Effect of nuclear factor κB inhibition on tumor cell sensitivity to natural killer-mediated cytolytic function
The first two authors contributed equally to this work.
Abstract
Inhibition of the transcription factor NF-κB has been reported to increase cell sensitivity to TNF and some cytotoxic drugs. We investigated the effect of NK-κB inhibition on the susceptibility of tumor cells to freshly isolated, nonactivated, human NK cells and to a TCRγ / δ T cell clone displaying an MHC-unrestricted "NK-like" lysis. Using electrophoretic mobility shift assay, we first demonstrated that NF-κB / DNA binding activity was induced in target cells following coculture with NK cells or TCRγ / δ T cell clone. To investigate the effect of target cell NF-κB inhibition on NK-mediated lysis, we blocked NF-κB translocation by introducing a human cDNA coding for a mutated IκB-α. Interestingly, our results indicated that inhibition of NF-κB did not induce any increase in either granzyme-dependent non-MHC-restricted cytotoxicity mediated by fresh non-stimulated NK cells and by TCR γ / δ T cell clone or in CD95-mediated lysis. These results emphasize that NF-κB expressed in target cells does not play a role in the molecular process related to the control of target cell susceptibility to NK-mediated lysis and suggest that the NF-κB pathway is not a general mechanism for controlling the cytotoxic response.
Abbreviations:
-
- NF-κB:
-
Nuclear factor κB
-
- EMSA:
-
Electrophoretic mobility shift assay
-
- FasL:
-
Fas ligand
-
- MnSOD:
-
Manganous superoxide dismutase
1 Introduction
The nuclear factor kappa B (NF-κB) is an ubiquitous transcription factor that is involved in the expression control of a variety of genes 1. It plays a central role not only in inflammation and immune response but also in oncogenesis and in cancer cell response to cytotoxic agents 2. In the majority of cells, NF-κB exists in an inactive form in the cytoplasm bound to the inhibitory protein, IκB. Treatment of the cells with various inducers results in degradation of IκB, thus releasing NF-κB, which translocates to the nucleus and up-regulates gene expression 3. Numerous experimental studies have established that NF-κB plays an important role in cell survival by regulating some anti-apoptotic genes 4, 5. In this context, recent studies have established cell type-specific anti-apoptotic functions for this transcription factor 6. In addition, death-inducing ligands such TNF or cytotoxic drugs have been shown to activate an NF-κB-dependent program that may rescue cells from apoptosis 7. Based on these observations, it has been suggested that inhibition of NF-κB activation may provide a molecular approach to increase apoptosis sensitivity in anti-cancer treatment 8. It has been shown that NK cells use the perforin / granzyme and the Fas / Fas ligand (FasL) pathway to induce target cell death 9. Both pathways share some components also involved in the triggering of TNF-induced cell death, including activation of caspases 10. Nevertheless, it should be noted that despite the reliance on common mediators, these pathways are governed by distinct regulatory mechanisms. The significance of NF-κB inhibition in the regulation of NK-induced cell death has not been defined. In the present study, we demonstrate that, unlike sensitization to drugs and TNF, the inhibition of NF-κB in tumor target cells does not result in an increase in NK-induced target cell killing. Our data suggest that cell death induced by these distinct pathways is differentially sensitive to NF-κB and that the inhibition of this transcription factor is not a general regulatory event of cell lysis.
2 Results and discussion
2.1 Induction of NF-κB activation in target cells upon interaction with NK cells
It has been suggested that NF-κB may mediate aspects of programmed cell death. In these studies, we first demonstrated that co-incubation of MCF7 target cells with freshly isolated NK cells (> 90 % CD3–, see Sect. 4.2) for 15 or 30 min resulted in the translocation of NF-κB (Fig. 1). Similar results were obtained with a TCRγ δ T cell clone, L6-1, displaying an MHC-unrestricted, "NK-like", lysis (Fig. 1 A 11. Integrity of the nuclear extracts was controlled by electrophoretic mobility shift assays (EMSA) using a probe that interacts with Oct 1 which is not inducible (Fig. 1 B). These data suggest that target cell interaction with NK cell effectors may utilize surface structures in initiating transmembrane signaling. In this regard, it should be noted that NK cells interact with tumor cells via some target cell membrane proteins, including MHC molecules. Whether there is a subset of receptors (i. e. TNFR, CD48, TRAIL / R) initiating second messengers in target cells that may regulate NK-induced cell killing has to be determined. Immunofluorescence analysis indicated Fas and TNFR expression on all tumor cel lines (data not shown). Recently, it has been reported that fresh NK cells express the membrane form of TNF 12, which may represent a potential structure involved in the induction of NF-κB. Future studies are, however, needed to delineate the exact structure involved and to characterize the putative target surface receptors that initiate NF-κB activation. Experiments are in progress to further delineate the signaling pathways activated in target cells and to examine their possible interference with the control of susceptibility to NK-induced cell death.
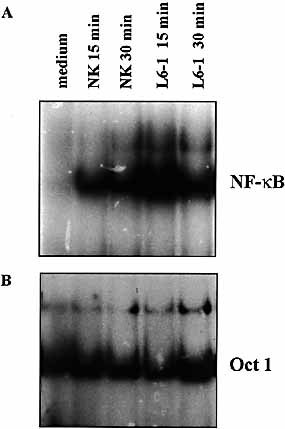
(A) Induction of NF-κB activation in MCF7 tumor cells following interaction with NK or L6-1 cells. Tumor cells (3 × 106) were incubated with freshly isolated NK cells (9 × 106) or TCRγ δ L6-1 T cells for the indicated times. Only nuclear extracts from adherent cells were prepared. Data are from one representative experiment out of five. (B) Integrity of the extracts was controlled under the same conditions as in (A) using Oct 1 probe.
2.2 Inhibition of NF-κB activation by cell transfection with mutated IκB-α cDNA
Mutations of the serine 32 and 36 of IκB-α protein have been described 13. These mutations, by disrupting the two main inducible IκB-α phosphorylation sites, abolish IκB-α degradation and thus prevent NF-κB activation. To inhibit NF-κB translocation, we therefore introduced the dominant negative human IκB-α mutant construct coding for a mutated IκB-α that is resistant to both phosphorylation and proteolytic degradation into MCF7, HCT116 and OVACAR-3 cells. As demonstrated in gel retardation assays performed with nuclear extracts from representative stable clones, NF-κB / DNA binding activity was potently induced in all cells tested after stimulation with TNF for 30 min (Fig. 2). In sharp contrast, TNF failed to induce the nuclear translocation of NF-κB in cells expressing mutated IκB (MCF7 / MT, HCT116-MT and slightly OVCAR-MT). No further activation of NF-κB could be detected after 24 h treatment with TNF, suggesting that NF-κB was inhibited by a stabilized association with the mutated IκB-α. Similarly, no NF-κB translocation was observed in Iκ-B-α-transfected cells when incubated with NK cells for 30 min (data not shown).
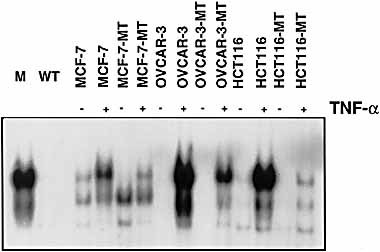
Inhibition of NF-κB induction in the presence of mutated IκB-α. MCF7, OVCAR-3 and HCT116 cells, either untransfected or stably transfected with an expression vector coding for the mIκB-α protein (MT cells), were left untreated or were stimulated for 30 min with TNF. Nuclear extracts were prepared and analyzed by EMSA using specific NF-κB probe.
2.3 Functional inhibition of NF-κB in mutated IκB-α-expressing cells
We then tested the functional effect of the inhibition of NF-κB translocation in IκB-α-transfected cells by studying the expression of one of the TNF-inducible genes, mitochondrial manganous superoxide dismutase (MnSOD). This gene presents potential κB sites in its promoter region and the induction of its expression is closely associated with NF-κB activation by TNF. Northern blot analysis indicated that, as opposed to MCF7, OVCAR and HCT116 parental cells, TNF failed to induce MnSOD transcription in mutated IκB-α-transfected equivalents demonstrating a functional inhibition of NF-κB in these cells (Fig. 3). Similar results were obtained with another NF-κB-regulated gene, ICAM-1 (data not shown).
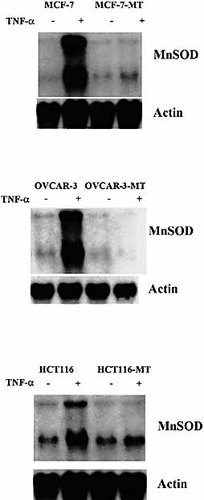
Northern blot analysis of mitochondrial MnSOD gene expression upon TNF treatment in MCF7 / MCF7-MT, OVCAR / OVCAR-MT and HCT116 / HCT116-MT tumor cells. Cells were incubated in medium or in the presence of 50 ng / ml TNF for 6 h at 37 °C. Total RNA (15 μg / lane) were electrophoresed in 1.2 % agarose gel and hybridized with 32P-labeled mitochondrial MnSOD-specific cDNA probe. A β-actin probe was used to confirm integrity and comparable loading of RNA sample.
2.4 NF-κB inhibition in target cells does not increase their sensitivity to NK-mediated killing
It is now well established that NK cell-mediated cytotoxicity is inhibited upon specific recognition of MHC class I molecules on target cells 14, 15. Given the fact that NF-κB has been shown to participate in the control of the MHC class I gene basal expression 16, initial experiments were performed to examine the effect of NF-κB inhibition on constitutive MHC class I expression on the target cells. Basal MHC class I expression was thus compared in parental cells and in their derivatives stably transfected with the mutated IκB-α gene. FACS analysis indicated comparable fluorescence intensity in HCT, MCF7 and OVCAR parental cells and in their equivalent transfected with mutated IκB-α (data not shown). These results indicate that stable transfection of the mutated IκB-α does not alter the constitutive expression of MHC class I in these cells.
Cytotoxicity assays were then performed to determine the sensitivity of the mutated IκB-α -transfected cells to NK-induced cytotoxicity. For this purpose, fresh NK cells (> 90 % CD3–) were isolated from healthy donor PBMC after depletion of T cells, B cells and macrophages. These non-stimulated NK cells were able to efficiently lyse all tumor cell lines in a non-MHC class I-restricted manner. Data shown in Fig. 4 A indicate the absence of significant difference in the cytotoxic response between the control tumor cells and the mutated IκB-α-transfected cells. This suggests that the activation of NF-κB by NK in target cells is not protective and fits with our previous work indicating the failure of NF-κB inhibition to increase sensitivity to cytotoxic drugs in these cells 17. To further confirm that inhibition of NF-κB does not influence the non-MHC-restricted cytotoxicity, we used the TCRγ δ T cell clone, L6-1. This clone displays an HLA-A1-restricted TCR-mediated cytotoxic activity toward its specific target cells as well as a MHC-unrestricted lysis against K562 and Daudi target cells 11. As shown in Fig. 4B, L6-1 T cell clone killed parental target cells and their equivalents, in which NF-κB activation was inhibited, in a similar manner.
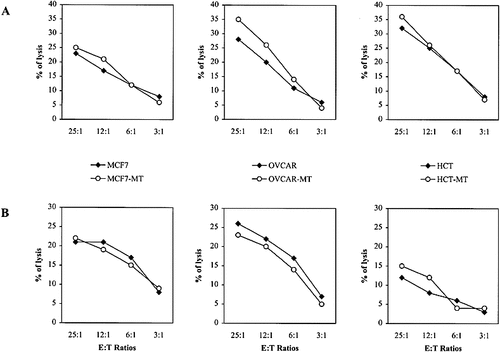
Cytotoxic activity of fresh NK cells (A) and TCRγ δ T cell clone (B) toward target cells transfected or not with mutated IκB-α. Cytolytic experiments were performed for 4 h at 37 °C at 25 : 1, 12 : 1, 6 : 1 and 3 : 1 E / T ratios DS were < 10 %. Data are from one representative experiment out of three.
In the following experiments, we examined the mechanisms used by both NK and TCRγ / δ cells to mediate their cytotoxic activity toward all tumor cell lines, transfected or not with IκB-α. For this purpose, blocking experiments were performed in the presence of ZB4 (anti-Fas neutralizing mAb), EGTA (used to inhibit the Ca2+-dependent perforin / granzyme-mediated lysis) or NKTa control mAb. Results presented in Fig. 5 clearly showed that both effector cells mainly use a granule exocytosis pathway to kill tumor targets expressing or not NF-κB. Furthermore, experiments performed either with agonistic anti-Fas mAb (CH11) or soluble FasL indicated that all IκB-α-transfected cells were as efficiently lysed as parental tumor cells (Fig. 6 A). This cytotoxicity was inhibited in the presence of ZB4 anti-Fas blocking mAb (Fig. 6 B). It should be noted that previous immunofluorescence experiments indicated that both parental and transfected tumor cells express similar intensity of CD95 on their surface (data not shown).
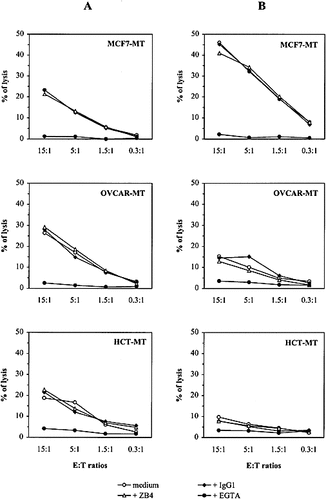
Cytotoxic activity of fresh NK and L6-1 cells toward MCF7-MT, OVCAR-MT and HCT116-MT tumor cells. Experiments were performed either in media or after preincubation of target cells for 1 h in the presence of anti-Fas ZB4 mAb, NKTa (isotypic control) or in the presence of 4 mM EGTA + 3 mM MgCl2. The E / T ratios were as indicated.
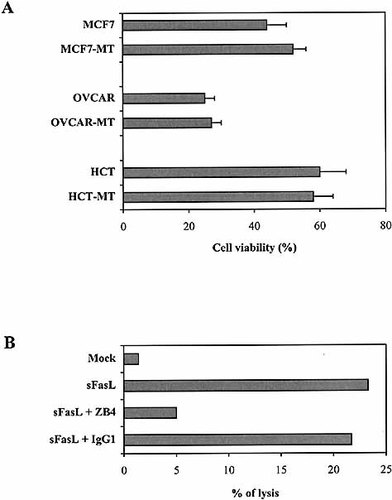
(A) MCF7 / MCF7-MT, OVCAR / OVCAR-MT and HCT116 / HCT116-MT tumor cell lysis induced by CH11 anti-Fas agonistic mAb. (B) OVCAR-MT tumor cell lysis induced by soluble FasL (sFasL). Mock empty vector was used as control. Experiments were peformed either in media or after preincubation of target cells with ZB4 anti-Fas blocking mAb or isotypic control.
3 Concluding remarks
The present data further point to the concept that the inhibition of NF-κB activation had no effect on non-MHC-restricted cytotoxicity. Although it has been suggested that inhibition of NF-κB activation may provide a molecular approach to increase apoptosis sensitivity in anti-cancer treatment, the present studies suggest that such inhibition may have no consequence on non-MHC-restricted cell-mediated cytotoxicity. Whether these differences are related to cell type specificity has to be determined. Our data suggest that although some malignant cells display a high level of constitutive nuclear NF-κB activity, which may stimulate proliferation and confers resistance to cytotoxic drugs 18, these cells remain similarly sensitive to innate immunity involving NK-mediated cytotoxicity. These studies also emphasize that although cell death mediated by drugs, TNF, CTL and FasL may have similarities in term of activation of proteases, differences in their regulation may exist. These differences might be due to differential contributions of the respective signaling cascades or the critical intermediates in cell death. Therefore, the distinct role of NF-κB in the control of cytotoxic pathways suggests that the death process induced by different agents is governed by distinct mechanisms related, at least in part, to the nature of cell death inducer. It would be of major interest to determine how receptor-mediated signaling in target cells is translated into the putative intracellular biochemical events and whether some membrane determinants on target cells are involved in the control of cell susceptibility to lysis.
4 Materials and methods
4.1 Target cell lines and reagents
HCT116 human colon carcinoma cells (ATCC CCL 247), MCF7 breast cancer cells, and OVCAR-3 ovarian carcinoma cells were described previously 19. MCF7 / MT cells were transfected with IκB-α cDNA as described 20. OVCAR-MT and HCT116-MT were obtained by stable transfection of parental cells with a linearized pcRV-CMV plasmid encoding the IκB-α protein mutated at Ser-32 and Ser-36 (a gift from Dr. A. Israël, Institut Pasteur, Paris, France) as described previously 17. L6-1 TCRγ / δ T cell clone was obtained from a healthy individual (A1 / A24, BW57 / B27, CW6) as previously reported 11. The human recombinant TNF (specific activity 6.63 × 106 U / mg protein) was kindly provided by Dr. I. Apfler (Bender Wien, Austria).
4.2 NK cell preparation and FACS analysis
PBL were isolated from healthy donors (Banque du sang, Hôpital Saint-Louis, Paris, France) by Ficoll-Hypaque gradient centrifugation followed by plastic adherence. NK cells were enriched by Percoll density gradient and incubated for 30 min at 4 °C with a cocktail of OKT3, OKT4 and OKT8 antibodies (Ortho Diagnostics Systems, Inc, Westwood, MA), which recognized CD3, CD4 and CD8 molecules, respectively, and MY4 (anti-CD14) and B4 (anti-CD20) mAb, (Coulter / Immunotech, Marseille, France). The cells were resuspended in RPMI complete medium supplemented with 10 % human serum, incubated with magnetic beads (Dynal, Oslo, Norway) for 2 min, and passed over a magnet. This separation was performed twice and the resulting cell preparation contained more than 90 % CD3– cells. For flow cytometry analysis of MHC class I expression, tumor cells were trypsinized, washed and resuspended in cold buffer consisting of PBS with 2 % FCS. Cells (2 × 105) were resuspended in 100 μl W6 / 32 mAb at a concentration of 1 μg / ml and then were incubated on ice for 25 min, washed twice in the above buffer and resuspended in 100 μl of a 1 / 200 dilution of FITC-labeled goat anti-mouse IgG.
4.3 Nuclear extracts and EMSA
Tumor cells (3 × 106) were incubated in medium in the presence of 50 ng / ml TNF or in the presence NK cells or TCRγ / δ T cells (9 × 106) for 15 or 30 min. When tumor cells were cocultured with the NK cells or L6-1 CTL clone, only adherent cells were used for nuclear extract preparation. The cells were then trypsinized and washed with PBS. Nuclear extracts were prepared according to the procedure of Dignam et al. 21.
4.4 Cytotoxicity assay
Serial dilutions of effector cells were distributed in triplicates (0.1 ml per well) into round-bottom microwell plates. Target cells were labeled with 200 mCi Na251CrO4 (5 μCi / ml; Amersham) for 1 h at 37 °C and washed three times. Cells (5.000 per well in 0.1 ml) were distributed in round-bottom microwells with 0.1 ml of effector cells at various E / T ratios. After 4 h of incubation at 37 °C, the plates were centrifuged at 2,000 g for 2 min, and cell-free supernatants were collected using a cell harvester (Skatron Inc, Sterling, VA). Supernatant radioactivity was assayed using an automated gamma counter (Packard Instrument Co, Meriden, CT). Spontaneous release was determined by incubating target cells in medium alone. Maximum release was determined by adding 0.1 ml of 1 M HCl to the target cell suspension. The percentage of specific lysis was calculated as follows: 100 × (experimental 51Cr release – spontaneous 51Cr release) / (maximum 51Cr release – spontaneous 51Cr release). SD were < 10 %. Inhibitory effect of the antibodies was tested by preincubating target cells for 1 h in the presence of ZB4 (anti-Fas mAb), isotype control mAb (NKTa) or EGTA + MgCl2, the effector cells were then added.
Acknowledgements
We thank Dr. A. Moretta for helpful discussion and G. Trinchieri for reading the manuscript. We also thank Y. Lecluse for FACS analysis and C. Leroy for secretarial assistance. This work was supported by grants from the Institut National de la Santé et de la Recherche Médicale (INSERM), the Association pour la Recherche contre le Cancer (ARC) and the Ligue Nationale Française de Recherche contre le Cancer. V. B. is research associate at the National Fund for Scientific Research (FNRS, Belgium).
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




