Human CD8+ T cells expressing HLA-DR and CD28 show telomerase activity and are distinct from cytolytic effector T cells
Abstract
Cycling lymphocytes may express the enzyme telomerase which is involved in maintenance of telomere length and cell proliferation potential. In CD8+ T cells freshly isolated from peripheral blood, we found that in vivo cycling cells expressed HLA-DR. Furthermore, CD28-positive cells are known to have longer telomeres than CD28-negative T cells. Therefore we used HLA-DR- and CD28-specific antibodies to sort CD8+ T cells and measure telomerase activity ex vivo. Relatively high levels of telomerase activity were found in HLA-DR / CD28 double-positive cells. In contrast, HLA-DR-negative and CD28-negative cells had almost no telomerase activity. In summary, HLA-DR expression correlates with proliferation, and CD28 expression with proliferative potential. We have previously identified that ex vivo cytolytic CD8+ T cells are CD56 (NCAM) positive. Here we show that HLA-DR+ cells were rarely CD56+ and vice versa. This demonstrates that telomerase-expressing and cytolytic CD8+ T cells can be separated on the basis of the cell surface markers HLA-DR and CD56. Thus, activated CD8+ T cells specialize and exert distinct functions correlating with surface molecule expression.
Abbreviation:
-
- TRAP:
-
Telomerase repeat amplification protocol
1 Introduction
During an immune response, T cells are activated upon antigen recognition and TCR triggering. Subsequently, the cells undergo clonal expansion and acquire effector functions such as cytolytic activity and cytokine production. In this process, T lymphocytes differentiate from naive cells to activated effector cells and / or memory cells, but this process is only partly understood, and molecular events associated with T cell differentiation need to be defined in more detail. Changes in the level of expression of the TCR, the co-receptors CD4 and CD8, CD28 and HLA class II molecules have been identified and shown to correlate with T cell activation and differentiation. The earliest event so far known to take place on the cell surface after T cell activation is the down-regulation of the α β TCR 1. Subsequently, CD69 and the IL-2R α-chain (CD25) are up-regulated, and other activation markers such as HLA-DR and CD38 (cyclic ADP-ribose hydrolase) appear later 2. Further events are changes in isoforms of CD45 3, and down-regulation of the co-stimulatory molecules CD28 4, 5 and CD27 6.
These cell surface molecules transmit various intracellular signals through Ras, mitogen-activated protein kinases, phospholipase Cγ, or PKC. Signaling results in cell cycle progression, proliferation, cytokine production, or cytotoxicity 7, 8. However, it has been difficult to establish correlations between cell surface molecule expression and cellular differentiation / function, in part because of considerable crosstalk in signaling pathways.
A precise characterization of the association between molecular phenotype and function would strongly facilitate the investigation of immune responses and would enhance immune diagnostics in patients. The long CD45RA and the short CD45RO isoforms of the common leukocyte antigen CD45 have been found to be expressed by "naive" and antigen-experienced T cells, respectively 3, 9. Antibodies specific for these two isoforms are thus frequently applied to separate T cell subsets. However, since activated T cells can revert from CD45RO back to CD45RA expression 10, the CD45RA-positive subset contains both naive and non-naive T cells. For CD8+ T cells, it was found that CD28– CD27– CD45RA+ cells are enriched for cytolytic effector cells 11, 12. More recently we have demonstrated that CD56 (NCAM) represents a better marker to characterize activated cytolytic effector cells, since CD56+ CD8+ T cells are highly enriched for ex vivo cytolytic function 13, regardless of their CD45RA / CD27 phenotype.
Another parameter that changes during T cell activation and differentiation is the mean telomere length. Telomeres are terminal chromosome structures of repetitive DNA that shorten with each successive round of cell division due to the inability of DNA polymerase to replicate the terminal portion. For some cell types it has been demonstrated that telomere shortening is tightly associated with cellular senescence 14. The telomerase enzymatic complex is able to add telomeric repeats to chromosome ends, and allows cells of the germ line and malignant cells to maintain telomere length 15 – 19. B and T lymphocytes are different from the other normal differentiated human cells because they are able to transiently express telomerase activity after in vitro activation 16 – 18 and during in vivo clonal expansion 20. However, this may not be enough to completely maintain telomere length in lymphocytes. As compared to naive T cells, CD45RO+ "memory" cells and CD28 negative / CD27 negative effector cells were found to have reduced telomere length 21, 22. Thus, despite their ability to express telomerase, lymphocytes reduce telomere length during differentiation. However, it remains unknown whether some long-lived lymphocytes may efficiently maintain telomere length and cell proliferation potential. Therefore, we searched for subpopulations of T cells with high telomerase activity in vivo. Here we show that telomerase activity was clearly present in HLA-DR+ CD28+ T cells, but hardly detectable in the CD8+ T cells negative for HLA-DR or CD28. Furthermore, the HLA-DR+ population contained proliferating cells expressing the cell cycle-associated molecules Ki-67 23, 24 and the transferrin receptor CD71 25 – 27. In contrast, the cytotoxic effector cells were HLA-DR negative and CD56 positive.
2 Results
2.1 CD8+ T cells expressing the cell cycle-associated molecule Ki-67 are preferentially HLA-DR+
To test for a possible association between HLA-DR expression and the cell cycle, we used antibodies specific for Ki-67 which is expressed in proliferating cells of human origin 23 and has a short half-life of approximately 1 h 24. From PBL of the melanoma patient LAU 337, CD8+ T cells were isolated by magnetic cell sorting, fixed, stained with mAb and analyzed by flow cytometry. Fig. 1 shows that the Ki-67+ cells were HLA-DR+, confirming previously published data for CD4+ cells 28. Analysis of 12 individuals confirmed that Ki-67+ CD8+ T cells were mostly HLA-DR+ (81 ± 11 %; mean ± SD; not shown). A second molecule associated with cell proliferation, namely the transferrin receptor CD71 25 – 27, was also expressed by the HLA-DR+ cells (Fig. 1). In parallel, expression of CD28 was analyzed. The data show that some but not all of the cycling cells expressed CD28. Together, the cycling Ki-67+ and CD71+ cells expressed HLA-DR+, while there was no correlation with expression of CD28.
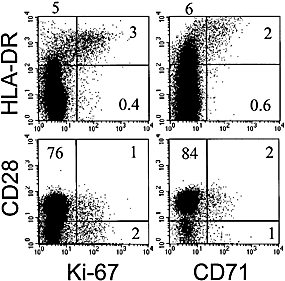
HLA-DR expression by circulating CD8+ T cells positive for the cell cycle-associated molecules Ki-67 and CD71. The numbers indicate the percentages of cells in the quadrants. CD8+ PBL from patient LAU 337 were analyzed by flow cytometry. MiniMACS sorting for CD8+ T cells and staining was done as described in Sect. 4.4.
2.2 In vivo expansion of tumor antigen-specific CD8+ T cells correlates with HLA-DR expression
In PBL from patient LAU 337 we observed a strong expansion of tumor antigen Melan-A-specific CD8+ T cells which occurred in parallel to immune therapy consisting of four vaccine (peptide + adjuvant) injections given in monthly intervals 29. Using fluorescent HLA-A*0201 / Melan-A tetramers, we found increasing percentages of Melan-A-specific cells, which were 0.1 % 1 week before immune therapy, 0.8 % after two vaccine injections, and 2.3 % after four vaccine injections (Fig. 2 A). Within these Melan-A-specific cells, HLA-DR expression was relatively low before the patient received immune therapy (Fig. 2 B). Afterwards, it increased significantly concomitant with cell expansion. Later blood samples revealed that the frequency of Melan-A-specific cells did not increase further, since relatively stable percentages (approximately 2 % of CD8+ T cells) were found during the following 2 months (not shown). Therefore, in vivo proliferative activity was probably highest around the time point of the second blood sample, i. e. after the first two vaccinations (Fig. 2 B, center histogram). At this time point, the percentage of Melan-A-specific cells expressing HLA-DR was highest, indicating that HLA-DR expression was associated with in vivo expansion of circulating CD8+ T cells. Interestingly, other "activation markers" such as CD69 and CD25 were not expressed by the Melan-A-specific cells of this patient (data not shown). Similar observations were made in a second melanoma patient with a significant albeit weaker CTL response to peptide immunization (data not shown).
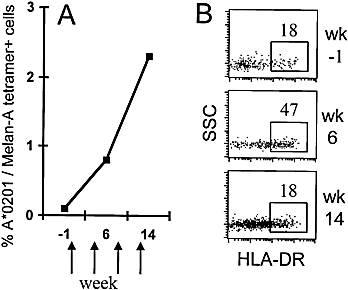
HLA-DR expression by tumor antigen-specific CD8+ T cells that expand in vivo. (A) In PBL from patient LAU 337, the percentages of CD8+ T cells positively stained with HLA-A*0201 / Melan-A tetramers increased over time, concomitant with immune therapy with Melan-A peptide given at weeks 0, 4, 8, 12 (arrows). (B) The dot plots show the cells gated for positive staining with HLA-A*0201 / Melan-A tetramers: HLA-DR expression increased from 18 % before immune therapy (top histogram; week-1) to 47 % during therapy (center; week 6). After therapy (bottom; week 14), the percentage was again low (18 %). Blood samples were taken before and 2 weeks after the second and fourth vaccine injections.
2.3 HLA-DR+ T cells express high levels of telomerase activity
Human lymphocytes transiently express telomerase activity after in vitro activation 16 – 18 and during in vivo clonal expansion 20. It can be expected that telomerase is preferentially expressed in proliferating T cell subpopulations. Furthermore, CD28+ cells have longer telomeres than CD28– cells. To test if there was a correlation between HLA-DR / CD28 expression and telomerase activity, we stained CD8+ T cells with antibodies specific for HLA-DR and CD28, sorted the resulting subpopulations and measured telomerase activity by telomerase repeat amplification protocol (TRAP). The results show that telomerase activity was found in the double-positive (HLA-DR+ CD28+) cells, but was hardly detectable in the HLA-DR– and CD28– cells (Fig. 3). Thus, circulating CD8+ T cells contain telomerase activity in vivo, and this activity is essentially confined to the HLA-DR+ CD28+ population.
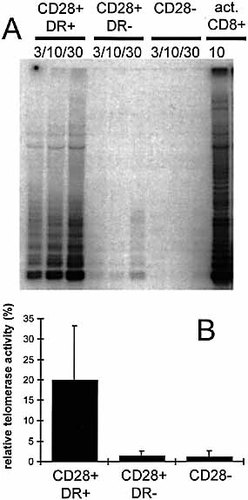
Telomerase activity in cell extracts of HLA-DR+ CD28+ T cells. MiniMACS-sorted CD8+ T cells were FACS sorted with HLA-DR- and CD28-specific mAb and analyzed directly (without in vitro activation) with a sensitive TRAP assay. (A) The three sorted populations were teted at 3,000 (3), 10,000 (10), and 30,000 (30) cells. As positive control, the same extract from CD8+ T cells activated in vitro with anti-CD3 and anti-CD28 antibodies (act. CD8+) was assayed in every experiment. (B) Quantification was done by measuring the OD and calculating the percent relative telomerase activity by setting the positive control as 100 %. For all the samples included in the quantification, a linear correlation between the signal strength and the amount of extract was demonstrated (data not shown) indicating that the signal obtained was proportional to the enzymatic activity. The results are mean values and SD from six independent experiments with six different healthy donors. Statistical evaluation (t-test): p = 0.013 for the comparison of CD28+DR+ with CD28+DR–; p = 0.028 for the comparison of CD28+DR+ with CD28–; and p = 0.408 for the comparison of CD28+DR– with CD28–. A TRAP assay was also performed with CD8+ sorted PBL from patient LAU 337: the relative telomerase activity was 11.2 % for the CD28+DR+ cells, 0.4 % for the CD28+DR– cells, and 1.2 % for the CD28– cells.
2.4 HLA-DR characterizes an activated CD8+ T cell population that is distinct from cytotoxic effector cells
The question arose whether all activated T cells express HLA-DR and whether HLA-DR-expressing cells exert cytotoxic effector function. Recently, we have demonstrated that CD8+ T cells with cytolytic activity ex vivo express the NCAM (CD56) 13. To assess HLA class II- and NCAM-positive cells, we analyzed PBL by multiparameter flow cytometry and gating for CD8+ T cells. The data from both normal individuals (Fig. 4 A; closed bars) and melanoma patients (open bars) show that the HLA-DR+ population was largely non-overlapping with the CD56+ lymphocytes, since cells positive for both markers were rare. Thus, although both markers can be expressed by activated CD8+ T cells, they are characteristic for distinct subpopulations.
To test for cytolytic function, we sorted CD8+ T cells with a FACS sorter and performed redirected cytotoxicity assays. As expected, the CD56+ DR– cells were strongly cytotoxic 13. In contrast, the CD56– DR+ CD28+ cells contained only low cytolytic activity, comparable to the CD56– DR– CD28+ (activation marker negative) cells (Fig. 4 B). CD56 / CD28 double-negative cells were also found to have only low cytolytic activity (data not shown).
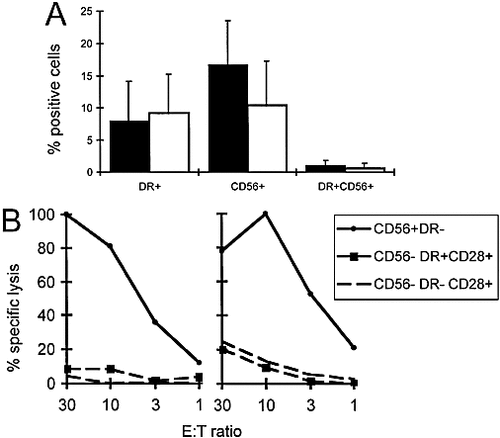
HLA-DR+ CD8+ T cells are distinct from CD56+ cytotoxic effector cells. (A) PBL from six healthy donors (closed bars) and six melanoma patients (open bars) were analyzed by flow cytometry and gating for CD8+ CD3+ T cells. Significant percentages of HLA-DR+ and of CD56+ cells were found in both groups. In contrast, CD8+ T cells expressing both markers simultaneously were rare. (B) Ex vivo CD8+ T cells were FACS sorted and tested in redirected cytolysis assays against 51Cr-labeled P815 target cells. While the CD56+ DR– cells showed strong cytolytic activity, only low level activity was observed in CD56– DR+ CD28+, and activation marker negative (CD56– DR– CD28+) cells. The results are from two healthy donors (HD400 left, and HD527 right), which are representative for four independent experiments with sorted PBL from a total of eight individuals, and confirm previously published data demonstrating that CD56+ T cells are highly enriched for ex vivo cytolytic activity 13.
Finally, CD56 expression was analyzed in patient LAU 337 whose Melan-A-specific cells expanded in vivo as shown in Fig. 2. A more detailed description of the Melan-A-specific cells of this patient is subject of a separate manuscript 29. These tumor antigen-specific T cells were CD56 negative and only weakly cytolytic ex vivo, but become highly cytotoxic after in vitro stimulation 29. Within the total CD8+ T cell pool, the CD56+ cells of this patient were largely negative for HLA-DR and for the cell cycle-associated markers Ki-67 and CD71 (Fig. 5). Analysis of 12 individuals revealed that CD56+ CD8+ T cells indeed expressed Ki-67 infrequently [7.4 ± 7.0 % (mean ± SD) of CD56+ cells were Ki-67 positive; not shown]. In conclusion, the HLA-DR-positive population containing cycling cells is distinct from the CD56-positive cells with cytolytic activity.
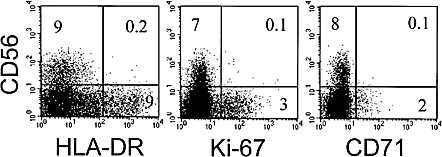
CD56+ CD8+ cytotoxic effector T cells are negative for HLA-DR, Ki-67 and CD71. PBL from patient LAU 337 were MiniMACS sorted for CD8+ cells and analyzed by flow cytometry. The numbers indicate the percentages of cells in the quadrants.
3 Discussion
The present study demonstrate three associations between surface molecule expression and cellular function in CD8+ T cells analyzed ex vivo: First, HLA-DR-expressing cells contained the proliferative pool. Second, HLA-DR / CD28 double-positive cells were enriched for telomerase activity. And third, HLA-DR+ cells were distinct from CD56+ cytolytic effector cells.
Our results are in agreement with the notion that HLA-DR is expressed by activated human T cells, but they specify more precisely that HLA-DR-expressing T cells contain the proliferative pool of T cells. Possibly, also other MHC class II molecules (HLA-DQ and -DP) may be expressed in association with cell cycle activity and proliferation. There is no explanation why human but not murine T cells can express MHC class II molecules, and their functional role remains unknown. Possibly, CD4+ Th cells may be activated upon recognition of peptide / MHC class II complexes on CD8+ T cells 30. As a consequence, CD8+ T cells may receive "help" through cell surface receptors or soluble factors. Another possibility is that HLA-DR directly transmits signals to CD8+ T cells 31, but it remains unclear whether this may affect the function of HLA-DR-expressing cells. Even less is known about whether HLA-DR signaling may influence cell cycle activity. It may simply be a coincidence that HLA-DR-expressing cells contain the cycling pool, as suggested by the finding that only a subpopulation of HLA-DR-positive cells expressed the cell cycle-associated molecules Ki-67 and CD71.
T cells show telomerase activity upon activation in vitro and in vivo 16 – 18. The expression of this enzyme has been proposed to allow T cells to undergo a high number of cell divisions without entering into replicative senescence. In unmanipulated human peripheral blood T cells, however, telomerase activity has been reported to be undetectable 17 or very low 15. Furthermore, telomerase activity could so far not be attributed to a particular T cell subset. For example, CD25 (IL-2R α-chain)-expressing T cells are not enriched for telomerase activity 15, indicating that CD25 expression is not tightly linked to telomerase activity. Therefore we have searched for other phenotypic characteristics of telomerase-positive T cells. To specifically investigate telomerase activity in cycling cells ex vivo, it would be useful to use the cell cycle marker Ki-67. However, staining for the intracellular Ki-67 requires cell fixation which precludes subsequent enzymatic assays such as the TRAP assay. In search for a surrogate marker expressed on the cell surface, we found that the Ki-67+ cells were HLA-DR positive, and used this information to investigate telomerase activity. Our results support the idea that telomerase is preferentially expressed in cycling cells.
In vitro activated T cells reduce telomere length despite high telomerase activity 18. Here we provide an explanation for this observation, since our data reveal the existence of distinct T cell subpopulations. While many T cells lack telomerase activity, some T cells have considerable telomerase activity presumably involved in the maintenance of telomere length. The fact that telomerase activity was found in unmanipulated human blood poses several questions. Is this activity related to proliferation of "memory" cells, and / or of naive cells to maintain T cell homeostasis? Or does it reflect low level activity immune responses in the healthy donors analyzed? Investigations of ongoing immune responses in vivo 20 will be useful to address these questions. However, appropriate human blood samples are difficult to obtain, and often the numbers of available lymphocytes are too low to perform functional (enzymatic) tests, as was the case for most patients analyzed in this study.
It is tempting to speculate that HLA-DR and CD28 signaling may directly influence the generation and maintenance of long-lived memory T cells. As stated above, the information for HLA-DR is scarce. More is known about the involvement of CD28 in T cell activation 4, 5. It will be interesting to investigate whether CD28 triggering may directly activate transcription of components of the telomerase enzymatic complex.
Another unresolved issue is the significance of CD28 down-regulation in human CD8+ T cells. While naive resting T cells are always CD28 positive, activated cells can be both positive or negative for CD28 11. Similar findings have been made for CD27, a TNFR family member with co-stimulatory functions 12. We found that CD28 and CD27 frequently down-regulate in parallel (not shown). For this study we focused on CD28, in part because its function is well characterized, and specifically because it has been suggested that expression of CD28 may correlate with proliferative potential 10. Several observations are compatible with the latter: (1) As compared to CD28+ T cells, the CD28-negative cells proliferate significantly less in vitro 11. (2) CD28-negative cells have been described to contain strongly expanded monoclonal populations in vivo 32 and (3) have short telomeres 21, 22, both of which indicate that they have undergone multiple cycles of proliferation in the past. Interestingly, the Melan-A-specific T cells in the melanoma patient described in Figs. 1 and 2 were 80 % CD28+ before expansion, and only 10 % CD28+ after vaccination and consecutive expansion 29. Nevertheless, many CD28-negative T cells continued to cycle, indicating that CD28-negative T cells can proliferate. Although CD28-negative T cells show reduced proliferation 11, many lymphocytes and T cell clones lacking CD28 can still proliferate considerably. However, the proliferative potential of CD28-negative T cells may be reduced, since these cells frequently stop dividing after a relative limited number of mitoses (unpublished data). Together, the current data indicate that it is the proliferative potential rather than the proliferative activity that correlates best with CD28 expression.
Our results differ from reports by Giorgi and colleagues 33 demonstrating that HIV-specific human cytotoxic T cells expressed HLA-DR. Although we saw some cytolytic activity in the HLA-DR-positive cells, this was not as strong as the cytotoxicity exerted by the CD56-positive cells. Possibly, cytotoxic T cells in viral infection may give a similar difference when CD56- and HLA-DR-expressing cells are analyzed separately. Another possibility is that the results with peptide antigen-specific killing assays differ from the results obtained with the redirected killing assay. Due to the limitations in the available number of antigen-specific T cells we could not address this question experimentally. Further studies are necessary to determine whether our observations also apply for antigen-specific cytotoxicity and for anti-viral immune responses.
The progressive reduction of CD28 and CD27 expression and the consequent reduced susceptibility to co-stimulatory signals of highly differentiated effector cells may be interpreted as follows. The absence of co-stimulatory molecules may help to avoid that immune responses become too strong in situations with sudden increase of antigen-specific stimulation. This may be particularly important for the clonally expanded CD28– CD27– CD8+ T cells with rapid cytotoxic activity and / or cytokine production. Many of these cells are specific for chronically infecting viruses such as EBV or CMV 34, 35. In persisting infections there is a homeostatic balance between viral replication and immune activity. This probably reflects the need to avoid that a sudden increase of antigen-specific stimulation leads to overwhelming immune activity that can be detrimental for the host 36. This situation is distinct from the classically defined secondary "memory" responses in acute (e. g. influenza virus) infections: the so-called memory cells are only present at relatively low numbers but express CD28 and have high proliferative potential. Rapid cellular expansion upon antigen re-exposure is crucial for successful elimination of pathogens causing acute non-persisting infections. Therefore, it appears that evolution has shaped the immune system such that cellular immune responses against persisting infections are functionally different (probably less aggressive) from those against acute non-persisting infections.
The results in this study were obtained with cells analyzed ex vivo. We avoided in vitro cultured cells since only few of the many possible differentiation stages develop and are maintained in cells that are kept in the usual in vitro culture systems (data not shown). Therefore, functional tests that are frequently used to analyze cell proliferation, cytokine production or cytotoxicity need to be complemented with ex vivo analyses. This becomes more feasible with increasing knowledge of phenotype-function relationships. Specific cellular markers as described in this study provide valuable tools and are especially meaningful in conjunction with fluorescent MHC / peptide-tetramers 37. Carefully performed studies in humans will improve our understanding of both the natural course of immune responses as well as the effects of vaccines and immune suppressive therapy.
4 Materials and methods
4.1 Healthy donors and melanoma patients
Peripheral blood was obtained from healthy donors and patients with informed consent. All patients had no irradiation, chemotherapy, or immune therapy during several weeks before blood withdrawal, except patient LAU 337 who had the following history. This patient was diagnosed with primary skin melanoma and underwent surgery in October 1996, and was treated with IFN-α until May 1998. After this, chemotherapy was given because of progressive disease. In early 1999, further progression (in lymph nodes and lungs) was diagnosed, and immune therapy with peptides plus adjuvant was initiated in April 1999 (the patient was HLA-A*0201 positive). The patient received a mixture of the two synthetic peptides Melan-A26 – 35 EAAGIGILTV and influenza matrix protein58 – 66 GILGFVFTL (each 100 μg), and 600 μl adjuvant SB-AS2 provided by SmithKline Beecham. This was given i. m. at weeks 0, 4, 8, and 12. By the end of 1999, there was complete regression of some subcutaneous metastases and partial remission of one pulmonary metastasis, while other lesions remained stable and some progressed. The patient died 59 weeks after initiation of immune therapy due to tumor progression.
4.2 Blood samples
PBL from patient LAU 337 were obtained before (week-1), and 14 days after two vaccinations (week 6) and after four vaccinations (week 14). The PBL used in Figs. 1 and 5 were obtained at week 6. The other patients had no immune therapy preceding blood withdrawal. PBL were separated from heparinized blood by centrifugation over Ficoll-Paque (Pharmacia), washed three times and cryopreserved in RPMI 1640, 40 % FCS and 10 % DMSO. Vials containing 5 × 106 – 10 × 106 cells were stored in liquid nitrogen.
4.3 mAb and tetramers
mAb specific for human CD3, CD8, CD28, CD56, CD71 and Ki-67 were obtained from Becton-Dickinson. Tetramer complexes were synthesized as described 37, 38.
4.4 Flow cytometry
For fluorescent stainings, frozen and thawed PBL were used. Where indicated, CD8+ T cells were purified in two rounds of positive sorting with magnetic beads and a MiniMACS device (Miltenyi Biotech Inc). The resulting cells were 95 – 100 % CD3+ CD8+. Cells (5 × 105 – 1 × 106) were stained with tetramers, FITC-, PE-, PerCP- or allophycocyanin-labeled mAb conjugates in 50 μl PBS, 2 % BSA and 0.2 % azide for 40 min at 4 °C (except tetramer incubation that was done at room temperature). Cells were washed once in the same buffer and analyzed immediately in a FACSCaliburTM machine (Becton Dickinson, Mountain View, CA). Data acquisition and analysis was performed using CELL QUESTTM software.
4.5 PCR-based telomerase assay
PBL were thawed, and MiniMACS-sorted cells (95 – 100 % CD3+ CD8+) were stained with CD28- and HLA-DR-specific antibodies and sorted using a FACSVantageTM machine (Becton Dickinson, Mountain View, CA). Cell extracts were prepared by incubation of the sorted cell populations in a CHAPS-containing lysis buffer, and the TRAP was performed according to reference 39 using the ACX anchored return primer. The PCR step was performed in two-step cycles (95 ° for 30 s and 60 ° for 30 s), and the number of amplification cycles was increased up to 32 in order to achieve a high sensibility. As the positive control, the same extract from activated lymphocytes was included in every experiment (CD8+ T cells in vitro activated with anti-CD3 plus anti-CD28 for 3 days as described 40). Relative signal intensity of the repeat bands was measured by phosphor imaging analysis. These data were normalized with the cell number, and relative telomerase activity was expressed as percent of the positive control. Three extract amounts corresponding to 3, 10 and 30 × 103 cells were assayed for every sample. The three values obtained were plotted against the cell number to check that there is a linear correlation between the band obtained in the TRAP and the amount of enzyme, as previously described. All the detectable ladder bands found in the experiments described in this study were in the linear range of correlation with the extract amount, and therefore they could be used for quantification.
4.6 Chromium release assay
MiniMACS-sorted CD8+ T cells were stained with CD28-, HLA-DR- and CD56-specific antibodies and sorted as described above. The cells were then cultured overnight in Iscove's Dulbecco's medium supplemented with 0.55 mM Arg, 0.24 mM Asn, 1.5 mM Gln and 10 % FCS. Cytolytic activity was tested in anti-CD3 mAb redirected 51Cr-release assays. Briefly, FcγR-expressing P815 mastocytoma cells were radiolabeled with Na51CrO4 for 1 h at 37 °C at 5 % CO2. After washing, 106 P815 cells were incubated with 300 ng / ml anti-CD3 OKT3 mAb 40 in 1 ml PBS containing 0.2 % BSA at room temperature and 15 min later diluted 100-fold in medium. Effector and target cells were co-incubated in V-bottom microwells at the indicated ratio, whereby each well contained 103 P815 target cells and 300 pg / ml anti-CD3 mAb. After 4 h at 37 °C, supernatants were collected and counted in a Top-countTM (Canberra Packard) gamma counter. Percent specific lysis was calculated as (experimental release – spontaneous release) × 100 / (total release – spontaneous release).
Acknowledgements
We are grateful to the patients and the healthy donors for blood donation. We thank Hans Acha-Orbea, Markus Nabholz and Anne Wilson for discussions and support; and Christian Knabenhans, Pierre Zaech and Pascal Batard for cell sorting. We also acknowledge the excellent technical and secretarial help of Andrée Porret, Danielle Minaïdis, Christine Geldhof, Nicole Montandon, and Martine van Overloop. This work was in part supported by a grant from the Swiss National Science Foundation to Markus Nabholz, and by a grant from the Leenaards Foundation.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




