Cilia propel the embryo in the right direction
Abstract
Cilia have long been suspected to play a role in the determination of left–right asymmetry. Humans with the dominantly inherited condition Kartagener syndrome have defective cilia and a 50% incidence of mirror-image positioning of their organs (situs inversus). Analysis of mouse mutations affecting ciliary biogenesis and motility has demonstrated that the molecular motors kinesin and dynein are required to establish normal handed organismal asymmetry. The cilia that propel formation of the embryonic left–right axis are not conventional cilia, but monocilia. They are found on the node, or organizer, of the gastrulation-stage mouse embryo where they drive net leftward movement of the fluid surrounding the node, and initiate left–right asymmetry. © 2001 Wiley-Liss, Inc.
In 1933, Kartagener [1933] noted an association between bronchiectasis, chronic sinusitis, and situs inversus totalis (mirror-image positioning of the organs across the left–right axis). Forty-two years later, Afzelius [1976] observed that a large percentage of men with Kartagener syndrome were sterile, and that their sperm were immotile and lacked dynein arms. The abnormal sperm suggested that the explanation for the association between male infertility and respiratory disease seen in patients with Kartagener syndrome lies in an intrinsic abnormality of cilia and flagella. Indeed, tracheal cilia in affected patients also had defective dynein arms and were immotile, leading to severely abnormal mucociliary clearance in the lungs [Camner et al., 1975]. Since the initial identification of absent outer dynein arms in the cilia of patients with Kartagener syndrome, a wide range of ciliary defects at the structural level has been shown to result in the clinical presentation of situs inversus, respiratory disease, and male infertility. These ciliary defects are collectively called primary ciliary dyskinesia (PCD). PCD is a heterogeneous genetic disease that is usually transmitted in an autosomal recessive manner, although cases of X-linked and autosomal dominant inheritance have also been reported [Narayan et al., 1994]. The most common ciliary defect in PCD is absence of the outer dynein arms, but defects including absent inner dynein arms, absent radial spokes, and abnormally arranged microtubules have also been reported [Meeks et al., 2000].
Situs inversus occurs in 50% of patients with PCD. It represents the failure of establishment of normal handed left–right asymmetry. Humans and all other vertebrates appear, on the outside, to be bilaterally symmetric. Underlying the external symmetry, however, is extensive left–right asymmetry of the internal organs. The thoracic organs in particular have a high degree of left–right asymmetry, and failure to establish normal left–right asymmetry is frequently associated with congenital heart disease. The three body axes along which the vertebrate body plan is organized are anteroposterior (AP), dorsoventral (DV), and left–right (LR), and among these, the LR axis is unique in several ways. LR is the dependent axis, existing only relatively to the previously established AP and DV axes. In addition, LR is a binary decision instead of a continuous gradient like the AP and DV axes. Finally, the handedness of LR asymmetry is consistent across all vertebrates. This means that the organism needs to not only have a mechanism by which it creates asymmetry along the LR axis, but that there also has to be a mechanism that aligns that asymmetry to the preexisting AP and DV axes to create consistent handedness. In patients with Kartagener syndrome, determination of cardiac and visceral handedness has become random: one half of affected patients have normal position of their organs, the other one half have a perfect mirror image.
What is the link between defects in the development of LR asymmetry during early embryonic development and abnormal ciliary function in the adult?
The enigmatic connection between ciliary abnormalities and defective LR development was further clouded by the observation that during the time in development when LR is established, mammalian embryos lacked ciliated tissues equivalent to tracheal or nasal epithelium. As it turns out, however, there are cilia on many embryonic tissues [Bancroft and Bellairs, 1974; Sulik et al., 1994; Bellomo et al., 1996]. Cilia in the embryo are not found on traditional ciliated epithelia carrying many cilia with a classic 9 + 2 microtubule organization, but instead they are observed on many cells carrying a single 9 + 0 monocilium. The cilia that are found on ciliated epithelia such as tracheal epithelial cells have a well-defined structure. They arise from a basal body near the surface of the polarized epithelial cell. Nine outer microtubule doublets surround a central pair of microtubules. The outer doublets are linked by force-producing outer and inner dynein arms. Radial spokes connect the outer doublets to the central pair microtubules. In contrast, the detailed structure of monocilia (also called primary cilia) is less well defined. Monocilia arise from the mature centriole, and a significant portion of the monocilium is frequently embedded within the cytoplasm [Poole et al., 1997]. Like 9 + 2 cilia, they have nine outer microtubule doublets. However, they lack a central pair microtubule. Whether or not some or all of the primary cilia have outer and/or inner dynein arms remains questionable, and it was long believed that all monocilia were immotile.
The embryonic node is a major organizing center in primitive streak-stage embryos that regulates pattern formation (Fig. 1). It is the mammalian equivalent of Hensen node in the chick and the Spemann organizer in Xenopus. Several genes such as nodal [Zhou et al., 1993], hnf-3β [Collignon et al., 1996] and fgf8 [Meyers and Martin, 1999], which are essential to the formation of the LR axis, are expressed at or around the node at the time of gastrulation. Furthermore, manipulation of the node in gastrulation-stage chick embryos is able to affect subsequent LR development [Pagan-Westphal and Tabin, 1998]. Taken together, these data suggest that the LR axis is established at the node at the time of gastrulation.
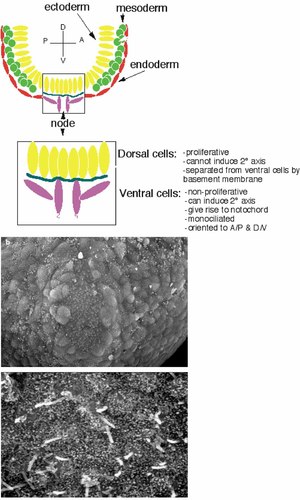
The mouse node. a: Schematic drawing of the mouse node. The ectoderm is shown in yellow, endoderm in red/pink, and mesoderm in green. b: Electron micrograph showing the triangular shape of the mouse node (outlined in red) (×500). c: Each of the central (pit) cells has a single cilium. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The mouse node forms at 7–7.5 days of embryonic development and is located at the distal tip of the embryo. At the node, the ectoderm (shown in yellow) and endoderm (shown in pink) (Fig. 1) make contact with each other. The proliferative ectodermal layer is separated from the nonproliferative endodermal layer by a basement membrane. The “organizer” function is contained within the endodermal cells: When they are transplanted to different parts of the embryo, they are able to induce a secondary axis [Beddington, 1994]. It is thought that positional information is acquired by the mesoderm as it contacts the node during gastrulation. Notably, each ventral node cell carries a monocilium pointing away from the embryo into the extracellular fluid [Sulik et al., 1994; Bellomo et al., 1996].
Ciliary structure or function is defective in several mouse mutants affecting LR axis development. Some of these mutations are in genes coding for ciliary structural proteins and others include mutations in a transcription factor and proteins known to have a role in ciliary assembly. Their only commonality, besides abnormal development of LR asymmetry, is their effect on cilia. This lends strong support to the argument that LR asymmetry is established by specific function(s) of cilia during embryogenesis.
Targeted mutagenesis of two members of the heterotrimeric kinesin family KIF3A [Marszalek et al., 1999; Takeda et al., 1999] and KIF3B [Nonaka et al., 1998] result in similar phenotypes including midgestation lethality and multiple severe developmental abnormalities. Kinesins are a large family of predominantly plus-end directed microtubule motors that are required for diverse intracellular transport functions. KIF3A and KIF3B proteins form a heterotrimeric complex with an associated protein KAP3 [Yamazaki et al., 1995, 1996]. Heterotrimeric kinesin homologues are found ubiquitously in many species including mouse, C. elegans, Chlamydomonas, and sea urchin. In sea urchin embryos, disruption of heterotrimeric kinesin function results in a failure to assemble motile ciliary axonemes [Morris and Scholey, 1997]. Studies in Chlamydomonas and C. elegans indicate that this is due to a requirement for heterotrimeric kinesin in intraflagellar transport (IFT); when IFT is defective, existing flagella resorb, and no new flagella can be assembled [Cole et al., 1998]. Notably, both KIF3A and KIF3B mutants display randomization of cardiac looping and abnormal LR expression patterns of genes such as lefty-2, implicating heterotrimeric kinesin in LR development. A striking feature of kif3A and kif3B homozygous mutants is that they have no node monocilia. Thus, heterotrimeric kinesin is essential both for assembly of node cilia and normal LR development.
The transcription factor Hfh4, a member of the forkhead/winged-helix family of transcription factors, also has a role, albeit unspecified, in ciliary morphogenesis [Chen et al., 1998]. Mice that are homozygous for a targeted mutation in Hfh4 have random development of LR asymmetry. They are devoid of all 9 + 2 cilia; however, monocilia on the node are present. Positioning of the basal bodies in Hfh4 −/− ciliated epithelia is abnormal, and the ultrastructure of their node monocilia is unknown.
Another link between cilia and LR development is provided by targeted mutagenesis of the mouse polaris gene. Polaris is the gene mutated in the Tg737 (mouse polycystic kidney) insertional mutant. When there is no functioning polaris protein, mice have absent node monocilia and defective LR development [Murcia et al., 2000]; Their phenotype is indistinguishable from that of the heterotrimeric kinesin mutations. Like heterotrimeric kinesin, the Chlamydomonas homolog of polaris functions in intraflagellar transport [Pazour et al., 2000]. Therefore, polaris is likely to be required to assemble functional node monocilia.
The recessive mouse iv (inversus viscerum) mutation, first described at the Jackson Laboratories in 1959 [Hummel and Chapman, 1959], results in random asymmetry. Fifty percent of liveborn mice homozygous for the iv mutation have situs solitus, and the other 50% have complete situs inversus. Expression of many of the asymmetrically expressed genes is perturbed [Collignon et al., 1996; Lowe et al., 1996], however, unlike the mice with KIF3A and KIF3B mutations described above, the abnormalities in iv/iv mice are entirely limited to defective LR development [Supp et al., 1999]. The iv mutant phenotype is caused by a missense mutation in the motor domain of an axonemal dynein heavy-chain gene named LR dynein (lrd) [Supp et al., 1997]. Dyneins are a family of minus-end directed microtubule-based motors that are commonly classified as either cytoplasmic or axonemal based on specific sequence characteristics of the component heavy chains. These proteins function as large multisubunit complexes made of up to three heavy chains in addition to intermediate (ICs) and light chains (LCs) [Holzbaur and Vallee, 1994]. The dynein heavy chain genes are large proteins (Mr ∼500 K) and are encoded by mRNAs ranging from 14 to 18 kb. The heavy chain consists of two major structural domains. The carboxy two thirds of the protein comprises four evenly spaced, highly conserved P-loop consensus sequence elements that correspond to sites of MgATPase activity, and a single coiled/coil domain that has microtubule binding ability [Gee et al., 1997; Gee and Vallee, 1998]. The amino terminal one third of the protein is more unique to each dynein and contains the region that binds to light and medium chains. Thus, this region is thought to tether dynein to its cargo [Kandl et al., 1995]. To date, at least 15 distinct dynein heavy chain genes have been identified in vertebrates, of which two are believed to be components of cytoplasmic dyneins, and the remainder are components of multiple axonemal dynein isoforms [Tanaka et al., 1995; Vaughan et al., 1996]. Cytoplasmic dyneins exist as homooligomers of two identical heavy chains along with multiple ICs and LCs. They are required for many cellular processes, including organelle transport and chromosome segregation. The target specificity is not defined by the dynein heavy chain itself, but by the attached medium and light chains. Axonemal dyneins, conversely, are heterooligomers of up to three different heavy chains in addition to the ICs and LCs, and are thus far thought to be involved only in the movement of cilia or flagella. lrd mRNA codes for an axonemal dynein and is expressed in the developing embryo in the ventral node cells that, as previously described, each possesses a monocilium [Supp et al., 1999]. In contrast to mice that are deficient in heterotrimeric kinesin, the node cilia in mice with abnormal lrd appear normal in size and distribution.
All of the mutations described above point to a pivotal role for node monocilia in the determination of LR asymmetry, but how do node monocilia initiate the LR axis? Recent observations of node monocilia in wild-type and mutant mice suggest that the underlying mechanism is based on directional, vortical motion of the node monocilia. Videomicroscopy of node monocilia in e7.5 wild-type mice demonstrate that contrary to earlier beliefs, the node monocilia are indeed motile [Nonaka et al., 1998]. They move in a directional, vortical manner that is distinct from the bending movement of 9 + 2 cilia. Motility of the node monocilia drives directional, leftward movement of the extracellular fluid surrounding the node. Mice with a targeted mutation in the lrd gene have normal-appearing node monocilia. However, their cilia are frozen in a rigorlike state, and there is no movement of the perinodal fluid. lrd −/− mice have random development of LR asymmetry in the absence of any other defects [Supp et al., 1999]. Thus, motile node monocilia are essential for the embryo to distinguish its left side from its right.
Net leftward flow of the extracellular fluid surrounding the node that is generated by directional vortical movement of node monocilia appears to be the event that breaks embryonic bilateral symmetry. How this flow activates asymmetric gene expression remains a mystery, however. The most obvious idea is that nodal flow directs a soluble morphogen toward the left side of the node, whereupon it interacts with its receptor and sets up the cascade of asymmetric gene expression that results in, for example, eventual left-sided nodal and PitX2 expression (Fig. 2a). Several candidates come to mind for the soluble asymmetry factor, including: sonic hedgehog, fgf8 [Meyers and Martin, 1999] and Vg1-related growth factors such as GDF1 (growth and differentiation factor 1) [Rankin et al., 2000]. In essence, all proteins secreted into the perinodal fluid will be moved to the left by nodal flow. The degree to which their action will be asymmetric will depend to a large extent on the relative kinetics of processing, interaction of a secreted factor with its receptor, and breakdown, compared to the velocity of nodal flow. In this model, when the soluble factor or its receptor is absent or defective, the result would be maintenance of bilateral symmetry (Fig. 2b). If fgf-8, GDF-1, or the activin 2B receptor are indeed involved at this level of determination of LR asymmetry, this could explain the finding of right atrial isomerism in mice with mutations in these genes. In contrast, when there are absent or defective node cilia, nodal flow would be absent, but the soluble factor and its receptor would remain intact. A current at the node could be generated randomly, perhaps through physical movement of the embryo within the uterus, and this movement will move the soluble factor and activate its receptor in a random manner, resulting in random asymmetry (Fig. 2c). This is precisely the phenotype observed in humans with Kartagener syndrome and mice with mutations in lrd: a random distribution between situs solitus and situs inversus among a population of affected individuals.
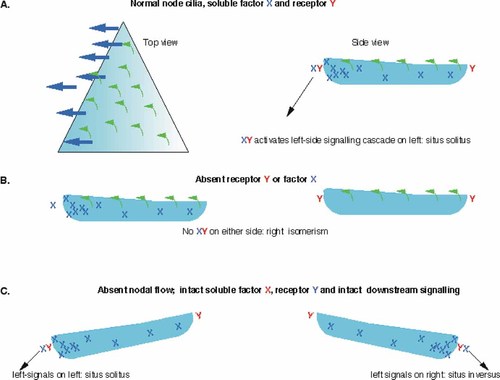
Model for the effect of abnormalities in nodal flow on left–right phenotype. a: Schematic drawing of a normal node, seen from the top and side. Cilia (shown in green) generate leftward flow (shown as blue arrows) of the perinodal fluid. Leftward nodal flow results in soluble factor “X” being concentrated on the left side of the node, where it activates receptor “Y” and starts the signaling cascade that results in “left” identity. b: When either factor X or receptor Y is defective or absent, “left” signaling cannot be activated, even in the face of normal nodal flow. The hypothetical result would be bilateral “right” identity, as seen in right isomerism. c: When the node cilia are immotile, directed leftward nodal flow is absent. However, because the soluble factor and receptor remain intact, “left” signaling can still be activated by randomly generated currents in the perinodal fluid. This would result in a random distribution of situs solitus (left signaling initiated on the left) and situs inversus (left signaling initiated on the right). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
It is also possible that the current generated by nodal flow activates a mechanosensor to the left of the node. Cilia found on the surface of the embryo outside of the node could be a candidate for this mechanosensor. This could reconcile the difference in phenotype observed between ciliary immotility mutants and ciliary assembly mutants. In the mutations that affect ciliary motility only, the mechanosensor remains intact. Mutations that affect ciliary assembly, however, result in a total absence of cilia. Here, even randomly generated nodal flow has no mechanosensor to activate, and the resultant phenotype would be expected to be maintenance of embryonic bilateral symmetry.
Twenty-five years ago, Afzelius [1976] observed that patients with Kartagener syndrome had defective cilia. At that time this observation led to the suggestion that “On the various epithelia of an embryo there are cilia that have determined positions and a fixed beat direction” [Loefberg, 1974] and that “Ciliary beating in normal embryos is assumed to be instrumental in pushing the heart to the left side”[Afzelius, 1976]. A quarter of a century and detailed analysis of many mouse mutants demonstrated that this idea was, in essence, true: Although the heart is not pushed directly by ciliary movement, ciliary movement initiates a cascade of signals that eventually converges on the heart assuming its normal, left-sided position.




