Indole-3-propionic acid, a melatonin-related molecule, protects hepatic microsomal membranes from iron-induced oxidative damage: Relevance to cancer reduction
Abstract
Excessive free iron and the associated oxidative damage are commonly related to carcinogenesis. Among the antioxidants known to protect against iron-induced oxidative abuse and carcinogenesis, melatonin and other indole compounds recently have received considerable attention. Indole-3-propionic acid (IPA), a deamination product of tryptophan, with a structure similar to that of melatonin, is present in biological fluids and is an effective free radical scavenger. The aim of the study was to examine the effect of IPA on experimentally induced oxidative changes in rat hepatic microsomal membranes. Microsomes were preincubated in presence of IPA (10, 3, 2, 1, 0.3, 0.1, 0.01 or 0.001 mM) and, then, incubated with FeCl3 (0.2 mM), ADP (1.7 mM) and NADPH (0.2 mM) to induce oxidative damage. Alterations in membrane fluidity (the inverse of membrane rigidity) were estimated by fluorescence spectroscopy and lipid peroxidation by measuring concentrations of malondialdehyde+4-hydroxyalkenals (MDA+4-HDA). IPA, when used in concentrations of 10, 3 or 2 mM, increased membrane fluidity, although at these concentrations it did not influence lipid peroxidation significantly. The decrease in membrane fluidity due to Fe3+ was completely prevented by preincubation in the presence of IPA at concentrations of 10, 3, 2 or 1 mM. The enhanced lipid peroxidation due to Fe3+ was prevented by IPA only at the highest concentration (10 mM). It is concluded that Fe3+-induced rigidity and, to a lesser extent, lipid peroxidation in microsomal membranes may be reduced by IPA. However, IPA in high concentrations increase membrane fluidity. Besides melatonin, IPA may be used as a pharmacological agent to protect against iron-induced oxidative damage to membranes and, potentially, against carcinogenesis. J. Cell. Biochem. 81:507–513, 2001. © 2001 Wiley-Liss, Inc.
Iron plays an ambivalent role in biology; on the one hand, it acts as a cofactor for many biological reactions, but, on the other, its toxicity alters cellular integrity leading to organelle dysfunction. Iron exerts its toxicity through a variety of reactions, mainly via the production of free radicals. Iron and oxidative stress are involved in the mechanisms of carcinogenesis [Aust and Eveleigh, 1999].
A complex process of carcinogenesis due to oxidative stress may be initiated after alterations in one of several macromolecules. Biological membranes, being a source of polyunsaturated fatty acids (PUFAs), constitute one of the main sites of free radical attack. Lipid peroxidation, resulting from the action of free radicals on PUFAs, is a chain reaction involving various radicals and reactive oxygen species (ROS) and the resulting metabolites (products of lipid peroxidation) which contribute to DNA damage and, consequently, to carcinogenesis [Burcham, 1998]. Structural changes in cellular membranes due to lipid peroxidation and, additionally, to oxidation of membrane proteins, cause cross-linking between adjacent lipid and protein molecules, thereby disrupting molecular motion in the membrane and changing membrane fluidity [Yu et al., 1992; Chen and Yu, 1994]. As a result, there are changes in the properties of membrane receptors, signal transduction mechanisms, transmembrane transport processes, the activities of enzymes associated with membranes, etc.
Melatonin has recently gained significance as a highly effective molecule in defense against oxidative damage and carcinogenesis [Reiter, 1997, 1998, 1999; Blask et al., 1999; Karbownik et al., 2000a; Karbownik and Reiter, 2000; Reiter et al., 2000]. Indole-3-propionic acid (IPA) is a deamination product of tryptophan, possessing, like melatonin, a heterocyclic aromatic ring structure, but its properties have not been as thoroughly examined as those of melatonin. IPA is found in plasma and cerebrospinal fluid [Young et al., 1980; Morita et al., 1992]. IPA is known to be an effective free radical scavenger and it protects against oxidative damage; to date, no pro-oxidative effects of IPA have been observed [Chyan et al., 1999; Poeggeler et al., 1999]. These findings suggest IPA may be a potential therapeutic agent in protecting against free radical damage and thereby reducing cancer.
Besides DNA, cellular membranes are considered to be important targets for both carcinogens and for anticancer drugs [Arancia and Donelli, 1991]. The above findings prompted us to design a study to evaluate the possible protective effects of IPA on iron-induced changes in hepatic microsomal membrane fluidity and lipid peroxidation.
MATERIALS AND METHODS
Chemicals
The LPO-586 kit for lipid peroxidation was obtained from Calbiochem (La Jolla, CA) and 1-[4-(trimethylammonium)phenyl]-6-phenyl-1, 3,5-hexatriene p-toluene sulfonate (TMA-DPH) from Molecular Probes (Eugene, OR). Indole-3-propionic acid (IPA), ferric chloride (FeCl3), adenosine 5′-diphosphate (ADP), nicotinamide adenine dinucleotide phosphate (NADPH), and ethylenediaminetetraacetic acid (EDTA) were purchased from Sigma (St. Louis, MO). Other chemicals used were of analytical grade and came from commercial sources. IPA was diluted in ethanol and TMA-DPH was diluted in tetrahydrofuran (THF) and water. The final concentrations of ethanol and THF in the incubation volume were 2.67 and 0.53%, respectively, and that of TMA-DPH was 88.9 nM.
Animals
The procedures used in the study were approved by the Institutional Animal Care and Use Committee. Male Sprague-Dawley rats, weighing at that time ∼250 g were purchased from Harlan (Houston, TX) and housed in plexiglas cages (3 animals per cage) in a windowless room with automatically regulated temperature (22 ± 2°C) and lighting (14 h light/10 h dark, with light on from 06:00 to 20:00). The animals received standard chow (Ralston Purina Co., Inc., St. Louis, MO) and water ad libitum. After 1 week of acclimatization, the rats were killed by decapitation and their livers collected, frozen on solid CO2 and stored at −80°C until assay.
Microsomal Membrane Isolation
Hepatocyte microsomal membranes were isolated according to a method described by Yu et al. [1992]. Liver was homogenized in 140 mM KCl/20 mM HEPES buffer (pH 7.4) (1:10 w/v) and, then, centrifuged at 1,000 × g for 10 minutes at 4°C. The pellets containing nuclei were removed, and the supernatant was centrifuged at 105,000 × g for 60 minutes at 4°C. Thereafter, the pellets containing both microsomes and mitochondria were re-suspended in the buffer and centrifuged at 10,000 × g for 15 minutes at 4°C. The supernatant, which contained only the microsomal fraction, was centrifugated at 105,000 × g for 60 minutes at 4°C. Following the last centrifugation, the final microsomal pellets were suspended in 140 mM KCl/20 mM HEPES buffer (1:1, v/v) and kept at −80°C until assay.
Measurement of Protein
Protein was measured using the method of Bradford [1976], with bovine albumin as the standard.
Incubation of Microsomal Membranes
Microsomes (0.5 mg/ml microsomal protein) were resuspended in 50 mM Tris–HCl buffer (pH 7.4) (3 ml of final volume) and were preincubated with IPA (10, 3, 2, 1, 0.3, 0.1, 0.01 or 0.001 mM) for 30 minutes at 37°C. Next, microsomes were incubated in the presence of FeCl3 (0.2 mM), ADP (1.7 mM) and NADPH (0.2 mM) for 20 minutes at 37°C. The reaction was stopped by addition of EDTA (2 mM).
Measurement of Membrane Fluidity
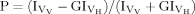
Measurement of Products of Lipid Peroxidation (LPO)
The concentrations of malondialdehyde+4-hydroxyalkenals (MDA+4-HDA), as products of LPO, were measured in hepatocyte microsomal membranes. Two hundred microliters of suspensions of microsomal membranes obtained after above incubations were mixed with 650 μl of methanol:acetonitrile (1:3, v/v) solution containing a chromogenic reagent, N-methyl-2-phenylindole, and vortexed. After adding 150 μl of 15.4 M methanesulfonic acid, incubation was continued at 45°C for 40 minutes. The reaction between MDA+4-HDA and N-methyl-2-phenylindole yields a chromophore, which is measured spectrophotometrically at the absorbance of 586 nm using a solution of 10 mM 4-hydroxynonenal as standard. The level of LPO is expressed as the amount of MDA+4-HDA (nmol) per mg microsomal protein.
Statistical Analyses
Results are expressed as means ± SE. The data were statistically analyzed using a one-way analysis of variance (ANOVA) followed by a Student–Newman–Keuls test. Statistical significance was determined at a level of < 0.05.
RESULTS
Effects of IPA on Fluidity and Lipid Peroxidation in Microsomal Membranes
The incubation of microsomal membranes in the presence of IPA resulted in the highly pronounced increase in membrane fluidity when the indole was used at concentrations of 10, 3 or 2 mM. IPA at a concentration of 1 mM and lower did not change significantly membrane fluidity (Fig. 1A). IPA, at none of the applied concentrations, influenced the amount of lipid peroxidation (Fig. 1B).
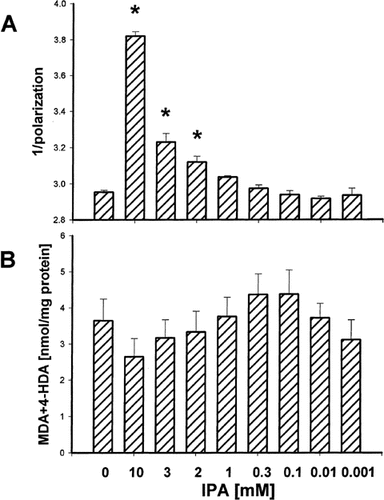
Membrane fluidity (the inverse of membrane rigidity), expressed as an inverse of polarization (1/polarization) (A) and the concentration of malondialdehyde+4-hydroxyalkenals (MDA+4-HDA) (B) in hepatic microsomal membranes incubated in the presence of indole-3-propionic acid (IPA). Bars represent the mean ± SE of four independent experiments. *P < 0.05 vs control (in the absence of IPA).
IPA Reverses Changes in Fluidity and Lipid Peroxidation in Microsomal Membranes Caused by Ferric Iron, ADP and NADPH
The incubation of microsomal membranes in the presence of FeCl3, ADP and NADPH resulted in a significant decrease in membrane fluidity. The preincubation of microsomes with IPA (10, 3, 2 or 1 mM) prevented the ferric iron-related reduction in membrane fluidity. However, IPA in the highest used concentration, i.e., 10 mM, even when it caused oxidative damage to lipids, still increased microsomal membrane fluidity (Fig. 2A). FeCl3, ADP and NADPH-induced lipid peroxidation in microsomal membranes was significantly protected by IPA but only at the highest concentration used, i.e., 10 mM (Fig. 2B).
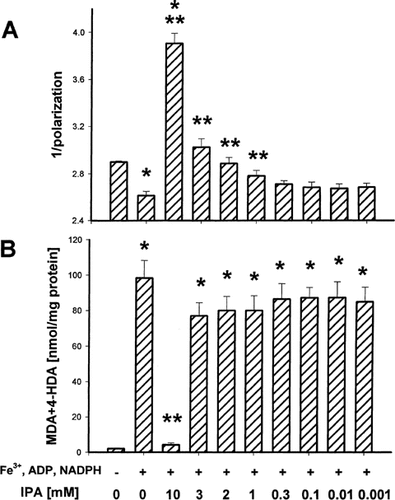
Membrane fluidity (the inverse of membrane rigidity), expressed as an inverse of polarization (1/polarization) (A) and the concentration of malondialdehyde+4-hydroxyalkenals (MDA+4-HDA) (B) in hepatic microsomal membranes preincubated in the presence of indole-3-propionic acid (IPA) and, then, incubated with FeCl3 (0.2 mM), ADP (1.7 mM) and NADPH (0.2 mM). Bars represent the mean ± SE of four independent experiments. * P < 0.05 vs control (in the absence of any treatment); ** P < 0.05 vs microsomes exposed to FeCl3, ADP and NADPH.
DISCUSSION
Iron, besides being of significant importance in oxidative stress and carcinogenesis, has been documented as a critical nutrient for tumor cells [Cazzola et al., 1990; Weinberg, 1996]. Both iron ions, ferrous (Fe2+) and ferric (Fe3+), may participate in oxidative reactions, triggering oxidative damage to cellular components, and, as a consequence, potentially leading to carcinogenesis. The Fenton reaction which is initiated by ferrous ion (Fe2++H2O2+H+→ Fe3++˙OH+H2O) is a well-known phenomenon and is commonly applied in experimental models to induce oxidative stress. Ferric ion participates in Fenton reaction indirectly, when reduced to Fe2+ · Fe3+ alone directly stimulates lipid peroxidation in phospholipid liposomes under acidic conditions, because of the accompanying generation of superoxide anion radical (O2−˙), hydrogen peroxide (H2O2) and the hydroxyl radical (˙OH) [Ohyashiki and Nunomura, 2000]. ˙OH have been found to be the main reactive species formed in the presence of Fe3+ and endogenous reductants [Oikawa and Kawanishi, 1998]. The results of in vitro studies in which ferric salts were used suggest that iron may be a co-factor in the development and/or progression of Kaposi's sarcoma [Simonart et al., 1998].
Hepatic microsomal membranes reveal high sensitivity to oxidative stress caused by Fe3+, ADP and NADPH [Garcia et al., 1997, 1998, 1999, 2000]. Melatonin [Garcia et al., 1997] as well as some structurally related compounds such as pinoline [Garcia et al., 1999], 5-methoxytryptophol [Garcia et al., 2000] and N-acetyl-serotonin [Garcia et al., unpublished] were found to be differentially protective against changes in membrane rigidity and lipid peroxidation in the model system used in the current study. Interestingly, whereas melatonin [Garcia et al., 1999] and N-acetyl-serotonin [Garcia et al., unpublished], applied without induced oxidative stress did not change membrane fluidity, pinoline and 5-methoxytryptophol by themselves decreased membrane fluidity when used in high pharmacological concentrations [Garcia et al., 1999, 2000]. Thus, the latter two compounds were actually pro-oxidant under the conditions of the experiment. In the present study, increased membrane fluidity as a consequence of the treatment with IPA in high pharmacological concentrations was unexpected. There are several potential explanations for this phenomenon. A direct pro-oxidative effect of IPA on membrane fluidity cannot be excluded, despite the fact that in another study IPA was found not to be converted to reactive intermediates with pro-oxidative activity [Chyan et al., 1999]. Indeed, either increased or decreased membrane fluidity can result from oxidative abuse. The factors that determine membrane fluidity, i.e., the cholesterol/phospholipid ratio, the composition of the phospholipid head group, the degree of unsaturation of the acyl chains and protein composition of the membranes, could be potentially influenced by IPA thereby causing an increase in membrane fluidity under the in vitro conditions used here. Thus, the present in vitro finding suggests that IPA may increase the fluidity of microsomal membranes in vivo, although this remains to be tested. Thus, unlike melatonin, side effects related to IPA treatment, which involve changes in membrane fuidity, is a consideration if the indole is to be used clinically.
In spite of the increased fluidity of microsomal membranes caused by IPA, it also prevented iron-induced rigidity in microsomal membranes. Comparing this effect to that caused by melatonin, however, suggests the latter indole to be superior to IPA on an equimolar basis [Garcia et al., 1997].
Changes in membrane rigidity and lipid peroxidation, as observed in the present study, did not run in parallel, since the effects of IPA on membrane rigidity were more obvious than those on lipid peroxidation. The relationship of lipid peroxidation to membrane fluidity is not always clear since both an increase [Galisteo et al., 2000] or a decrease [Karbownik et al., 2000b,c] in membrane fluidity due to external toxins has been reported, even though oxidative damage to lipids is always apparent. The present as well as previous findings are consistent with the fact that lipid peroxidation is exclusively due to the attack of free radicals on PUFAs, whereas membrane fluidity depends on lipid peroxidation as well as on the direct oxidation of membrane proteins, which are especially abundant in microsomal membranes [Engelke et al., 1994], on changes in the membrane concentration of cholesterol, pH and ATPase activity, and on the possible intercalation of some of the molecules into the membrane itself.
To what extent our findings can be extrapolated to in vivo conditions remains to be determined. It is worth mentioning that under in vivo conditions melatonin has been shown to prevent changes in microsomal membrane fluidity due to other carcinogens and to ionizing radiation [Karbownik and Reiter 2000], δ-aminolevulinic acid [Karbownik et al., 2000b,c] and phenylhydrazine [Karbownik et al., 2000d]; the latter agents also involve iron when they induce oxidative damage. Both melatonin and N-acetyl-serotonin have been found to protect against the reduction in hepatic microsomal membrane fluidity caused by injections of α-naphthylisothiocyanate into rats [Calvo et al., 2000].
The mechanisms by which IPA protects microsomal membranes from oxidative abuse are likely complex. Recently, IPA was reported to readily protect primary neurons, neuroblastoma cells and rat brain against oxidative damage due to β-amyloid protein, to H2O2, to an inhibitor of superoxide dismutase (a key antioxidative enzyme), and to effectively scavenge ˙OH with a rate constant of 7.8–8.0 × 1010 M−1s−1; this is roughly the same rate constant for the scavenging of the ˙OH by melatonin [Chyan et al., 1999; Poeggeler et al., 1999]. Besides directly scavenging the ˙OH, IPA acts synergistically with a well-known intracellular antioxidant, glutathione [Poeggeler et al., 1999] to reduce oxidative damage. When compared under the same in vitro conditions, IPA effectively protected against chromium(III)-induced damage to DNA (damage which depends on the generation of ˙OH), but its efficacy in doing so was less than that of melatonin [Qi et al., 2000]. Nevertheless, IPA seems to be a worthy molecule in protecting against ˙OH-induced oxidative damage. Besides scavenging the ˙OH, a chemiluminescence study revealed that IPA also quenches the superoxide anion radical (O2˙−) [Hardeland et al., 1999]. However, IPA appears to be a poor chain-breaking antioxidant, since it was only found effective in reducing lipid peroxidation in millimolar concentrations [Poeggeler et al., 1999]. That is consistent with the present findings in which the iron-induced increase in MDA+4-HDA concentrations was reduced by IPA only when the indole was used at a high pharmacological concentration (10 mM).
Both melatonin and IPA act as endogenous electron donors, primarily detoxifying reactive radicals, but, at the same time they do not undergo autooxidation in the process of redox-recycling or in the presence of transition metals [Tan et al., 2000]. This is believed to be due to the fact that they lack a free hydroxyl group [Candeias et al., 1995].
The similarities in structural and antioxidative properties of melatonin and IPA suggest that the latter compound, like melatonin [Blask et al., 1999; Reiter, 1999; Reiter et al., 2000], may be a pharmacologically useful agent in reducing damage to macromolecules, which, as a result, could lower carcinogenesis. It is concluded that IPA is effective in protecting rat hepatic microsomal membranes against rigidity and, to a lesser extent, against lipid peroxidation caused by iron. On the basis of the previous as well as present findings, IPA, like melatonin, may be considered for therapeutic use.




