Inhibition of monocyte and macrophage chemotaxis by hypoxia and inflammation – a potential mechanism
Abstract
Macrophages accumulate in areas of inflammation and necrosis that are likely to be hypoxic. Chemotaxis of monocytes and macrophages towards chemokines is rapidly (within 60–90 min) inhibited by hypoxia. Exposure to the inflammatory cytokine TNF-α has a similar effect on monocyte migration. We report here that neither changes in mitochondrial respiration nor intracellular pH are involved in migration arrest. However, hypoxic inhibition of migration was mimicked using chemical activators of hypoxia-inducible factor-1 and reversed by transcriptional inhibition. We used RNA arbitrarily primed PCR, a differential display technique, to investigate which genes were up-regulated within 90-min exposure to hypoxia. Of several thousand mRNA screened, only one was consistently up-regulated by hypoxia and this was identified as MAPK phosphatase 1 (MKP-1), which modulates MAPK activity. Levels of MKP-1 mRNA and protein were rapidly elevated in monocytic cells and primary macrophages after hypoxia or TNF-α treatment. The functional significance of MKP-1 was illustrated by hypoxia-induced decreases in phosphorylated MAPK in these cells and arrest of chemotaxis by MAPK inhibitors. We suggest that one of the important events in an 'emergency stop' response in monocytic cells and macrophages may be inhibition of the chemoattractant signaling cascade.
Abbreviations:
-
- HIF-1:
-
Hypoxia-inducible factor 1
-
- HRE:
-
Hypoxic response element
-
- MKP-1:
-
MAPK phosphatase 1
-
- MCP-1:
-
Monocyte chemoattractant protein 1
-
- MIP-1α:
-
Macrophage inflammatory protein 1α
-
- RAP-PCR:
-
RNA arbitrarily primed PCR
1 Introduction
Inflamed tissues and solid tumors are likely to have areas of low oxygen tensions (<1% oxygen) 1. We reported previously that chemokine-induced migration of THP-1 monocytic cells and human macrophages was inhibited by hypoxia 2, 3. The inhibition was rapid and reversible and not specific to a single chemoattractant; neither was it caused by induction of soluble factors nor decreased expression of the chemokine receptor CCR2 2.
Inhibition of chemotaxis by hypoxia may explain how macrophages accumulate in areas of necrosis or inflammation: cells migrate along a chemotactic gradient until they reach an area of hypoxia where they are rapidly inhibited from progressing further. Once within these areas, macrophages function as phagocytes clearing up cell debris and also have a role in extracellular matrix remodeling and angiogenesis 4–6.
It is of interest that the inflammatory stimulators bacterial lipopolysaccharide (LPS) and TNF-α also cause a rapid (<1 h) inhibition of chemotactic migration of monocytes with similar kinetics to hypoxia 7.
The cellular response to hypoxia is a complex process involving numerous transcription factors 8. A well-characterized hypoxic response of many cell types is the induction of gene transcription by hypoxia inducible factor 1 (HIF-1), a heterodimeric transcription factor which is post-translationally stabilized by hypoxia. The stabilized transcription factor binds to the hypoxic response element (HRE) in the promoter of hypoxia responsive genes, such as GAPDH or VEGF, up-regulating expression of that gene 9–13. Nuclear accumulation of HIF-1 is slow compared with other transcription factors 14 and up-regulation of HIF/HRE-dependent genes may take several hours 10. Nuclear factor κB and nuclear factor IL-6 have also been implicated in the hypoxic response of several cell types 15–17 and induction of several members of the bZIP (basic/leucine zipper domain) transcription factor class is seen in fibroblasts during anoxia 18.
In this study we used the THP-1 monocytic cell line and primary macrophages from human blood to investigate the mechanism by which hypoxia inhibits chemotactic migration. We tested three hypotheses: (i) that the inhibition of chemotaxis by hypoxia is a metabolic event caused, for example, by a lack of oxygen for aerobic respiration; (ii) that the mechanism of migration inhibition is similar for hypoxia and TNF-α; and (iii) that inhibition is an orchestrated event involving a transcriptional mechanism.
We present evidence that hypoxia- and TNF-mediated inhibition of chemotaxis involves a rapid transcriptional response of at least one gene that impinges on a chemokine-induced signaling cascade, and de-activation of that cascade.
2 Results
2.1 Hypoxic inhibition of THP-1 cell chemotaxis to MCP-1 is not due to respiratory suppression
We have shown previously that hypoxia prevents chemotaxis of THP-1 cells and primary human macrophages to monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α) and the chemoattractant fMLP 3. Fig. 1 shows a typical example. In the following series of experiments we used THP-1 cells as a model to investigate the mechanism of this effect and verified our key findings with primary human macrophages.
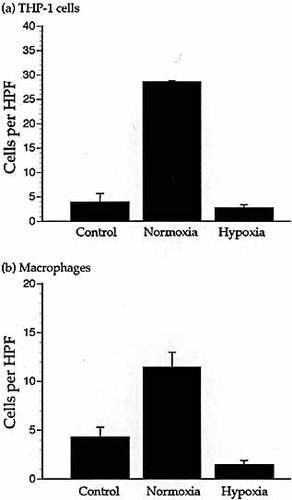
Chemotaxis of THP-1 cells and macrophages during hypoxia. Migration of the THP-1 cells (a) and primary macrophages (b) in response to 10 ng/ml MCP-1 in a microchemotaxis assay under normoxia and hypoxia. Unstimulated cells were assayed as a control for random migration. Results are the mean (±SEM) of ≥three experiments and n≥eight wells of the microchemotaxis chamber per parameter. Migration is significantly decreased by hypoxia (THP-1 cells p <0.0001. Macrophages p<0.001). Random migration was not significantly affected by hypoxia. HPF = high power field.
Hypoxic inhibition of chemotaxis could be a nonspecific event; insufficient mitochondrial respiration during hypoxia may induce metabolic changes in the cell. To test this, THP-1 cells were incubated in medium containing one of several electron transport chain or mitochondrial inhibitors for 2 h and then subjected to chemotaxis towards 10 ng/ml MCP-1 in the presence of these inhibitors.
After 2-h incubation with mitochondrial inhibitors (2 mM azide, 10 mg/ml diphenylene iodonium or 300 μM neopterin) THP-1 cells were still able to migrate towards MCP-1 under normoxic conditions and in the continued presence of the inhibitor (Fig. 2a).
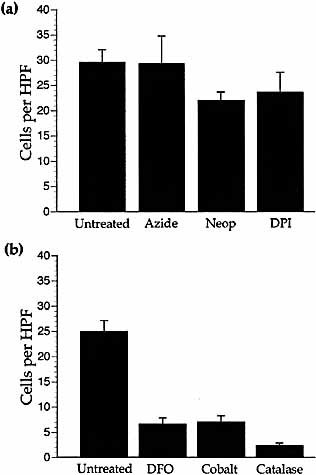
Chemotaxis in the presence of mitochondrial inhibitors or HIF-1 activators. (a) Migration of THP-1 cells to 10 ng/ml MCP-1 under normoxic conditions in the presence of mitochondrial inhibitors 2 mM azide, 300 μM neopterin (Neop) or 10 mg/ml diphenylene iodonium (DPI) compared to untreated cells. Results are the mean (±SEM) of ≥three experiments and n≥9 wells per parameter. Migration was not significantly reduced by any metabolic inhibitor and random migration was unaffected. (b) Migration of THP-1 cells to 10 ng/ml MCP-1 in the presence of 200 μM desferrioxamine (DFO), 100 μM cobalt chloride or 4000 U/ml catalase compared to untreated cells. Results are the mean (±SEM) of ≥three experiments and n≥12 wells per parameter. Migration was significantly reduced by DFO (p <0.001), cobalt (p <0.001) and catalase (p <0.001) compared to untreated cells. Random migration was unaffected by HIF activators.
2.2 Hypoxic inhibition of monocyte chemotaxis to MCP-1 is not due to decreased intracellular pH
Hypoxia may cause cellular acidosis and decreased intracellular pH can detrimentally effect neutrophil migration 19. We investigated whether reduced intracellular pH affects THP-1 cell migration. Internal pH was manipulated by exposure to the weak acid sodium acetate (50 mM) at constant extracellular pH (7.4). Sodium acetate did not mimic the hypoxic inhibition of migration (data not shown).
2.3 Cobalt, desferrioxamine and catalase mimic the chemotactic inhibitory effects of hypoxia
We next studied whether known chemical activators of hypoxia-inducible transcription factors, e.g. HIF-1 (for reviews see 20, 21), could mimic hypoxic inhibition of chemotaxis. Cobalt (100 μM), desferrioxamine (200 μM) or catalase (4000 U/ml) inhibited THP-1 cell chemotaxis to MCP-1 under normoxic conditions (Fig. 2b). To ensure that cells were not being killed by the addition of such chemicals, THP-1 cells were incubated for 4 h with these chemicals before viability testing using Trypan blue staining. Cells retained almost 100% viability (data not shown).
2.4 Transcriptional inhibitors can reverse hypoxia- or TNF-mediated inhibition of chemotaxis
Having shown that transcriptional activators such as cobalt could inhibit chemotaxis in a similar way to hypoxia, we incubated THP-1 cells or primary macrophages with a transcriptional inhibitor (50 μg/ml actinomycin D) before the cells were assayed for chemotaxis under hypoxia. Actinomycin D reversed the hypoxic inhibition of THP-1 cell and macrophage chemotaxis to MCP-1 (Fig. 3a and b).
When THP-1 cells are treated with the inflammatory stimulator TNF-α, a rapid, reversible inhibition of chemotactic migration similar to hypoxic inhibition occurs 7. TNF-α inhibition of THP-1 cell chemotaxis (under normoxia) could be reversed by 50 μg/ml actinomycin D (Fig. 3c).
Because TNF-α is hypoxia responsive in many cell types, including macrophages 22–24, it was possible that hypoxia stimulated TNF-α expression which then inhibited chemotaxis. However, TNF-neutralizing antibodies did not reverse the hypoxic inhibition of chemotaxis (data not shown).
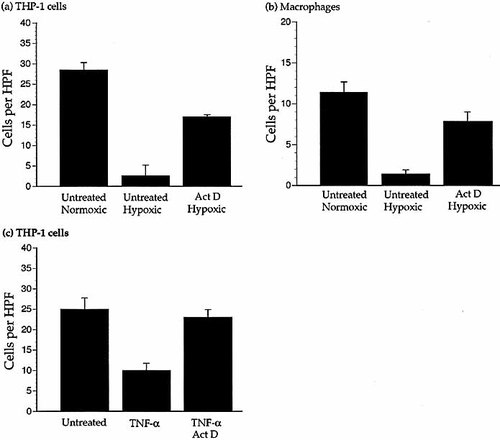
A transcriptional inhibitor reverses the hypoxic- or TNF-α-mediated inhibition of chemotaxis. (a and b) Migration of THP-1 cells (a) and primary macrophages (b) to 10 ng/ml MCP-1 under normoxic and hypoxic conditions in the presence of 50 μg/ml actinomycin D (Act D) compared to untreated cells. Results are the mean (±SEM) of several experiments and n≥8 wells per parameter. Migration under hypoxia of cells treated with actinomycin D was significantly increased (THP-1 cells p<0.001. Macrophages p<0.05) compared to untreated hypoxic cells. (c) Migration of THP-1 cells to 10 ng/ml MCP-1 in the presence of TNF-α (10 ng/ml) with or without 50 μg/ml actinomycin D. Actinomycin D was added to the cells 2 h prior to migration and TNF-α was added to the cells immediately before migration. Results are the mean (±SEM) of ≥four experiments and n≥18 wells per parameter. Migration was significantly reduced by TNF-α (p <0.001) compared to untreated cells and restored by actinomycin D (p <0.001). Actinomycin D did not significantly affect random migration or migration to MCP-1 under normoxic conditions without TNF-α treatment.
2.5 RNA arbitrarily primed PCR and MKP-1
Having found that transcriptional activation was required for the hypoxic inhibition of chemotaxis, we used RNA arbitrarily primed PCR (RAP-PCR) to identify genes that were up-regulated in THP-1 cells within 90 min of exposure to hypoxia. Unstimulated and MCP-1-stimulated THP-1 cells were hypoxically incubated for 90 min before total RNA was extracted. This RNA was used for RAP-PCR and hypoxically up-regulated genes were sought. Of the several thousand RAP-PCR products analyzed, only one repeatedly indicated increased mRNA abundance after 90 min hypoxia compared to normoxia (Fig. 4a). This band was up-regulated by hypoxia in both unstimulated and MCP-1-stimulated cells. The cDNA was amplified and sequenced and was found to be identical to the MAPK phosphatase 1 mRNA (MKP-1, also known as CL100. Accession number X68277 S46269).
2.6 Time-course of MKP-1 induction by hypoxia or TNF-α
Differential expression of MKP-1 was confirmed by RT-PCR. MKP-1 mRNA expression by MCP-1 stimulated THP-1 cells could be detected by RT-PCR within 10–20 min of hypoxia (Fig. 4b). A similar time-course of MKP-1 mRNA induction was seen for unstimulated cells (data not shown). The PCR products were purified and sequenced to confirm identity. MKP-1 mRNA expression was too low (during normoxia or hypoxia) to be accurately quantified by Northern blotting. Increased MKP-1 protein could be seen after 30 min hypoxia of unstimulated and MCP-1 stimulated THP-1 cells (Fig. 4c). Accumulation of MKP-1 mRNA within 20 min could also be shown by incubating THP-1 cells with TNF-α (Fig. 4d).
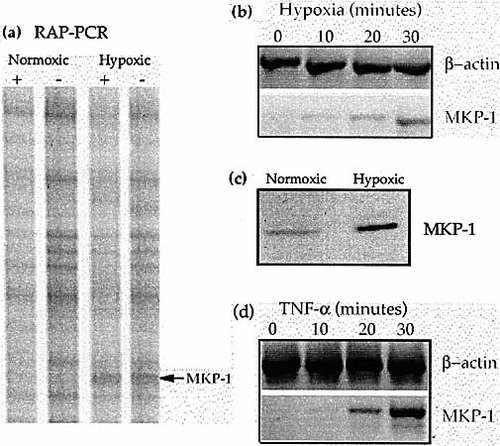
MKP-1 induction by hypoxia. (a) A section of a RAP-PCR autoradiogram showing a differentially expressed band later identified as MKP-1. + = MCP-1 stimulated cells. – = Unstimulated cells. (b) Time-course of induction of MKP-1 mRNA in MCP-1-stimulated THP-1 cells by hypoxia as shown by RT-PCR (n=3). A similar time-course of induction was seen using unstimulated cells. (c) Induction of MKP-1 protein in THP-1 cells by 30 min of hypoxia as shown by Western blotting. (d) Time-course of THP-1 cell MKP-1 induction by 10 ng/ml TNF-α as shown by RT-PCR (n=3).
2.7 Western analysis of MAPK and phosphorylated MAPK
To further study the functional significance of MKP-1 up-regulation by hypoxia, we analyzed the phosphorylation status of MAPK (p42 and p44) in THP-1 cells under normoxia and hypoxia (with and without stimulation by 10 ng/ml MCP-1) by Western blotting (Fig. 5a). MCP-1 stimulation increased phosphorylated MAPK but phosphorylated MAPK was decreased to unstimulated levels by 60-min hypoxia. Non-phosphorylated MAPK was increased or unchanged during hypoxia. Phosphorylated MAPK was also decreased by 60-min hypoxia in MCP-1-stimulated primary human macrophages (Fig. 5b). To test whether the dephosphorylation of MAPK was a specific event, the tyrosine phosphorylation status of THP-1 cells during hypoxia and normoxia was examined. The tyrosine phosphorylation status of the cell was not altered by hypoxia (Fig. 5c).
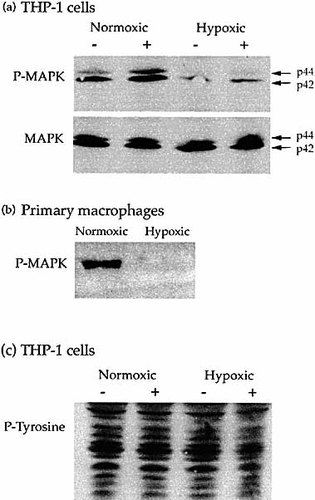
Dephosphorylation of MAPK by hypoxia. (a) Western blot of MAPK and phospho-MAPK proteins in the extract of THP-1 cells incubated in either normoxia or hypoxia for 60 min; + indicates cells stimulated with 10 ng/ml MCP-1; – indicates unstimulated cells. Stimulation by MCP-1 under normoxia led to the phosphorylation of MAPK (P-MAPK), and phosphorylated p42 and p44 MAPK were decreased by hypoxia. Nonphosphorylated MAPK (MAPK) was increased or unchanged by hypoxia. (b) Western blot of phospho-MAPK proteins in the extract of 10 ng/ml MCP-1 stimulated macrophages incubated in either normoxia or hypoxia for 60 min. Phosphorylated p42 and p44 MAPK were decreased by hypoxia. (c) Western blot of phosphotyrosine levels in the extract of THP-1 cells incubated in either normoxia or hypoxia for 60 min; + indicates cells stimulated with 10 ng/ml MCP-1; – indicates unstimulated cells. Phosphotyrosine levels (P-tyrosine) in the cells were unchanged by hypoxia.
2.8 Inhibition of chemotaxis by MAPK kinase inhibitors
To further investigate the functional relevance of MAPK activation in THP-1 cells and human primary macrophages during chemotaxis to MCP-1, we used the MAPK kinase (MAPKK) inhibitor PD98059 (2′-amino-3prime;-methoxyflavone). Cells were incubated with 20 μM PD98059, or DMSO as a control, for 60 min prior to chemotaxis. PD98059 significantly inhibited chemotaxis of THP-1 cells and primary macrophages to MCP-1 (Fig. 6). PD98059 also significantly inhibited normoxic THP-1 cell chemotaxis towards 10 ng/ml MIP-1α, a CCR1 chemokine (p < 0.0005. Data not shown).
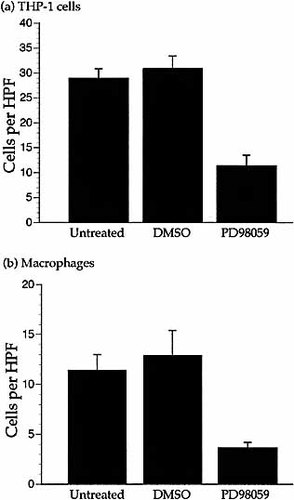
Inhibition of chemotaxis by an MAPKK inhibitor. Migration of THP-1 cells (a) and primary macrophages (b) to 10 ng/ml MCP-1 under normoxic conditions in the presence of 20 μM PD98059. Cells treated with 1% DMSO were included as a control. Results are the mean (±SEM) of ≥three experiments and n≥12 wells per parameter. DMSO did not affect migration. PD98059 did not affect random migration. Migration of THP-1 cells and primary macrophages was significantly reduced by PD98059 (THP-1 cells p <0.0005. Macrophages p <0.001) compared to untreated cells. PD98059 also inhibited chemotaxis of THP-1 cells towards MIP-1α (p<0.0005. Data not shown).
Cells treated with the inhibitor for 2.5 h maintained viability similar to untreated cells.
3 Discussion
Hypoxic inhibition of macrophage and monocyte migration to MCP-1 is a rapid event and this inhibition is found with other chemokines and the chemoattractant fMLP 3. The rapidity and apparent non-specificity of this inhibition led us to suggest that it may be caused by cellular metabolic changes during anaerobic respiration. However, we found that mitochondrial and electron transport chain inhibitors did not impede monocyte chemotaxis; thus a metabolic event is unlikely to be the cause of the inhibition. Neither did decreased intracellular pH mimic the hypoxic inhibition of chemotaxis.
Reversal of inhibition by actinomycin D indicates a transcriptional event. Chemical inducers of hypoxic transcriptional activity 25 inhibited chemotaxis suggesting a HIF-like response. However, the speed with which hypoxia inhibited migration suggests that HIF-1 itself may not be responsible as it is not thought to activate transcription this quickly 10, 14. The archetypal HIF-1 responsive gene, GAPDH, is not up-regulated by monocytes within 90-min hypoxia (our unpublished data), again suggesting that it is not an HIF response.
It is probable that the HIF-mimetics impinge on transcription factor pathways other than the HIF/HRE pathway. An unidentified hypoxia responsive transcription factor, such as one that activates early growth response-1 gene transcription within 15 min, may be involved 26. Alternatively, a transcription factor already present may be rapidly activated. For instance, NFκB is implicated in the hypoxic response in tumor cells 15.
Having found that transcription was required for the hypoxic inhibition of chemotaxis we compared the expression profiles of hypoxic and normoxic cells by RAP-PCR. Although numerous genes are hypoxically responsive, many of these genes are only up-regulated after several hours. We would expect few changes in expression after only 90-min hypoxia. Indeed, of several thousand RAP-PCR products analyzed only one repeatedly indicated mRNA accumulation after 90-min hypoxia. This up-regulated gene was identified as MKP-1.
Up-regulation of MKP-1 mRNA was confirmed by RT-PCR and was shown to occur within 10–20 min of hypoxia in both MCP-1-stimulated and unstimulated cells. Increased MKP-1 protein was seen after 30 min of hypoxia. MKP-1 is a rapidly responsive, stress response gene that encodes a dual specificity phosphatase which attenuates p42 and p44 MAPK (Erk2 and Erk1, respectively) activation and activity 27–29. That MKP-1 is rapidly up-regulated in both chemoattractant-stimulated and unstimulated cells may explain the apparent `nonspecificity' of the hypoxic inhibition of migration to several chemoattractants that signal through different chemoreceptors. MKP-1 is hypoxically responsive in the SiHa human cervical carcinoma cell line and up-regulation occurred within 2 h 30.
Similar to Yen et al. 31, we have shown that phosphorylation of MAPK is required for THP-1 cell and primary macrophage chemotaxis towards MCP-1 and other chemoattractants. MAPK activation is necessary for chemotaxis of several cell types 32–36. Western analysis showed that phosphorylation of MAPK in response to MCP-1 was prevented or reversed by 60-min hypoxia. We compared TNF-α inhibition of chemotaxis to hypoxic inhibition. TNF-neutralizing antibodies did not stop the hypoxic inhibition of chemotaxis, showing that it was not increased TNF-α expression during hypoxia that inhibited migration. However, TNF-α inhibition of chemotactic migration could be reversed by actinomycin D, suggesting a similar transcriptional response. Indeed, exposure of THP-1 cells to TNF-α caused MKP-1 mRNA to accumulate within 10–20 min. TNF-α increases HIF-1 activation in hepatoma cells under normoxia 37 suggesting that this cytokine may stimulate a transcriptional response using similar cellular machinery to hypoxia.
One mechanism proposed for inhibition of chemotactic migration by LPS is the down-regulation of the chemokine receptor CCR2 although this may occur after migration has ceased. In a study by Sica et al. 7, CCR2 mRNA was down-regulated 4 h after addition of LPS although migration was inhibited before this (<1 h). Xu et al. 38 found that LPS induced a marked reduction in CCR2 cell surface protein levels within 2 h. This reduction in chemokine receptor expression could be blocked by tyrosine kinase inhibitors that block Erk1 and Erk2, and may represent a similar pathway to the one proposed in this paper. As measured by calcium flux, hypoxia did not decrease surface CCR2 protein expression of monocytes 3.
The data presented here indicate that inhibition of monocyte and macrophage chemotaxis by hypoxia or other inflammatory stimulators is not a nonspecific metabolic event but an orchestrated event involving a rapid mRNA accumulation. HIF-1 may be involved although it is probable that other transcription factors may be responsible. One of the primary steps in the orchestrated 'emergency stop' cascade may be the up-regulation of MKP-1 expression that encodes a phosphatase that dephosphorylates MAPK. Phosphorylation of MAPK is required for monocyte and macrophage chemotaxis towards MCP-1 and hypoxia and inflammatory cytokines prevent this. Thus monocytes and macrophages may accumulate in areas of hypoxia and inflammation as a result of inhibition of the chemoattractant signaling cascade.
4 Material and methods
4.1 Cell lines and culture
The human monocytic cell line, THP-1, was purchased from the American Type Culture Collection (Rockville, MD) and grown in a humidified atmosphere at 37 °C and 5% CO2 in endotoxin-free RPMI 1640 medium supplemented with 10% fetal bovine serum and 50 μM β-mercaptoethanol.
4.2 Primary cell isolation and culture
Peripheral blood mononuclear cells (PBMC) were isolated from whole human blood using a Ficoll-Paque gradient (Amersham Pharmacia Biotech, Bucks, GB). CD14+ monocytes were isolated from the PBMC using MACS super-paramagnetic MicroBeads conjugated with monoclonal mouse anti-human CD14 antibodies and the MidiMACS isolation kit in combination with LS separation columns from Miltenyi Biotec, Germany. Isolated cells were cultured in AIM-V medium (Gibco BRL Life Technologies, Paisley, GB) supplemented with 2% human AB serum (Sigma-Aldrich Company Ltd., Poole, GB) in teflon bags (Süd-Laborbedarf GmbH, Germany) until the cells took on a typical macrophage morphology (7–14 days).
4.3 Microchemotaxis assay
THP-1 cells (2.5×106 cells/ml in RPMI supplemented with 1% BSA) or primary macrophages (1×106 cells/ml in RPMI supplemented with 1% BSA) were subjected to chemotaxis assays toward 10 ng/ml human recombinant MCP-1 or 10 ng/ml human recombinant MIP-1α (PeproTech Inc., NJ) as described in Turner et al. 3. Migration was stopped after 90 min for each experiment. Cells were counted in five high power fields (HPF. ×400 magnification) per well. The efficiency of THP-1 cell migration is dependent upon passage number (unpublished data) causing some variation between experiments.
4.4 Culture with chemical hypoxia-mimics or other additives
Sigma-Aldrich Company Ltd. supplied hypoxia-mimicking chemicals. Cells (2.5×106 THP-1 cells/ml or 1×106 macrophages/ml ) were incubated in RPMI 1% BSA supplemented with the hypoxia mimic or other additive at 37 °C under normoxia (5% CO2, atmospheric O2) for 2 h prior to and during the microchemotaxis assay. Primary monocytes were incubated in teflon pots (Pierce Chemical Company, IL) to prevent adherence.
4.5 Hypoxic culture and RNA extraction
THP-1 cells (2 ml; 2.5×106 cells/ml in RPMI medium with 1% BSA) in six-well plates or 2 ml primary macrophages (1×106 cells/ml in RPMI medium with 1% BSA) in Teflon pots were gassed with nitrogen with 5% CO2 in a modular incubation chamber. Cells were spun down in a microcentrifuge (4 °C, 1 min) and total RNA was prepared using Tri-Reagent (Sigma) following the manufacturer's instructions. Total RNA was DNase treated before being re-suspended in DEPC treated water.
4.6 RAP-PCR
Enzymes and reagents were supplied by Gibco BRL Life Technologies. RAP-PCR was carried out using total RNA and 20-mer arbitrary primers following the method described by Welsh et al. 39 except that a second arbitrary primer B was used in the PCR reaction in 10 molar excess of the primer A that was used in the RT reaction. Briefly, 1 μl arbitrary primer A (25 μM) was added to 1 μg DNase-treated total RNA in 11.6 μl DEPC-treated dH2O and incubated at 70 °C for 10 min and on ice for 1 min. Four microliter 5× RT buffer, 2 μl 0.1 M DTT, 0.4 μl 25 mM dNTP and 1 μl Superscript II RT enzyme were added, mixed and incubated at room temperature (10 min), 37 °C (90 min), 94 °C (5 min) and finally on ice (10 min).
cDNA were amplified by PCR using primer A and a further arbitrary primer B. The following PCR reaction was made up: 5 μl 10× PCR buffer, 3 μl 50 mM MgCl2, 0.1 μl 25 mM dNTP mix, 1 μl [35S]dCTP, 0.2 μl 25 μM primer A, 2 μl 25 μM primer B, 0.5 μl Taq polymerase, 1 μl cDNA and dH2O to 50 μl. The mixture was cycled in a thermal cycling machine as follows: one 'low stringency' cycle of 94 °C (1 min), 42 °C (5 min), 72 °C (5 min) followed by 40 'high stringency' cycles of 94 °C (1 min), 52 °C (2 min), 72 °C (2 min) and then 1 cycle of 72 °C (10 min).
4.7 PAGE analysis of RAP-PCR products
PAGE separation was carried out using the method described in Sambrook et al. 40. Kodak BioMax MS film was exposed to the gel overnight using a BioMax 35S intensifying screen. After developing, bands were analyzed visually on a lightbox. Bands that reproducibly differed in intensity between oxygen tensions were deemed to be differentially regulated.
4.8 Amplification of differentially expressed bands
The band was cut out of the gel and the fragment was placed in 100 μl TE buffer then incubated at 98 °C for 60 min. Eluted DNA was recovered by ethanol/glycogen precipitation and resuspended in 20 μl TE buffer. The DNA was amplified by PCR in a reaction mixture similar to that in the RAP-PCR reaction but containing 0.5 μM A and B primers. The PCR mixture was cycled as in the `high stringency' round of RAP-PCR.
4.9 Analysis of sequences
Gel purified PCR products were sequenced in each direction using A or B primers by automated dideoxy cycle sequencing. Sequences were analyzed as EditView files (Perkin-Elmer Automated Sequence Editor) and a contiguous sequence generated from the forward and reverse sequences using SeqMan software from DNASTAR inc. The consensus sequences were compared to the BLAST (Basic Local Alignment Search Tool) database at the National Centre for Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST/).
4.10 RT-PCR
Primers were designed from the published human MKP-1 sequence (5′ – ccaaccattttgagggtcac, 3prime; – caatgggatgtgaagagcct). DNase-treated total RNA was reverse transcribed using the Ready-To-Go kit from Amersham Pharmacia Biotech Inc., NJ. cDNA (1 μl) was amplified by PCR using primers for MKP-1 and also for β-actin as a positive control.
4.11 Western analysis
4.11.1 MKP-1
Whole cell extracts were made from unstimulated THP-1 cells or cells stimulated with 10 ng/ml MCP-1 (2.5×106 cells/ml in RPMI 1% BSA) incubated either normoxically or hypoxically for 30 min. Cell extract (10 μg) was run on an SDS 10% acrylamide gel and transferred to a nylon membrane. The membrane was probed using an anti-MKP-1 antibody from Santa Cruz Biotechnology Inc., CA (0.1 μg/ml in 5% BSA PBS with 0.1% Tween; overnight at 4 °C). After washing with PBS, the membrane was incubated in 5% milk powder PBS with 0.1% Tween containing an HRP-conjugated secondary antibody (1/5000 dilution; room temperature for 1 h). The secondary antibody was detected using the Western Blot Chemiluminescence Reagent Plus kit (NEN Life Science Products, MA).
4.11.2 MAPK and phospho-MAPK
Whole cell extracts were made from unstimulated cells or cells stimulated with 10 ng/ml MCP-1 (2.5×106 THP-1 cells/ml or 1×106 macrophages/ml in RPMI 1% BSA) and incubated either normoxically or hypoxically for 1 h. Cell extract (10 μg) was run on an SDS 10% acrylamide gel and transferred to a nylon membrane. The membrane was probed using anti-nonphosphorylated-MAPK or anti-phosphorylated MAPK antibodies from New England Biolabs Inc. MA (1/1500 dilution in 5% BSA PBS with 0.1% Tween; overnight at 4 °C). After washing with PBS, an HRP-conjugated secondary antibody (1/5000 dilution in 5% milk powder PBS with 0.1% Tween; 1 h at room temperature) was used and detected using the Western Blot Chemiluminescence Reagent Plus kit.
4.11.3 Phosphorylated tyrosine
Whole cell extracts were made from unstimulated THP-1 cells or cells stimulated with 10 ng/ml MCP-1 (2.5×106 cells/ml in RPMI 1% BSA) incubated either normoxically or hypoxically for 1 h. Cell extract (10 μg) was run on an SDS 10% acrylamide gel and transferred to a nylon membrane. The membrane was probed using an anti-phosphotyrosine antibody from Cell Signaling Technology, MA (1/2000 dilution in 5% BSA PBS with 0.1% Tween; overnight at 4 °C). An HRP-conjugated secondary antibody (1/5000 dilution in 5% milk powder PBS with 0.1% Tween; 1 h at room temperature) was used and detected using the Western Blot Chemiluminescence Reagent Plus kit.
Protein concentration equivalence was confirmed after probing by amido black staining
4.12 Statistical analysis
Results were tested for statistical significance using InStat Version 2.01 software.
Acknowledgements
We thank Dr. Stuart Naylor (Oxford BioMedica (UK) Limited) and Dr. Julian Downward (ICRF, London, GB) for helpful discussions. We also thank Dr. Caroline Arnott (ICRF), for assistance with Westerns and other signaling matters, and Dr. Julia Wilson and Chris Scotton for aid in the preparation of this manuscript. MG was funded by Oxford BioMedica (UK) Ltd., Oxford, GB
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




