NcoI, KpnI and EcoRI. With EcoRI, three to four bands were revealed, and among these bands, a 9.5-kb band.
These unexpected results may be explained in several ways. First, some β1 exons could be linked to highly divergent β2 exons unrecognized by our β2 probe. Such an unrelated class II β2 domain has already been described in Xiphophorus (locus DXB compared to DAB) 16. However, if these divergent loci were present and functional, their transcripts by cross-hybridization with the DAB β1 probe should be detected by Northern blot on various axolotl tissues, which is not the case. Second, the 9.5-kb band revealed by the β2 probe may contain several superimposed restriction fragments, each containing a recently duplicated β2 exon that could be associated with β1 exons located in several different restriction fragments (including a 9.5-kb fragment), hypothesizing that three to four complete loci would be defined. It would however be unrealistic to imagine RFLP with five different restriction enzymes for each of the β1 exons and none for the β2 exons. Furthermore, the fact that no distinct class II B 3prime;UTR cDNA sequences were found also stands against this hypothesis. Third, a variable number of β1 orphan exons or partial MHC class II B pseudogenes (two to three) could be present in the axolotl MHC. Such pseudogenes have been already described in the zebrafish MHC DFB locus which is made up of a single β1 exon 17.
A phylogenetic analysis has estimated from 1 to 13 the number of MHC class II B loci in different species and among different individuals of the same species 18. To date, we have evidenced only one complete and functional MHC class II B locus in the axolotl: Amme-DAB.
All the MHC class II β chain clones from axolotls of the European breeding stocks have identical sequences. These axolotls came all from a small founder population of six individuals (including a single female) that were imported to France from lake Xochimilco about 130 years ago. Thus, there is no doubt that these inbred animals passed through a strong population bottleneck. However, the same β1 sequence was identified in two wild animals recently caught in lake Xochimilco. On the whole, among the 52 class II β1 sequences obtained from axolotls of different strain origin, only one has three substitutions. This is far below the average of substitutions observed between two HLA-DRB alleles in humans (between 10 and 20) 19. The absence of polymorphism at the Amme-DAB locus by cDNA sequences of wild and inbred individuals can be compared to similar situations observed in several mammalian species 20 – 22. It can be ascribed to a situation where the species is subjected to a strong population bottleneck, due to its ecological niche or to domestication. Axolotls presently live in a single Mexican lake isolated in an environment that is non-permissive for animal migration and this may account for the genetic homogeneity of their MHC class II B gene. However, this predominance of one class II B allele strikingly contrasts with the considerable variability of their MHC class I genes 7, both studies being performed on the same batch of axolotl individuals. This axolotl MHC pattern is reversed, compared to that of Xenopus, where a single polymorphic class Ia locus and three polymorphic class II B loci have been characterized so far 23, 24. Low allelic polymorphism has been noticed in the axolotl at the protein level by isoelectric focusing analysis of class II immunoprecipitates 12, 25. Yet, despite their ability to bind superantigens 26, a limited set of antigenic peptides would be presented by axolotl class II molecules.
The presence of two MHC class II β chain transcripts in the axolotl is an unexpected result. The simplest hypothesis to explain this finding is alternative splicing of a common mRNA transcript. Indeed, the full-length transcripts Amme-DAB and Amme-DABmB1 have the same overlapping sequence even in the 5′ and 3prime; UTR regions. Furthermore, only one β2 locus can be evidenced by Southern blot. From the known genomic organization of the MHC class II B genes in different species, and from the alignments of both Amme-DAB isoforms, we can speculate that the shorter transcript results from the splicing of exon 1 to exon 3. Exon 1 may contain the leader peptide coding sequence plus three codons and the first base of the fourth codon of the β1 domain. Such a genomic architecture has already been described for Xenopus MHC class II B genes where four codons of the β1 domain are located in the first exon 23. Exon 3 may start with the second nucleotide of the first codon of the β2 domain. This exon 1 to exon 3 alternative splicing may thus produce a mature transcript encoding a presumptive 16.8-kDa protein (versus 27.4 kDa for the complete β chain), including a complete signal peptide sequence followed by the β2 domain, the connecting peptide, and the transmembrane and cytoplasmic domains. Our hypothesis would be reinforced by the genomic analysis of the axolotl class II B genes. However, due to the size of the axolotl genome which is about ten times larger than the human genome 27, gDNA analysis presents serious technical problems and to data no genomic sequence showing exon-intron organization has been cloned in the axolotl.
Among MHC genes, alternative splicing has been described for classical class Ia loci and class Ib loci. To our knowledge, such alternative splicing has rarely been described regarding a class II B locus. The cases reported concerned splicing of transmembrane or cytosolic exons or aberrant splice signals 28 – 30.
The two axolotl MHC class II β chain isoforms are efficiently produced at the transcription level. The results obtained by Northern blotting (one Amme-DABmB1 transcript for two Amme-DAB transcripts) are correlated with the thymic cDNA library screening results (five Amme-DABmB1 clones for eight Amme-DAB clones). The presence of a leader sequence associated to the shorter mRNA transcript argues for the production of the corresponding polypeptide isoform in spleen and in thymus. Our results are archetypal and one may wonder about the function of this PBD-deficient β chain in the axolotl. Possible interference of this short isoform with the classical MHC class II antigen processing pathway and with T helper cell activation and repertoire development cannot be excluded. Moreover, this very unexpected splicing is not restricted to the A. mexicanum species since it was evidenced also in A. tigrinum. This characteristic should therefore be ancestral to the A. mexicanum / A. tigrinum species divergence, some 30 millions years ago 31. It is possible that the shorter isoform observed in ambystomidae represents an ancestral state of the class II molecule formed of one IgC1 domain completed ultimately by exon shuffling to form the complete β chain with a β1 PBR domain 32, 33.
Overall, the MHC organization and expression in the axolotl seems to be oriented toward maximal polylocism and variability with regard to class I, associated with a non-polymorphic class II B locus coding for two class II β chain isoforms, one being unable to present antigens. The evolutionary forces resulting in this peculiar feature are unknown but could lead to the impaired axolotl immune response.
4 Materials and methods
4.1 Animals
Axolotls of the Ax 6 strain (Paris-Black) were bred in our laboratory colony in 19 °C tap water and fed with trout pellets. They represent the F5 generation of brother-sister matings (J. Charlemagne, personal communication). Twenty-six BAm animals coming from three F1 families (A, C, D) were analyzed.
Wild animals captured in Mexico were also used in this study, 19 WAm, and four WAt.
4.2 Genomic DNA extractions
Blood cells were incubated in lysis buffer (0.1 M EDTA, 0.05 M Tris-HCl, 0.1 % SDS) at 65 °C for 45 min, supplemented with proteinase K (100 μg / ml lysis buffer) and incubated at 45 °C for 12 h. After salt precipitation of proteins, genomic DNA was recovered from the lysate, precipitated with 0.6 volume isopropanol and ethanol washed.
4.3 RNA extractions and cDNA synthesis
Total RNA were isolated from axolotl spleen cells using the RNA PLUSTM protocol (Bioprobe, Montreuil-sous-bois, France). PolyA+ RNA were extracted on PolydT Sephadex beads (Pharmacia, Piscataway, NJ). The first strand cDNA synthesis was performed as already described 6.
4.4 Southern and Northern blot analyses
gDNA (20 μg) was digested with 240 U of restriction enzyme (40 U / μl, Promega, Madison, WI) in 100 μl for 20 h at 37 °C. After heat denaturation, samples were loaded on a 0.8 % agarose gel and separated for 18 h at 40 V. DNA in the gel was treated with 0.25 N HCl before denaturation with 0.4 N NaOH. Samples (0.5 μg) of mRNA isolated from spleen were separated in a 2 % agarose TAE denaturing gel at 30 V for 18 h in MOPS running buffer. After blotting, membranes were hybridized with Amme-DAB.010 β1 or β2 probes as previously described 7. A final wash was carried out at 65 °C with 2 × SSC, 0.025 % SDS. Autoradiography was performed at − 80 °C using Kodak films (X-O MAT AR, Stuttgart, Germany) or PhosphorImager (Amersham, Freiburg, Germany) exposure.
4.5 Screening of axolotl thymic cDNA library
An unamplified cDNA library (kindly provided by Dr. Fellah) was built from polyA+ RNA extracted from thymic lobes of 80 Ax-6 axolotls aged 6 to 12 months and inserted into the λZAPII vector (Stratagene, La Jolla, CA). One million PFU were screened by hybridization using a mix of β1 and β2 probes. Positive clones were purified and excised in vivo with the R408 helper phage (Stratagene).
4.6 DNA sequencing and sequence analysis
Nucleotide sequencing was performed with the sequenase version 2.0 kit (US Biochemicals, Cleveland, OH) on double-strand DNA. All sequences were checked on double-strand at the MWG (Ebersberg, Germany) company. Nucleotide and deduced aa sequence alignments were performed using FASTA, BLAST, LALIGN and CLUSTAL W computer softwares with Infobiogen (www.infobiogen.fr /) facilities.
4.7 RT-PCR, 5′RACE-PCR analyses and labeled PCR probes
RT-PCR was carried out in a total volume of 20 μl containing 1 % of the total cDNA, 50 μM of each dNTP, 1 μM of each primer and 1 U Goldstar DNA polymerase (Eurogentec, Seraing, Belgium) in PCR buffer. Thirty cycles of 1 min at 94 °C, 1 min at 60 °C and 1 min at 72 °C were performed. 5′ RACE-PCR were performed on splenic tailed cDNA 6 using an antisense primer located in the conserved region of the β2 domain NGDW (5′ CTGCCCGGTCCAGTCCCCGTT 3′) and a sense primer corresponding to the universal adapter primer (5′ CGGAATTCTCGAGATCGA 3prime;). β1 and β2 probes were first PCR amplified from the plasmid Amme-DB.010 clone with specific sense and antisense primers of β1 (5′ GATGATTTCGTGACTCAGTGG 3′ and 5′ TCTGCGGCGCTGCATGAAGTC 3′) and of β2 (5′ GTGAAGCCCAAAGTGAAAGTG 3′ and 5′ CCAGTTTCTCCTGAGGGGCTC 3′) domains, respectively. The sense primer used for the β1 probe starts with the fourth codon of β1. One nanogram of amplified and purified DNA fragment was then labeled with [α-32P] dCTP by PCR with 5 mM MgCl2, 3 μM of each dATP, dGTP, dTTP, 0.25 μM primers, and 2.5 U Taq polymerase.
4.8 Construction of dendrograms
The inferred protein sequence of the Amme-DAB.021 cDNA clone was aligned with other vertebrate MHC class II sequences using the CLUSTAL W computer program 34. The percentages of aa identity between aligned sequences were used to construct distance matrices 35. The evolutionary relationships were then calculated by the neighbor-joining algorithm 36 and presented by the Njplot software (Manolo Gouy, Laboratoire de Biométrie, Lyon, France). One thousand bootstrap replications were performed to determine the reliability of the branching order.
Acknowledgements
We are grateful to L. Du Pasquier and J. Charlemagne for their constructive advices and contributions to this work and to P. Ducoroy, L. Pichon, F. Salvadori, B. Sammut, and L. Schreiber for their helpful contribution to this manuscript. We would like to thank in Mexico V. Graue, M. Léon-Oléa and O. Vindrola for their contribution to animal supply. This study was supported by Université de Bourgogne, Centre National de la Recherche Scientifique (CNRS UMR 5548), ECOS, the Mexican Consejo National de Cienca y Technologia and ANUIES and Conseil Régional de Bourgogne.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




