Polymetallic Cobalt and Manganese Cages with Phosphinate and Phosphonate Ligands
Euan K. Brechin Dr.
Department of Chemistry The University of Edinburgh (UK) Fax: (+44) 131-650-4743
Search for more papers by this authorRobert A. Coxall Dr.
Department of Chemistry The University of Edinburgh (UK) Fax: (+44) 131-650-4743
Search for more papers by this authorAndrew Parkin
Department of Chemistry The University of Edinburgh (UK) Fax: (+44) 131-650-4743
Search for more papers by this authorSimon Parsons Dr.
Department of Chemistry The University of Edinburgh (UK) Fax: (+44) 131-650-4743
Search for more papers by this authorPeter A. Tasker Prof.
Department of Chemistry The University of Edinburgh (UK) Fax: (+44) 131-650-4743
Search for more papers by this authorRichard E. P. Winpenny Prof.
Department of Chemistry The University of Manchester Oxford Road, Manchester M13 9PL (UK) Fax: (+44) 161-275-4616
Search for more papers by this authorEuan K. Brechin Dr.
Department of Chemistry The University of Edinburgh (UK) Fax: (+44) 131-650-4743
Search for more papers by this authorRobert A. Coxall Dr.
Department of Chemistry The University of Edinburgh (UK) Fax: (+44) 131-650-4743
Search for more papers by this authorAndrew Parkin
Department of Chemistry The University of Edinburgh (UK) Fax: (+44) 131-650-4743
Search for more papers by this authorSimon Parsons Dr.
Department of Chemistry The University of Edinburgh (UK) Fax: (+44) 131-650-4743
Search for more papers by this authorPeter A. Tasker Prof.
Department of Chemistry The University of Edinburgh (UK) Fax: (+44) 131-650-4743
Search for more papers by this authorRichard E. P. Winpenny Prof.
Department of Chemistry The University of Manchester Oxford Road, Manchester M13 9PL (UK) Fax: (+44) 161-275-4616
Search for more papers by this authorThis work was supported by the EPSRC (UK).
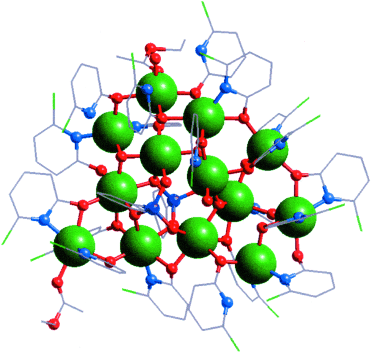
Graphical Abstract
Addition of a coligand in reactions of phosphonates with salts of late 3d metals can lead to more soluble and tractable materials, such as the {Co13} cage shown (Co: green; P: purple). The structure contains two central PhPO32− ligands, surrounded by a hexanuclear cobalt helix, capped by seven further cobalt sites.
References
- 1
- 1a R. Sessoli, H.-L. Tsai, A. R. Schake, S. Wang, J. B. Vincent, K. Folting, D. Gatteschi, G. Christou, D. N. Hendrickson, J. Am. Chem. Soc. 1993, 115, 1804–1816;
- 1b Z. M. Sun, D. Ruiz, E. Rumberger, C. D. Incarvito, K. Folting, A. L. Rheingold, G. Christou, D. N. Hendrickson, Inorg. Chem. 1998, 37, 4758–4759;
- 1c C. Sangregorio, T. Ohm, C. Paulsen, R. Sessoli, D. Gatteschi, Phys. Rev. Lett. 1997, 78, 4645–4648;
- 1d Z. Sun, C. M. Grant, S. L. Castro, D. N. Hendrickson, G. Christou, Chem. Commun. 1998, 721–722;
- 1e M. W. Wemple, D. M. Adams, K. S. Hagen, K. Folting, D. N. Hendrickson, G. Christou, J. Chem. Soc. Chem. Commun. 1995, 1591–1593;
- 1f E. K. Brechin, J. Yoo, M. Nakano, J. C. Huffman, D. N. Hendrickson, G. Christou, Chem. Commun. 1999, 783–784;
- 1g A. L. Barra, A. Caneschi, D. Gatteschi, D. P. Goldberg, R. Sessoli, J. Solid State Chem. 1999, 145, 484–487;
- 1h A. L. Barra, A. Caneschi, A. Cornia, F. F. deBiani, D. Gatteschi, C. Sangregorio, R. Sessoli, L. Sorace, J. Am. Chem. Soc. 1999, 121, 5302–5310.
- 2
- 2a J. R. Friedman, M. P. Sarachik, J. Tejada, R. Ziolo, Phys. Rev. Lett. 1996, 76, 3830–3832;
- 2b L. Thomas, F. Lionti, R. Ballou, D. Gatteschi, R. Sessoli, B. Barbara, Nature 1996, 383, 145–147.
- 3 R. E. P. Winpenny, Adv. Inorg. Chem., in press, and references therein.
- 4 M. I. Khan, J. Zubieta, Prog. Inorg. Chem. 1995, 43, 1–149, and references therein.
- 5 M. G. Walawalker, H. W. Roesky, R. Murugavel, Acc. Chem. Res. 1999, 32, 117–126, and references therein.
- 6
V. Chandrasekhar, S. Kingsley, Angew. Chem. 2000, 112, 2410–2412;
10.1002/1521-3757(20000703)112:13<2410::AID-ANGE2410>3.0.CO;2-A Google ScholarAngew. Chem. Int. Ed. 2000, 39, 2320–2322.10.1002/1521-3773(20000703)39:13<2320::AID-ANIE2320>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 7
- 7a W. F. Ruettinger, D. M. Ho, G. C. Dismukes, Inorg. Chem. 1999, 38, 1036–1037;
- 7b W. F. Ruettinger, G. C. Dismukes, Inorg. Chem. 2000, 39, 1021–1027.
- 8 A. Clearfield, Prog. Inorg. Chem. 1998, 47, 371–510.
- 9
Crystal data for 1: C119H98Cl19Co13N19O36P2, Mr=3872, triclinic, P
 , a=16.880(5), b=17.978(5), c=29.874(10) Å, α=104.47(2), β=102.391(15), γ=94.077(18)°, V=8499(4) Å3, Z=2, T=220.0(2) K, crystal size 0.39×0.35×0.19 mm, μ(MoKα)=1.619 mm−1. Crystal data for 2: C139.5H135Cl21.5Co13N23.5O37.5P2, Mr=4331, triclinic, P
, a=16.880(5), b=17.978(5), c=29.874(10) Å, α=104.47(2), β=102.391(15), γ=94.077(18)°, V=8499(4) Å3, Z=2, T=220.0(2) K, crystal size 0.39×0.35×0.19 mm, μ(MoKα)=1.619 mm−1. Crystal data for 2: C139.5H135Cl21.5Co13N23.5O37.5P2, Mr=4331, triclinic, P , a=16.964(7), b=21.564(9), c=25.142(10) Å, α=98.556(7), β=98.280(7), γ=101.171(7)°, V=8780(6) Å3, Z=2, T=150.0(2) K, crystal size 0.15×0.13×0.08 mm, μ(MoKα)=1.615 mm−1. Crystal data for 3: C95H79.5Mn6N4.5O28P4, Mr=2186, triclinic, P
, a=16.964(7), b=21.564(9), c=25.142(10) Å, α=98.556(7), β=98.280(7), γ=101.171(7)°, V=8780(6) Å3, Z=2, T=150.0(2) K, crystal size 0.15×0.13×0.08 mm, μ(MoKα)=1.615 mm−1. Crystal data for 3: C95H79.5Mn6N4.5O28P4, Mr=2186, triclinic, P , a=12.440(3), b=13.640(3), c=17.010(4) Å, α=96.511(4), β=109.479(3), γ=112.445(4)°, V=2417.2(9) Å3, Z=1 (the molecule lies on an inversion center), T=150.0(2) K, crystal size 0.20×0.05×0.02 mm, μ(MoKα)=0.906 mm−1. Data for 1 were collected with a Stoe Stadi-4 diffractometer; data for 2 and 3 were collected on a Bruker Smart APEX CCD area detector. Both diffractometers were equipped with an Oxford Cryosystems low-temperature device. Absorption corrections were applied to all data by face-indexing for 1 (min./max. transmission: 0.624/0.829), by using ψ scan data for 2 (min./max. transmission: 0.658/0.928), and by using Sadabs (area-detector absorption correction; Siemens Industrial Automation Inc., Madison, WI, 1996) for 3 (min./max. transmission: 0.806/0.928). The structures were solved by direct methods using SHELXS-97 for 1 and 2 (
G. M. Sheldrick, SHELX97, Programs for Crystal Structure Analysis, University of Göttingen, 1998), and SIR92 for 3 (
A. Altomare, G. Cascarano, C. Giacovazzo, A. Guagliardi, J. Appl. Crystallogr. 1993, 26, 343), and completed by iterative cycles of ΔF syntheses and full-matrix least-squares refinement against F 2 (
G. M. Sheldrick, SHELX97, Programs for Crystal Structure Analysis, University of Göttingen, 1998). In 2 there is one chp ligand disordered over two orientations, with a common oxygen position, while in 3 the crystallographically unique PhPHO2 ligand is disordered over two orientations with common O sites. In 1 and 2 there were diffuse regions of solvent of crystallization, which was treated by the method of van der Sluis and Speck (
P. van der Sluis, A. L. Spek, Acta Crystallogr. Sect. A 1990, 46, 194–201). A half-occupancy molecule of Hchp was also found in the lattice of 2. Hydrogen atoms were included in calculated positions, riding on parent carbon atoms, with U(H)=1.2 Ueq(C) for aromatic H atoms and U(H)=1.5 Ueq(C) for methyl hydrogen atoms. All full-weight non-hydrogen atoms were refined with anisotropic displacement parameters to give: for 1, for 995 parameters, wR2=0.1959 for 15 739 unique data (2θ≤40°), R1=0.0854 for 6002 data with Fo>4σ(F), max./min. residual electron density 0.724/−0.540 e Å3; for 2, for 1955 parameters, wR2=0.2456 for 30 480 unique data (2θ≤50°), R1=0.0868 for 13 202 data with Fo>4σ(F), max./min. residual electron density 1.327/−1.233 e Å3; for 3, for 622 parameters, wR2=0.1597 for 4510 unique data (2θ≤40°), R1=0.0673 for 2297 data with Fo>4σ(F), max./min. residual electron density 0.401/−0.419 e Å3. Crystallographic data (excluding structure factors) for the structures reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC-158429, CCDC-158430, and CCDC-158431. Copies of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: (+44) 1223-336-033; e-mail: [email protected]).
, a=12.440(3), b=13.640(3), c=17.010(4) Å, α=96.511(4), β=109.479(3), γ=112.445(4)°, V=2417.2(9) Å3, Z=1 (the molecule lies on an inversion center), T=150.0(2) K, crystal size 0.20×0.05×0.02 mm, μ(MoKα)=0.906 mm−1. Data for 1 were collected with a Stoe Stadi-4 diffractometer; data for 2 and 3 were collected on a Bruker Smart APEX CCD area detector. Both diffractometers were equipped with an Oxford Cryosystems low-temperature device. Absorption corrections were applied to all data by face-indexing for 1 (min./max. transmission: 0.624/0.829), by using ψ scan data for 2 (min./max. transmission: 0.658/0.928), and by using Sadabs (area-detector absorption correction; Siemens Industrial Automation Inc., Madison, WI, 1996) for 3 (min./max. transmission: 0.806/0.928). The structures were solved by direct methods using SHELXS-97 for 1 and 2 (
G. M. Sheldrick, SHELX97, Programs for Crystal Structure Analysis, University of Göttingen, 1998), and SIR92 for 3 (
A. Altomare, G. Cascarano, C. Giacovazzo, A. Guagliardi, J. Appl. Crystallogr. 1993, 26, 343), and completed by iterative cycles of ΔF syntheses and full-matrix least-squares refinement against F 2 (
G. M. Sheldrick, SHELX97, Programs for Crystal Structure Analysis, University of Göttingen, 1998). In 2 there is one chp ligand disordered over two orientations, with a common oxygen position, while in 3 the crystallographically unique PhPHO2 ligand is disordered over two orientations with common O sites. In 1 and 2 there were diffuse regions of solvent of crystallization, which was treated by the method of van der Sluis and Speck (
P. van der Sluis, A. L. Spek, Acta Crystallogr. Sect. A 1990, 46, 194–201). A half-occupancy molecule of Hchp was also found in the lattice of 2. Hydrogen atoms were included in calculated positions, riding on parent carbon atoms, with U(H)=1.2 Ueq(C) for aromatic H atoms and U(H)=1.5 Ueq(C) for methyl hydrogen atoms. All full-weight non-hydrogen atoms were refined with anisotropic displacement parameters to give: for 1, for 995 parameters, wR2=0.1959 for 15 739 unique data (2θ≤40°), R1=0.0854 for 6002 data with Fo>4σ(F), max./min. residual electron density 0.724/−0.540 e Å3; for 2, for 1955 parameters, wR2=0.2456 for 30 480 unique data (2θ≤50°), R1=0.0868 for 13 202 data with Fo>4σ(F), max./min. residual electron density 1.327/−1.233 e Å3; for 3, for 622 parameters, wR2=0.1597 for 4510 unique data (2θ≤40°), R1=0.0673 for 2297 data with Fo>4σ(F), max./min. residual electron density 0.401/−0.419 e Å3. Crystallographic data (excluding structure factors) for the structures reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC-158429, CCDC-158430, and CCDC-158431. Copies of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: (+44) 1223-336-033; e-mail: [email protected]).
- 10 Harris notation describes the binding mode as [X.Y1Y2Y3…︁Yn], where X is the overall number of metals bound by the whole ligand, and each value of Y refers to the number of metal atoms attached to the different donor atoms. Therefore for chp, there will be two values for Y, while for PhPO32− there will be three values of Y. The ordering of Y is listed by the Cahn–Ingold–Prelog priority rules, hence O before N. See: R. A. Coxall, S. G. Harris, D. K. Henderson, S. Parsons, P. A. Tasker, R. E. P. Winpenny, Dalton Trans. 2000, 2349–2356.
- 11
E. K. Brechin, S. G. Harris, S. Parsons, R. E. P. Winpenny, Angew. Chem. 1997, 109, 2055–2057;
10.1002/ange.19971091810 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 1967–1969.
- 12
A. Tsohos, S. Dionyssopoloulou, C. P. Raptopoulou, A. Terzis, E. G. Bakalbassis, S. P. Perlepes, Angew. Chem. 1999, 111, 1036–1038;
10.1002/(SICI)1521-3757(19990401)111:7<1036::AID-ANGE1036>3.0.CO;2-E Google ScholarAngew. Chem. Int. Ed. 1999, 38, 983–985.10.1002/(SICI)1521-3773(19990401)38:7<983::AID-ANIE983>3.0.CO;2-K CAS Web of Science® Google Scholar
- 13
- 13a
C. Benelli, A. J. Blake, E. K. Brechin, S. J. Coles, A. Graham, S. G. Harris, S. Meier, A. Parkin, S. Parsons, A. M. Seddon, R. E. P. Winpenny, Chem. Eur. J. 2000, 6, 883–896;
10.1002/(SICI)1521-3765(20000303)6:5<883::AID-CHEM883>3.0.CO;2-0 CAS PubMed Web of Science® Google Scholar
- 13b A. Graham, S. Meier, S. Parsons, R. E. P. Winpenny, Chem. Commun. 2000, 811–812.
- 14 E. K. Brechin, A. Graham, A. Parkin, S. Parsons, A. M. Seddon, R. E. P. Winpenny, Dalton Trans. 2000, 3242–3252.
- 15 E. K. Brechin, S. G. Harris, A. Harrison, S. Parsons, A. G. Whittaker, R. E. P. Winpenny, Chem. Commun. 1997, 653–654.
- 16 V. Calvo-Perez, M. Shang, G. P. A. Yap, A. L. Rheingold, T. P. Fehlner, Polyhedron 1999, 18, 1869–1880.
- 17 J. B. Vincent, H.-R. Chang, K. Folting, J. C. Huffman, G. Christou, D. N. Hendrickson, J. Am. Chem. Soc. 1987, 109, 5703–5711.
- 18
- 18a A. R. E. Baikie, A. J. Howes, M. B. Hursthouse, A. B. Quick, P. Thornton, J. Chem. Soc. Chem. Commun. 1986, 1587–1588;
- 18b A. S. Batsanov, Yu. T. Struchkov, G. A. Timco, N. V. Gérbéléu, O. S. Manole, S. V. Grebenko, Koord. Khim. 1994, 20, 604–606;
- 18c A. R. Schake, J. B. Vincent, Q. Li, W Boyd, K. Folting, J. C. Huffman, D. N. Hendrickson, G. Christou, Inorg. Chem. 1989, 28, 1915–1923.
- 19 A. Caneschi, D. Gatteschi, J. Laugier, P. Rey, R. Sessoli, C. Zanchini, J. Am. Chem. Soc. 1988, 110, 2795–2799.
- 20 G. Aromi, M. J. Knapp, J.-P. Claude, J. C. Huffman, D. N. Hendrickson, G. Christou, J. Am. Chem. Soc. 1999, 121, 5489–5499.
- 21 E. Libby, J. K. McCusker, E. A. Schmitt, K. Folting, D. N. Hendrickson, G. Christou, Inorg. Chem. 1991, 30, 3486–3495.



40:14<>1.0.co;2-e.cover.gif)
