An electron localization function investigation of bonding in some simple four-coordinate nitrogen and phosphorus compounds
Corresponding Author
D. B. Chesnut
P. M. Gross Chemical Laboratory, Duke University, Durham, North Carolina 27708
P. M. Gross Chemical Laboratory, Duke University, Durham, North Carolina 27708Search for more papers by this authorCorresponding Author
D. B. Chesnut
P. M. Gross Chemical Laboratory, Duke University, Durham, North Carolina 27708
P. M. Gross Chemical Laboratory, Duke University, Durham, North Carolina 27708Search for more papers by this authorAbstract
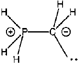
The bonding in some simple four-coordinate species involving nitrogen and phosphorus has been studied by the electron localization function (ELF) approach and compared to that in their conventionally singly and doubly bonded counterparts. Despite evidence suggesting the presence of a conventional multiple bond in certain cases of the four-coordinate species, the ELF study shows this not to be the case. Rather, the situation is better pictured as, for example, in the case of H3PCH2as
REFERENCES
- 1 Gilheany, D. G. In The Chemistry of Organophosphorus Compounds; F. R. Hartley, Ed.; Wiley: New York, 1992; Vol. 2, Chapter 1.
- 2 Gilheany, D. G. Chem Rev 1994, 94, 1339.
- 3 Magnusson, E. J Am Chem Soc 1990, 112, 7940.
- 4 Magnusson, E. J Am Chem Soc 1993, 115, 1051.
- 5 Reed, A. E.; Schleyer, P. v. R. J Am Chem Soc 1990, 112, 1434.
- 6 Schleyer, P. v. R.; Kos, A. J. Tetrahedron 1983, 39, 1141.
- 7 Schmidt, M. W.; Yabushita, S.; Gordon, M. S. J Phys Chem 1984, 88, 382.
- 8 Schmidt, M. W.; Gordon, M. S. J Am Chem Soc 1985, 107, 1922.
- 9 Schmidt, M. W.; Gordon, M. S. Can J Chem 1985, 63, 1909.
- 10 Guest, M. F.; Hillier, I. H.; Saunders, V. R. J Chem Soc Faraday Trans 1972, 2, 867.
- 11 Wallmeier, H.; Kutzelnigg, W. J Am Chem Soc 1979, 101, 2804.
- 12 Messmer, R. P. J Am Chem Soc 1991, 113, 433.
- 13 Nyulászi, L.; Tamás, V.; Réffy, J. J Phys Chem 1995, 99, 10142.
- 14 Chesnut, D. B. J Am Chem Soc 1998, 120, 10504.
- 15 Bader, R. F. W. Acc Chem Res 1985, 18, 9.
- 16
Bader, R. F. W.
Atoms in Molecules: A Quantum Theory;
Clarendon Press: Oxford,
1990.
10.1093/oso/9780198551683.001.0001 Google Scholar
- 17 Cisolowski, J. Int J Quant Chem Quant Chem Symp 1990, 24, 15.
- 18 Cisolowski, J. J Math Chem 1991, 8, 169.
- 19 Cioslowski, J.; Mixon, S. T. J Am Chem Soc 1991, 113, 4142.
- 20 Rai, U. S.; Symons, M. C. R. J Chem Soc Faraday Trans 1994, 90, 2649.
- 21 Power, W. P. J Am Chem Soc 1995, 117, 1800.
- 22 Dixon, D.; Smart, B. J Am Chem Soc 1986, 108, 7172.
- 23 Bachrach, S. J Org Chem 1992, 57, 4367.
- 24 MacDougall, P. J.; Hall, M. B. Trans Amer Cryst Assoc 1990, 26, 105.
- 25 Dobado, H.; Martinez-Garcia, H.; Molina, J. M.; Sundberg, M. R. J Am Chem Soc 1998, 120, 8461.
- 26 Chesnut, D. B.; Savin, A. J Amer Chem Soc 1999, 121, 2335.
- 27 Becke, A. D.; Edgecombe, K. E. J Chem Phys 1990, 92, 5397.
- 28 Savin, A.; Becke, A. D.; Flad, J.; Nesper, R.; Preuss, H.; von Schnering, H. G. Angew Chem Int Ed Engl 1991, 30, 409.
- 29 Silvi, B.; Savin, A. Nature 1994, 371, 683.
- 30 Savin, A.; Silvi, B.; Colonna, F. Can J Chem 1996, 74, 1088.
- 31 Kohout, M.; Savin, A. Int J Quantum Chem 1996, 60, 875.
- 32 Savin, A.; Nesper, R.; Wengert, S.; Fässler, T. Angew Chem Int Ed Engl 1997, 36, 1808.
- 33 Marx, D.; Savin, A. Angew Chem Int Ed Engl 1997, 36, 2077.
- 34 Noury, S.; Colonna, F.; Savin, A.; Silvi, B. J Mol Struct 1998, 450, 59.
- 35 Kouhout, M.; Savin, A. J Comput Chem 1997, 18, 1431.
- 36 Fourré, I.; Silvi, B.; Chaquin, P.; Savin, A. J Comput Chem 1999, 20, 897.
- 37 Gaussian 98, Revision A.7, Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Zakrzewski, V. G.; Montgomery, J. A., Jr.; Stratmann, R. E.; Burant, J. C.; Dapprich, S.; Millam, J. M.; Daniels, A. D.; Kudin, K. N.; Strain, M. C.; Farkas, O.; Tomasi, J.; Barone, V.; Cossi, M.; Cammi, R.; Mennucci, B.; Pomelli, C.; Adamo, C.; Clifford, S.; Ochterski, J.; Petersson, G. A.; Ayala, P. Y.; Cui, Q.; Morokuma, K.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Cioslowski, J.; Ortiz, J. V.; Baboul, A. G.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Gomperts, R.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Gonzalez, C.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Andres, J. L.; Gonzalez, C.; Head-Gordon, M.; Replogle, E. S.; and Pople, J. A., Gaussian, Inc., Pittsburgh PA, 1998.
- 38 Noury, S.; Krokidis, X.; Fuster, F.; Silvi, B. Computers and Chemistry 1999, 23, 597.
- 39 Becke, A. D. J Chem Phys 1993, 98, 5648.
- 40 Lee, C.; Yang, W.; Paar, R. G. Phys Rev 1988, B37, 785.
- 41 Ditchfield, R. Mol Phys 1974, 27, 789.
- 42 Chesnut, D. B. Chem Phys Lett 1995, 246, 235.
- 43 Chesnut, D. B. Heteroat Chem 2000, 11, 73.
- 44 Curtiss, L. A.; Raghavachari, K.; Refern, P. C.; Rassolov, V.; Pople, J. A. J Chem Phys 1998, 109, 7764.
- 45 Curtiss, L. A.; Refern, P. C.; Raghavachari, K.; Rassolov, V.; Pople, J. A. J Chem Phys 1999, 110, 4703.
- 46 Ochterski, J. W.; Petersson, G. A. J Chem Phys 1996, 104, 2598.
- 47 Curtiss, L. A.; Raghavachari, K.; Pople, J. A. J Chem Phys 1993, 98, 1293.
- 48 Withnall, R.; Andrews, L. J Phys Chem 1987, 91, 784.
- 49 Absar, I.; van Wazer, J. R. J Am Chem Soc 1972, 94, 2382.
- 50 Lischka, H. J Am Chem Soc 1977, 99, 353.
- 51 Eades, R. A.; Gassman, P. G.; Dixon, D. A. J Am Chem Soc 1981, 103, 1066.
- 52 Streitwieser, A.; Rajca, A.; Mcdowell, R. S.; Glaser, R. J Am Chem Soc 1987, 109, 4184.




