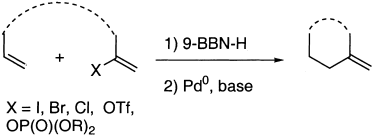
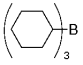
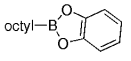
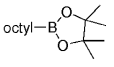
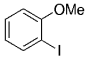
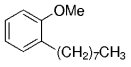
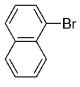
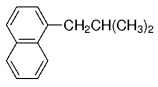
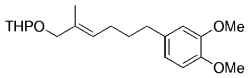
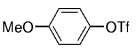
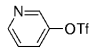
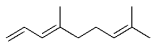
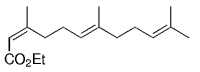
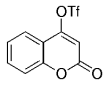
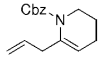
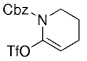
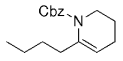
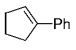
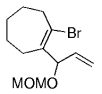
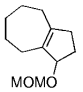
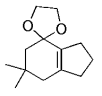
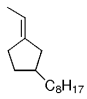
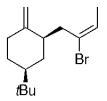
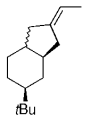
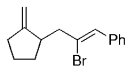
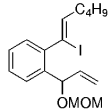
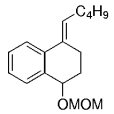
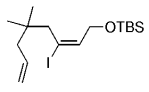
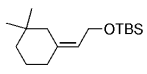
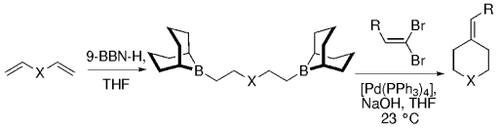
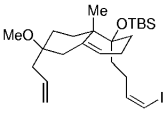
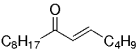
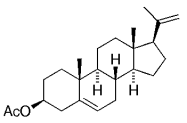
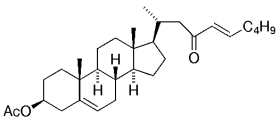
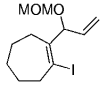
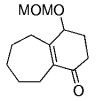
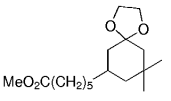
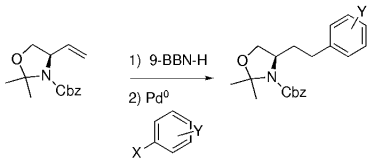
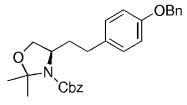
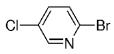
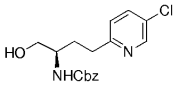
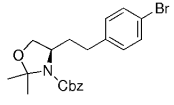
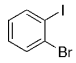
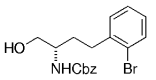
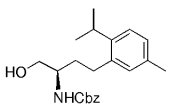
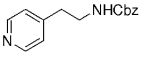
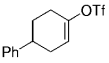
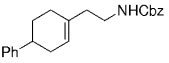
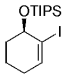
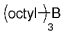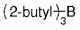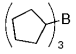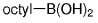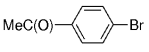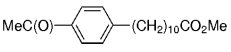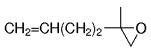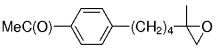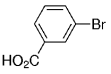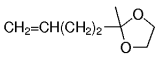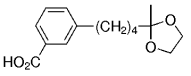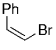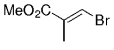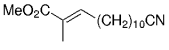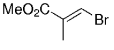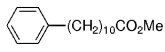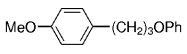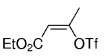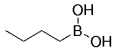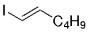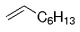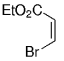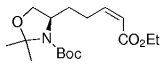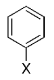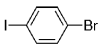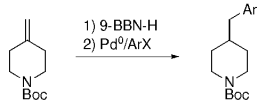The B-Alkyl Suzuki–Miyaura Cross-Coupling Reaction: Development, Mechanistic Study, and Applications in Natural Product Synthesis
A list of abbreviations can be found at the end of the article.
Graphical Abstract
An extremely attractive method for forming C(sp3)−C(sp2) bonds in complex molecular settings is the B-alkyl Suzuki–Miyaura reaction (see scheme; 9-BBN-H = 9-borabicyclo[3.3.1]nonane; the dashed line indicates that intramolecular reactions are also possible). The regio-, chemo-, and stereoselective nature of this reaction has been used in a number of syntheses of natural and non-natural compounds. An intricate knowledge of the mechanism of this reaction provides the basis for future advancements in this field.
Abstract
The development of new reactions that facilitate the creative and efficient synthesis of molecular structures with desirable properties continues to fascinate chemists. The test of a significant contribution is its acceptance over time by the scientific community. The B-alkyl Suzuki–Miyaura cross-coupling reaction appears to be one such reaction. Since its disclosure by Suzuki and Miyaura in 1986, this reaction has been an attractive solution to challenging synthetic problems.
1. Introduction
Transition metal mediated cross-coupling reactions have revolutionized organic synthesis. Many highly efficient and mild protocols for bond construction have emerged by mastery of such reactions, particularly in multifunctional settings.1, 2 Perhaps the most utilized C−C bond-forming cross-coupling reactions are the Heck,1 Stille,3, 4 and Suzuki–Miyaura reactions.5, 6 In recent years, the olefin metathesis reaction has also emerged as a particularly powerful method, especially for ring formation.7–12 The B-alkyl Suzuki–Miyaura reaction is distinguished from other Suzuki–Miyaura cross-coupling reactions in that a reaction occurs between an alkyl borane (as opposed to a vinyl or aryl borane) and an aryl or vinyl halide, triflate, or enol phosphate. We will show that this important variation, which involves an sp3 carbon in the coupling event, fills a growing niche. This reaction, which occurs in the presence of a base and a Pd0 catalyst, was first reported by Suzuki and Miyaura in 1986.13 The various transformations that are accessible through these valuable cross-coupling methods are depicted in Equations (1)–(4)1 (the dashed lines represent intramolecular variations).
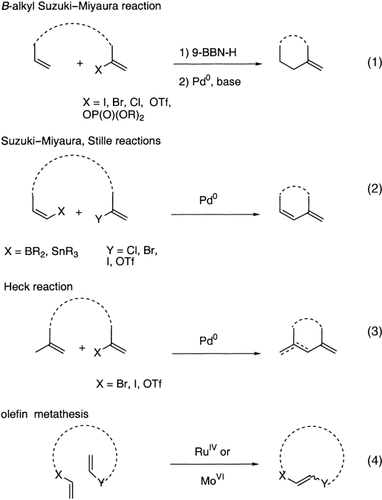
Other cross-coupling methods for C(sp3)−C(sp2) bond formation are available,1, 2, 14 most notably the Negishi protocol,15–19 which utilizes an alkylzinc derivative as the organometallic component. By comparison, the strength of the B-alkyl Suzuki–Miyaura cross-coupling lies in the mild and versatile methods for the synthesis of the alkyl borane component, the ease of incorporation of nontransferable boron ligands, and the manageable toxicity of the boron-derived by-products (e.g. R2B(OH)2−).20 Another advantage of this reaction over similar methods is its tolerance of water, which is often a beneficial additive.
Our interest in the B-alkyl Suzuki–Miyaura reaction first emerged in our program involving the synthesis of natural products with challenging structures. As will be demonstrated in Section 11, we have often found this reaction to be a rather valuable resource for the coupling of complex molecular fragments. In this review, a detailed account of the mechanism of the B-alkyl Suzuki–Miyaura reaction is presented. Factors that affect its rate, and thereby its efficiency, are summarized. The application of this coupling method to the development of new synthetic protocols as well as illustrations of its growing use in natural product synthesis are highlighted.
2. Synthesis of the Alkyl borane Component
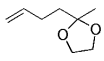
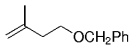
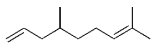
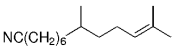
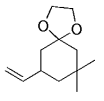
The alkyl borane component of the B-alkyl Suzuki reaction can be derived from the hydroboration of the corresponding olefin [Eqs. (5) and (6)1]21 or, less frequently, from the alkylation of a boron-based electrophile with an alkyllithium or Grignard reagent [Eqs. (7) and (8)1].22–24 More generally one takes advantage of the fact that alkyl boranes can be synthesized in a highly chemo-, regio-, and diastereoselective manner by means of the hydroboration reaction. In the hydroboration route, the terminal alkyl borane regioisomer is the highly favored adduct (anti-Markovnikov addition). The rate of hydroboration is sensitive to electronic as well as steric factors, although steric effects tend to predominate. Electron-rich, unhindered olefins generally react most rapidly. Thus, a substrate that contains two different olefin moieties often undergoes hydroboration at one double bond, with a high degree of selectivity (Figure 1).25–27
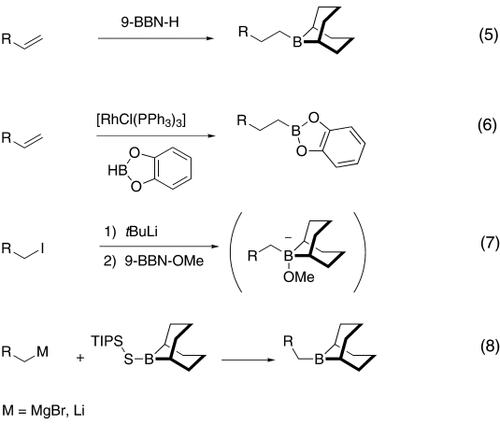
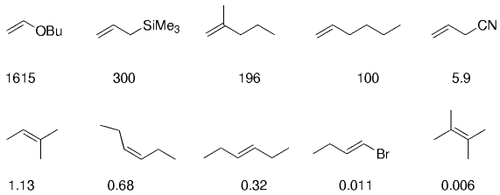
Relative rate of hydroboration with 9-BBN-H.
3. Catalytic Cycle
As in other cross-coupling reactions, the catalytic cycle of the Suzuki–Miyaura reaction is thought to involve a sequence consisting of an oxidative addition, a transmetalation, and a reductive elimination (Figure 2).5, 28
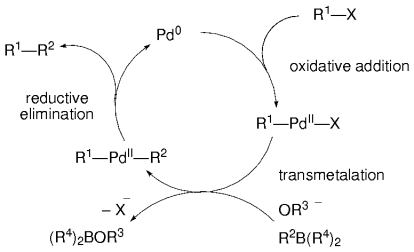
General Suzuki–Miyaura catalytic cycle.
The oxidative addition is often the rate-limiting step in a cross-coupling catalytic cycle. Under appropriate reaction conditions, alkenyl, alkynyl, allyl, benzyl, aryl, and alkyl halides may participate in the Suzuki–Miyaura cross-coupling reaction. Aryl and 1-alkenyl halides that are activated by electron-withdrawing groups are more reactive to the oxidative addition step than those with electron-donating groups. Through a competition reaction, Oh-e et al. found bromobenzene to be 2.2 times more reactive than phenyl triflate as a coupling partner in the B-alkyl Suzuki reaction with 9-octyl-9-BBN, whereas iodobenzene was consumed exclusively in the presence of phenyl triflate.29 In a separate study, Molander and Ito determined that the reaction of p-ClC6H4OTf with a benzyl borate led to exclusive coupling at the triflate position.20 Therefore, the order of reactivity of the electrophilic partner has been established as:30
I≫Br>OTf≫Cl
Although alkyl halides that have a hydrogen atom in the β position are considered to be problematic substrates as a result of potentially competing β-hydride elimination processes,5 C(sp3)−C(sp3) and carbonylative coupling reactions of alkyl iodides with alkyl boranes have been reported.31, 32, 101 The nature of the organoborane, the aryl, vinyl, or alkyl halide, the palladium catalyst, and the base all influence the overall rate of the cross-coupling reaction.33–37
4. Experimental Factors that Affect the B-Alkyl Suzuki–Miyaura Reaction
In the initial reaction development reports, Suzuki and co-workers surveyed various conditions (solvent, base, temperature, and catalyst) for the cross-coupling reaction of B-octyl-9-BBN with iodobenzene (Table 1).13, 21
|
Entry |
Catalyst |
Base [equiv] |
Solvent |
Temp. [°C] |
Yield [%] |
|---|---|---|---|---|---|
|
1 |
[PdCl2(dppf)] |
NaOH (3) |
THF/H2O (5:1) |
65 |
99 |
|
2 |
[PdCl2(dppf)] |
TlOH (1.5) |
THF/H2O (5:1) |
20 |
79 |
|
3 |
[PdCl2(dppf)] |
NaOMe (1.5) |
THF |
65 |
98 |
|
4 |
[PdCl2(dppf)] |
NaOMe (1.5) |
THF/MeOH (5:1) |
65 |
18 |
|
5 |
[PdCl2(dppf)] |
K2CO3 (2) |
DMF |
50 |
98 |
|
6 |
[PdCl2(dppf)] |
K3PO4 (2) |
DMF |
50 |
94 |
|
7 |
[Pd(PPh3)4] |
NaOH (3) |
THF/H2O (5:1) |
65 |
84 |
|
8 |
[Pd(PPh3)4] |
NaOH (3) |
Benzene/H2O |
80 |
97 |
- [a] Reactions were carried out for 16 h with 3 mol % catalyst.
4.1. Influence of the Catalyst
Substoichiometric amounts (3 mol %) of [PdCl2(dppf)] or [Pd(PPh3)4] were found to be viable catalysts for the B-alkyl Suzuki–Miyaura reaction (Table 1). Subsequent to these initial studies, complexes with an electron-rich Pd0 center, or precursors thereof, continued to be the most widely used catalysts for this reaction.5
One problem commonly associated with the coupling reactions of organometallic compounds that are metalated at sp3 C atoms and that possess hydrogen atoms in the β position, is the proclivity of the alkyl–palladium complex to undergo β-hydride elimination instead of reductive elimination.13 The bidentate bis(diphenylphosphino)ferrocene ligand on the palladium catalyst [PdCl2(dppf)] is thought to help favor reductive elimination by enforcing a cis geometry between the vinyl and alkyl groups on the square-planar PdII complex.1 Thus, this catalyst would be most effective if the reductive elimination process is part of the rate-determining step in the coupling reaction. (If the reactive groups are initially trans to each other on the PdII complex, the complex must rearrange to a cis geometry before reductive elimination can occur.) The “bite angle” of the bidentate ligand also affects the rate of the reductive elimination: a large bite angle of the ligand forces the two alkyl groups closer to each other about the PdII center, thus promoting the reductive elimination process.35
Recent studies by Buchwald and co-workers on the effects of ligand structure on the rate of oxidative addition have led to the development of reaction protocols that enable the coupling of alkyl boranes with aryl chlorides. These were previously an unreactive class of substrates [(Eqs. (9) and (10)1].38, 39
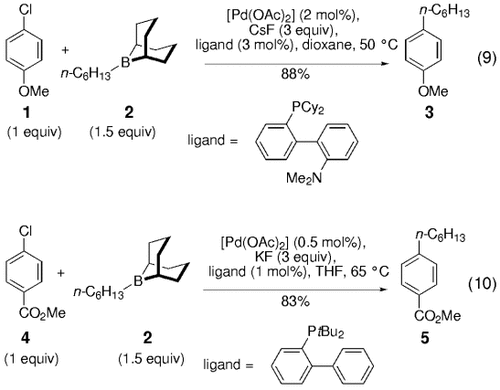
Fürstner and Leitner have also developed a ligand for such coupling partners.40
4.2. Influence of the Base and Solvent
A carefully selected base is essential in the projected applications of the B-alkyl Suzuki–Miyaura reaction. The base has been proposed to be involved in several steps of the catalytic cycle, most notably the transmetalation process (see Section 5). Miyaura and co-workers found that stronger bases such as NaOH, TlOH, and NaOMe performed well in THF/H2O solvent systems, whereas weaker bases such as K2CO3 and K3PO4 were more successful in DMF (Table 1).13, 21 These reactions were generally conducted at 50–80 °C, although the use of TlOH as the base made the reaction viable at 20 °C (Tl2CO3 and TlOEt are commercial available and are thus more commonly used than TlOH). Other research groups have subsequently introduced their own variations on the reaction conditions (base, solvent, ligand additives) in their applications of this reaction to methodologies and natural product syntheses (see Sections 7–11).
4.3. Influence of the Borane Substituents
Generally, unhindered electron-rich organoboranes and electron-deficient vinyl or aryl halides or triflates are the most reactive partners for the B-alkyl Suzuki reaction. A survey on the effect of various borane substituents on the coupling reaction is presented in Table 2.21, 41 A variety of hydroborating reagents such as 9-BBN-H, disiamylborane, dicyclohexylborane, and borane can be used in this reaction (Table 2, entries 1–4), although 9-BBN-H is the most commonly used. The rate of transmetalation of a primary alkyl group on boron is much higher than that of a secondary alkyl group. The coupling reactions between secondary alkyl boron compounds and iodobenzene can occur in moderate yields when aqueous KOH (3 M) is used as the base (Table 2, entries 5–7). However, there are no examples of B-alkyl Suzuki reactions that involve secondary alkyl boranes in preparative synthetic organic chemistry.
|
Entry |
Organoborane |
Base (equiv) |
Solvent |
Yield [%][b] |
|---|---|---|---|---|
|
1 |
NaOH (3) |
THF/H2O |
99 |
|
|
2 |
NaOH (3) |
THF/H2O |
82 |
|
|
3 |
NaOH (3) |
THF/H2O |
93 |
|
|
4 |
NaOH (3) |
THF/H2O |
98 |
|
|
5 |
KOH (3) |
THF/H2O |
40[c] |
|
|
6 |
KOH (3) |
THF/H2O |
65[c] |
|
|
7 |
KOH (3) |
THF/H2O |
55[c] |
|
|
8 |
KOH (3) |
THF/H2O |
75 |
|
|
|
Tl2CO3 (1.5) |
THF |
60 |
|
|
|
TlOH (3) |
Benzene/H2O |
93[d] |
|
|
9 |
KOH (3) |
THF/H2O |
trace |
|
|
|
TlOEt (3) |
THF/H2O |
41 |
|
|
|
Tl2CO3 (1.5) |
THF |
93 |
|
|
|
TlOH (3) |
Benzene |
84[e] |
|
|
10 |
TlOH (3) |
THF/H2O |
34 |
|
|
|
Tl2CO3 (1.5) |
THF |
trace |
|
|
11 |
TlOH (3) |
THF/H2O |
trace |
- [a] Reactions were carried out at 50 °C with [PdCl2(dppf)] catalyst (3 mol %), organoborane (1.1 equiv), and iodobenzene (1.0 equiv) unless otherwise noted. [b] yields (GC) based on iodobenzene. [c] Biphenyl was also obtained as a by-product (10–30 %). [d] [PdCl2(dppe)] was used as the catalyst. [e] [PdCl2(dppf)] was used as the catalyst.
Alkyl boronic esters can also be viable substrates in the Suzuki reaction when thallium salts such as TlOH or Tl2CO3 are used as the base (Table 2, entries 8–11).41 In contrast, other bases such as KOH are ineffective in these reactions. The special rate-enhancing effect of thallium salts in the C(sp2)−C(sp2) Suzuki reaction was first observed by Kishi and co-workers.42 Thallium salts have since provided a useful solution for difficult Suzuki couplings in numerous instances.5, 43–45 Unfortunately, the toxicity of the thallium salts restricts their widespread use.
5. Reaction Mechanism of the Suzuki–Miyaura Reaction
5.1. Stereochemistry of the Oxidative Addition
The oxidative addition process in the Suzuki–Miyaura cross-coupling reaction is thought to proceed through the broadly applicable mechanistic framework that accompanies many other cross-coupling processes. The oxidative addition of alkyl and alkenyl halides occurs with retention of configuration, whereas inversion of configuration is observed in the case of allylic and benzylic halides.2, 31, 46, 47
5.2. Stereochemistry of the Transmetalation Step
Independent studies by the groups of Soderquist33 and Woerpel34 revealed that transmetalation (R−B→R−Pd) occurs with retention of stereochemistry. Both groups used an NMR spectroscopic technique that takes advantage of the preference of bulky substituents on 1,2-disubstituted ethanes to adopt an antiperiplanar conformation.48 The stereochemistry can then be determined by analysis of the coupling constants of the vicinal protons.
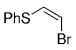
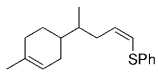
Soderquist and co-workers prepared the known syn and anti derivatives of tBuCHDCHDPh, 8 and 11 (Schemes 1 and 2).49 Hydroboration of 3,3-dimethyl-1-butyne with 9-BBN-D, followed by treatment with CD3CO2D gave the dideuterated olefin 6. Subsequent hydroboration of 6 with 9-BBN-H followed by Suzuki coupling with PhBr gave 8, whose Ha–Hb coupling constant of 4.8 Hz is consistent with syn stereochemistry (Scheme 1). Hydroboration is a syn addition process,50 and reductive elimination of the Pd intermediate is known to occur with retention of stereochemistry.51 Soderquist and co-workers thus concluded that the transmetalation step also occurs with retention of stereochemistry. By reversing the order in which the reagent is added, the anti isomer 11 was produced; the Ha–Hb coupling constant of 12.5 Hz confirms that transmetalation occurred with retention of configuration (Scheme 2).
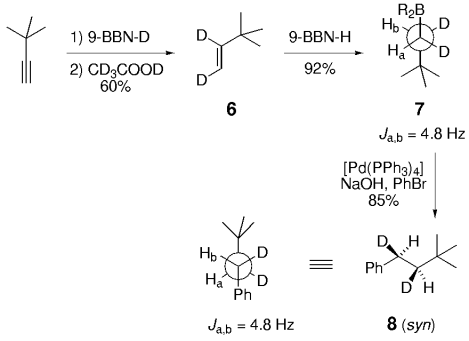
Soderquist and co-workers demonstrated that hydroboration of a cis dideuterated olefin followed by cross-coupling gives a syn adduct.
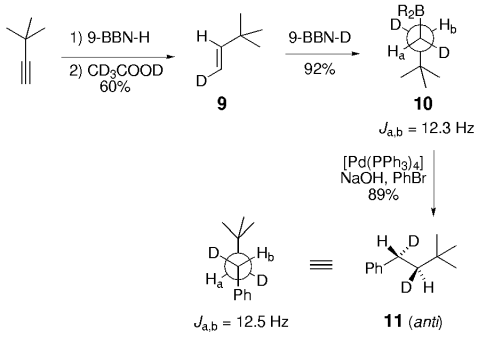
The hydroboration of a trans dideuterated olefin followed by cross-coupling gives the anti adduct. These studies show that transmetalation occurs with retention of configuration at the carbon atom.
Soderquist and co-workers argued that the four-centered μ2-hydroxo-bridged transition state model 14 (Scheme 3) is consistent with a transmetalation that proceeds with retention of configuration. The transition state can arise from either the hydroxyborate–palladium complex 12 or the borane–palladium hydroxide complex 13. Both structures had previously been suggested as possible intermediates by Miyaura and Suzuki.5 Soderquist and co-workers determined that the mechanism depends on the Lewis acidity of the organoborane (see Section 5.3). In a concurrent and similar study, Woerpel and Ridgway also concluded that transmetalation occurs with retention of stereochemistry (Schemes 4 and 5).
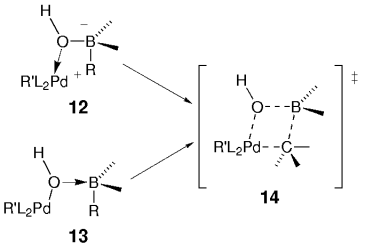
Mechanism of the transmetalation.
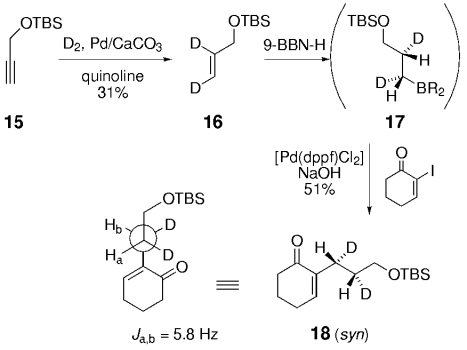
Woerpel and co-workers also demonstrated that the hydroboration of a cis-dideuterated olefin followed by cross-coupling gives a syn adduct.
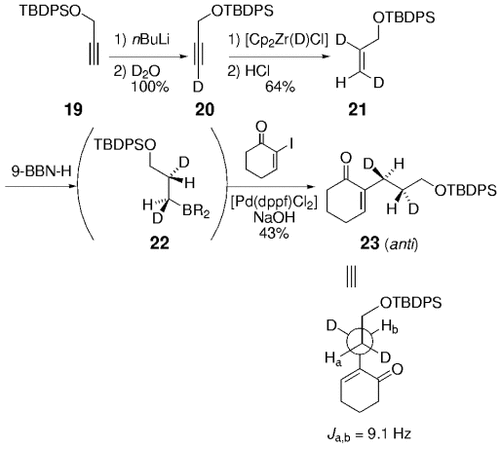
The hydroboration of a trans dideuterated olefin followed by cross-coupling provides the anti adduct. These studies also demonstrate that transmetalation occurs with retention of configuration at the carbon atom.
5.3. Role of the Boron Substituents in the Coupling Process
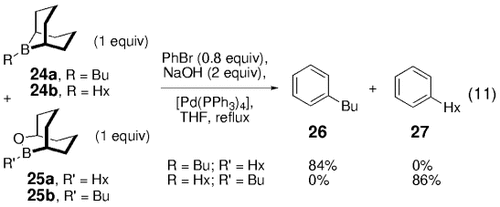
To evaluate the effects of the boron substituents on the rate of the coupling reaction, Soderquist and co-workers performed a competition experiment between the B-alkyl-9-BBN derivative 24 and its B-alkyl OBBD counterpart 25 [Eq. (11)-4]. The 9-BBN derivative 24 was found to be significantly more reactive than the R-OBBD derivative 25, as shown by the exclusive formation of phenylbutane (26) in an experiment in which 24 a (R=butyl) and 25 a (R′=hexyl) competed for bromobenzene. This finding was supported by the exclusive formation of phenylhexane (27) in the analogous competition reaction with 24 b (R=hexyl) and 25 b (R′=butyl).
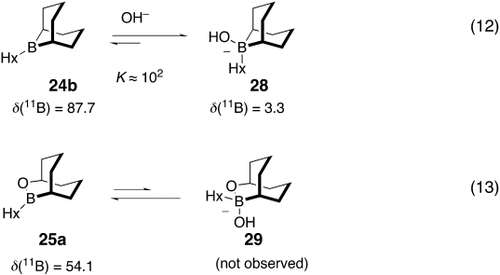
Soderquist and co-workers hypothesized that the difference in reactivity between the two boranes was related to their different Lewis acidity. This proposal was tested by the study of the borane⇄borate equilibria [Eqs. (12) and (13)-4]. The boranes were titrated with NaOH and the changes in chemical shift in their 11B NMR spectra were noted. It was found that one equivalent of NaOH shifts the 11B NMR signal of the 9-BBN derivative 24 b from δ=87.7 to δ=12.0, two equivalents causes a shift to δ=6.0, and three equivalents shifts the signal to δ=3.3 (the spectrum did not change with additional NaOH). In contrast, titration of the OBBD derivative 25 a with NaOH led to no observable change in the 11B NMR spectrum. These studies support the assertion that the 9-BBN derivative 24 b is much more Lewis acidic than 25 a. Thus, under the Suzuki reaction conditions, it is highly probable that the species involved in the transmetalation step in the case of 24 b, is its corresponding borate 28, whereas the OBBD species 25 a is probably involved in the transmetalation step, rather than its borate derivative 29. This then implies that transmetalation of the 9-BBN derivative 24 a occurs via intermediate 12, whereas the transmetalation of the OBBD derivative 25 a occurs via 13 (Scheme 3).
5.4. Role of Base in the Coupling Process
Soderquist and co-workers have shown that the base is involved in as many as five mechanistically distinct events in the B-alkyl Suzuki reaction. Arguably the most essential role of the base is the conversion of the alkyl 9-BBN species into the more reactive borate species [R-9-BBN-OH]−. The other four roles include: 1) hydrolysis of the PdIIX intermediate to the more reactive PdIIOH species; 2) complexation of HOBR2 by-products that can compete for base with the trialkyl borane (hence the need for two equivalents of base in the reaction); 3) accelerated coupling rates for the OBBD derivatives; and 4) regeneration of the catalyst.
Based on such studies, Soderquist and co-workers proposed a more detailed catalytic cycle for the B-alkyl Suzuki reaction that includes the important role played by the base both for the 9-BBN derived organoborane manifold (top cycle of Figure 3) as well as for the OBBD organoboranes (bottom cycle of Figure 3).
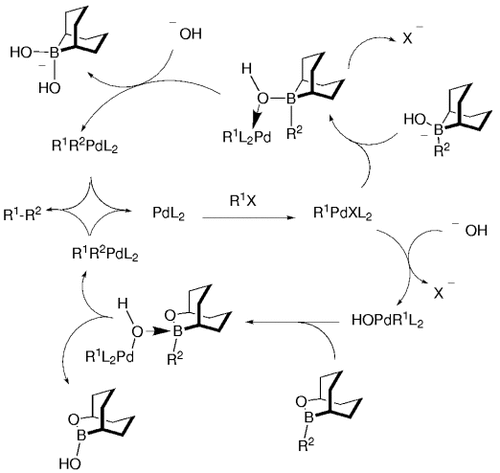
Modified Suzuki–Miyaura catalytic cycle.
Direct evidence for the hydrolysis of a PdIIX intermediate to a PdIIOH intermediate was observed by means of 31P NMR spectroscopy. As shown in Scheme 6, the [Pd(PPh3)4] (broad singlet, δ=18.0) reacts cleanly with PhBr to produce the oxidative addition adduct [BrPdPh(PPh3)2] (30; δ=26.1) and PPh3 (δ=−3.2). When this mixture was treated with two equivalents of NaOH, 30 was partially converted into the hydrolysis product [HOPdPh(PPh3)2] (31; δ=23.6). Also, the amount of triphenylphosphane oxide (δ=27.0) in the mixture increased significantly after addition of the base, a consequence of PdII→Pd0 reduction. Heating the mixture at reflux (THF) increased the rate of conversion of 30 into 31.
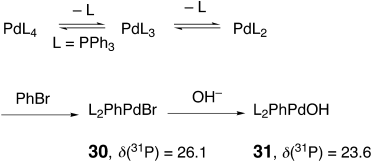
Evidence for the hydrolysis of the PdIIBr intermediate was obtained by means of 31P NMR spectroscopy.
5.5. Reaction Kinetics
Soderquist and co-workers also determined the kinetics of the reaction for both Bu-9-BBN and Bu-OBBD. For the reaction of PhBr with [(HO)Bu-9-BBN]−, they determined that the reaction rate was independent of the concentration of the borate, but was first order in [PhBr]. Therefore, the oxidative addition step in the catalytic cycle is rate limiting for the coupling of Bu-9-BBN derivatives with PhBr. In contrast, the rate of the reaction of Bu-OBBD with PhBr was found to be independent of [PhBr] and [Bu-OBBD], but first order in base. Therefore, the hydrolysis of [BrPdPh(PPh3)2] is rate-determining for the coupling of Bu-OBBD with PhBr.
6. Substrate Generality
6.1. Cross-Coupling Reactions of Vinyl or Aryl Halides
In their original studies,13 Miyaura and co-workers found that a combination of [PdCl2(dppf)] and sodium hydroxide in refluxing THF/water provides adequate cross-coupling conversions, as long as there were no base-sensitive functional groups on either the alkyl boranes or on the aryl halides (Table 3, entries 1–2). Potassium carbonate or phosphate suspended in DMF at 50 °C provided superior results for base-sensitive substrates (Table 3, entries 3–6).
|
Entry |
Aryl halide |
Alkene |
Product |
Yield [%] |
|---|---|---|---|---|
|
1 |
1-octene |
90, 71[b] |
||
|
2 |
88, 71[b] |
|||
|
3 |
CH2=CH(CH2)8CO2Me |
88[c] |
||
|
4 |
CH2=CH(CH2)8CN |
98[c] |
||
|
5 |
52[c] |
|||
|
6 |
77[c] |
- [a] Reactions were run as follows unless otherwise noted: 1) alkene (1 equiv) in THF was treated with 9-BBN-H (1.1 equiv); 2) the borane was added to a solution of aryl halide (1 equiv), [PdCl2(dppf)] (3 mol %), and NaOH (3 equiv) in THF, and the solution was heated at reflux. [b] NaOMe (1.5 equiv) was used instead of NaOH. [c] K2CO3 (2 equiv) was used instead of NaOH and the reaction was conducted in DMF/THF at 50 °C.
The study included the cross-coupling reactions of alkyl boranes with a variety of vinyl bromides (Table 4).42, 52 These reactions again occur with complete retention of olefin geometry.
|
Entry |
Vinyl bromide |
Alkene |
Product |
Yield [%] |
|---|---|---|---|---|
|
1 |
1-octene |
85 |
||
|
2 |
1-octene |
90 |
||
|
3 |
CH2=CH(CH2)8CO2Me |
92,[c] 69[b] |
||
|
4 |
1-octene |
98 |
||
|
5 |
80 |
|||
|
6 |
CH2=CH(CH2)8CN |
80[b] |
||
|
7 |
73,[b] 79[c] |
|||
|
8 |
CH2=CH(CH2)8CN |
81,[b] 72[c] |
||
|
9 |
90 |
- [a] Reactions were run as follows unless otherwise noted: 1) alkene (1 equiv) in THF was treated with 9-BBN-H (1.1 equiv); 2) the borane was added to a solution of vinyl halide (1 equiv), [PdCl2(dppf)] (3 mol %), and NaOH (3 equiv) in THF and the solution was heated at reflux. [b] K2CO3 (2 equiv) was used instead of NaOH, and the reaction was conducted in DMF/THF at 50 °C. [c] K3PO4 (1 equiv) was used instead of NaOH, and the reaction was conducted in DMF/THF at 50 °C.
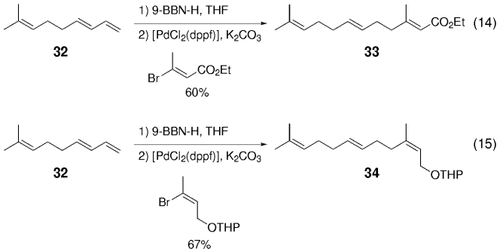
The high degree of chemo- and stereoselectivity of the initial hydroboration phase is efficiently utilized in the B-alkyl Suzuki reaction. As an example of chemoselective hydroboration, coupling products derived from triene 32 are obtained in good yields [Eqs. (14) and (15)-5].21
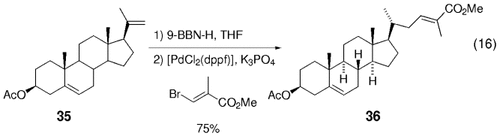
The stereoselective hydroboration of 20-(21)-methylene steroid 35, which in turn was derived from pregnenolone acetate, produced predominantly the (20R)-21-boryl steroid intermediate.53, 54 This intermediate was cross-coupled with ethyl (E)-β-bromomethacrylate to give 36 in 75 % yield [Eq. (16)-5].21 (For another example, see Table 4, entry 7.) Corey and Roberts have also conducted a study on the synthesis of trisubstituted double bonds through the cross-coupling reactions of alkyl boranes and vinyl iodides.55
6.2. Cross-Coupling Reactions with Aryl or Vinyl Triflates
Organoboranes can also undergo cross-coupling reactions with aryl or vinyl triflates.29 Such triflates can be easily accessed from phenols or enolates.56, 57 A representative substrate pool is shown in Table 5. These cross-coupling reactions are catalyzed by [Pd(PPh3)4] or [PdCl2(dppf)] in the presence of K3PO4 in dioxane or THF.
|
Entry |
Triflate |
Alkene |
Product |
Yield [%] |
|---|---|---|---|---|
|
1 |
CH2=CH(CH2)8CO2Me |
87, 82[b] |
||
|
2 |
CH2=CHCH2OPh |
92, 70[b] |
||
|
3 |
65 |
|||
|
4 |
1-octene |
67[b] |
||
|
5 |
CH2=CH(CH2)8CO2Me |
89, 86[b] |
||
|
6 |
65 |
|||
|
7 |
64 |
- [a] Reactions were run as follows, unless otherwise noted: 1) alkene (1 equiv) in THF was treated with 9-BBN-H (1.1 equiv); 2) the borane was added to a solution of triflate (1 equiv), [PdCl2(dppf)] (2.5 mol %), and K2PO4 (1.5 equiv) in dioxane, and the solution was heated at 85 °C. [b] The reaction was carried out in THF at reflux.
Molander and Ito recently reported the coupling reactions of aryl or vinyl triflates with potassium alkyl trifluoroborates (Table 6).20 The potassium alkyl trifluoroborates are solid, crystalline, air- and water-stable reagents that are easily prepared from the corresponding alkyl boronic acids or esters. They undergo Suzuki–Miyaura coupling with the vinyl or aryl triflates in the presence of [PdCl2(dppf)] and Cs2CO3 in refluxing THF/H2O. As shown in Table 6, a variety of functional groups are tolerated.
|
Entry |
Alkyl borane |
Triflate |
Product |
Yield [%] |
|---|---|---|---|---|
|
1[b] |
PhCH2BF3K |
p-IC6H4OTf |
p-TfOC6H4CH2Ph |
16 |
|
2[b] |
PhCH2BF3K |
p-BrC6H4OTf |
p-TfOC6H4CH2Ph |
70 |
|
3[b] |
PhCH2BF3K |
p-ClC6H4OTf |
p-ClC6H4CH2Ph |
57 |
|
4[c] |
NC(CH2)5BF3K |
p-NO2C6H4OTf |
p-NO2C6H4(CH2)5CN |
73 |
|
5[c] |
Me(CO)(CH2)4BF3K |
p-AcC6H4OTf |
p-AcC6H4(CH2)4(CO)Me |
79 |
|
6[c] |
CH3(CH2)7BF3K |
m-CNC6H4OTf |
m-CNC6H4(CH2)6Br |
65 |
|
7[c] |
Br(CH2)6BF3K |
p-AcC6H4OTf |
p-AcC6H4(CH2)6Br |
61 |
|
8[c] |
BzO(CH2)6BF3K |
68 |
||
|
9[c] |
CH3(CH2)7BF3K |
75 |
- [a] The potassium alkyl trifluoroborate (1 equiv) was treated with the triflate (1 equiv) in the presence of [Pd(Cl2)(dppf)] (9 mol %) and Cs2CO3 (3 equiv) in THF/H2O, and the mixture was heated at reflux for 18 h. [b] Borates were prepared by using the corresponding Grignard reagent. [c] Borates were prepared by means of the catalytic hydroboration method.
In a particularly challenging coupling reaction of an azulene-derived triflate, Danheiser and co-workers found the Buchwald ligand (o-biphenyl)PCy2 to be essential for its cross-coupling reaction with B-ethyl-9-BBN [Eq. (17)-5].58 Occhiato et al. found that vinyl triflates derived from six- and seven-membered N-alkoxycarbonyl lactams undergo cross-coupling with allyl or alkyl boronic acids and esters (Table 7). Whereas the allyl boronic esters underwent coupling under standard conditions (substoichiometric [(Ph3P)2PdCl2], Na2CO3, THF, H2O, reflux), the alkyl boronic acids required the addition of Ag2O for an acceptable rate of conversion. Interestingly, the six-membered lactam-derived triflates gave much higher yields than the corresponding seven-membered triflates.59
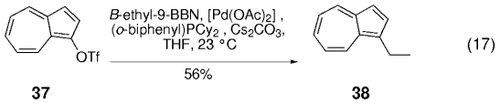
|
Entry |
Borate |
Triflate |
Product |
Yield [%] |
|---|---|---|---|---|
|
1[a] |
65 |
|||
|
2[b] |
93 |
|||
|
3[a] |
27 |
|||
|
4[b] |
22 |
- [a] The reactions were run at 80 °C in THF/ Na2CO3 (aq., 2 M) with the vinyl triflate (1 equiv) and the borate (1.5 equiv) in the presence of [(Ph3P)2PdCl2] (5 mol %). [b] The reactions were run in toluene at 80 °C with the vinyl triflate (1 equiv) and the borate (2 equiv) in the presence of K2CO3 (3 equiv), Ag2O (2 equiv), and [PdCl2(dppf)] (3 mol %).
6.3. Intramolecular Cross-Coupling
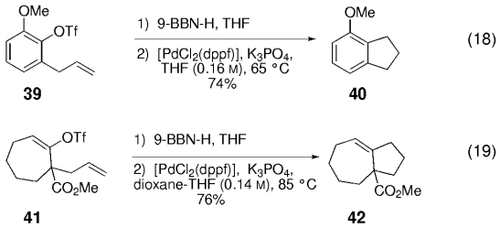
Miyaura and co-workers demonstrated that five- and six-membered rings can be accessed through the B-alkyl Suzuki reaction of aryl or vinyl halides that are functionalized with terminal olefins (Table 8).21 The cross-coupling reactions were carried out in THF/H2O solution (0.2 M) by using catalytic [PdCl2(dppf)] and NaOH (3 equiv). The authors did note, however, that they were unable to gain access to larger rings by using this cyclization protocol. Clearly, competing oligomerization processes constitute risk factors that complicate prospects for cyclization. As will be shown in Section 6.4, the macrocyclization reaction can be achieved at lower substrate concentration (thus favoring intramolecular over intermolecular cross-coupling). Oh-e et al. subsequently found that both aryl and vinyl triflates are also good substrates for the intramolecular B-alkyl Suzuki reaction [Eqs. (18) and (19)-5].29
- [a] Conditions: 1) Olefin (1 equiv), 9-BBN-H (1.05 equiv), THF, 0→23 °C; 2) [PdCl2(dppf)] (1.5 mol %), NaOH (3 equiv), THF/H2O (0.2 M).
Compounds that contain vinyl halide and terminal olefin functional groups linked through a two- or three-carbon tether can also be cyclized to form five- and six-membered exocyclic alkenes (Table 9).60 In an interesting extension of this idea, Soderquist and co-workers developed a protocol which couples vinyl geminal dibromides with symmetrical bis-boranes, which are derived from the corresponding bis-olefins (Table 10).61 This process involves tandem intermolecular–intramolecular cross-coupling reactions. The authors noted that attempts to apply the methodology to the synthesis of five-membered ring systems starting from 1,4-dienes were unsuccessful.
|
Entry |
Alkyl halide |
Product |
Yield [%] |
|---|---|---|---|
|
1 |
71,[a] 80[b] |
||
|
2 |
68[a,c] |
||
|
3 |
83,[a] 72[b] |
||
|
4 |
51,[a] 69[b] |
||
|
5 |
60[a] |
- [a] 1) 9-BBN-H (1.05 equiv), THF, 0→23 °C; 2) [PdCl2(dppf)] (1.5 mol %) and NaOH (3 M, 3 equiv) in THF (0.14 M) at 60 °C. [b] Hydroboration as above, followed by [Pd(PPh3)4] (3 mol %), K3PO4 (1.5 equiv), dioxane/THF (0.12 M), 60 °C. [c] Diastereomeric mixture (3:7).
- [a] The olefin (1 equiv) was treated with 9-BBN-H in THF at 25 °C. This solution was subsequently treated with vinyl bromide (1 equiv), NaOH (4 equiv), THF/H2O (0.09 M), and [Pd(PPh3)4] (6 mol %) at 25 °C.
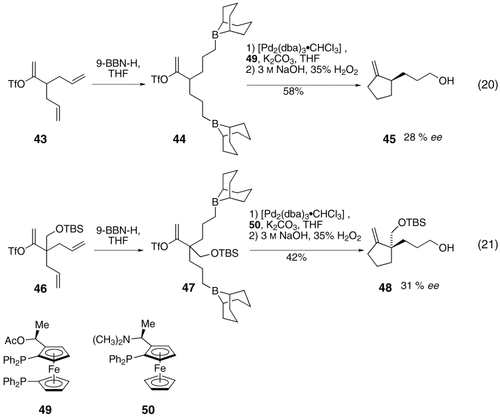
In an extension of this cyclization protocol, Cho and Shibasaki reported the asymmetric synthesis of cyclopentanes 45 and 48 by using chiral ligands 49 and 50, respectively, in the B-alkyl Suzuki–Miyaura reaction [Eqs. (20) and (21)-5].62 Although the level of asymmetric induction is moderate (28 and 31 % ee, respectively), this work constitutes a significant advancement in the field.
6.4. Macrocyclizations
The first moderately successful cyclizations to form rings with more than six members by means of the B-alkyl Suzuki–Miyaura reaction were reported by Kwochka and co-workers en route to silametacyclophanes [Eqs. (22) and (23)-5] and [6.6]metacyclophanes [Eq. (24)-5].63, 64
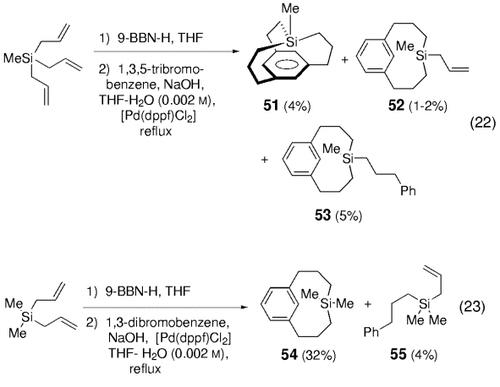
The hydroboration of methyltriallylsilane followed by coupling with 1,3,5-tribromobenzene gave the target 4-methyl-4-sila[34,10][7]metacyclophane 51 in 4 % yield [Eq. (22)-5]. Silacycles 52 (1–2 %) and 53 (5 %) were obtained as undesired side products. A higher yield of macrocyclization adduct 54 was achieved in the coupling reaction of diallyldimethylsilane with 1,3-dibromobenzene [Eq. (23)-5]. Kwochka et al. noted that neither 1,2- nor 1,4-dibromobenzene yielded the corresponding silacyclophanes under similar conditions. Compounds 51–54 were used to study anisotropic effects by means of NMR spectroscopic analysis.
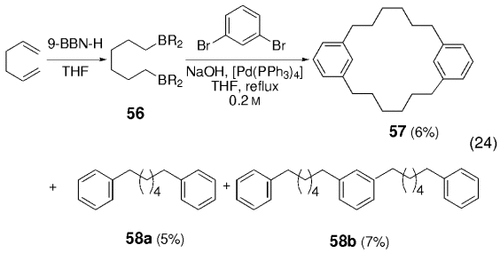
In an analogous study, coupling of the bis-borane 56 with 1,3-dibromobenzene led to the desired [6.6]metacyclophane 57 in 6 % yield, with adducts 58 a and 59 b as side products [Eq. (24)-5].
A macrocyclization study which is potentially more relevent to natural product synthesis was subsequently carried out by Danishefsky and Chemler (Table 11).45 These reactions were carried out under dilute conditions (0.003 M) and with slow addition of the alkyl borane intermediates to the catalyst solution. In the first set of reaction conditons, the borane was added to a dilute solution of Cs2CO3, [PdCl2(dppf)], and AsPh3 in THF/DMF/H2O.65 Under a second set of reaction conditions, the borane was treated with TlOEt and the resulting mixture was added dropwise to a dilute solution of [PdCl2(dppf)] and AsPh3 in THF/DMF/H2O.43, 44 Although ortho-iodobenzenes gave relatively poor yields of cyclization adducts (Table 11, entries 1 and 2), the yields could be improved by extending the centers of reactivity away from the aryl ring and by increasing the ring size (Table 11, entry 3). Transannular macrocyclization was also a viable process (Table 11, entries 4 and 5), especially when TlOEt was used as the base. Apparently, the rate enhancement offered by the thallium base increased the rate of the desired macrocyclization reaction relative to competing oligomerization processes.
|
Entry |
Substrate |
Product |
Yield [%] |
|---|---|---|---|
|
1 |
22[b] |
||
|
2 |
23 |
||
|
3 |
41 |
||
|
4 |
40, 60[b] |
||
|
5 |
46[b] |
- [a] All reactions were carried out as follows, unless otherwise noted: 1) The terminal olefins were hydroborated with 9-BBN-H (1.5 equiv) for 1.5 h at 23 °C in THF (0.5 M); 2) the borane solution was diluted with THF and added over 3–5 h by means of a syringe pump to a solution of [PdCl2(dppf)] (0.2 equiv), AsPh3 (0.2 equiv), Cs2CO3 (3 equiv), and H2O (40 equiv) in a 10:1 THF/DMF solution (0.003 M with respect to the substrate). [b] Water (5 equiv) and TlOEt (3 equiv) were added to the borane solution prior to the addition to the catalyst solution (Cs2CO3 and additional H2O were omitted from these reactions).
6.5. Carbonylative Cross-Coupling Reactions
Another important application of the B-alkyl Suzuki reaction is the synthesis of unsymmetrical ketones by means of the palladium-catalyzed reaction of alkyl boranes and vinyl or alkyl halides under a carbon monoxide atmosphere.31, 32 The synthesis of α,β-unsaturated ketones by means of the carbonylative cross-coupling reaction of alkyl boranes and vinyl iodides proceeded smoothly in benzene or dioxane under a carbon monoxide atmosphere (1–3 atm), when K3PO4 was used as the base and either [Pd(PPh3)4] or [PdCl2(PPh3)2] as the catalyst (Table 12).32 Iodobenzene and benzyl bromide are also feasible partners in the carbonylative coupling with 9-octyl-9-BBN (82 and 72 % yields, respectively, not shown).
|
Entry |
Vinyl iodide |
Alkene |
Product |
Yield [%] |
|---|---|---|---|---|
|
1 |
99[a] |
|||
|
2 |
78[b] |
|||
|
3 |
68[b] |
|||
|
4 |
53[b] |
|||
|
5 |
90[c] |
|||
|
6 |
48[a] |
|||
|
7 |
77[a] |
|||
- [a] Reactions conditions: 1) The alkene (1.1 equiv) was treated with 9-BBN-H (1.1 equiv) in THF, 0 °C→RT; 2) benzene, iodoalkene (1 equiv), K2PO4 (3 equiv), and [Pd(PPh3)4] (5 mol %) were added, and the reaction was conducted under CO (1 atm). [b] Same conditions as above except in dioxane under CO (3 atm).
Alkyl iodides are viable substrates for the carbonylative Suzuki coupling reaction with alkyl boranes (Table 13).31 These reactions were efficiently conducted in the presence of K3PO4 and substoichiometric ammounts of [Pd(PPh3)4] in dioxane under a carbon monoxide atmosphere. The reaction is greatly accelerated by visible light (100-W tungsten lamp), which suggests that a radical process may be involved in the oxidative addition. Generally, cross-couplings of alkyl halides that have a hydrogen atom in the β position often suffer from competitive side reactions such as β-hydride elimination, thus leading to alkenes or to the isomerization of alkyl groups. However, the carbonylative couplings do not suffer from these problems, probably because the CO insertion is very fast.
|
Entry |
Alkyl iodide |
Alkene |
Product |
Yield [%] |
|---|---|---|---|---|
|
1 |
C6H13I |
1-octene |
C6H13C(O)C8H17 |
67 |
|
2 |
1-octene |
65 |
||
|
3 |
tBuCH2I |
1-octene |
tBuCH2C(O)C8H17 |
69 |
|
4 |
4-allylveratrole |
73 |
||
|
5 |
65 |
|||
|
6 |
MeO2C(CH2)3I |
CH2=CH(CH2)8CN |
MeO2C(CH2)3C(O)(CH2)10CN |
65 |
|
7 |
MeI |
50 |
- [a] Reaction conditions: 1) the alkene (1 equiv) in THF was treated with 9-BBN-H (1.05 equiv); 2) the borane was added to a solution of iodoalkene (1.5 equiv), CO (1 atm), [Pd(PPh3)4] (3 mol %), and K3PO4 (3 equiv) in benzene, and the solution was irradiated with a 100-W tungsten lamp for 24 h.
6.6. C(sp3)−C(sp3) Cross-Coupling Reactions
Alkyl halides are not common substrates for the Suzuki reaction as a result of their slow rate of oxidative addition and their fast β-hydride elimination from the derived σ-alkylpalladium intermediate. However, Ishiyama and co-workers found that iodoalkanes react with alkyl boranes in the presence of K3PO4 and catalytic amounts of [Pd(PPh)4] in dioxane to generate the corresponding coupled adducts in moderate to good yields (45–71 %, Table 14). They noted that [PdCl2(dppf)] did not act as an efficient catalyst in these reactions. Also, this reaction was not enhanced by light.
|
Entry |
Alkyl iodide |
Alkene |
Product |
Yield [%] |
|---|---|---|---|---|
|
1 |
MeI |
CH2=CH(CH2)8CO2Me |
CH3(CH2)10CO2Me |
71 |
|
2 |
CH3(CH2)5I |
CH2=CH(CH2)5CH3 |
C14H30 |
64 |
|
3 |
CH2=CH(CH2)8CO2Me |
45 |
||
|
4 |
CH3(CH2)5I |
58 |
||
|
5 |
CH3(CH2)3I |
CH2=CH(CH2)8CO2Me |
CH3(CH2)15CO2Me |
54 |
|
6 |
CN(CH2)3I |
61 |
||
|
7 |
MeO2C(CH2)3I |
57 |
- [a] Reactions conditions: 1) the alkene (1 equiv) in THF was treated with 9-BBN-H (1.0 equiv); 2) this solution was diluted with dioxane and treated with [Pd(PPh3)4] (3 mol %), K3PO4 (3 equiv), and the alkyl iodide (1.5 equiv), and the solution was heated at 60 °C.
7. Synthesis of Non-natural Amino Acids
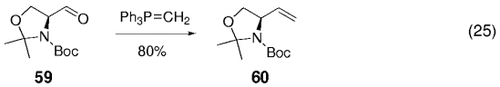
The B-alkyl Suzuki reaction has been used effectively in the synthesis of non-natural amino acids. Taylor and co-workers were first to recognize the usefulness of this sort of construction.66 Starting amino olefin 60 was prepared by means of Wittig olefination of the Garner aldehyde (59), which in turn can be obtained from the corresponding L- or D-serine [Eq. (25)-5]. Hydroboration of 60 with 9-BBN-H followed by Suzuki–Miyaura coupling of the derived alkyl borane with a variety of vinyl bromides, iodides, and triflates gave the corresponding non-natural amino acid synthons in good yields (Table 15).

|
|||
|---|---|---|---|
|
Entry |
Substrate |
Product |
Yield [%] |
|
1 |
70 |
||
|
2 3 |
79 (X=I) 72 (X=Br) |
||
|
4 |
69 |
||
|
5 |
76 |
||
|
6 |
ca. 70 |
||
|
7 8 |
72 (X=I) 11 (X=OTf) |
||
- [a] 1) The olefin (1 equiv) in THF (0.22 M) was treated with 9-BBN-H (2 equiv) at 0 °C, and then warmed to room temperature; 2) the solution was treated with aqueous K2PO4 (3 M, 2.1 equiv), vinyl or aryl halide (1.1 equiv) in DMF, and [PdCl2(dppf)] (5 mol %).
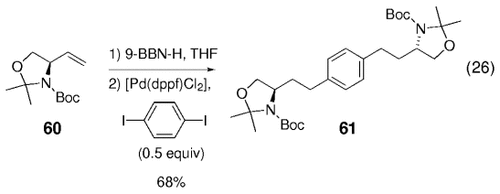
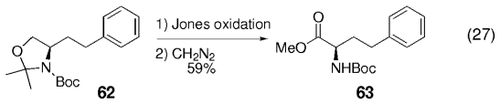
para-Diiodobenzene was coupled twice with 60 as well, to yield diamide 61 in 68 % yield [Eq. (26)-5]. Some of the cross-coupled adducts were converted into their methyl ester amino acid derivatives by treatment with Jones' reagent followed by methylation of the resulting acids with diazomethane, for example, [Eq. (27)-5].
Sabat and Johnson subsequently reported a similar method for the synthesis of α-amino acids and α-amino alcohols that contain a variety of side chains that do not occur naturally.67 Yields for the coupling of various aryl iodides and bromides ranged from 66–89 % (Table 16). The rate of coupling of iodides is much faster than that of bromides (Table 16, entries 3 and 4). All these adducts could be converted into their corresponding amino acid derivatives.
- [a] 1) 9-BBN-H, toluene, 80 °C; 2) NaOH (3.2 n), [Pd(PPh3)4] (3 mol %), 99 °C. [b] The product mixture was subsequently treated with p-toluenesulfonic acid in MeOH.
8. Methods for the Synthesis of Phenethyl and Homoallylic Amines
Along similar lines, Overman and Kamatani reported an efficient β-aminoethylation procedure with the B-alkyl Suzuki–Miyaura reaction, starting from benzyl vinyl carbamate 64 (Scheme 7).68 Vinyl carbamate 64, in turn, was prepared by means of a Curtius rearrangement of acryloyl chloride. Hydroboration of 64 (1.1–1.5 equiv) with 9-BBN-H in THF followed by cross-coupling with the aryl or vinyl halide/triflate component (1 equiv) in the presence of a substoichiometric amount of [PdCl2(dppf)] and excess aqueous NaOH (3 M) produced a variety of homoallylic amines in excellent yields (77–97 %, Table 17).
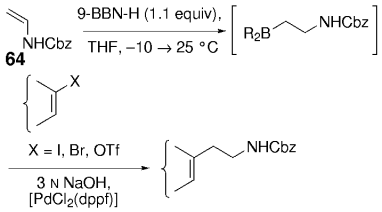
β-Aminoethylation starting from benzyl vinyl carbamate precursors.
|
Entry |
Substrate |
Product |
Yield [%] |
|---|---|---|---|
|
1 |
87 |
||
|
2 |
80 |
||
|
3 |
94 |
||
|
4 |
88 |
||
|
5 |
97 |
||
|
6 |
94 |
||
|
7 |
77 |
- [a] The coupling reactions were conducted in THF at 25 °C with the borane (1.1–1.5 equiv), the vinyl or aryl halide/triflate (1 equiv), and [PdCl2(dppf)] (9–15 mol %) for 1–24 h. The borane residues were then oxidized by treatment of the reaction mixtures with buffered aqueous H2O2 (30 %).
The products obtained by using this method have been used in the synthesis of decahydroisoquinoline rings, which are structural components of isoquinoline alkaloids.69 Condensation of the primary amine 65 with n-butanal under Lewis acid catalysis and ethylation/ethoxycarbonylation of the resulting imine was followed by a pinacol rearrangement. The subsequent hydride migration generated the trans-decahydroquinoline 69 (87 % ee) with an axial substituent at C1 (Scheme 8).
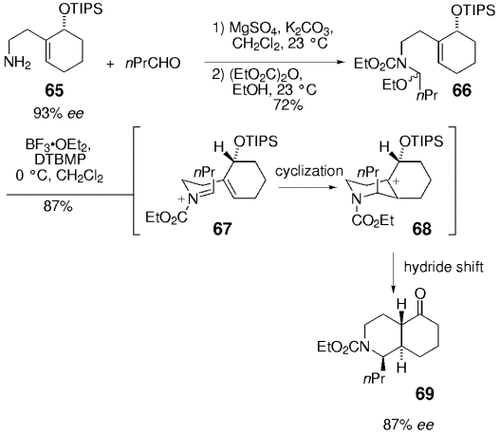
Synthesis of trans-hydroquinolines by Overman and co-workers.
9. Carbapenemes
Narukawa et al. have demonstrated the used of the B-alkyl Suzuki–Miyaura coupling for the preparation of highly functionalized carbapeneme antibiotics.70 In one example, a stereoselective hydroboration of proline derivative 70 set the stage for a coupling with enol triflate 71 to afford 72 [Eq. (28)-7]. Other functionalized alkenes have also been employed. The hydroboration step generates a secondary stereocenter in this case.
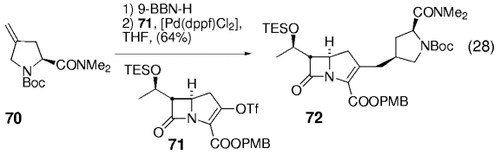
10. (4-Arylmethyl)piperidines
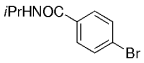
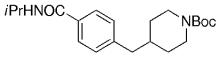
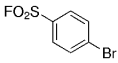
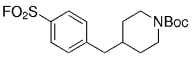
An interesting study of the B-alkyl Suzuki–Miyaura coupling for the synthesis of 4-arylmethyl piperidines was performed by Vice et al. at Schering-Plough Research Institute en route to pharmacologically active compounds.71 Many functional groups are tolerated under the reaction conditions (Table 18).
- [a] All reactions were run as follows: The unsaturated piperidine (1 equiv) was treated with 9-BBN-H (1 equiv) in THF and heated at reflux for 1 h. The resulting borane was added to a mixture of the aryl halide (0.91 equiv), [PdCl2(dppf)] (3 mol %), and K2CO3 (3 equiv) in DMF, and the mixture was heated at 60 °C for 3 h.
11. Application of the B-Alkyl Suzuki–Miyaura Reaction in the Total Syntheses of Natural Products
The B-alkyl Suzuki–Miyaura reaction has proven to be of considerable value in natural product syntheses, especially in the coupling reactions of complex fragments. This review will conclude with a comprehensive summary of such synthetic applications and covers the timespan 1990–2001. The synthetic applications are organized according to the sp2-hybridized coupling partner: aryl or vinyl halides, triflates, and finally, enol phosphates.
11.1. (R)-(+)-Quadrilure
Mori and Puapoomchareon reported the first application of the B-alkyl Suzuki–Miyaura coupling reaction to the synthesis of (R)-(+)-quadrilure in 1990.72 (R)-(+)-Quadrilure is an aggregation pheromone produced by the square-necked grain beetle. Hydroboration of the olefin moiety of intermediate 73 with 9-BBN-H, followed by Pd0-mediated coupling with vinyl bromide 74 and subsequent removal of the THP protecting group afforded alcohol 75 in 58 % yield. Acetylation of alcohol 75 provided the natural product (Scheme 9).
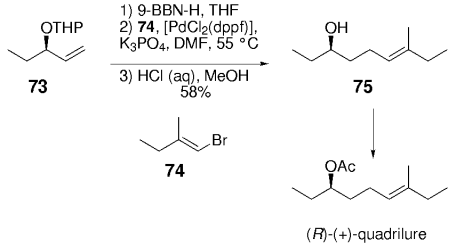
Mori and co-workers were the first to demonstrate the utility of the B-alkyl Suzuki–Miyaura reaction in natural product synthesis.
11.2. (±)-Dihydroxyserrulatic Acid
Also in 1990, Uemura and co-workers used the B-alkyl Suzuki–Miyaura cross-coupling reaction as a key step in the synthesis of (±)-dihydroxyserrulatic acid,73, 74 which is a member of the serrulatane class of diterpenoids. The diterpenoids are known for their potent anti-inflammatory and analgesic properties. Hydroboration of the advanced intermediate 76 with 9-BBN-H, followed by cross-coupling with (E)-methyl β-bromomethacrylate (77) in the presence of [PdCl2(dppf)], K2CO3, and H2O afforded 78 in 77 % yield (Scheme 10). Subsequent functional group manipulations transformed intermediate 78 into the natural product.
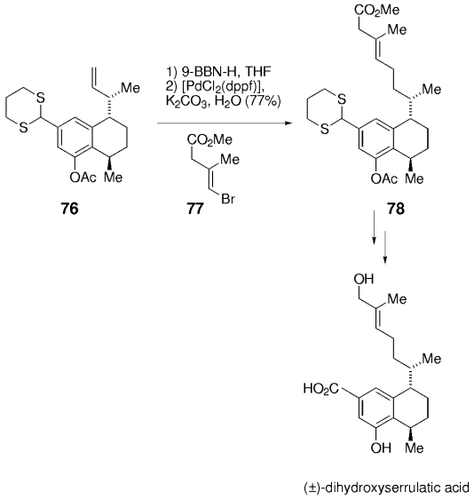
Synthesis of (±)-dihydroxyserrulatic acid (Uemura and co-workers).
11.3. Prostaglandin E1
The B-alkyl Suzuki–Miyaura cross-coupling was incorporated into a new variant of the three-component synthesis of prostaglandins by Johnson and co-workers in 1993. They used very mild conditions ([PdCl2(dppf)], AsPh3, Cs2CO3, DMF/H2O) to effect a highly efficient coupling of vinyl iodide 80 with the alkyl borane derived from olefin 79 (Scheme 11).65 Subsequent stereoselective conjugate addition of cuprate 82 to enone 81 afforded 83, which was then converted into prostaglandin E1. The Johnson protocols for the Suzuki–Miyaura reaction were used in the majority of later applications of this reaction in the synthesis of natural products.
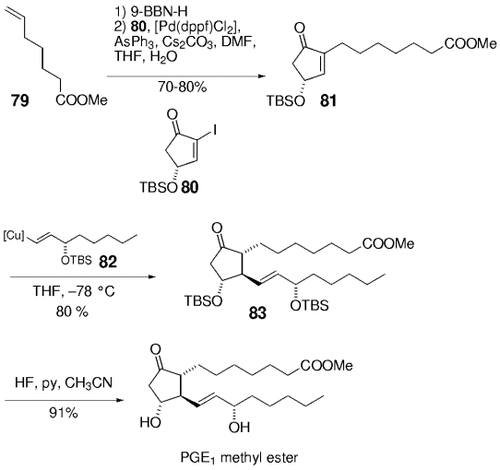
Mild cross-coupling conditions were used in the synthesis of PGE1 methyl ester by Johnson and co-workers.
11.4. Aza-C-disaccharides
In 1994, Johnson and co-workers reported the synthesis of an aza-C-disaccharide 88, a potential glycosidase inhibitor, by means of the B-alkyl Suzuki–Miyaura method.75 This class of sugar mimetics, in which the oxygen atom of the glycoside is replaced by a methylene unit, could potentially preserve the binding properties of the parent azasugar, but be more inert to acidic or enzymatic hydrolysis. The key disaccharide coupling step involved the alkyl borane derived from olefin 84 (obtained in several steps from D-galactose) and vinyl bromide 85. Oxidative cleavage of the resulting olefin by means of ozonolysis, followed by reduction and deprotection, afforded the desired mimetic 88 (Scheme 12).

Synthesis of aza-C-disaccharides by Johnson and co-workers.
11.5. CP-263,114
Danishefsky and co-workers employed a sequence similar to the Johnson prostaglandin work in the synthesis of CP-263,114, which is a structurally unique fungal metabolite that inhibits squalene synthase and farnesyl transferase.76, 77 Coupling of iodo-enone 90 with the borane derived from alkene 89 afforded enone 91. A Sakurai-type conjugate addition then introduced a handle for the second side chain and installed the desired trans relative stereochemistry of the side chains. The resulting intermediate 92 was subsequently converted into CP-263,114 (Scheme 13).

Synthesis of CP-263,114 (Danishefsky and co-workers).
11.6. Agelasimine A
The B-alkyl Suzuki coupling served as a key step in the synthesis of the marine diterpenoid agelasimines A by Ohba et al.78 A key step involved the elongation of the vinyl side chain in compound 93 (Scheme 14). Although the hydroboration of the neopentyl vinyl group with 9-BBN-H required harsh conditions (THF, reflux, 2 h), it proceeded with a high degree of chemoselectivity with respect to the even more hindered trisubstituted double bond. Subsequent palladium-catalyzed cross-coupling with (E)-iodoalkenoate 94 then gave α,β-unsaturated ester 95, which was transformed into agelasimin A in a series of straightforward steps.
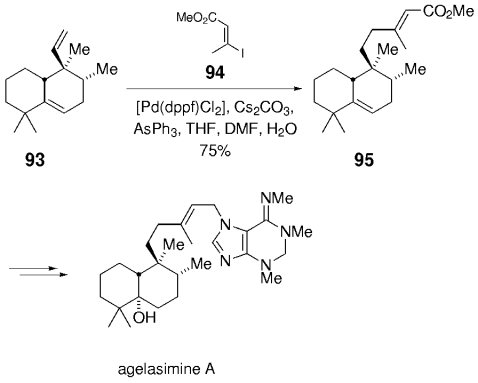
Synthesis of agelasimine A (Ohba and co-workers).
11.7. (+)-Halichlorine
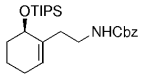
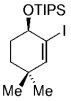
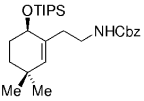
The elongation of an unsaturated side chain by B-alkyl Suzuki coupling set the stage for a key step in a synthesis of the marine alkaloid (+)-halichlorine by Danishefsky and Trauner.79, 80 Contrary to the case in the synthesis of agelasimin A (Section 11.6), the alkene delivered by the vinyl iodide partner was not retained but served as an acceptor in a subsequent conjugate addition. Hydroboration of the protected amino alkene 96, followed by palladium-mediated Suzuki-coupling with methyl (Z)-3-iodoacrylate (97), afforded α,β-unsaturated ester 98 (Scheme 15). Upon deprotection of the amino function with TFA and subsequent basification, 98 underwent a highly stereoselective intramolecular 1,4-addition to afford piperidine 99 as the only isolated isomer. Intermediate 99 was subsequently converted into the VCAM-1 inhibitor (+)-halichlorine in eight steps (VCAM=Vascular Cell Adhesion Molecule).
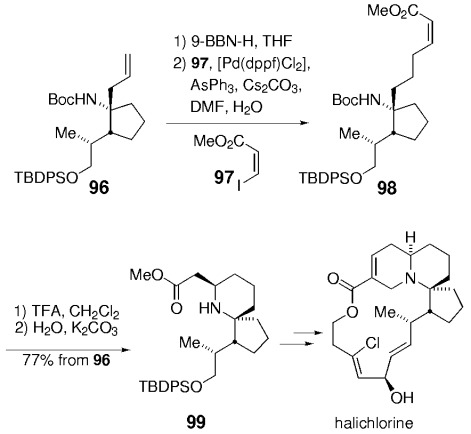
Synthesis of halichlorine (Danishefsky and co-workers).
11.8. Pinnaic Acid
The strategy described in Section 11.7 was expanded further in a synthesis of the related marine natural product pinnaic acid, an inhibitor of cytosolic phospholipase A2.81 Hydroboration of 96 followed by coupling with iododienoic ester 100 afforded compound 101 in excellent yield. This intermediate was subsequently converted into pinnaic acid (Scheme 16).
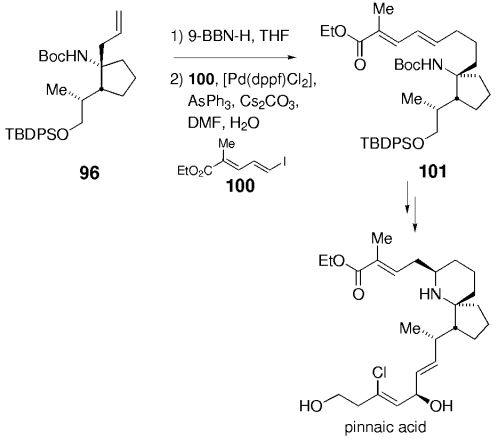
Synthesis of pinnaic acid (Danishefsky and co-workers).
11.9. Sphingofungin F
Trost and Lee used the B-alkyl Suzuki coupling in a synthesis of the serin palmitoyl transferase inhibitor sphingofungin F.82 Hydroboration of the terminal alkene 102 and cross-coupling with the densely functionalized (E)-iodoalkene 103 gave 104. Global deprotection of this intermediate then yielded the natural product (Scheme 17). A synthesis of sphingofungin E that uses a similar Suzuki–Miyaura cross-coupling sequence was subsequently reported by Nakamura and Shiozaki.83
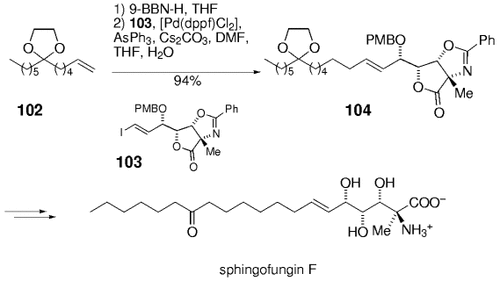
Synthesis of sphingofungin F (Trost and co-workers).
11.10. Discodermolide
An alternative version of the B-alkyl Suzuki coupling was used by Marshall and Johns in an elegant synthesis of the immunosuppressant discodermolide.22 Lithiation of primary iodide 105, followed by addition of 9-methoxy-BBN gave the intermediate borate 106, which was coupled with the trisubstituted iodoalkene 107 in high yield (Scheme 18). An alternative synthesis of the alkyl borane by means of hydroboration of the corresponding 1,1-disubstituted alkene would not have been compatible with the (Z,E)-diene functionality in 105. This efficient coupling enabled a highly convergent synthesis of discodermolide.
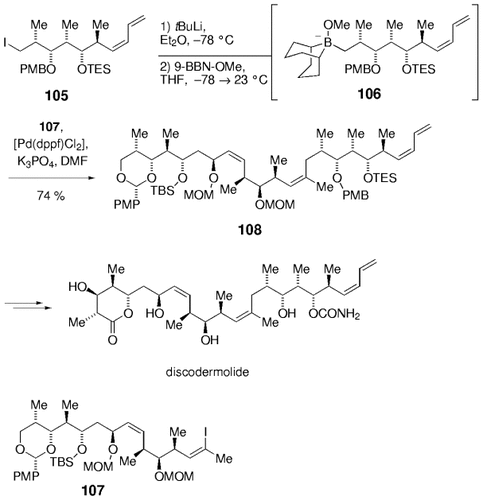
Synthesis of discodermolide with an alkyl borate derived from a primary iodide (Marshall and co-workers).
11.11. Epothilones
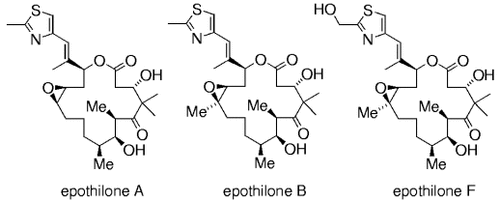
B-alkyl Suzuki coupling reactions were used extensively by Danishefsky and co-workers in the syntheses of the anticancer agents epothilone A, B, and F.-1784–88
During an early approach to epothilone A, vinyl iodide 110 was coupled with an organoborane derived from alkene 109. The product 111 was converted into 12,13-desoxyepothilone A by using an intramolecular aldol addition. Chemo- and stereoselective epoxidation then gave epothilone A (Scheme 19).
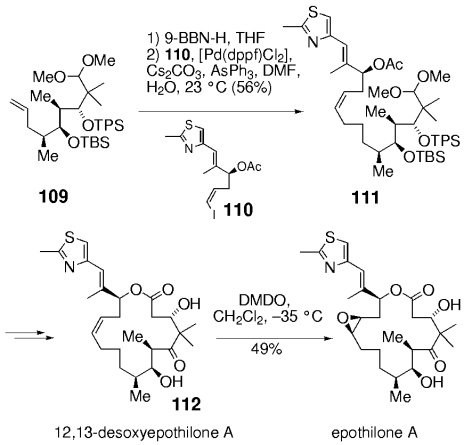
An early synthesis of epothilone A (Danishefsky and co-workers).
A closely related reaction was used by Zhu and Panek in the synthesis of epothilone A (Scheme 20).89
Panek and co-workers used a similar method in their synthesis of epothilone A.
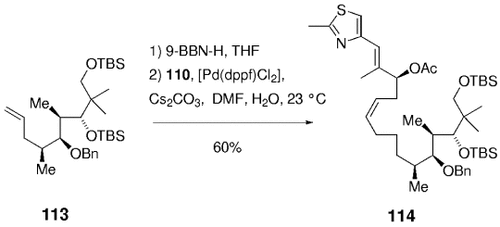
In later syntheses of the epothilones by Danishefsky and co-workers, this chemistry was extended to more elaborate and sensitive substrates (Scheme 21). Hydroboration of 115 and coupling with 116, followed by treatment of the crude product with dilute hydrochloric acid afforded 117. The two carbonyl groups and the ester functionality remain unaffected under these conditions. Stereoselective reduction of the β-ketone (relative to the ester moiety), macrolactonization, and subsequent deprotection gave 12,13-desoxyepothilone B, whose selective epoxidation finally afforded epothilone B. A similar sequence was later used in the synthesis of epothilone F (Scheme 22).90
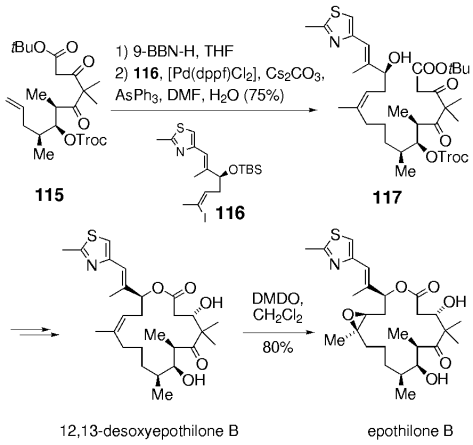
Synthesis of epothilone B (Danishefsky and co-workers).
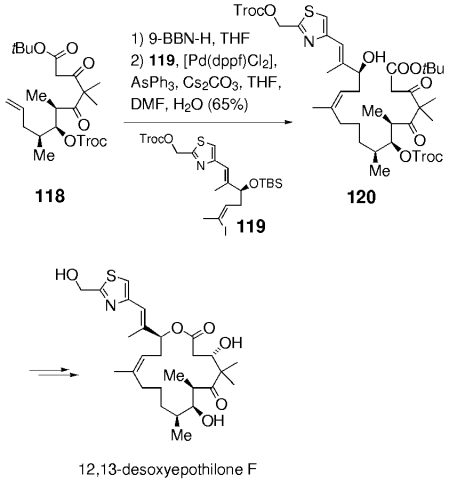
Synthesis of 12,13-desoxyepothilone F (Danishefsky and co-workers).
Aryl iodides were successfully used in related coupling reactions. Compound 121 was converted into epothilone derivative 122 by using the same overall strategy (Scheme 23).84 Unfortunately, several attempts to effect Suzuki-macrocyclizations in this context (e.g. 123→124) have thus far been unsuccessful (Scheme 24).
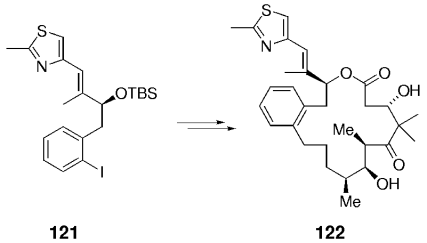
Synthesis of an epothilone analogue.
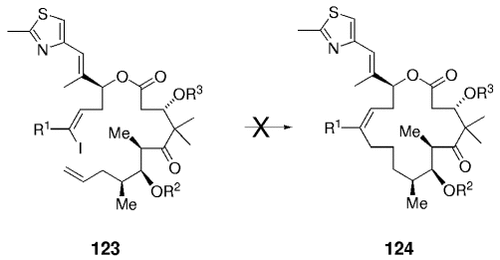
Macrocyclization by means of a B-alkyl Suzuki–Miyaura coupling failed in the synthesis of epothilones.
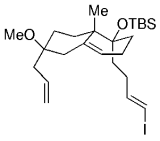
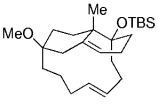
11.12. Phomactin D
The application of a ring-closing intramolecular B-alkyl Suzuki couplings in natural product synthesis was recently described by the Halcomb group.91 Regioselective hydroboration of the diene moiety in 125 followed by intramolecular cross-coupling afforded the bicyclic product 126. A highly strained 12-membered ring is formed in the process (Scheme 25). Compound 126 is a possible intermediate en route to phomactin D, which is an antagonist of platelet activating factor (PAF).
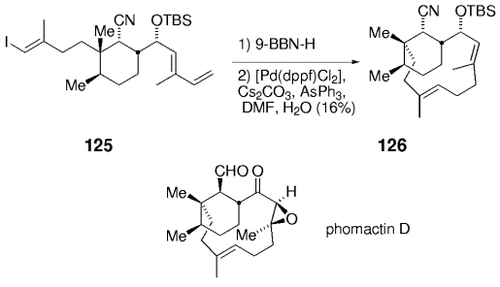
Halcomb and co-workers used a B-alkyl Suzuki–Miyaura macrocyclization in their work towards the synthesis of phomactin D.
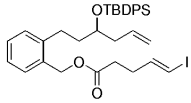
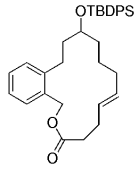
Simultaneously, a related model study was undertaken by Danishefsky's group to assess whether this chemistry is suitable for the synthesis of the related natural product phomactin A (127→128, Scheme 26).45
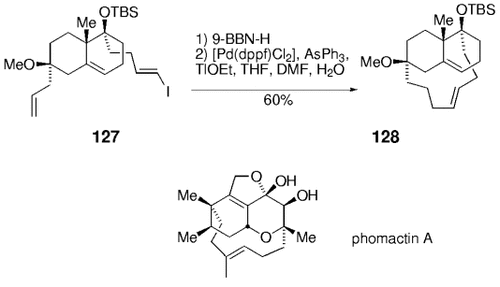
Danishefsky and co-workers used a transannular B-alkyl Suzuki–Miyaura coupling in their work towards the synthesis of phomactin A.
11.13. Squalene Epoxidase Inhibitor
As a final example of the Suzuki–Miyaura coupling reactions between alkyl boranes and vinyl halides, Moore et al. demonstrated that vinyl iodide 129, which has a geminal fluoride substituent, couples with methyl-OBBD to generate the squalene epoxidase inhibitor 130 in good yield (Scheme 27).92
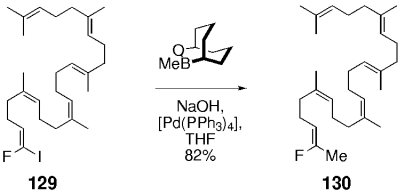
Moore and co-workers used methyl-OBBD to complete the synthesis of a squalene epoxidase inhibitor.
11.14. Caloporoside
Fürstner and Konetzki used the B-alkyl Suzuki coupling in a synthesis of the phospholipase C inhibitor caloporoside (134).93 The (16R)-hydroxyheptadecylsalicylic acid part of the natural product was efficiently prepared by means of a palladium-catalyzed cross-coupling: aryl triflate 132 was coupled with the 9-BBN derivative of alkene 131, to give 133 (Scheme 28).
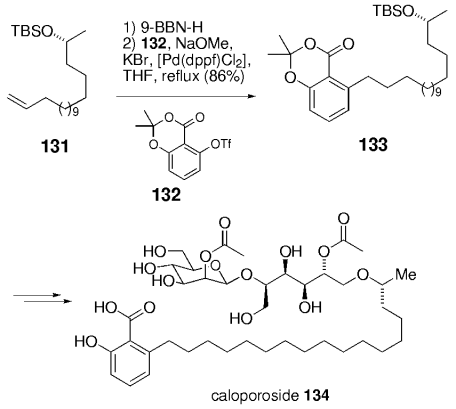
Synthesis of caloporoside (Fürstner and co-workers).
11.15. 5-Alkylresorcinols
Fürstner's group also described very concise syntheses of several biologically active 5-alkylresorcinols.94 For example, threefold hydroboration of dienyne 135 afforded the trisborane 136 (Scheme 29). Selective hydrolysis of the less stable vinylic borane and activation of the two remaining alkyl boranes as borate complexes to form 137 then set the stage for a twofold Suzuki coupling with 3,5-dimethoxyphenol triflate (138). Overall, this stereoselective one-pot transformation yielded aromatic tetra-O-methyl ether 139. Subsequent deprotection afforded bis-resorcinol 140, a natural product with DNA-cleaving properties.

Fürstner and co-workers used the B-alkyl Suzuki–Miyaura coupling in the synthesis of a C2-symmetric resorcinol derivative.
11.16. Halenaquinol
In an elegant synthesis of the bioactive sponge metabolite halenaquinol, Shibasaki and co-workers demonstrated that an intermolecular B-alkyl Suzuki–Miyaura coupling can be combined with an asymmetric intramolecular Heck-reaction (Scheme 30).95 Selective hydroboration of diene 141 followed by Pd-mediated cross-coupling with the symmetrical aromatic bis-triflate 142 gave intermediate alkene 143, which immediately underwent cyclization to afford enol ether 144. In the presence of a chiral phosphane (BINAP), this reaction afforded the cyclization product (85 % ee). Although the chemical yield is still unsatisfactory, this is the first example of a tandem reaction that involves a Suzuki coupling. Since many other useful reactions (e.g. allylic alkylations or cycloisomerizations) are catalyzed by Pd0, further applications and other combinations can be expected to appear in the literature.
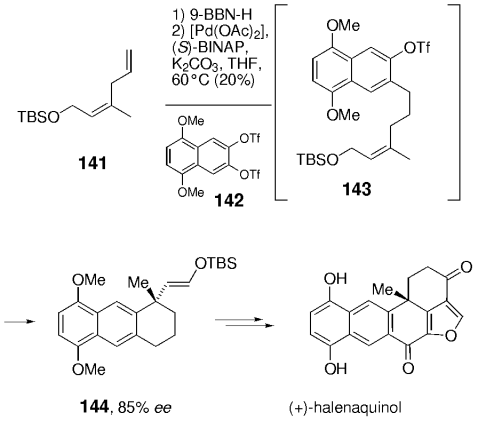
The first tandem Suzuki–Heck reaction was used by Shibasaki and co-workers in their synthesis of halenaquinol.
11.17. Ciguatoxin
Recently, Sasaki, Tachibana, and co-workers began to apply the B-alkyl Suzuki reaction to the synthesis of complex marine polycyclic ethers such as the brevetoxins, ciguatoxin, or gambierol (Schemes 31–33).96–99 This work is unusual in several respects. On the one hand, an exocyclic enol ether, typically derived from a sugar, is used as a borane precursor. Its stereoselective hydroboration proceeds syn to the α-alkoxy or α-silyloxy group and generates a tertiary stereocenter. On the other hand, cyclic ketene acetal triflates or phosphates serve as coupling partners. Thus, hydroboration of enol ether 145 furnished 146. This borane was coupled with ketene acetal triflate 147 to afford the endocyclic enol ether 148 (Scheme 31).
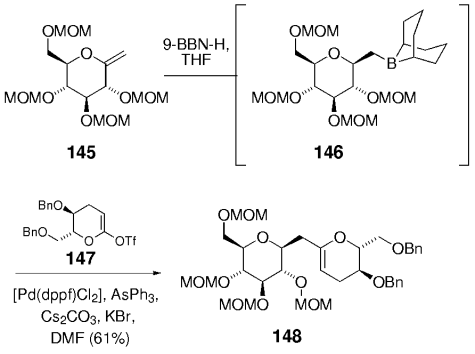
Sasaki and co-workers relied heavily on the B-alkyl Suzuki–Miyaura coupling for the synthesis of polyether natural products.
A considerable portion of the marine-derived polycyclic ether ciguatoxin-30 has been synthesized by using this chemistry. The method has been extended to the synthesis of seven- and eight-membered rings (Schemes 32 and 33). Rings A–D of this molecule were constructed as shown in Scheme 32. The hydroboration of 149 followed by palladium-mediated coupling with enol phosphate 150 gave compound 151, which was converted into tetracycle 152 in a few steps. Rings G–M of ciguatoxin were assembled in a similar way (Scheme 33).
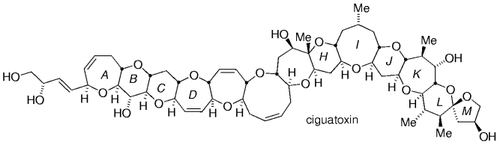
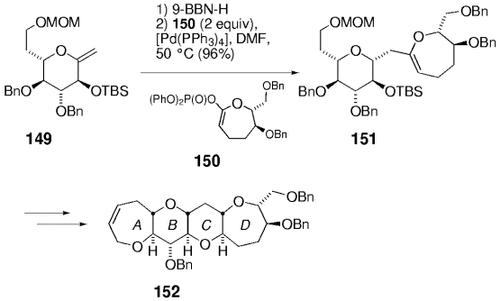
Synthesis of rings A–D of ciguatoxin (Sasaki and co-workers).
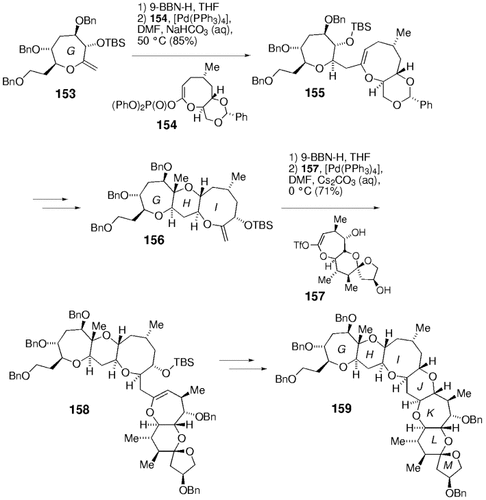
Synthesis of rings G–M of ciguatoxin (Sasaki and co-workers).
11.18. Gambierol
The application of this general strategy to the synthesis of the marine polyether toxin gambierol is shown in Scheme 34.100 Hydroboration of 160 and palladium-catalyzed reaction with the seven-membered cyclic ketene acetal phosphate 161 afforded the coupling product 162. This intermediate was converted into tetracycle 163, an intermediate of the F–H portion of gambierol. The Buchwald ligand (o-biphenyl)P(tBu)2 greatly improves the efficiency of the coupling reaction.
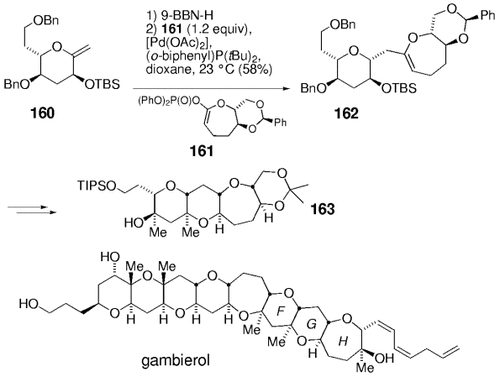
Work towards the synthesis of gambierol.
12. Summary and Outlook
As demonstrated throughout this review, the B-alkyl Suzuki–Miyaura cross-coupling reaction has proven to be an extraordinarily useful tool for the construction of carbon frameworks. These products may ultimately be converted into useful compounds, including biologically active natural and non-natural products. It is probable that this area will develop further. Potential developments include the incorporation of reagent-directed asymmetry in the hydroboration reaction, improvement in catalyst-derived asymmetry in the C−C bond-forming step, improvement of coupling yields, and synthetic applications for secondary and even tertiary alkyl boranes.
Note added in proof: Impressive sp3–sp3 B-alkyl Suzuki–Miyaura couplings between alkyl boranes and alkyl bromides were published by Fu and co-workers shortly after this review was prepared.101
Abbreviations
9-BBN-H 9-borabicyclo[3.3.1]nonane
BINAP 2,2′-bis(diphenylphosphanyl)-1,1′-binaphthyl
Bn benzyl
Boc tert-butoxycarbonyl
Bz benzoyl
Cbz carbobenzoxy (benzyloxycarbonyl)
Cp cyclopentadienyl
dba dibenzylideneacetone
DMDO dimethyldioxirane
DMS dimethyl sulfide
dppe bis(diphenylphosphanyl)ethane
dppf bis(diphenylphosphanyl)ferrocene
DTBMP 2,6-di-tert-butyl-4-methylpyridine
Hx hexyl
MOM methoxymethyl
OBBD 9-oxa-10-borabicyclo[3.3.2]decane
PMB p-methoxybenzyl
PMP p-methoxyphenyl
py pyridine
TBDPS tert-butyldiphenylsilyl
TBS tert-butyldimethylsilyl
TES triethylsilyl
Tf trifluoromethanesulfonyl
TFA trifluoro acetic acid
THP tetrahydropyranyl
TIPS triisopropylsilyl
TPS triphenylsilyl
Troc trichloroethoxycarbonyl
Acknowledgements
We acknowledge the financial support of the National Institutes of Health (postdoctoral fellowship to S.R.C.) and the Schering Research Foundation, Berlin (postdoctoral fellowship to D.T.).
Dedicated to Professor Akira Suzuki
Biographical Information
Samuel J. Danishefsky was born in 1936. Under the tutelage of his father, he was exposed at an early age to the elements of logical though and critical analysis through the study of the Talmud. He received a B.S. degree at Yeshiva University (1956). In keeping with the example of an older brother, Isadore, he took an interest in chemistry at college. A life-long fascination with organic chemistry followed from absorption of two introductory treatments of the subject—-one by Rayond Brewster and the other by Louis and Mary Fieser. This exposure led him to pursue organic studies at Harvard University where he received a Ph.D. (1962) under the direction of Professor Peter Yates. From 1961–1963 he was an NIH-sponsored Postdoctoral Fellow at Columbia University under the mentorship of Gilbert Stork. His first independent academic position, which started in 1963, was at the University of Pittsburgh where he became Professor in 1971 and University Professor in 1979. In 1980 he moved to Yale University and served as chairman of the department from 1981–1987. He was named Eugene Higgins Professor in 1984 and Sterling Professor in 1990. In 1993 he returned to New York as Professor of Chemistry at Columbia University and as Kettering Professor and first Head of the Laboratory for Bioorganic Chemistry at the Memorial Sloan–Kettering Cancer Center. His research interests have been in the areas of synthetic strategy, cytotoxic natural products, and, most recently, fully synthetic carbohydrate-based tumor antigens. In 1996 he shared the Wolf Prize in Chemistry with Gilbert Stork.
Biographical Information
Sherry R. Chemler was born in Chicago, Illinois, in 1972. She received a B.A. from Boston University in 1994 and a Ph.D. in 1999 from Indiana University. Her doctoral research, under the guidance of Prof. William R. Roush, focused on the development of methodology for the synthesis of polypropionate-derived natural products. As an NIH Postdoctoral Fellow with Professor Danishefsky at the Memorial Sloan–Kettering Cancer Center, she has undertaken the total synthesis of phomactin A. Her research interests include the design and study of novel chemical reactions and the synthesis of molecules with beneficial functional properties.
Biographical Information
Dirk Trauner was born in Linz, Austria, in 1967. He received his Diplom in 1994 from the Freie Universität in Berlin, Germany. In 1997 he obtained a Ph.D. from the University of Vienna, Austria, where he worked with Professor Johann Mulzer on the synthesis of alkaloids. He held an Ernst Schering Research Foundation postdoctoral fellowship at the Memorial Sloan–Kettering Cancer Center, where he completed a synthesis of halichlorine under the supervision of Professor Danishefsky. He is currently an assistant professor of chemistry at the University of California, Berkeley. His interests include neurochemistry, the total synthesis of biologically active natural products, and the development of new synthetic methods based on transition metal catalysis.