Iron-Catalyzed Regio- and Stereoselective Carbolithiation of Alkynes
Makoto Hojo Dr.
Department of Chemistry Graduate School of Pure and Applied Sciences University of Tsukuba, Tsukuba, Ibaraki 305-8571 (Japan) Fax: (+81) 298-53-6503
Search for more papers by this authorYoshio Murakami
Department of Chemistry Graduate School of Pure and Applied Sciences University of Tsukuba, Tsukuba, Ibaraki 305-8571 (Japan) Fax: (+81) 298-53-6503
Search for more papers by this authorHidenori Aihara Dr.
Department of Chemistry Graduate School of Pure and Applied Sciences University of Tsukuba, Tsukuba, Ibaraki 305-8571 (Japan) Fax: (+81) 298-53-6503
Search for more papers by this authorRie Sakuragi
Department of Chemistry Graduate School of Pure and Applied Sciences University of Tsukuba, Tsukuba, Ibaraki 305-8571 (Japan) Fax: (+81) 298-53-6503
Search for more papers by this authorYu Baba
Department of Chemistry Graduate School of Pure and Applied Sciences University of Tsukuba, Tsukuba, Ibaraki 305-8571 (Japan) Fax: (+81) 298-53-6503
Search for more papers by this authorAkira Hosomi Prof. Dr.
Department of Chemistry Graduate School of Pure and Applied Sciences University of Tsukuba, Tsukuba, Ibaraki 305-8571 (Japan) Fax: (+81) 298-53-6503
Search for more papers by this authorMakoto Hojo Dr.
Department of Chemistry Graduate School of Pure and Applied Sciences University of Tsukuba, Tsukuba, Ibaraki 305-8571 (Japan) Fax: (+81) 298-53-6503
Search for more papers by this authorYoshio Murakami
Department of Chemistry Graduate School of Pure and Applied Sciences University of Tsukuba, Tsukuba, Ibaraki 305-8571 (Japan) Fax: (+81) 298-53-6503
Search for more papers by this authorHidenori Aihara Dr.
Department of Chemistry Graduate School of Pure and Applied Sciences University of Tsukuba, Tsukuba, Ibaraki 305-8571 (Japan) Fax: (+81) 298-53-6503
Search for more papers by this authorRie Sakuragi
Department of Chemistry Graduate School of Pure and Applied Sciences University of Tsukuba, Tsukuba, Ibaraki 305-8571 (Japan) Fax: (+81) 298-53-6503
Search for more papers by this authorYu Baba
Department of Chemistry Graduate School of Pure and Applied Sciences University of Tsukuba, Tsukuba, Ibaraki 305-8571 (Japan) Fax: (+81) 298-53-6503
Search for more papers by this authorAkira Hosomi Prof. Dr.
Department of Chemistry Graduate School of Pure and Applied Sciences University of Tsukuba, Tsukuba, Ibaraki 305-8571 (Japan) Fax: (+81) 298-53-6503
Search for more papers by this authorThis work was partly supported by Grants-in-Aid for Scientific Research and Grants-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports, and Culture, Japan.
Abstract
Katalytische Mengen günstiger Eisen(III)-Salze lassen bei der Reaktion von 3-Pentinylethern mit Butyllithium bei −20°C in Toluol die (E)-4-Methyl-3-octenylether in hohen Ausbeuten und ohne die sonst zu erwartenden Nebenreaktionen entstehen. Eine anschließende Addition von Elektrophilen (E+, siehe Schema; acac = Acetylacetonat) liefert stereochemisch definierte tetrasubstituierte Alkene.
Supporting Information
Supporting information for this article is available on the WWW under http://www.angewandte.com or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 B. J. Wakefield in Organolithium Methods ( ), Academic Press, London, 1988.
- 2 Zr:
- 2a E. Negishi, T. Takahashi, Synthesis 1988, 1–19; Ti:
- 2b D. C. Brown, S. A. Nichols, A. B. Gilpin, D. W. Thompson, J. Org. Chem. 1979, 44, 3457–3461; Cu:
- 2c J. F. Normant, A. Alexakis, Synthesis 1981, 841–870; Zn:
- 2d F. Bernadou, L. Miginiac, Tetrahedron Lett. 1976, 3083–3086;
- 2e M. Bellassoued, Y. Frangin, Synthesis 1978, 838–840; Ni:
- 2f B. B. Snider, R. S. E. Conn, M. Karras, Tetrahedron Lett. 1979, 1679–1682; See also:
- 2g P. Knochel in Comprehensive Organic Synthesis, Vol. 4 ( ), Pergamon, Oxford, 1991, pp. 865–911.
- 3
- 3a J. E. Mulvaney, Z. G. Gardlund, S. L. Gardlund, J. Am. Chem. Soc. 1963, 85, 3897;
- 3b J. E. Mulvaney, D. J. Newton, J. Org. Chem. 1969, 34, 1936–1939;
- 3c W. Bauer, M. Feigel, G. Müller, P. von R. Schleyer, J. Am. Chem. Soc. 1988, 110, 6033–6046;
- 3d L.-I. Olsson, A. Claesson, Tetrahedron Lett. 1974, 2161–2162.
- 4 In the absence of a catalyst, a complex mixture without a normal product 2 b was produced.
- 5
- 5a M. Tamura, J. K. Kochi, J. Am. Chem. Soc. 1971, 93, 1487–1489;
- 5b S. M. Neumann, J. K. Kochi, J. Org. Chem. 1975, 40, 599–606;
- 5c R. S. Smith, J. K. Kochi, J. Org. Chem. 1976, 41, 502–509.
- 6 Conditions for all reactions reported here are not fully optimized. A sufficient amount of butyllithium per iron catalyst (5 mol %) seems to be required during the reaction for the successful carbolithiation. Therefore, 3 equivalents used in reactions are a large enough amount for the present carbolithiation, and, indeed, a reaction of 1 b with 2 equivalents of butyllithium gave essentially the same result (95 % of 2 b, cf. Table 1, entry 6).
- 7 This product was possibly obtained by the addition of butyllithium to pent-1-en-3-yne, produced by the β-elimination of phenylpropanol from 1 a.
- 7a A. A. Petrov, V. A. Kormer, T. V. Yakovleva, Zh. Obshch. Khim. 1960, 30, 2238;
- 7b A. A. Petrov, Yu. I. Porfir'eva, V. A. Kormer, Zh. Obshch. Khim. 1961, 31, 1518.
- 8 M. Nakamura, A. Hirai, E. Nakamura, J. Am. Chem. Soc. 2000, 122, 978–979.
- 9 BuMgBr was prepared in diethyl ether, and most of the solvent was replaced with toluene before use.
- 10 Only allylmagnesium bromide successfully added to 1 b (in Et2O at RT for 7 h using 20 mol % of [Fe(acac)3]) and the corresponding allylated product was obtained in quantitative yield.
- 11
The produced vinyl anion seems to be unstable at room temperature. Yield of 2 b and its deuterium content were decreased to 90 % and 82 %-D, respectively when the reaction mixture was kept at ambient temperature for 3 h before quenching with DCl/D2O, the reaction was conducted under the same conditions as in Equation (5)
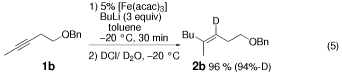 .
.
- 12 Ethyllithium and hexyllithium were also added to 1 b to afford alkenes in high yields (Et: 96 %, Hex: 92 %).
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.



113:3<>1.0.co;2-j.cover.gif)
