Localization and controls of aromatase in the quail spinal cord
Abstract
In adult male and female Japanese quail, aromatase-immunoreactive cells were identified in the spinal dorsal horns from the upper cervical segments to the lower caudal area. These immunoreactive cells are located mostly in laminae I–III, with additional sparse cells being present in the medial part of lamina V and, at the cervical level exclusively, in lamina X around the central canal. Radioenzyme assays based on the measurement of tritiated water release confirmed the presence of substantial levels of aromatase activity throughout the rostrocaudal extent of the spinal cord. Contrary to what is observed in the brain, this enzyme activity and the number of aromatase-immunoreactive cells in five representative segments of the spinal cord are not different in sexually mature males or females and are not influenced in males by castration with or without testosterone treatment. The aromatase activity and the numbers of aromatase-immunoreactive cells per section are higher at the brachial and thoracic levels than in the cervical and lumbar segments. These experiments demonstrate for the first time the presence of local estrogen production in the spinal cord of a higher vertebrate. This production was localized in the sensory fields of the dorsal horn, where estrogen receptors have been identified previously in several avian and mammalian species, suggesting an implication of aromatase in the modulation of sensory (particularly nociceptive) processes. J. Comp. Neurol. 423:552–564, 2000. © 2000 Wiley-Liss, Inc.
In the brain, estrogens control many physiologic and behavioral processes that are or are not related to reproduction (McEwen et al., 1997) through a combination of genomic and nongenomic mechanisms (McEwen, 1994; Joëls, 1997; Moss et al., 1997). Estrogens are produced by the enzymatic transformation of aromatizable androgens, such as testosterone (T), in a variety of tissues (e.g., gonads, placenta, fat, bone, etc.), including the brain (Naftolin and MacLusky, 1984; Sasano and Harada, 1998). This transformation is catalyzed by aromatase (or estrogen synthase), a P450 enzyme encoded by the gene CYP19 that has been cloned recently in several species (Simpson et al., 1994).
Aromatase activity (AA) was identified in the brain in the early 1970s (Naftolin et al., 1975). It is increased by a synergistic action of androgens (enzyme substrate) and estrogens (enzyme product) and is located primarily in the limbic system (Roselli et al., 1997; Balthazart and Ball, 1998). Groups of aromatase-containing cells often are located adjacent to areas that contain estrogen receptors (Balthazart et al., 1991).
Estrogen-concentrating cells have been identified in the dorsal horns of the spinal cord in mammals (see, e.g., Stumpf, 1970) and birds (Martinez-Vargas et al., 1976), in which estrogens presumably modulate sensory perception. More recent studies have confirmed the presence of specific estrogen receptors at this location by using in situ hybridization or immunocytochemistry (visualization of the corresponding mRNA or of the protein, respectively; Simerly et al., 1990; Amandusson et al., 1995; Williams and Papka, 1996; Shurghrue et al., 1997; VanderHorst et al., 1997; unpublished results from our laboratory also demonstrate the presence of estrogen receptor α in the quail spinal dorsal horns).
Estrogens potentiate lordosis behavior in female rats by enlarging the sensory field of the pudendal nerve, which contributes to an enhanced perception of the male-derived, lordosis-inducing stimulus (Komisaruk et al., 1972; Kow and Pfaff, 1973). In canaries, estrogens increase tactile sensitivity at the level of the ventral incubation patch (Hinde and Steel, 1964).
Moreover, estrogens have been reported to modulate nociception. Some hyperalgesic effects of estrogens have been described (e.g., effects resulting from interactions with catecholamines or dehydroepiandrosterone; Levine and Taiwo, 1989; Frye and Duncan, 1994; Kayser et al., 1996), although most of the literature on pain and steroids suggests that estrogens are analgesic (see, e.g., Drury and Gold, 1978; Ryan and Maier, 1988; McCarthy et al., 1990; Negus and Mello, 1999). In fact, estrogens enhance the analgesia produced by the direct action on the spinal dorsal horns of compounds such as opioids (Dwson-Basoa and Gintzler, 1998; Amandusson et al., 1999), γ-aminobutyric acid (GABA; McCarthy et al., 1991), progesterone and its metabolites (Frye and Duncan, 1994), or norepinephrine acting through the α2 noradrenergic receptors (Liu and Gintzler, 1999). Accordingly, the rise in estrogen levels during the rat estrus cycle has been reported to increase pain thresholds, and these changes are abolished by ovariectomy and are mimicked by estradiol injections (Ryan and Maier, 1988; Martinez-Gomez et al., 1994). The high estrogen (and progesterone) levels associated with pregnancy also are associated with elevated pain thresholds (Gintzler, 1980; Dawson-Basoa and Gintzler, 1998). Thus, estrogens modulate sensory perception in a complex, behaviorally relevant manner at least in part by actions at the spinal level.
Estrogens acting on spinal perception are thought commonly to be of gonadal origin. Although AA has been identified in the spinal cord of a few fish species (Pasmanik and Callard, 1985; Callard et al., 1988), no activity could be detected in the spinal cord of higher vertebrates, particularly in mammals (Hauser et al., 1987; MacLusky et al., 1987). Because brain aromatase often is associated closely with cells that contain estrogen receptors (Balthazart et al., 1991; Dellovade et al., 1995), and because we recently observed a group of aromatase-immunoreactive (ARO-ir) cells in the quail myelencephalon (at the level of the descending nucleus of the trigeminal nerve; unpublished observation) that seems to extend farther caudally, we investigated the presence of aromatase in the quail spinal cord, a species in which brain aromatase is particularly active and can be detected reliably by using immunocytochemistry (Balthazart and Ball, 1998; Balthazart et al., 1998a,b). Immunocytochemical and radioenzyme assays were used in parallel to visualize the aromatase protein and to measure its enzymatic activity. Possible gender differences and controls by using steroids also were investigated.
MATERIALS AND METHODS
Subjects
The studies described in this paper were conducted on male (n = 40 birds) and female (n = 16 birds) Japanese quail (Coturnix japonica) that were obtained from a local breeder (C. Dujardin, Liernu, Belgium) at the age of approximately 2 weeks. Quail were maintained in heterosexual groups in brooding cages until they reached the age of 4 weeks. They were then isolated in individual cages. Food and water were always provided ad libitum, and birds were kept under a long-day photoperiod (16 hours light, 8 hours dark). All experimental procedures were in agreement with the Belgian laws on the protection and welfare of animals and the protection of experimental animals and followed the “International Guiding Principles for Biomedical Research Involving Animals” published by the Council for International Organizations of Medical Sciences. The protocols were approved by the Supervisor of Animal Care for the University of Liège.
Experiment 1: Cytoarchitectonic organization of the spinal gray matter and distribution of ARO-ir structures
These studies were performed on a total of four males and four females that were sexually mature at the time they were killed, as indicated by the presence of enlarged cloacal glands in males and daily egg laying in females. All subjects were injected first with heparin (20 mg/ml; H-7005; Sigma, St. Louis, MO) and then deeply anesthetized with an intramuscular injection of Hypnodil™ (50 mg/kg body weight; Janssen Pharmaceutica, Beerse, Belgium). They were perfused through the heart with saline solution (0.9%; 0.15 M) followed by ≈500 ml of fixative (4% paraformaldehyde and 0.1% glutaraldehyde in 0.1 M phosphate buffer, pH 7.2). The spinal cord was dissected out of the spine immediately and cut into five segments based on anatomic landmarks corresponding to the cervical (1–11), brachial (1–4), thoracic (1–5), lumbar (1–7), and sacral (sacral 1 to the pygostil) vertebrae. These dissections were based on our unpublished analysis of the gross anatomy of the spinal column and spinal cord of the Japanese quail that were shown to be very similar to the anatomy described previously for the pigeon and the domestic fowl (Baumel and Witmer, 1993; Dubbeldam, 1993). These samples were placed overnight in a 20% sucrose solution in 0.1 M phosphate buffer, frozen on powdered dry ice, and stored in a freezer at −76°C until they were used.
All spinal cord segments were cut on a cryostat at 40 μm thickness. The samples from two males and two females were cut in the transverse plane, those from the last two males and two females were cut sagittally. All sections were collected in 0.01 M phosphate buffer containing 0.125 M NaCl, pH 7.2 (PBS). The transverse sections from the two males and two females were stained alternatively by using immunocytochemistry for aromatase and by toluidine blue to visualize Nissl bodies. All sagittal sections were stained by using immunocytochemistry for aromatase.
Transverse spinal sections to be stained for Nissl bodies first were incubated for 45 seconds in Walpole acetate buffer, pH 4, containing toluidine blue (2 g/liter) and then rinsed twice for 15 minutes in Walpole buffer. The toluidine blue coloration was fixed by a 2.5-minute incubation in molybdate solution (50 g/liter). Sections were then dehydrated, mounted in Eukitt™ medium (O. Kinder GmbH, Freiburg, Germany), and coverslipped.
For immunocytochemistry, sections were pretreated for 20 minutes at room temperature with 0.6% hydrogen peroxide in PBS and then washed three times in PBS containing 0.1% Triton X-100 (PBST). Sections were incubated overnight at 4°C with the primary polyclonal, affinity-purified antibody raised in rabbit against quail recombinant aromatase diluted 1:500 in PBST (for a description of the preparation of this antibody and its validation for use in quail, see Foidart et al., 1995). The specificity of this antibody has been demonstrated by Western blot analysis, Ouchterlony double-diffusion tests, and liquid preadsorbtion (Foidart et al., 1995). Previous studies also have confirmed that immunocytochemical staining of aromatase with this antibody detects immunoreactive cells specifically and exclusively in the parts of the quail brain that are known to contain AA (Foidart et al., 1995). The next day, sections were rinsed three times in PBST and incubated for 90 minutes at room temperature in secondary antibody (goat anti-rabbit; provided by Professor F. Vandesande, Catholic University of Leuven, Leuven, Belgium) at 1:400 dilution in PBST. Sections were rinsed again three times in PBST and then reacted for 90 minutes at room temperature with the peroxidase-antiperoxidase complex (Dako A/S, Copenhagen, Denmark) at a dilution of 1:1,000 in PBST. After three more washes in PBST, the bound peroxidase was revealed by immersing sections for 10 minutes in a solution of 3,3′diaminobenzidine tetrahydrochloride (D-5637; Sigma; 20 mg in 50 ml PBST containing 20 μl of hydrogen peroxide at 30%). Finally, sections were mounted in a gelatin medium and coverslipped.
Sections that were stained by using the Nissl method were observed, and the different laminae were identified based on the shape, cytoarchitectonic organization, density of Nissl staining, and relative position with respect to the gray matter surface with the help of the cytoarchitectonic study of the domestic fowl spinal cord (Martin and Brinkman, 1970; Brinkman and Martin, 1973). This species is closely related to the Japanese quail and, thus, is likely to present a similar cytoarchitectonic organization.
All sections were examined with a Leica DMBR microscope (Leica, Wetzlar, Germany). Photomicrographs of representative sections were obtained through an Olympus C-35AD-4 camera (Olympus, Tokyo, Japan) equipped with an automatic exposure-control unit. The localization of aromatase-immunoreactive structures was identified in the sagittal sections and then confirmed in transverse sections based on the laminar organization of the gray matter in adjacent Nissl-stained sections.
Experiment 2: Effects of T and sex differences on ARO-ir cells
One week after their arrival in the laboratory, 12 males were castrated under total anesthesia (Hypnodil; Janssen Pharmaceutica; 15 mg/kg body weight). The two testes were removed in males through a unilateral incision on the left side just behind the last rib (Balthazart and Schumacher, 1984; Balthazart et al., 1998a). Six females and six other males were submitted to a sham operation (anesthesia and exposure of gonads).
Two weeks after castration or sham operation, six of the castrated males were submitted to a treatment with exogenous T by the implantation under the skin in the neck region of two 20-mm-long Silastic™ capsules (Silclear Tubing; catalog no. 20301502431; Degania Silicone, Israel; 1.57 mm inner diameter, 2.41 mm outer diameter) filled with crystalline T (86500; Fluka, Buchs, Switzerland). This dose of T was shown previously to produce plasma levels in castrated male and female quail similar to those observed in sexually mature males (Schumacher and Balthazart, 1986; Balthazart et al., 1987), which was confirmed in this experiment by the restoration of the cloacal gland, an androgen-dependent structure the size of which is correlated directly with plasma T levels (Sachs, 1967; Delville et al., 1985). The other six castrated males, sham-operated intact males, and females received empty capsules of the same size. The capsules were closed with Silastic glue (Silastic brand medical adhesive, silicone type A no. 891; Dow Corning, Midland, MI). Before implantation, capsules were washed and incubated at 40°C for 24 hours in isotonic saline solution (0.9%) to initiate steroid diffusion through the tube walls and to avoid an initial surge of steroids at implantation.
This combination of the sex (male/female), surgery (castrated/sham operated), and type of Silastic implants (filled with T or empty) defined four experimental groups: intact males (sham-operated males with empty implants; MI; n = 6 birds), intact females (sham-operated females with empty implants; FI; n = 6 birds), castrated males (castrated males with empty implants; CX; n = 6 birds), and castrated T-treated males (castrated males with implants filled with T; CX+T; n = 6 birds). Immediately before and at the end of the treatment with T, birds were weighed to the nearest gram, and the size of their cloacal gland was measured with a caliper.
The Silastic capsules were left in place for 3 weeks. It has been shown previously that this is more than sufficient time to restore in castrated male quail the morphologic, physiologic, and behavioral features (including fully stimulated AA in the preoptic area of the brain) that are observed normally in gonadally intact, sexually mature males (Balthazart et al., 1990). All birds were then perfused with 4% paraformaldehyde and 0.1% glutaraldehyde in 0.1 M phosphate buffer, pH 7.2, as described for Experiment 1.
The spinal cord was dissected out of the spine immediately and frozen on dry ice after being cryoprotected overnight in sucrose. Sections through the rostral part of each of the five anatomic segments described for the cytoarchitectonic study were cut on a cryostat at 40 μm thickness in the transverse plane in all subjects (in the cervical, brachial, thoracic, lumbar, and sacral segments). All sections were collected in PBS and stained by using immunocytochemistry for aromatase, as described above.
All sections were examined in an Olympus BH2-BS microscope and were drawn schematically with the help of a camera lucida attached to the microscope. For each level in each bird, the number of ARO-ir cells, as defined by the presence of a brown-stained cytoplasm (clearly darker than surrounding background) and a clear, unstained nucleus, was counted separately in four sections that were 80 μm apart. This criteria (the presence of a nucleus) associated with the large thickness of the sections (40 μm) compared with the cell size (10–20 μm) minimized the potential overestimation of cell numbers due to double counting. An individual mean (± S.E.) of positive cells per section was calculated for each level in each subject, and these means were analyzed by a mixed two-way analysis of variance (ANOVA) with the five rostrocaudal levels of the spinal cord used as the repeated factor and the experimental groups used as the independent factor.
Experiment 3: Distribution, sex differences, and effects of T on spinal AA
One final experiment was carried out to determine whether the aromatase-immunoreactive structures correspond to enzymatically active material and to determine whether the activity of this enzyme is affected by the sex (by comparing adult, sexually mature males and females) and the endocrine condition of the subjects (by comparing castrated males with or without treatment with T). Intact males (MI; n = 6 birds) and intact females (FI; n = 6 birds) were bought from the local breeder at the age of about 7 weeks and were maintained until they were killed in the laboratory in isolation cages, as described above. Castrated males (CX; n = 6 birds) and castrated males that were treated with exogenous T (CX+T; n = 6 birds) were bought from the local breeder at about 3 weeks of age, castrated, and treated with T as described for Experiment 2.
All birds were killed by decapitation at the age of 9 weeks. The CX+T group at that time had been treated with T for about 3 weeks. Spinal cords were dissected out of the spine immediately, cut in to four segments, and frozen on powdered dry ice. From the most rostral level to the most caudal level, these four segments corresponded to the cervical (cervical 1– 11), brachial (brachial 1–4), thoracic (thoracic 1–5) and lumbosacral (lumbar 1 to the pygostil) parts of the spinal cord. For these enzyme assays, the lumbar and sacral segments were pooled, as described above for the cytoarchitectonic and immunocytochemical studies, because the small weight of the sacral part would have prevented an accurate measurement of enzymatic activity. All segments were then stored in the freezer at −76°C until they were assayed for AA.
AA was quantified separately for each subject at each rostrocaudal level (cervical, brachial, thoracic, and lumbosacral) by measuring the release of tritiated water during aromatization of [1β-3H]-androstenedione, as described by Roselli and Resko (1991), with minor modifications. The assay and its validation for use in the quail brain have been described previously (Baillien and Balthazart, 1997). Briefly, spinal cord samples were homogenized in a buffer containing 150 mM KCl, 1 mM ethylenediamine tetraacetic acid, and 10 mM Tris-HCl, pH 7.2. Duplicate aliquots (100 μl) of homogenate containing ± 4 mg wet weight were added to 50 μl of 100 nM [1β-3H]-androstenedione (24.3 Ci/mmol). To initiate the assay, 50 μl of nicotinamide adenine dinucleotide phosphate were added to reach the final concentration of 1.2 mM. All of these steps were conducted at 4°C in 1.5 ml Eppendorf tubes that were capped quickly and incubated for 15 minutes at 37°C. The reaction was stopped by cooling the samples in an ice bath and adding 0.4 ml ice-cold 10% trichloroacetic acid containing 2% activated charcoal. After centrifugation at ×1,200 g for 15 minutes, supernatants were applied to small columns made of Pasteur pipettes plugged with glass beads and filled (3 cm high) with a Dowex cation exchange resin (AG 50W-X4) 100–200 mesh (BioRad, Richmond, CA). The columns were then eluted with 3.0 ml × 0.6 ml distilled water. Effluents were collected in scintillation vials, and, finally, 10 ml Ecoscint A (National Diagnostics, Atlanta, GA) were added. Vials were counted for 3 minutes on a Packard Tri-Carb 1600 TR liquid scintillation analyzer.
Within each assay, blanks were obtained by processing selected samples in the presence of an excess (final concentration, ≈ 40 μM) of the potent and specific aromatase inhibitor, R76713 (Racemic vorozole™; Janssen Pharmaceutica). These blank values never exceeded 300 dpm, whereas active control samples had radioactivities equal or superior to 1,000 dpm. The protein content of the homogenates was determined by a micromodification of the Bradford method (Bradford, 1976), using commercial Coomassie Plus Protein Assay reagent (Pierce, Rockford, IL). Enzyme activity was expressed in fmol/mg protein.hour after correction of the counts for quenching, recovery, blank values were obtained in the presence of the aromatase inhibitor, and the percentage of tritium in the β position in the substrate. The means of these duplicate determinations were analyzed by a mixed, two-way ANOVA with the four rostrocaudal levels of the spinal cord used as the repeated factor and the experimental groups used as the independent factor.
It clearly is established that the amount of tritiated water released during aromatization of [1β-3H]-androstenedione is equal in molar concentration to the amount of estrone produced. The kinetic characteristics of aromatase, as measured by using the tritiated-water method, also are similar to the characteristics measured during a product-formation assay (Roselli and Resko, 1991; Baillien and Balthazart, 1997). The fact that the production of tritiated water was abolished by the addition of a specific aromatase inhibitor further confirms that the enzymatic activity measured in this assay corresponds to the aromatization of androstenedione into estrone.
RESULTS
Experiment 1: Cytoarchitectonic organization of the spinal gray matter and distribution of ARO-ir structures
Cytoarchitectonic organization.
The analysis of transverse sections stained by toluidine blue and their comparison with previous studies on the closely related domestic fowl (Martin and Brinkman, 1970; Brinkman and Martin, 1973) allowed us to identify at each of the five rostrocaudal levels (cervical, brachial, thoracic, lumbar, and sacrocaudal) and the ten subdivisions (laminae) that have been described in the organization of the spinal gray matter in many vertebrate species. These subdivisions were found to correspond closely to those described in the domestic fowl and, thus, were named laminae I–X like in the fowl. The most prominent features of these laminae are illustrated at the five distinct rostrocaudal levels in Figure 1 and are described below.
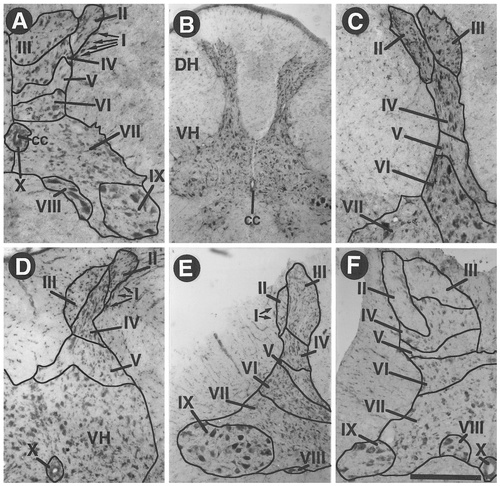
Photomicrographs of Nissl-stained, 40 μm-thick sections at different levels of the spinal cord illustrating the divisions of the gray matter in ten distinct laminae. Each photomicrograph illustrates a specific rostrocaudal level: cervical (A), brachial (B), brachial at higher magnification (C), thoracic (D), lumbar (E), and sacral (F). The gray matter and different laminae have been outlined, and laminae are numbered I–X except when a given lamina cannot be distinguished at a given level. Laminae of the ventral horn could not be distinguished at the thoracic level (D). cc, Central canal; DH, dorsal horn, VH, ventral horn. Scale bar = 200 μm in A,C,D,F; 400 μm in B,E.
The most remarkable feature of this laminar organization at all levels concerned laminae I–III located in the dorsal horns. In quail, as in fowl and contrary to what has been described previously in mammals (Rexed, 1954), laminae II and III face one another at the top of the dorsal horn, and the line separating these two divisions runs almost along the dorsoventral axis. This line is materialized by a thin invagination of the white matter at the dorsal edge of the gray matter. These two laminae are comprised of the same kind of small (10–15 μm), ovoid perikarya.
A line of elongated, spindle-shaped cells separates the lateral border of lamina II from the white matter and entirely caps the dorsolateral edge of lamina II. These cells that form lamina I, as in fowl, are oriented tangential to the border of lamina II and reach a size of ≈ 25–30 μm. This single layer of cells is visible clearly at the cervical and thoracic levels and, to a lesser extent, at the lumbar level (Fig. 1A,D,E). The layer of cells that constitutes lamina I often is discontinuous; therefore, lamina II sometimes is difficult to locate, especially in the sections from the sacral level. All other laminae are ventral to laminae I–III and are organized as described previously for domestic fowl (Martin and Brinkman, 1970; Brinkman and Martin, 1973). Because this study was focused on ARO-ir cells that are located almost exclusively in the dorsal horn (see below), the description of the most ventral laminae in quail is not reiterated here. These laminae are indicated clearly in Figure 1, and more details of their organization can be found in the studies by Martin and Brinkman (1970) and Brinkman and Martin (1973).
Localization of the ARO-ir structures.
All sections that were treated with the antibody raised against quail recombinant aromatase revealed the presence of numerous ARO-ir structures (Figs. 2, 3). In sagittal sections, ARO-ir perikarya and fibers were observed in the dorsal part of the spinal cord without discontinuity from its rostral tip to its caudal end (Fig. 2). At all levels, ARO-ir structures were confined exclusively to the dorsal horn. The ventral horn also could be differentiated clearly from the white matter in these sections; however, it did not contain any immunoreactive structures (Figs. 2A, 3A).
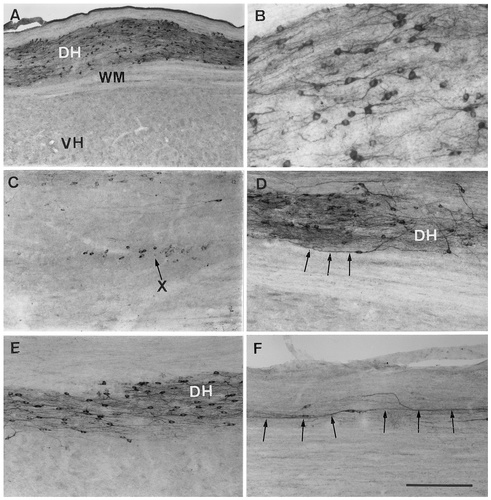
A–F: Photomicrographs of 40 μm-thick sagittal sections through the quail spinal cord illustrating the distribution of aromatase-immunoreactive (ARO-ir) cells and the orientation of their processes. B is a higher magnification of A showing cellular processes with more detail. C shows ARO-ir cells located in lamina X in addition to the cells identified in the dorsal horn. Arrows in D and F indicate long cellular processes that run along the longitudinal axis of the spinal cord. Sections were collected at the thoracic level (A,B,D) or at the cervical level (C,E,F). DH, dorsal horn; VH, ventral horn; WM, white matter; X, lamina X. Scale bar = 400 μm in A; 200 μm in C–F; 100 μm in B.
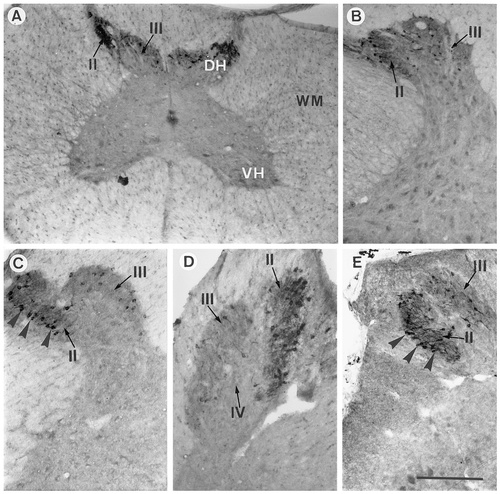
Photomicrographs of 40 μm-thick transverse sections through the quail spinal cord that were stained by immunocytochemistry for aromatase. A: Section at the cervical level photographed at low magnification to illustrate the exclusive localization of ARO-ir material in the dorsal horns. B–E: Hemisection at the brachial, thoracic, lumbar, and sacral levels, respectively, showing the presence of ARO-ir cells in laminae II and III. The arrowheads in C and E point to ARO-ir cells located in lamina I. II–IV, laminae II–IV; DH, dorsal horn; VH, ventral horn; WM, white matter. Scale bar = 125 μm in A,B; 200 μm in C–E.
At higher magnification, the ARO-ir structures displayed a neuronal morphology that was characterized by immunopositive perikarya with clear nuclei surrounded by immunopositive cell processes (Fig. 2B). Some of these processes were very short, whereas others (axons and/or dendrites) ran for several hundreds of microns (Fig. 2D–F). Most of these processes were oriented preferentially along the longitudinal axis of the spinal structure. In sagittal sections that were very close to the medial plane, another cluster of ARO-ir cells could be observed around the central canal in lamina X (Fig. 2C). These cells were seen only at the cervical level. At all rostrocaudal levels, scarce, immunoreactive perikarya also were seen occasionally in lamina V.
Transverse sections were used subsequently to analyze in more detail the anatomic localization of the ARO-ir structures and to compare this localization with the cytoarchitectonic organization of the gray matter, as identified in Nissl-stained sections. Because sagittal sections had not revealed a segmental organization of the ARO-ir structures and suggested a relative homogeneity along the rostrocaudal axis, investigations in the transverse plane were limited to the study of representative sections selected in the rostral segments of five levels in the rostrocaudal axis that represent the main anatomic specializations of the spinal cord.
ARO-ir cells and fibers, as also observed in sagittal sections, were found almost exclusively in the dorsal horns at all levels of the spinal cord (Fig. 3). In transverse sections, it became clear that these cells are located mostly in lamina II and, to a lesser extent, in lamina III. ARO-ir cells were particularly numerous at the dorsolateral edge of lamina II, where this lamina meets lamina I. Some of the immunopositive cells located at this level were ovoid, and their longest axis was parallel, running along the external edge of dorsal horn, as described previously for the neurons of lamina I that were stained by toluidine blue. Thus, based on their location, shape, and orientation, it can be assumed that a significant fraction of these most lateral cells actually are part of lamina I (see Fig. 3C,E, cells indicated by arrows). In addition to these dense populations of ARO-ir cells located in laminae II and III, a few immunopositive cells also were found at the medial junction between the right and left part of the gray matter within the most central part of the lamina V. These cells were observed at low densities all along the spinal cord from the cervical segments to the sacral segments; however, they were not observed in all transverse sections due to their very low density. In addition, a small number of ARO-ir cells (one to three per section) also were present in the ventral part of the lamina X around the central canal. However, this cell group is present only in cervical segments (cervical 1–11), contrary to the other groups described above that are found throughout the full extent of the spinal cord.
When they were observed in transverse sections, ARO-ir cells often were found to be bipolar or, more rarely, tripolar. Bipolar cells also often were elongated in the medioventral-to-dorsolateral axis. Long processes were observed very rarely, contrary to what could be seen in sagittal sections.
In the current study, this staining was shown to be specific, as reported previously for quail brain aromatase (Foidart et al., 1995). Sections that were treated with primary antibody that was preincubated with an excess of pure antigen (recombinant quail aromatase) showed no immunoreactive material, whereas adjacent sections that were treated with the antibody alone contained conspicuously labeled structures.
Experiment 2: Changes in aromatase immunoreactivity as a function of sex or T plasma levels
ARO-ir cells were identified reliably in all sections obtained at each level from the four experimental groups (Fig. 4). In the other groups (CX, CX+T, and FI), as observed previously in sexually mature males (MI), these cells were located in the dorsal horns of the gray matter. The largest numbers of immunopositive perikarya were present in laminae I–II and III.
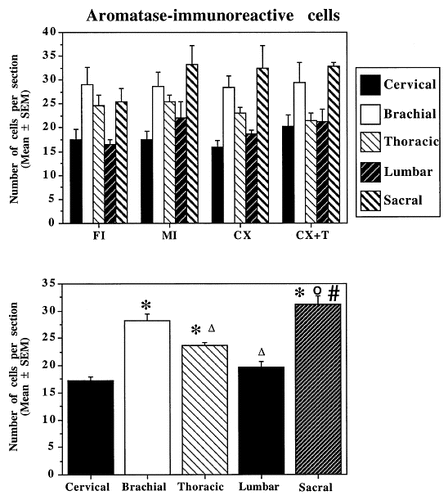
Top: Bar graph illustrating the mean number of ARO-ir cells per section at five rostrocaudal levels in the spinal cord of the four experimental groups. Bottom: Bar graph illustrating the numbers of ARO-ir cells per section counted in the cervical, brachial, thoracic, lumbar, and sacral segments in the entire population of subjects irrespective of their experimental group. MI, intact males; FI, intact females; CX, castrated males; CX+T, testosterone-treated, castrated males. In B, the symbols above the bars indicate the results of post-hoc tests comparing the numbers of cells at different levels as follows: asterisk, P < 0.05 compared with the cervical level; Δ, P < 0.05 compared with the brachial level; number sign, P < 0.05 compared with the thoracic level; and circle, P < 0.05 compared with the lumbar level.
The analysis with a mixed-design, two-way ANOVA (with group as the independent factor and level as the repeated factor) of the mean number of ARO-ir cells per section counted at each level in the four groups of subjects revealed no sex difference and no effect of the T treatment (F3,15 = 0.570; P = 0.644) but a significant effect of the anatomic position of the sample (F4,60 = 25.788; P < 0.001). There was no interaction between group and position (F12,60 = 0.939; P = 0.516; Fig. 4A).
Because no group difference and no interaction between group and level was observed, cell counts also were reanalyzed as a function only of the position in the spinal cord without taking into account the experimental group (Fig. 4B). These mean values at the five rostrocaudal levels were compared two-by-two with post-hoc Student's tests adapted for multiple comparisons and using the residual mean square of the ANOVA as a basis of comparison. These tests indicated the origin of the overall effect in the ANOVA: ARO-ir cells were significantly more numerous in the brachial, thoracic, and sacral segments than at other levels (for details of the significant differences, see Fig. 4B).
Experiment 3: AA in the spinal cord
A substantial level of AA, as expected from the results of the immunocytochemical studies, was detected in the four different anatomic levels of the spinal cord that were studied. This enzymatic activity was present and was measurable in the four experimental groups with mean values ranging between 250 fmol/mg protein.hour and 450 fmol/mg protein.hour (Fig. 5A). The analysis of these data by a mixed-design, two-way ANOVA (sex as the independent factor and levels as the repeated factor) indicated an absence of sex difference (F1,9 = 0.055; P = 0.821) but a significant effect of the anatomic position of the sample (F3,27 = 3.550; P = 0.028). There was no interaction between sex and position (F3,27 = 0.616; P = 0.611; Fig. 5A).
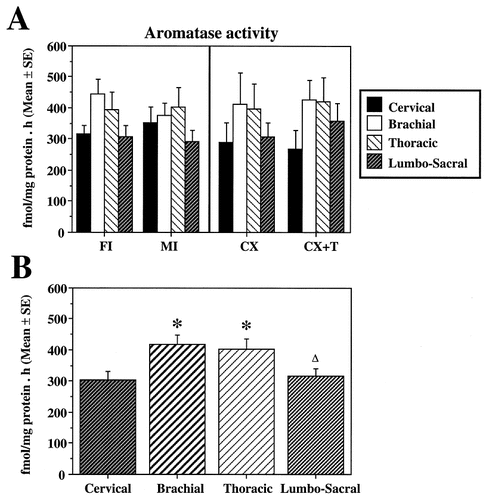
A: Bar graph illustrating the aromatase activity measured at four rostrocaudal levels in the spinal cord of the four experimental groups. B: Mean levels of aromatase activity measured at four rostrocaudal levels in the entire population of subjects irrespective of their experimental group. FI, intact females; MI, intact males; CX, castrated males; CX+T, testosterone-treated, castrated males. In B, the symbols above the bars indicate the results of post-hoc tests comparing the enzymatic activity at different levels as follows: asterisk, P < 0.05 compared with the cervical level; Δ, P < 0.05 compared with the brachial level.
Similarly, analysis by using the same procedure with data concerning the CX and CX+T groups revealed no effect of the T treatment (F1,10 = 0.043; P = 0.840), a significant effect of the anatomic position of the sample (F3,30 = 7.461; P < 0.001), and no interaction between treatment and position (F3,30 = 0.372; P = 0.773; Fig. 5A). An additional analysis including the four groups of birds also revealed no group difference and no interaction between groups and positions but a very significant effect of the position of the sample in the rostrocaudal axis (respectively, F3,19 = 0.029, P = 0.993; F9,57 = 0.669, P = 0.7329; and F3,57 = 9.537, P < 0.0001).
Because no group differences and no interactions between groups and levels were observed, data also were reanalyzed as a function only of the position in the spinal cord without taking into account the experimental groups (Fig. 5B). These means at the four rostrocaudal levels were compared two-by-two with post-hoc Student's tests adapted for multiple comparisons and by using the residual mean square of the ANOVA as a basis of comparison. These tests indicated the origin of the overall position effect in the ANOVA: AA was significantly higher in the brachial and thoracic segments compared with the cervical segment, and it also was significantly higher in the brachial segment compared with the lumbosacral segment (P < 0.05 in each case; see Fig. 5B).
DISCUSSION
This study identified for the first time in a higher vertebrate the widespread distribution of aromatase in the spinal cord. ARO-ir perikarya were observed in the dorsal spinal horns (primarily ion laminae I–III) from the cervical level to the sacral level. Radioenzyme assays confirmed that this ARO-ir material corresponds to enzymatically active protein that produces equimolar concentrations of estrogen and tritiated water by aromatization of tritiated androstenedione. Substantial levels of AA were detected throughout the spinal cord. ARO-ir cell numbers and aromatase activity were not affected by sex or by changes in plasma T levels, contrary to what has been reported for the brain. These data demonstrate that estrogens are produced in the spinal dorsal horns in the vicinity of ERs. Laminae I and II represent primary nociceptive fields; therefore, locally produced estrogens may modulate pain perception at the level of primary afferents from the dorsal root ganglia.
Comparisons with other species and anatomic organization
To our knowledge, there have been four studies that investigated the presence of AA in the spinal cord. Some enzyme activity was identified in three fish species (goldfish, toadfish, and Tilapia; Pasmanik and Callard, 1985; Callard et al., 1988); however, no measurable level was detected in fetal mouse spinal cultures (Hauser et al., 1987) or in homogenates of adult rats spinal cord (MacLusky et al., 1987). ARO-ir cells and fibers have been observed in sensory systems and in their primary afferents of the embryonic rat brain, including the sensory nucleus of the trigeminal nerve (Horvath and Wikler, 1998). This immunoreactive material apparently extends in the rostral spinal cord. Whether it is present in the entire spinal cord and in adults has not been investigated.
In quail, large populations of ARO-ir cells are found in the dorsal spinal horns, and they correspond to enzymatically active protein. Why AA was not detected in previous studies on rodents remains unclear but probably relates to technical limitations rather than to species differences. The spinal cord indeed is well conserved during evolution (Butler and Hodos, 1996), and recent studies suggest the presence of immunoreactive aromatase in the rostral part of the embryonic rat spinal cord (Horvath and Wikler, 1998).
AA measured in the quail spinal cord is substantially lower (5–10 times) than in the most active brain sites, such as the medial preoptic area, but it is in the same range or higher than the values obtained for other brain regions by using the same technique (Balthazart et al., 1998b). Therefore, spinal aromatase produces physiologically relevant levels of estrogens.
There is a slight rostrocaudal variation in the AA levels measured throughout the spinal cord: Enzymatic activities are higher at the brachial and thoracic levels than at the cervical and lumbosacral levels. This variation is parallel to the changes in ARO-ir cell numbers observed from the cervical level to the lumbar level. It may appear to be surprising that large numbers of ARO-ir cells were detected by using immunocytochemistry in the sacral segment of the spinal cord, whereas low enzymatic activity was measured in the sample corresponding to the pooled lumbar and sacral levels. This apparent discrepancy presumably is due to the fact that the sacral segment is much smaller than the lumbar segment. Consequently, the enzyme activity present at the sacral level is diluted during the assays by the large volume of lumbar tissue that contains lower levels of AA. We pooled these samples because we thought that enzyme activity could not be measured in the sacral segment due to its very small size. In future studies, it may be worth reconsidering this decision given that many ARO-ir cells and presumably a high level of AA are present at this level.
Irrespective of these minor variations in enzyme distribution along the rostrocaudal axis of the spinal cord, it is interesting to note that ARO-ir cells and AA are present throughout this very long structure, which measures approximately 12 cm in the adult quail. We cannot exclude the presence of localized variations in aromatase expression that would not have been detected by using our systematic screening of the five representative levels; however, it remains clear that the local production of estrogens is a constant feature of the dorsal horns, the physiologic significance of which should be investigated. It is difficult at present to determine whether changes in AA and aromatase immunoreactivity along the rostrocaudal extent of the spinal cord simply reflect changes in the size of this structure at different levels or reflect specializations with a precise functional significance. Indeed, it may be functionally important to modulate the sensory afferent inputs with estrogens in a region-specific manner (see Functional implications, below).
Controls of spinal aromatase
In the quail preoptic area, T and its metabolite estradiol (E2) increase dramatically AA (300–500%), the concentration of aromatase mRNA, and the number of ARO-ir cells (Panzica et al., 1996; Balthazart and Ball, 1998. Preoptic AA is controlled mostly by steroid-induced changes in the transcription of the CYP19 gene (Balthazart and Ball, 1998). In contrast, castration or treatment of male quail with T (which also increases local E2) affected neither the number of ARO-ir cells in the different segments that were studied nor the activity of the enzyme measured at four anatomically defined levels.
Although steroids regulate aromatase in the brain of several species, some exceptions have been reported. In the rat cortical amygdala and in the zebra finch telencephalon, T does not increase AA, whereas the enzyme located in the preoptic area of these species shows a normal steroid-induced up-regulation (Roselli et al., 1985; Vockel et al., 1990). Similarly, in human, different effectors modulate AA in different tissues (ovary, uterus, breast, adipose tissue; Means et al., 1991). The recent analysis of the molecular controls of CYP19 expression identifies mechanisms that may mediate this differential regulation (Bulun and Simpson, 1994; Simpson et al., 1994). The CYP19 gene is comprised of ten exons, including a first tissue-specific, untranslated exon that is excised by alternative splicing. This first exon contains a variety of consensus sequences that bind control factors that regulate aromatase transcription in a tissue-specific manner. The current work suggests that steroids such as T or E2 do not play a major role in the control of spinal aromatase, contrary to what is observed generally in the brain. We speculated previously that, in the brain, aromatase transcription is controlled in part by neurotransmitters such as the catecholamines or by neuropeptides through the activation of second-messenger systems such as cyclic AMP (Balthazart and Ball, 1998). Recently, we also collected evidence showing that the activation of glutamate receptors profoundly inhibits brain AA (Balthazart et al., 1999). Therefore, it is interesting to note that the dorsal spinal horns contain dense networks of fibers that are immunoreactive for a variety of peptides in close association with ARO-ir cells. Furthermore, afferent C fibers originating in the dorsal root ganglion and terminating in the dorsal horns in the vicinity of ARO-ir cells corelease glutamate together with substance P (Juranek and Lembeck, 1997). Thus, these amino acids and peptides are potential candidates for the control of both the synthesis and the activity of spinal aromatase. Their specific role should be investigated.
Functional implications
The discovery of brain AA dramatically changed our view of steroid action in the brain. Similarly, the presence of the widespread production of estrogens in the dorsal spinal horns raises new, exciting possibilities about how estrogens may modulate pain and sensory perception. In a variety of animal models, estrogens have been found to decrease pain sensitivity but also to increase tactile sensitivity in behaviorally relevant areas (perineum of rat, incubation patch of canaries; see above). Estrogen receptors located in the spinal dorsal horns (see above) presumably mediate changes in perception through the regulation of various transmitters and neuropeptides. Nociceptive information enters the dorsal horns by Aδ and C. afferent fibers. Aδ and C fibers originating in the skin terminate primarily in laminae I and II, whereas C fibers originating in the viscera terminate in laminae V and X (Light and Perl, 1979; Willis, 1988). Peptides and transmitters, such as substance P and somatostatin on the one hand or opioids and GABA on the other hand, respectively enhance or decrease the transmission of this information (see, e.g., Yaksh and Henry, 1978; Kuraishi et al., 1985; Wiesenfeld-Hallin, 1986; Dirig and Yaksh, 1996; Nadeson et al., 1996). In the central nervous system, the activity of these transmitters and peptides is modulated at the presynaptic level or the postsynaptic level by estrogens (Coslovsky et al., 1984; McCarthy et al., 1991; Debeljuk et al., 1992; Medina et al., 1993; Amandusson et al., 1996; Dawson-Basoa and Gintzler, 1998) that, in this manner, can control nociception or (somato)sensory perception.
The existence of AA in dorsal horns opens the possibility that estrogen production and action can be regulated locally, so that sensory perception also can be modulated on a local basis. This, for example, makes it possible to enhance tactile sensitivity in the genital area but to leave it unchanged or to decrease it in the neck region, where grabbing by the male could be a painful stimulus during copulation. Local estrogen production also may decrease vaginal sensitivity and pain perception during parturition but leave sensitivity intact in other body parts. Such responses would be functionally appropriate. The local aromatization also means that the controls of perception by estrogens can occur in females that have high circulating levels of estrogens as well as in males in which here local production would be the main source of E2.
In addition, estrogens produced by aromatization in presumably high local concentrations may modulate neurotransmission by nongenomic mechanisms in a variety of ways similar to what has been described in the brain (Mermelstein et al., 1996; Ramierz et al., 1996; Joëls, 1997; Moss et al., 1997). We showed that, in the brain, ARO-ir material is found at the level of presynaptic terminals (Naftolin et al., 1996). This probably also is the case in the spinal cord: Immunoreactive material was found throughout cellular processes, although its presence has not yet been confirmed in synaptic boutons by electron microscopy. It is known that glutamate and substance P coexist in afferent C fibers and interact to modulate spinal excitability (Urban et al., 1994). Substance P potentiates glutamate-mediated responses in spinal dorsal horn neurons and is able to release glutamate from spinal cord slices (Rusin et al., 1993; Juranek and Lembeck, 1997). Along with the potential genomic effects on substance P synthesis (see above), locally produced estrogens may modulate glutamate response at the receptor level (mostly kainate receptor subtype) by nongenomic mechanisms, as demonstrated in the brain (Joöls, 1997; Moss et al., 1997). The presence of aromatase in the dorsal spinal horns, therefore, opens up a large number of possibilities for specific controls of somatosensory perception (and pain perception in particular). These new avenues for research have both theoretical and obvious practical implications that now should be explored.
Acknowledgements
A.F. is Research Associate of the Belgian Fonds National de la Recherche Scientifique.




