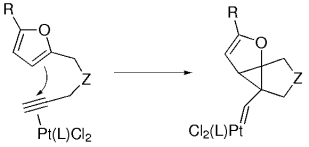
PtII-Catalyzed Intramolecular Reaction of Furans with Alkynes
Belén Martín-Matute
Departamento de Química Orgánica Universidad Autónoma de Madrid Cantoblanco, 28049 Madrid, Spain, Fax: (+349) 1-3973966
Search for more papers by this authorDiego J. Cárdenas Dr.
Departamento de Química Orgánica Universidad Autónoma de Madrid Cantoblanco, 28049 Madrid, Spain, Fax: (+349) 1-3973966
Search for more papers by this authorAntonio M. Echavarren Prof. Dr.
Departamento de Química Orgánica Universidad Autónoma de Madrid Cantoblanco, 28049 Madrid, Spain, Fax: (+349) 1-3973966
Search for more papers by this authorBelén Martín-Matute
Departamento de Química Orgánica Universidad Autónoma de Madrid Cantoblanco, 28049 Madrid, Spain, Fax: (+349) 1-3973966
Search for more papers by this authorDiego J. Cárdenas Dr.
Departamento de Química Orgánica Universidad Autónoma de Madrid Cantoblanco, 28049 Madrid, Spain, Fax: (+349) 1-3973966
Search for more papers by this authorAntonio M. Echavarren Prof. Dr.
Departamento de Química Orgánica Universidad Autónoma de Madrid Cantoblanco, 28049 Madrid, Spain, Fax: (+349) 1-3973966
Search for more papers by this authorThis work was supported by the DGICYT (project PB97-0002) and the MCyT (predoctoral fellowship to B.M.-M.). We also acknowledge Johnson Matthey PLC for a generous loan of PtCl2.
Graphical Abstract
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2001/z17658_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a M. Méndez, M. P. Muñoz, A. M. Echavarren, J. Am. Chem. Soc. 2000, 122, 11 549–11 550;
- 1b M. Méndez, M. P. Muñoz, C. Nevado, D. J. Cárdenas, A. M. Echavarren, J. Am. Chem. Soc. 2001, 123, 10 511–10 520.
- 2
- 2a B. M. Trost, M. J. Krische, Synlett 1998, 1–16;
- 2b N. Chatani, K. Kataoka, S. Murai, N. Furokawa, Y. Seki, J. Am. Chem. Soc. 1998, 120, 9104–9105;
- 2c A. Fürstner, H. Szillat, F. Stelzer, J. Am. Chem. Soc. 2000, 122, 6785–6786;
- 2d S. Oi, I. Tsukamoto, S. Miyano, Y. Inoue, Organometallics 2001, 20, in press.
- 3 A. S. K. Hashmi, T. M. Frost, J. W. Bats, J. Am. Chem. Soc. 2000, 122, 11 553–11 554.
- 4
For a highlight on the catalytic applications of gold, see G. Dyker, Angew. Chem. 2000, 112, 4407–4409;
10.1002/1521-3757(20001201)112:23<4407::AID-ANGE4407>3.0.CO;2-K Google ScholarAngew. Chem. Int. Ed. 2000, 39, 4237–4239.10.1002/1521-3773(20001201)39:23<4237::AID-ANIE4237>3.0.CO;2-A CAS PubMed Web of Science® Google Scholar
- 5 N. Chatani, H. Inoue, T. Ikeda, S. Murai, J. Org. Chem. 2000, 65, 4913–4918.
- 6 See the Supporting Information for experimental details and characterization data.
- 7 The reaction of 7 with AuCl3 as catalyst afforded exclusively phenol 8 (69 %).[3] In the platinum(II)-catalyzed reaction, traces (ca. 1 %) of a phenol tentatively assigned as 1,3-dihydro-6-methyl-5-isobenzofuranol were also obtained.
- 8
- 8a The calculations were performed with Gaussian 98.[8b] The geometries of all complexes were optimized at the DFT level of theory with the generalized gradient approximation and the B3LYP hybrid functional.[8c, d] The standard 6-31G(d) basis set was used for C, H, O and Cl, and the default LANL2DZ pseudorelativistic potential and basis set were used for Pt. Harmonic frequencies were calculated at B3LYP level to characterize the stationary points and to determine the zero-point energies (ZPE). The starting approximate geometry for the transition states (TS) were located graphically;
- 8b Gaussian 98 (Revision A.7), M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P. M. W. Gill, B. G. Johnson, W. Chen, M. W. Wong, J. L. Andres, M. Head-Gordon, E. S. Replogle, J. A. Pople, Gaussian, Inc., Pittsburgh, PA, 1998.;
- 8c P. J. Stephens, F. J. Devlin, C. F. Chabalowski, M. J. Frisch, J. Phys. Chem. 1994, 98, 11 623–11 627;
- 8d W. Kohn, A. D. Becke, R. G. Parr, J. Phys. Chem. 1996, 1000, 12 974–12 980.
- 9 Bond lengths, bond angles, and atomic coordinates for the structures of Figure 1 are given in the Supporting Information.
- 10
- 10a A mechanistic hypothesis for the formation of dihydrofurans from XI is outlined in the Supporting Information;
- 10b we have previously observed the formation of an aldehyde from a related platinum carbene intermediate;[1b]
- 10c for the related oxidation of a nickel carbene intermediate, see: S. K. Chowdhury, K. K. D. Amarasinghe, M. J. Heeg, J. Montgomery, J. Am. Chem. Soc. 2000, 122, 6775–6776.
- 11 Rhodium carbenes, generated in situ from [Rh2(OAc)4] and diazo compounds, react with aldehydes to form epoxides. See, for example,
- 11a V. K. Aggarwal, H. Abdel-Rahman, R. V. H. Jones, H. Y. Lee, B. D. Reid, J. Am. Chem. Soc. 1994, 116, 5973–5974;
- 11b M. Hamaguchi, H. Matsubara, T. Nagai, Tetrahedron Lett. 2000, 41, 1457–1460.
- 12 Trapping of an intermediate alkenylmetal intermediate with allyl chloride: C. Fernández-Rivas, M. Méndez, A. M. Echavarren, J. Am. Chem. Soc. 2000, 122, 1221–1222.
- 13
- 13a K. Kaneda, T. Uchiyama, Y. Fujiwara, T. Imanaka, S. Teranishi, J. Org. Chem. 1979, 44, 55–63;
- 13b J.-E. Bäckvall, Y. I. M. Nilsson, R. G. P. Gatti, Organometallics 1995, 14, 4242–4246, and references therein.