Metal Complexes of an N-Confused Calix[4]phyrin Derivative—The First X-ray Structure of an Organometallic Compound of Divalent Copper
Hiroyuki Furuta Prof.
Department of Chemistry Graduate School of Science Kyoto University, Kyoto 606-8502 (Japan) Fax: (+81) 75-753-3970
Search for more papers by this authorTomoya Ishizuka
Department of Chemistry Graduate School of Science Kyoto University, Kyoto 606-8502 (Japan) Fax: (+81) 75-753-3970
Search for more papers by this authorAtsuhiro Osuka Prof.
Department of Chemistry Graduate School of Science Kyoto University, Kyoto 606-8502 (Japan) Fax: (+81) 75-753-3970
Search for more papers by this authorYoshiya Uwatoko Prof.
Faculty of Science Saitama University, Saitama 338-8570 (Japan)
Search for more papers by this authorYuichi Ishikawa Prof.
Department of Applied Chemistry Faculty of Engineering, Oita University Oita 870-1192 (Japan) Fax: (+81) 97-554-7907
Search for more papers by this authorHiroyuki Furuta Prof.
Department of Chemistry Graduate School of Science Kyoto University, Kyoto 606-8502 (Japan) Fax: (+81) 75-753-3970
Search for more papers by this authorTomoya Ishizuka
Department of Chemistry Graduate School of Science Kyoto University, Kyoto 606-8502 (Japan) Fax: (+81) 75-753-3970
Search for more papers by this authorAtsuhiro Osuka Prof.
Department of Chemistry Graduate School of Science Kyoto University, Kyoto 606-8502 (Japan) Fax: (+81) 75-753-3970
Search for more papers by this authorYoshiya Uwatoko Prof.
Faculty of Science Saitama University, Saitama 338-8570 (Japan)
Search for more papers by this authorYuichi Ishikawa Prof.
Department of Applied Chemistry Faculty of Engineering, Oita University Oita 870-1192 (Japan) Fax: (+81) 97-554-7907
Search for more papers by this authorGraphical Abstract
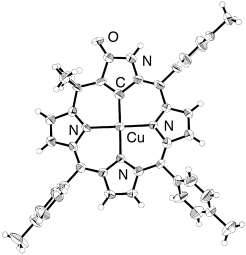
Rare organometallic complexes can be stabilized by using an N-confused system as a carbon ligand. A stable organocopper(II) compound of N-confused calix[4]phyrin has been characterized by X-ray crystallography (see picture). Complete π conjugation was found not to be a prerequisite for the formation of the metal–carbon bond.
Supporting Information
Supporting information for this article is available on the WWW under http://www.angewandte.com or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 The Porphyrin Handbook, Vol. 2 ( ), Academic Press, San Diego, 1999, pp. 1–421.
- 2
- 2a
P. J. Chmielewski, L. Latos-Grażyński, K. Rachlewicz, T. Głowiak, Angew. Chem. 1994, 104, 805–807;
10.1002/ange.19941060721 Google ScholarAngew. Chem. Int. Ed. Engl. 1994, 33, 779–781;
- 2b P. J. Chmielewski, L. Latos-Grażyński, Inorg. Chem. 1997, 36, 840–845;
- 2c P. J. Chmielewski, L. Latos-Grażyński, I. Schmidt, Inorg. Chem. 2000, 39, 5475–5482.
- 3
- 3a H. Furuta, T. Ogawa, Y. Uwatoko, K. Araki, Inorg. Chem. 1999, 38, 2676–2682;
- 3b H. Furuta, N. Kubo, H. Maeda, T. Ishizuka, A. Osuka, H. Nanami, T. Ogawa, Inorg. Chem. 2000, 39, 5424–5425;
- 3c H. Furuta, H. Maeda, A. Osuka, J. Am. Chem. Soc. 2000, 122, 803–807.
- 4
- 4a C. Bucher, D. Seidel, V. Lynch, V. Král, J. L. Sessler, Org. Lett. 2000, 2, 3103–3106;
- 4b
J.-M. Benech, L. Bonomo, E. Solari, R. Scopelliti, C. Floriani, Angew. Chem. 1999, 111, 2107–2109;
10.1002/(SICI)1521-3757(19990712)111:13/14<2107::AID-ANGE2107>3.0.CO;2-R Google ScholarAngew. Chem. Int. Ed. 1999, 38, 1957–1959. Formally, calix[4]phyrin can be classified as a substituted porphyrin derivative or a porphomethene.10.1002/(SICI)1521-3773(19990712)38:13/14<1957::AID-ANIE1957>3.0.CO;2-1 CAS Web of Science® Google Scholar
- 5
N-Confused calix[4]pyrroles have been reported: S. Depraeter, M. Smet, W. Dehaen, Angew. Chem. 1999, 111, 3556–3558;
10.1002/(SICI)1521-3757(19991115)111:22<3556::AID-ANGE3556>3.0.CO;2-I Google ScholarAngew. Chem. Int. Ed. 1999, 38, 3359–3361.10.1002/(SICI)1521-3773(19991115)38:22<3359::AID-ANIE3359>3.0.CO;2-K CAS PubMed Web of Science® Google Scholar
- 6
E. Nakamura, S. Mori, Angew. Chem. 2000, 112, 3902–3924;
Angew. Chem. Int. Ed. 2000, 39, 3750–3771.
10.1002/1521-3773(20001103)39:21<3750::AID-ANIE3750>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
- 7 The spectroscopic and electrochemical studies of CuII complexes of N-confused porphyrin derivatives have been reported recently, see ref. [2 c].
- 8 G. R. Geier III, D. M. Haynes, J. S. Lindsey, Org. Lett. 1999, 1, 1455–1458.
- 9 The formal nomenclature of 1 is 5,5-dimethyl-3-oxo-10,15,20-tris-p-tolyl-2-aza-21-carbaporphyrin.
- 10 The spectral data of 1–3 and the NMR spectra of 1 and 2 are given in the Supporting Information.
- 11 Crystal data for 2: C43H34N4ONi⋅0.5 C7H8⋅CH2Cl2, Mr=814.49, monoclinic, space group C2/c (no. 15), a=24.617(3), b=19.584(2), c=18.278(2) Å, β=113.475(2)°, V=8083(1) Å3, ρcalcd=1.339 g cm−3, Z=8, R=0.101, wR=0.106, GOF=10.04. Crystal data for 3: C43H34N4OCu⋅H2O, Mr=704.33, monoclinic, space group P21/a (no. 14), a=14.763(2), b=10.368(1), c=24.206(2) Å, β=102.790(3)°, V=3613.0(6) Å3, ρcalcd=1.295 g cm−3, Z=4, R=0.061, wR=0.040, GOF=2.470. Crystallographic data (excluding structure factors) for the structures reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication nos. CCDC-155130 (2) and -155131 (3). Copies of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: (+44) 1223-336-033; e-mail: [email protected]).
- 12
R. B. Roof, Jr., A. C. Larson, D. T. Cromer, Acta Crystallogr. Sect. B 1968, 24, 269–273.
10.1107/S0567740868002074 Google Scholar
- 13 The dimerization constant Kd for 1 was determined from 1H NMR measurements in CDCl3 at 25 °C as 14 M−1.
- 14 The temperature dependence of the magnetic moment and the crystal packing diagram of 3 are shown in the Supporting Information.
- 15 G. J. Hathaway in Comprehensive Coordination Chemistry, Vol. 3 ( ), Pergamon, Oxford, 1987, pp. 662–673.
- 16 The introduction of an electron-withdrawing group at the outer α-position of the confused pyrrole ring in the CuII-NCTTP complex raised the E1/2 value to 120 mV (versus Fc/Fc+) and, consequently, made the complex stable enough for column chromatography on silica gel. (Y. Ishikawa, H. Dejima, H. Furuta, unpublished results.)



40:12<>1.0.co;2-f.cover.gif)
