Abstract
Ssu1p, a plasma membrane protein involved in sulphite metabolism in Saccharomyces cerevisiae, was found to be required for efficient sulphite efflux. An SSU1 null mutant accumulated significantly more sulphite than wild-type, whereas cells expressing multicopy SSU1 accumulated significantly less. Cells expressing FZF1-4, a dominant allele of a transcriptional activator of SSU1 that confers sulphite resistance, also accumulated less sulphite. β-galactosidase activity in the FZF1-4 strain carrying an SSU1::lacZ fusion was found to be 8.5-fold higher than in a strain carrying wild-type FZF1, confirming that the heightened resistance was correlated with hyperactivation of SSU1. Multicopy SSU1 was also found to increase the sulphite resistance of a number of unrelated sulphite-sensitive strains by a factor of 3- to 8-fold. Rates of efflux of free sulphite from cells expressing multicopy SSU1 or FZF1-4 were significantly greater than that from wild-type or from a SSU1 null mutant. Rates of efflux of bound sulphite from wild-type, a SSU1 null mutant, a FZF1-4 mutant, or cells expressing multicopy SSU1 were not significantly different, suggesting that Ssu1p specifically mediates efflux of the free form of sulphite. Copyright © 2000 John Wiley & Sons, Ltd.
Introduction
Sulphite is a widely used bifunctional preservative in foods, beverages and pharmaceuticals as it has both antimicrobial and antioxidant activities (Taylor et al., 1986). Sulphite causes ATP depletion in S. cerevisiae (Schimz and Holzer, 1979) through inactivation of glyceraldehyde-3-phosphate dehydrogenase and alcohol dehydrogenase (Hinze and Holzer, 1986; Prakash et al., 1986; Maier et al., 1986). Sulphite is also a potentially toxic but normal yeast metabolite which occurs as an intermediate in the reductive sulphate assimilation pathway.
Because sulphite is used as a preservative in winemaking, wine strains of S. cerevisiae have been selected that have enhanced tolerance. Metabolic and genetic studies suggest that an important means of protection against sulphite is formation of a non-toxic adduct with acetaldehyde, 1-hydroxyethane sulphonate (Stratford et al., 1987; Pilkington and Rose, 1988; Casalone et al., 1992). We speculate that other protection mechanisms exist, because differences found in sulphite tolerance among a number of sulphite-sensitive and a resistant strain do not correlate with acetaldehyde production (Xu et al., 1994).
SSU1 encodes a plasma membrane protein previously implicated in sulphite metabolism (Avram and Bakalinsky, 1997). Mutations in SSU1 cause sensitivity (Xu et al., 1994), whereas overexpression confers heightened resistance (Park et al., 1999; Goto-Yamamoto et al., 1998), suggesting a role for SSU1 in sulphite detoxification. Sulphite resistance has also been ascribed to particular dominant alleles of the SSU1 transcriptional activator FZF1 and to overexpression of wild-type FZF1 (Park et al., 1999; Avram et al., 1999; Casalone et al., 1992, 1994). FZF1 (five zinc finger) shares homology to human WT1, Wilms' tumour gene, (P=e−20) (Fourny, 1997) which was recently shown to transcriptionally activate amphiregulin, an epidermal growth factor involved in kidney development (Lee et al., 1999).
The present study was undertaken to understand how SSU1 and FZF1-4 mediate sulphite resistance. We show that Ssu1p is involved in sulphite efflux, and that FZF1-4 confers sulphite resistance through hyperactivation of SSU1.
Materials and methods
Media, yeast strains, and reagents
YEPD is 2% Bacto peptone (Difco, Detroit, MI), 1% Bacto yeast extract (Difco) and 2% dextrose. SM is glucose-based synthetic complete medium (SD plus required amino acids and bases at the prescribed conditions), and drop-out media are SM lacking the indicated amino acid or base (Kaiser et al., 1994). YEPD+TA (tartaric acid) and SM-met+TA are YEPD and SM-met containing 75 mM L-tartaric acid buffered at pH 3.5, respectively. YEPD+TA or SM-met+TA plates were prepared as described (Park et al.1999). Yeast strains are listed in Table 1. All chemicals were reagent grade.
| Strains | Genotype | Source |
|---|---|---|
| 3163-1b | a FZF1-4 ura3-52 leu2-3, 112 | This study |
| 3090-9d | α ura3-52 leu2-3, 112 | Avram and Bakalinsky ( ) |
| 3090-9d-T4-L1 | α ssu1Δ ura3-52 leu2-3, 112 | Avram and Bakalinsky ( ) |
| 3090-9d-T6-L1 | α grr1Δ ura3-52 leu2-3, 112 | Avram and Bakalinsky ( ) |
| 3090-9d-T10-L1 | α fzf1 Δ99-182 ura3-52 leu2-3, 112 | Avram and Bakalinsky ( ) |
| 3090-9d-MC | 3090-9d transformed with pHP18 (multicopy SSUI) | This study |
| 3089-1d | a ssu3-7 ura3-52 leu2-3, 112 | Avram and Bakalinsky ( ) |
| CC359-OL2 | α ura3 leu2 his3 | Y. Surdin-Kerjan |
| CC501-2 | α ura3 leu2 met10 | Y. Surdin-Kerjan |
| CC363-20B | a ura3 leu2 his3 met18 | Y. Surdin-Kerjan |
Sulphite accumulation
Cells were grown to an OD600 ∼1.0 in 200 ml of YEPD+TA, washed twice with 75 mM L-tartaric acid, pH 3.5, containing 2% glucose, suspended in the same buffer to give a final cell concentration of about 20 mg dry weight per ml, and allowed to equilibrate for 10 min at 25°C. To 20 ml of cell suspension, sodium sulphite was added to a final concentration of 0–4 mM, and the mixture was incubated at 25°C with stirring. At appropriate time intervals, 1 ml aliquots were taken and rapidly vacuum filtered through a 0.45 µm membrane (Gelman Science, MI) and washed with five volumes of 75 mM cold tartaric acid (pH 3.5). Cells were suspended in 0.25 M phosphate buffer containing 5 mM EDTA (pH 7.3) by placing the 1 cm diameter filter—cell-side down—in buffer in a 1.7 ml microfuge tube. The cell pellet was recovered by centrifugation and resuspended in 50 µl of the same buffer (the cell-free filter remained on the buffer surface after centrifugation). A volume of acid-washed glass beads equal to that of the cell suspension was added and the mixture was vortexed six times at high speed in 30 s intervals on ice. The liquid fraction was centrifuged at 12 000×g for 20 min at 4°C to remove cell debris. Free and total sulphite were determined in the supernatant by the pararosaniline method (Grant, 1947) as described (AOAC, 1990). ‘Free’ sulphite refers to any form of sulphurous acid including sulphur dioxide. ‘Total’ sulphite refers to the sum of the free and bound forms where the latter are sulphite adducts, covalent products formed between sulphite and other compounds with which sulphite is reactive, i.e. carbonyl compounds (Taylor et al., 1986). Endogenous sulphite was not detected in control cells incubated without sodium sulphite. The limit of detection was found to be 0.07 nmol sulphite/mg dry weight cells. Sulphite was always assayed in freshly prepared samples, although no loss of sulphite was observed in samples stored at −80°C for a week.
Sulphite efflux
Cells were loaded with sulphite by incubation in external sulphite concentrations of up to 4 mM sodium sulphite in 20 ml, as described above, at 25°C for 5 min with stirring. The cell suspension was rapidly vacuum filtered through a 0.45 µm membrane, and washed with 20 ml of 75 mM cold tartaric acid (pH 3.5). Sulphite efflux was initiated by resuspending the cell pellet in 20 ml of 75 mM tartaric acid (pH 3.5) with stirring. At appropriate time intervals, 1 ml aliquots were taken and rapidly filtered through a 0.45 µm membrane. Initial efflux rates were based on three measurements of extracellular free and total sulphite in the supernatant at 10, 20, and 30 s, where rates were found to be linear. To determine the percentage reduction of intracellular sulphite by efflux, 1 ml aliquots were taken, rapidly vacuum filtered through a 0.45 µm membrane, and washed with five volumes of 75 mM cold tartaric acid (pH 3.5). The cell pellet was recovered by centrifugation and resuspended in 50 µl of 0.25 M phosphate buffer containing 5 mM EDTA (pH 7.3). Cell extracts were prepared by glass-bead disruption, and intracellular free and total sulphite were determined in the supernatant.
Subcloning, PCR and yeast transformation
Standard procedures (Sambrook et al., 1989) were used to manipulate plasmid DNA and to transform E. coli DH5α (Hanahan, 1983). Subcloning involved YEplac181 (Gietz and Sugino, 1988), and pDA2 carrying SSU1 and the proximal 658 bp of the GLR1 ORF (unpublished data, D. Avram, 1995). PCR was performed using Pfu (Invitrogen) in an Easycycler (Ericomp, Inc). Yeast transformation was performed as described (Gietz et al., 1992).
Construction of multicopy SSU1
The SSU1 ORF and proximal 540 bp of promoter were generated as described (Park et al., 1999). Only the proximal 540 bp of the 1 kb promoter were used because this region was previously shown to be sufficient for activation of SSU1 by multicopy FZF1, and for in vitro binding by Fzf1p (Avram et al., 1999).
β-galactosidase assay
The SSU1 promoter (−1016 to −1) was fused to lacZ in pDA6 (Avram et al., 1999), and integrated at URA3 in strain 3090-9d (FZF1), in 3090-9d-T10-L1 (fzf1Δ), and in 3163-1b (FZF1-4). Transformants were selected on SM-ura and screened for the ability to form blue colonies on X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactoside) indicator medium. Overnight cultures were inoculated in minimal medium (Adams et al., 1997) and grown to an OD600 of 2.0. β-Galactosidase activity was assayed in permeabilized cells (Kippert, 1995) and expressed in Miller units as the means of three assays of each of three independent transformants±SD.
Sulphite reductase assay
NADPH-dependent sulphite reductase activity was determined as described (Jiranek et al., 1996) with the following modification. Cell extracts were prepared from log-phase cells grown in 100 ml of SM-met, followed by glass-bead extraction. For each assay, 0.9 ml of reaction mixture (Jiranek et al., 1996) was dispensed into test tubes, and 100 µl of cell extract were added. The tubes were capped, gently inverted several times, and incubated at 30°C for 1 h. H2S was derivatized by adding 100 µl of reaction mixture to 110 µl of freshly-prepared HEPES-monobromobimane (Calbiochem, Lajolla, CA) mixture (Avram and Bakalinsky, 1996). The resulting fluorescent adduct was detected following separation by HPLC (Fahey and Newton, 1987; as modified by Vetter et al., 1989). Enzyme activity is expressed as µmoles of H2S produced/min/mg protein, and data are the means of three independent cultures±SD.
Results
Ssu1p decreases intracellular sulphite accumulation
In order to determine whether SSU1 and FZF1-4 are involved in sulphite transport, sulphite accumulation was determined in wild-type (3090-9d carrying empty YEplac181), an ssu1Δ mutant (3090-9d-T4-L1), an FZF1-4 mutant (3163-1b), and in 3090-9d carrying multicopy SSU1 (3090-9d-MC) at a representative sulphite concentration, 1 mM (Figure 1). In all strains, accumulation was rapid, reaching a plateau after approximately 2 min. In the absence of glucose, approximately 50% less sulphite was taken up (data not shown). The ssu1Δ mutant accumulated significantly more sulphite than wild-type, whereas cells expressing multicopy SSU1 accumulated significantly less. Cells expressing FZF1-4 also accumulated significantly less sulphite than wild-type, consistent with previous observations of a different resistant allele of FZF1 (Casalone et al., 1992, 1994). Free and total sulphite accumulation in the FZF1-4 and multicopy SSU1 strains were not significantly different. Table 2 shows that strains that accumulated the least amount of sulphite, 3163-1b and 3090-9d-MC, were also the most sulphite-resistant.
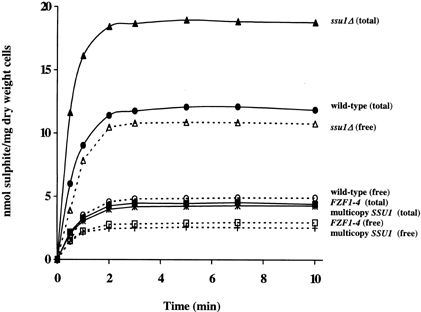
Sulphite accumulation in 3090-9d carrying empty YEplac181 (wild-type), 3090-9d-T4-L1 (ssu1Δ), 3163-1b (FZF1-4) and in 3090-9d carrying multicopy SSU1 (3090-9d-MC). Sulphite uptake was determined in log-phase cells suspended in tartrate buffer (pH 3.5) containing 2% glucose in the presence of 1 mM Na2SO3 for 10 min. Data points are means of duplicates. Standard deviations were less than 5% of the means. ‘Free’ refers to free or unbound sulphite, whereas ‘total’ refers to the sum of the free and bound forms
| Strain | Relevant genotype | Sulphite concentration (mM) | |||||
|---|---|---|---|---|---|---|---|
| 0; 0.5 | 1 | 2 | 3; 4; 5 | 6 | 7 | ||
| 3090-9d | Wild-type | + | + | ± | − | − | − |
| 3090-9d-T4-L1 | ssu1Δ | + | − | − | − | − | − |
| 3090-9d-MC | Multicopy SSU1 | + | + | + | + | + | − |
| 3163-1b | FZF1-4 | + | + | + | + | − | − |
- +, Normal growth; −, no growth; ±, poor growth as scored after 24 h.
- Sulphite sensitivity was determined on YEPD+TA containing of 0–8 mM Na2SO3 by replicating cells grown on YEPD. The sulphite tolerance of 3090-9d was the same as for 3090-9d carrying the empty vector, YEplac181.
Fzf1-4p hyperactivates SSU1 expression
In order to test the possibility that Fzf1-4p hyperactivates SSU1 expression, β-galactosidase was measured in wild-type, in a fzf1Δ mutant, and in a FZF1-4 mutant carrying an integrated SSU1 promoter–lacZ fusion construct. Activity in the FZF1-4 mutant was found to be 8.5- and 20-fold higher than in wild-type and in the fzf1Δ strain, respectively, indicating that FZF1-4 confers sulphite resistance due to hyperactivation of SSU1. (Mean activities±SD in wild-type, in the fzf1Δ mutant and in the FZF1-4 mutant were 1.2±0.1, 0.5±0.2, and 10.1±0.5, respectively.) The magnitude of activation by the dominant allele FZF1-4 was very similar to what was observed previously by a construct carrying multiple copies of wild-type FZF1 (Avram et al., 1999).
Sulphite reductase activity
Sulphite reductase activity was measured in log-phase cells grown in methionine- and cysteine-free medium (SM-met), where enzyme activity is required for growth. Activity in wild-type, 1.36±0.38, was not significantly different than that in the ssu1Δ mutant, 1.47±0.36 (p<0.05), ruling out the possibility that differential sulphite reductase activity might explain the differences in sulphite accumulation. In fact, consumption of sulphite by the enzyme was not observed during the accumulation assay, as more than 95% of the initial sulphite added was recovered as the sum of intracellular and extracellular sulphite at the end of experiment (data not shown).
SSU1 is involved in sulphite efflux
Strains 3090-9d (wild-type), 3090-9d-T4-L1 (ssu1Δ), 3163-1b (FZF1-4), and 3090-9d-MC (multicopy SSU1) were incubated with 0.8, 0.4, 3.0, and 4.0 mM sodium sulphite, respectively, for 5 min to yield a final total intracellular sulphite concentration of 10.5±1.0 nmol/mg (dry wt) cells per strain. Cell viability was not reduced by these concentrations over the course of the assay (data not shown). Sulphite efflux was then determined in the sulphite-loaded cells suspended in buffer by measuring the percentage reduction of intracellular free sulphite over time (Figure 2a). Efflux of free sulphite from cells expressing multicopy SSU1 or the dominant allele, FZF1-4, was significantly faster and greater than from wild-type or from an ssu1Δ mutant, as ≤20% free sulphite remained after 4 min in both 3163-1b and 3090-9d-MC, whereas 40% and 75% remained after 10 min in 3090-9d and 3090-9d-T4-L1, respectively. A similar pattern of reduction in intracellular sulphite was also observed in all strains incubated with the same amount of extracellular sulphite, 1 mM, which yielded different initial intracellular levels (data not shown). Efflux of bound sulphite from wild-type, an ssu1Δ mutant, an FZF1-4 mutant, or cells expressing multicopy SSU1 was not significantly different, suggesting that SSU1 is specifically involved in efflux of free sulphite (Figure 2b).
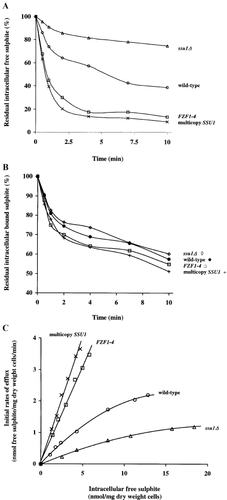
(A) Efflux of free sulphite from 3090-9d (wild-type), 3090-9d-T4-L1 (ssu1Δ), 3163-1b (FZF1-4), and 3090-9d-MC (multicopy SSU1). Cells were loaded for 5 min with varying amounts of sulphite to yield a final total intracellular concentration of 10.5±1.0 nmol/mg (dry wt) cells and an intracellular free sulphite concentration of 4.6±0.3 nmol/mg (dry wt) cells. Sulphite efflux was determined in sulphite-loaded cells suspended in tartrate buffer (pH 3.5) for 10 min by measuring the percentage reduction of intracellular free sulphite. Data points are means of duplicates. Standard deviations were less than 10% of the means. (B) Efflux of bound sulphite from 3090-9d (wild-type), 3090-9d-T4-L1 (ssu1Δ), 3163-1b (FZF1-4) and 3090-9d-MC (multicopy SSU1). Sulphite efflux was determined as described above. Cells were loaded for 5 min with varying amounts of sulphite to yield a final intracellular bound sulphite concentration of 6.1±0.5 nmol/mg (dry wt) cells. Data points are means of duplicates. Standard deviations were less than 10% of the means. (C) Efflux rates of free sulphite from 3090-9d (wild-type), 3090-9d-T4-L1 (ssu1Δ), 3163-1b (FZF1-4) and 3090-9d-MC (multicopy SSU1). Cells were loaded with 0 to 4 mM sodium sulphite for 5 min and initial efflux rates of free sulphite were measured, as described in Materials and methods. Data points are means of duplicates. Standard deviations were less than 10% of the means
Initial efflux rates of free sulphite were determined as a function of intracellular free sulphite from the sulphite-loaded cells (Figure 2c). Initial rates from an FZF1-4 mutant or cells expressing multicopy SSU1 were three- and four-fold higher, respectively, than from wild-type, whereas the rate from an ssu1Δ mutant was three-fold lower than from wild-type, confirming that Ssu1p mediates efflux of free sulphite. Because slow and saturable efflux of free sulphite was observed in the ssu1Δ mutant (at initial intracellular free sulphite concentrations >10 nmol/mg (dry wt) cells, Figure 2c), other low affinity transporter(s) appear to be functional.
Multicopy SSU1 suppresses sensitivity of unrelated sulphite mutants
To determine whether SSU1 plays a role in sulphite detoxification, a number of sulphite-sensitive strains were transformed with multicopy SSU1 (Table 3). Multicopy SSU1 increased the sulphite resistance of wild-type and all sulphite-sensitive strains by a factor of 3- to 8-fold. The grr1 mutant is defective in acetaldehyde production (Xu et al., 1994), and the ssu3 mutant is partially defective in sulphite reductase, but not to the extent that methionine auxotrophy is evident (unpublished data, H. Park, 1998). The met10 mutant is defective and met18 mutant is partially defective in sulphite reductase (Thomas et al., 1992). Suppression of the sulphite sensitivity of these strains by multicopy SSU1 is consistent with Ssu1p playing a significant role in sulphite detoxification.
| Strain | Relevant genotype | Sulphite concentration (mM) | ||||
|---|---|---|---|---|---|---|
| 0; 0.5 | 1 | 1.5 | 2; 3 | 4 | ||
| 3090-9d | Wild-type | + (+) | + (+) | + (+) | + (−) | + (−) |
| 3090-9d-T4-L1 | ssu1Δ | + (+) | + (−) | + (−) | + (−) | ± (−) |
| 3090-9d-T6-L1 | grr1Δ | + (+) | + (−) | + (−) | + (−) | ± (−) |
| 3090-9d-T10-L1 | fzf1Δ | + (+) | + (+) | + (−) | + (−) | + (−) |
| 3089-1d | ssu3 | + (+) | + (−) | + (−) | + (−) | − (−) |
| CC359-OL2 | Wild-type | + (+) | + (+) | + (+) | + (−) | + (−) |
| CC501-2 | met10 | + (+) | + (+) | + (−) | + (−) | + (−) |
| CC363-20B | met18 | + (+) | + (+) | + (±) | + (−) | + (−) |
- +, Normal growth; −, no growth; ±, poor growth as scored after 24 h.
- Values in parentheses indicate the tolerance of strains carrying vector alone (YEplac181) and are preceded by values for the same strains transformed with multicopy SSU1. Transformants were selected on SM-leu. Sulphite sensitivity was determined on SM-met+TA supplemented with 0.1 mM methionine in the presence of up to 8 mM Na2SO3 by replicating cells grown on SM-leu. No strains grew on plates containing ≥5 mM sulphite.
Discussion
Sulphite efflux mediated by Ssu1p is a major detoxification pathway
Metabolic and genetic studies in yeast suggest distinct mechanisms for protection against exogenous sulphite: (a) formation of a non-toxic adduct with acetaldehyde (Stratford et al., 1987; Pilkington and Rose, 1988; Casalone et al., 1992); (b) sulphite consumption by sulphite reductase (Thomas et al, 1992); and (c) sulphite efflux (present study). Because multicopy SSU1 significantly increased the resistance of a number of sulphite-sensitive strains including sulphite reductase mutants, and a mutant defective in acetaldehyde production, sulphite efflux appears to be a major detoxification pathway. This is also supported by the findings that lower sulphite tolerance in SSU1 mutants was not correlated with acetaldehyde production (Xu et al., 1994), or sulphite reductase activity (present study). Because transcription of genes involved in sulphate assimilation, including sulphite reductase, is negatively regulated by methionine and/or S-adenosylmethionine (Thomas and Surdin-Kerjan, 1997), it seems unlikely that sulphite reductase could function independently to detoxify excess sulphite.
Physiologic role of Ssu1p
Ssu1p lacks the nucleotide binding sequence typical of ABC transporters, but resembles the general structure of facilitator/transporter proteins (Balzi and Goffeau, 1994), suggesting that it is a member of the major facilitator superfamily involved in efflux of toxic compounds. While sulphite is a normal metabolite, it is not a natural sulphur source for yeast, nor is it likely that yeast would encounter significant concentrations of sulphite in nature. Thus, the question arises as to whether sulphite is the physiologic substrate for Ssu1p. Strain 3090-9d transformed with multicopy SSU1 was found to have a six-fold increase in selenite resistance relative to the untransformed parent (unpublished data, H. Park, 1999), consistent with a role in selenite efflux as well.
Jelinsky and Samson (1999) recently reported on the global response of the S. cerevisiae genome upon exposure to the alkylating agent methyl methanesulphonate (MMS). Of 6218 ORFs, 5% were induced more than four-fold, while 0.5% showed more than a 10-fold increase. Interestingly, induction of SSU1 and FZF1 increased about 18- and five-fold, respectively, suggesting that SSU1 might be involved in detoxification of the alkylating agent. It is likely that SSU1 is induced via FZF1, rather than directly by MMS or an MMS metabolite, such as methyl sulphonate, as SSU1 and FZF1 were both induced significantly (>two-fold) by other non-sulphur-containing DNA-damaging agents: 1-methyl-3-nitro-1-nitrosoguanidine (MNNG), 1,3-bis[2-chloroethyl]-1-nitrosourea (BCNU), and 4-nitroquinoline N-oxide, but not by gamma rays or tert-butyl hydroperoxide (Jelinsky and Samson, unpublished data, 1999). While no targets of FZF1 other than SSU1 have yet been identified in yeast, its human homologue, WT1 (Fourny, 1997), transcriptionally activates amphiregulin, a renal growth factor (Lee et al., 1999).
It will be of interest to determine what additional roles FZF1 and SSU1 play in yeast metabolism. Both null mutants are viable and neither exhibits striking phenotypes other than sulphite sensitivity, although a particular dominant FZF1 allele conferring sulphite resistance (FZF1-2) was previously shown to cause a 3.5-fold longer lag phase than wild-type following growth in minimal, but not rich medium (Xu et al., 1994). Ssu1p has no yeast homologues, while Fzf1p shares similarity to a subset of other yeast C2H2-type zinc finger transcription factors.
Acknowledgements
We thank Mike Penner for valuable discussions and Gary Merrill for critically reviewing the manuscript.




