Polymerizability of Cycloalkenes in “Living” Ring-Opening Metathesis Polymerization Initiated by Schrock Complexes, 2. Effect of Initiator Structure
Abstract
The kinetics of ring-opening metathesis polymerization of norbornene were investigated in cyclohexane using several Schrock complexes as initiators. Three different Schrock complexes were used: Mo(CHC(CH3)2Ph)(NAr)(O-t-Bu)2 (NAr = Ph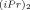 ) (I), Mo(CHC(CH3)2Ph)(NAr)(OC(CF3)2CH3)2 (NAr = Ph
) (I), Mo(CHC(CH3)2Ph)(NAr)(OC(CF3)2CH3)2 (NAr = Ph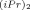 ) (II) and W(CH-t-Bu)(NAr)(O-t-Bu)2 (NAr = Ph
) (II) and W(CH-t-Bu)(NAr)(O-t-Bu)2 (NAr = Ph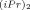 ) (III). The presence of alkoxy-fluorinated ligands on II and on the corresponding propagating species enhances the polymerizability of norbornene and modifies the kinetic scheme compared to that of polymerizations initiated by the tert-butoxy complex. The greater electropositive character of II compared to that of I results in a majority of metalla-alkylidenic growing species being complexed to monomer; therefore, the coordination of the monomer is not the rate-determining step for the process. In contrast, the major part of growing species, which are derived from the tert-butoxy complex are in a non-coordinated state and, thus, the ability of the initiator to complex the monomer is decisive. In addition, this study allows to quantify the stronger reactivity of W-based complexes compared to that of Mo-based ones. These results were all corroborated by prior theoretical studies using ab initio calculations.
) (III). The presence of alkoxy-fluorinated ligands on II and on the corresponding propagating species enhances the polymerizability of norbornene and modifies the kinetic scheme compared to that of polymerizations initiated by the tert-butoxy complex. The greater electropositive character of II compared to that of I results in a majority of metalla-alkylidenic growing species being complexed to monomer; therefore, the coordination of the monomer is not the rate-determining step for the process. In contrast, the major part of growing species, which are derived from the tert-butoxy complex are in a non-coordinated state and, thus, the ability of the initiator to complex the monomer is decisive. In addition, this study allows to quantify the stronger reactivity of W-based complexes compared to that of Mo-based ones. These results were all corroborated by prior theoretical studies using ab initio calculations.