Concave Butterfly-Shaped Organometallic Hydrocarbons?
Matthew Laskoski
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax (+1) 803-777-9521
Search for more papers by this authorGaby Roidl Dr.
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax (+1) 803-777-9521
Search for more papers by this authorMark D. Smith Dr.
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax (+1) 803-777-9521
Search for more papers by this authorUwe H. F. Bunz Prof. Dr.
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax (+1) 803-777-9521
Search for more papers by this authorMatthew Laskoski
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax (+1) 803-777-9521
Search for more papers by this authorGaby Roidl Dr.
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax (+1) 803-777-9521
Search for more papers by this authorMark D. Smith Dr.
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax (+1) 803-777-9521
Search for more papers by this authorUwe H. F. Bunz Prof. Dr.
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax (+1) 803-777-9521
Search for more papers by this authorU.H.F.B. and M.L. thank the NSF for generous support (CAREER, CHE 9981765, 2000–2004). U.H.F.B. is Camille Dreyfus Teacher-Scholar (2000–2004).
Graphical Abstract
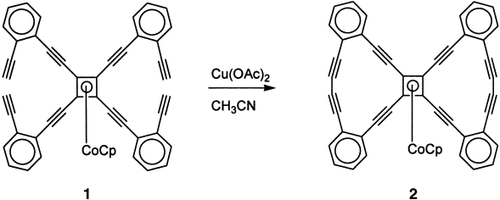
A metamorphosis of the tetraethynyl precursor 1 through double Cu-catalyzed ring closure leads to the corresponding butterfly-shaped cyclobutadiene complexes 2 in high yield (94 %). Single-crystal X-ray diffraction shows that the large organic ligand is distinctly nonplanar with a concave topology. Cp=cyclopentadiene.
References
- 1 F. Diederich, Y. Rubin, Angew. Chem. 1992, 104, 1123; Angew. Chem. Int. Ed. Engl. 1992, 31, 1101; F. Diederich, Nature 1993, 369, 199; R. R. Tykwinski, F. Diederich, Liebigs Ann. 1997, 649; F. Diederich, L. Gobbi, Top. Curr. Chem. 1999, 201, 43.
- 2 M. M. Haley, J. J. Pak, S. C. Brand, Top. Curr. Chem. 1999, 201, 81; M. M. Haley, Synlett 1998, 557.
- 3
T. Bartik, B. Bartik, M. Brady, R. Dembinski, J. A. Gladysz, Angew. Chem. 1996, 108, 467;
10.1002/ange.19961080415 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 414; U. H. F. Bunz, Angew. Chem. 1994, 106, 1127; Angew. Chem. Int. Ed. Engl. 1994, 33, 1073.
- 4
W. B. Wan, S. C. Brand, J. J. Pak, M. M. Haley, Chem. Eur. J. 2000, 6, 2044.
10.1002/1521-3765(20000602)6:11<2044::AID-CHEM2044>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 5 U. H. F. Bunz, Y. Rubin, Y. Tobe, Chem. Soc. Rev. 1999, 28, 107; U. H. F. Bunz, Top. Curr. Chem. 1999, 201, 131.
- 6 J. M. Hunter, J. L. Fye, E. J. Roskamp, M. F. Jarrold, J. Phys. Chem. 1994, 98, 1810.
- 7 K. P. Baldwin, A. J. Matzger, D. A. Scheiman, C. A. Tessier, K. P. C. Vollhardt, W. J. Youngs, Synlett 1995, 1215.
- 8
M. Laskoski, W. Steffen, M. D. Smith, U. H. F. Bunz, Chem. Commun., submitted;
M. Altmann, G. Roidl, V. Enkelmann, U. H. F. Bunz, Angew. Chem. 1997, 109, 1133;
10.1002/ange.19971091016 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 1107.
- 9 F. Vögtle, R. Berscheid, Synthesis 1992, 58.
- 10 U. H. F. Bunz, G. Roidl, R. D. Adams, J. Organomet. Chem. 2000, 600, 56.
- 11 U. H. F. Bunz, G. Roidl, M. Altmann, V. Enkelmann, K. D. Shimizu, J. Am. Chem. Soc. 1999, 121, 10 719.
- 12 For 4 b: A red plate of 4 b (0.25×0.2×0.08 mm) was grown from dichloromethane. Data were obtained on a Bruker SMART APEX CCD diffractometer at 211 K. The structure was solved and refined by the programs SAINT+ and SHELXTX by using heavy atom (Patterson) methods. Hydrogen atoms were localized and refined in the riding mode. The crystal was fixed in a capillary. CoC65H53⋅CH2Cl2 (Mr=977.93); MoKα radiation λ=0.71073 Å, graphite monochromator; 2Θmax=22.50°, tetragonal, space group I41; a=19.156(5), b=19.156(5), c=15.842(7) Å, V=5814(3) Å3, Z=48, ρcalcd=1.117 g cm−3, μ=0.424 mm−1, 12 129 reflections were measured and 4807 reflections with I>2σ(I) observed, R=0.0491, Rw=0.1031. Crystallographic data (excluding structure factors) for the structure reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC-154093. Copies of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: (+44) 1223-336-033; e-mail: [email protected]).
- 13 U. H. F. Bunz, V. Enkelmann, Organometallics 1994, 13, 3823.
- 14
- 14a D. L. Mohler, K. P. C. Vollhardt, S. Wolff, Angew. Chem. 1990, 102, 1200; Angew. Chem. Int. Ed. Engl. 1990, 29, 1151;
- 14b
for a discussion of the crystal-packing-induced nonplanarity of the [N]phenylenes see: D. Holmes, S. Kumaraswamy, A. J. Matzger, K. P. C. Vollhardt, Chem. Eur. J. 1999, 5, 3399.
10.1002/(SICI)1521-3765(19991105)5:11<3399::AID-CHEM3399>3.0.CO;2-V CAS Web of Science® Google Scholar
- 15 R. H. Mitchell, Y. S. Chen, N. Khalifa, P. Z. Zhou, J. Am. Chem. Soc. 1998, 120, 1785.
- 16
Y. Rubin, T. C. Parker, S. J. Pastor, S. Jalisatgi, C. Boulle, C. L. Wilkins, Angew. Chem. 1998, 110, 1353;
10.1002/(SICI)1521-3757(19980504)110:9<1353::AID-ANGE1353>3.0.CO;2-6 Google ScholarAngew. Chem. Int. Ed. 1998, 37, 1226.10.1002/(SICI)1521-3773(19980518)37:9<1226::AID-ANIE1226>3.0.CO;2-H CAS PubMed Web of Science® Google Scholar
- 17 Y. Tobe, N. Nakagawa, K. Naemura, T. Wakabayashi, T. Shida, Y. Achiba, J. Am. Chem. Soc. 1998, 120, 4544.
- 18 J. J. Pak, T. J. R. Weakley, M. M. Haley, J. Am. Chem. Soc. 1999, 121, 10 719.



40:8<>1.0.co;2-k.cover.gif)
