Clinical and molecular-cytogenetic studies in seven patients with ring chromosome 18
Abstract
We report the results of detailed clinical and molecular-cytogenetic studies in seven patients with ring chromosome 18. Classical cytogenetics and fluorescence in situ hybridization (FISH) analysis with the chromosome 18 painting probe identified five non-mosaic and two complex mosaic 46,XX,dup(18)(p11.2)/47,XX,dup(18)(p11.2),+r(18) and 46,XX,dup(18)(p11.32)/47,XX,dup(18)(p11.32),+r(18) cases. FISH analysis was performed for precise characterization of the chromosome 18 breakpoints using chromosome 18–specific short-arm paint, centromeric, subtelomeric, and a panel of fifteen Alu- and DOP-PCR YAC probes. The breakpoints were assessed with an average resolution of ∼2.2 Mb. In all r(18) chromosomes, the 18q terminal deletions ranging from 18q21.2 to 18q22.3 (∼35 and 9 Mb, respectively) were found, whereas only in four cases could the loss of 18p material be demonstrated. In two cases the dup(18) chromosomes were identified as inv dup(18)(qter→p11.32::q21.3→qter) and inv dup(18)(qter→p11.32::p11.32→p11.1: :q21.3→qter)pat, with no evidence of an 18p deletion. A novel inter-intrachromatid mechanism of formation of duplications and ring chromosomes is proposed. Although the effect of “ring instability syndrome” cannot be excluded, the phenotypes of our patients with characteristic features of 18q- and 18p- syndromes are compared and correlated with the analyzed genotypes. It has been observed that a short neck with absence of cardiac anomalies may be related to the deletion of the 18p material from the r(18) chromosome. © 2001 Wiley-Liss, Inc.
INTRODUCTION
r(18), del(18q), and del(18p) are considered the most frequently occurring autosomal deletions. The phenotype of the 18q- syndrome (MIM 601808) is highly variable and probably depends on the extent of terminal or interstitial 18q deletions [Kline et al., 1993; Strathdee et al., 1994; Silverman et al., 1995; Kohonen-Corish et al., 1996; Cody et al., 1997a; Strathdee et al., 1997; Brkanac et al., 1998]. It is characterized by mental retardation, hypotonia, foot deformity, proximally placed thumbs, atretic/stenotic ear canals, short stature, long tapering digits, microcephaly, abnormal male genitalia, flat midface, prominent antihelix and antitragus, and carp-shaped mouth [de Grouchy et al., 1964; Wilson et al., 1979; Schinzel, 1984; Strathdee et al., 1995; Cody et al., 1999]. Kline et al. [1993] observed some phenotype/genotype trends in 18q- syndrome patients, and recently, Cody et al. [1999] showed a correlation between the extent of 18q deletion and head circumference, external ear length, proximal or anomalous thumbs, and metatarsus adductus. However, results of other studies did not reveal such relationships [Silverman et al., 1995; Strathdee et al., 1995; Mahr et al., 1996].
The 18p- syndrome also presents with a wide variety of clinical features among which the more common are speech delay, hypotonia, short stature, ptosis of eyelids, mental retardation, midline defects, including holoprosencephaly, IgA deficiency, small mandible, dental caries, and short neck [Schinzel, 1994; Israels et al., 1996].
Most patients with the ring chromosome 18 share the symptoms of the 18q- syndrome [Schinzel, 1984; Aritaki et al., 1996; Nakayama et al., 1997], whereas a minority of them appear to be like 18p- syndrome [Bird et al., 1997] or a combination of these two syndromes [Schinzel, 1984]. Interestingly, several atypical manifestations have been observed in patients with r(18), including features of van der Woude syndrome [Kalker et al., 1988] and insulin-dependent diabetes mellitus [Dacou-Voutetakis et al., 1999], as well as agammaglobulinaemia [Litzman et al., 1998], and only facial anomalies [Los et al., 1996]. Too few mosaic r(18) chromosomes have been reported to date to draw a clinical distinction between mosaic and non-mosaic cases [Fryns et al., 1986; Jenderny et al., 1993].
To our knowledge, no molecular-cytogenetic analyses of ring chromosomes 18 have been previously published. Here we report the detailed clinical studies in seven patients with r(18) chromosomes and correlate them with their karyotypes defined by the fluorescence in situ hybridization (FISH) technique.
PATIENTS AND METHODS
Clinical Reports
The detailed clinical findings in our patients are shown in Tables I (cases 1–5) and II (cases 6 and 7).
| Characteristics | Case 1 (W.M.) | Case 2 (J.Z.) | Case 3 (L.S.) | Case 4 (M.M.) | Case 5 (A.T.) |
|---|---|---|---|---|---|
| Age (years) | 7/12 | 3/12 | 9 | 2 | 4/12 |
| Gender | Female | Female | Male | Male | Female |
| Gestation (hbd) | 38 | 40 | 38 | 38 | 35 |
| Birth weight (g) (low < 3rd centile) | 2,500 | 2,850 | 2,700 | 3,500 | 2,400 |
| Birth length (cm) | 56 | 49 | 49 | 53 | 50 |
| Head circumference (cm) | 31 | 32 | 33 | 34 | 33 |
| Psychomotor delay | + | + | + | + | + |
| Mental retardation | Severe | Mild | Moderate | Moderate | Moderate |
| Hypotonia | + | hypertonia | + | + | + |
| Short stature | + | + | + | ||
| Disproportionate build | + | ||||
| Brain | Asymmetric, dilated lateral ventricles with rounded anterior horns | Dilated anterior horns | Abnormal myelinization | ||
| Cranium | Asymmetric | Asymmetric | |||
| Microcephaly | + | + | + | + | |
| Dolichocephaly | + | + | |||
| Flattened occiput | + | + | + | ||
| Forehead | Convex | High, narrow | Convex | ||
| Over-ridged cranial sutures | + | + | |||
| Face | Asymm. triangular | Broad | |||
| Midface | Flat | Flat | Flat | Flat | |
| Micrognathia | + | + | + | + | + |
| Ears | Asymmetric | Asymmetric | Long | Asymmetric | |
| Low set | + | + | + | + | |
| Prominent upper part | + | ||||
| Atretic external ear canals | + | ||||
| Prominent anti-helix | + | ||||
| Deep concha | + | ||||
| Broad Incisura intertragica | + | ||||
| Eyes | |||||
| Deep set | + | + | + | + | + |
| Hypertelorism | + | + | + | + | + |
| Palpebral fissures slant | Down | Down | Down | Up | Down |
| Epicanthic folds | + | + | + | ||
| Sparse eyebrows and eyelashes | + | + | |||
| Nystagmus | + | + | + | ||
| Strabismus | + | + | + | + | + |
| Pale optic disc | + | ||||
| Myopia | + | ||||
| Nose | Bird like | Long, bird-like | Short | ||
| Broad base | + | + | + | + | |
| Bridge | Depressed/flat | Broad, saddle | Depressed | Broad, saddle | |
| Broad tip | + | + | + | ||
| Broad nares | + | + | |||
| Low set alae nasi | + | ||||
| Mouth | Microstomia | Macrostomia | Microstomia | ||
| Carp-like down-turned corners | + | + | + | + | |
| Thin upper and lower lips | + | + | + | Thin upper, thick lower | + |
| Cleft lip | + | ||||
| Macroglossia | + | ||||
| Philtrum | Long | Short | Long | Short | |
| Large frenulum | + | ||||
| Palate abnormality | |||||
| High | + | + | + | + | |
| Narrow | + | + | |||
| Cleft | + | ||||
| Teeth | Dental caries, malocclusion | ||||
| Neck | |||||
| Short | + | + | |||
| Long | + | + | |||
| Webbed | + | ||||
| Low posterior hairline | + | ||||
| Thorax | Narrow, long | Asymmetric, broad | Broad | ||
| Congenital heart defect | PDA + PS | VSD | AS + IA | ||
| Pectus excavatum | + | ||||
| Scoliosis | + | + | |||
| Short clavicles | + | ||||
| Winged scapulae | + | ||||
| Wide spaced nipples | + | + | + | +, hypoplastic supernurnerary | + |
| Haemangiectasies | + | ||||
| Abdomen | |||||
| Umbilical hernia | + | + | + | + | + |
| Inguinal hernia | + | + | + | + | |
| Frog belly | + | + | |||
| Upper limbs | |||||
| Long | + | ||||
| Hands | |||||
| Small | + | + | |||
| Proximally placed thumbs | + | + | |||
| Long tapering fingers | + | + | |||
| Clinodactyly | +V | + | + | + | |
| Camptodactyly | + | +V | + | ||
| Short fingers | + | + | + | + | |
| Broad thumbs | + | ||||
| Single transverse palmar crease | + | + | |||
| Lower limbs | |||||
| Long | + | ||||
| Genu valgum | + | + | |||
| Foot deformity | |||||
| Club | Varus | Valgus | Valgus | ||
| Over-riding II and III toes | + | + | + | + | |
| Toes | Long and broad | Long and broad | Long and broad | Short | |
| Genitourinary | |||||
| Abnormal male genitalia | Cryptorchismus, hypoplastic scrotum, small penis | Phimosis | |||
| Hypoplastic labia minora | + | ||||
| Renal agenesis | + | + | |||
| Blurring of renal structure | + | ||||
| Vesicoureteral reflux | + | ||||
| Hearing impairent | + | + | Conductive | ||
| Speech delay | + | + | |||
| Low IgA | + | ||||
| Dry skin | + | + | + | + | |
| Sparse hair | + | + | + |
| Characteristics | Case 6 (A.F.) | Case 7 (Z.A.) |
|---|---|---|
| Age (years) | 11 | 10 |
| Gender | Female | Female |
| Gestation (hbd) | 37 | 36 |
| Birth weight (g), low < 3rd centile | 2,650 | 2,450 |
| Birth length (cm) | 47 | 53 |
| Head circumference (cm) | 32 | 32 |
| Psychomotor delay | + | + |
| Mental retardation | Mild | Moderate |
| Short stature | + | |
| Brain | Cerebral cortex atrophy, internal hydrocephalus | |
| Cranium | ||
| Microcephaly | + | |
| Dolichocephaly | + | + |
| Narrow short convex forehead | + | |
| Face | ||
| Micrognathia | + | + |
| Long | + | + |
| Narrow | + | |
| Triangular | + | |
| Ears | ||
| Low set | + | |
| Antihelix crus | + | |
| Preauricular pits | + | |
| Eyes | ||
| Deep set | + | + |
| Hypertelorism | + | |
| Palpebral fissures slant down | + | |
| Palpebral fissures slant up | + | |
| Epicanthic folds | + | + |
| Strabismus | + | + |
| Coloborna | + | |
| Hypermetropia | + | |
| Myopia | + | |
| Nose | Large | |
| Bridge | High/prominent, broad | High/prominent, narrow |
| Broad base | + | |
| Broad nares | + | + |
| Broad tip | + | |
| Mouth | ||
| Carp-like, down-turned corners | + | |
| Thin upper and lower lips | + | |
| Microstomia | + | + |
| Short philtrum | + | |
| Palate abnormality | High, narrow | High, narrow |
| Teeth, general abnormalities | ||
| Present at birth | + | |
| Irregular | + | |
| Malocclusion | + | + |
| Dental caries | + | |
| Neck | Short, webbed | |
| Flaccidity of larynx | + | |
| Low posteror hairline | + | |
| Thorax | ||
| Narrow | + | |
| Pectus excavatum | + | + |
| Scoliosis | Thoracic | + |
| Wide spaced nipples | + | |
| Winged scapulae | + | + |
| Congenital heart defect | VSD | |
| Upper limbs | ||
| Cubitus valgus | + | |
| Hands | ||
| Small | + | |
| Clinodactyly V | + | + |
| Wide finger tips | + | + |
| Short fingers | + | |
| Hypoplastic nails | + | |
| Lower limbs | ||
| Genu valgum | + | |
| Foot deformity | Flat | Long |
| Sandal crease | + | |
| Halux valgus | + | |
| Genitourinary | ||
| Hypoplastic labia majora | + | |
| Vesicoureteral reflux | + | |
| Delayed skeletal maturation | + | Not analyzed |
| Hearing impairment | Conductive | |
| Speech delay | + | + |
| Dry skin | + |
- In bold are the traits found in ≥ 1/4 of subjects with dup(18)(q21qter) and in italic are the traits found in ≥ 1/4 of individuals with dup(18p) [Schinzel, 1994].
Case 1 (W.M.)
This female was the first child of non-consanguineous, healthy parents. At birth the mother was 25, and the father was 31 years old. Prenatal ultrasonography showed disproportion of the body with relatively long legs and a small head. The girl was born after the 38th week of pregnancy by spontaneous vaginal delivery. The delivery was complicated by abnormal fetal position and green amniotic fluid. The Apgar score was 6 at 1 min, and she required resuscitation. At birth the patient was noted to have multiple congenital defects and distinct craniofacial anomalies. On physical examination at the age of 5 months, her weight was 5,100 g (< 3rd centile); length, 68 cm (50th centile); OFC, 37 cm (< 3rd centile); and chest circumference, 37 cm (< 3rd centile). She was noted to have developmental delay, hypotonia, absence of deep tendon response, and numerous dysmorphic features. Her appearance was notable for a narrow trunk with relatively long limbs. The patient's development was definitely delayed: at age 5 months, when held in ventral suspension, she was unable to lift her head. Facial grimacing was noted in response to loud sounds, but visual fixation was not observed. At the age of 18 months she did not speak. She could sit, but was not able to stand alone.
Case 2 (J.Z.)
This female was born after a normal term pregnancy by spontaneous vaginal delivery. She was the first child of healthy parents—at birth the mother was 26, and the father was 33 years old. The Apgar score was 8 at 1 min. On physical examination at the age of 3 months, her weight was 4,000 g (< 3rd centile); length, 56 cm (3rd centile); OFC, 37 cm (< 3rd centile); and chest circumference, 36 cm (< 3rd centile). She was noted to have developmental delay, marked diffuse hypertonia, and several minor anomalies. When held in ventral suspension she was unable to lift her head. The infant was examined again at the age of 1 year. She continued to grow in weight and length, although remaining below the 10th centile. A mild muscular hypertonia was still present. She could sit, stand with support, and pronounce first syllables. Her development has been continuous, although slow.
Case 3 (L.S.)
This male patient was the first child of nonconsanguineous parents. At birth the mother was 27, and the father was 29 years old. He was born at 38 weeks of gestation by Cesarean section. The Apgar scores were 5, 5, 7, and 10 at 1, 3, 5 and 10 min, respectively. His parents and 15-month-old sister were healthy. The patient showed delay of mental development and his speech was impaired. He was able to maintain a sitting position at the age of 8 months, could walk at 3 years, and pronounced his first syllables at 4 years. On examination at the age of 10 years, his weight was 32 kg and his height was 131 cm. He had eunuchoid stature, slight hypotonia, and dysmorphic face. His psychomotor development was delayed, especially speech and motor development. He understood and fulfilled orders but could not express complete sentences.
Case 4 (M.M.)
A male of non-consanguineous, healthy parents was born to a 32-year-old mother and a 32-year-old father. The Apgar score was 10 at 1 min. At the age of 22 months he was admitted to the hospital because of psychomotor developmental delay and hypotonia. His weight was 9,700 g (< 3rd centile); height, 80 cm (< 3rd centile); and OFC, 44 cm (< 3rd centile). He was unable to walk without support. Clinical examination revealed several minor anomalies. The neurological examination found psychomotor developmental delay, hypotonia, muscular atrophy of the shoulder and pelvic girdle, and absence of knee tendon reflexes. Ophthalmologic examination revealed pigmentary degeneration of the right eye retina. Psychological evaluation (Brunet-Lezine test) showed a D.Q. of 45. Magnetic resonance imaging (MRI) of the brain demonstrated dysmyelination. Electromyogram revealed slightly neurogenic abnormalities (Fig. 1a).
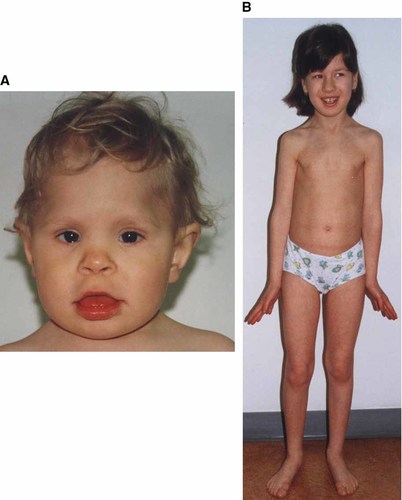
Patients M.M. (case 4), aged 22 months (a), and Z.A. (case 7), aged 10 years (b).
Case 5 (A.T.)
This female is the third child of the non-consanguineous marriage of healthy parents. At birth the mother was 24, and the father was 25 years old. The patient was born after a 35-week uncomplicated pregnancy. The Apgar score was 10 at 1 min. She was referred to us at the age of 3 months because of developmental delay and multiple congenital anomalies. Clinical examination showed a marked diffuse hypotonia, hyporeflexia, and minor facial anomalies. Ultrasonography and electrocardiography did not show any cardiac defect. Abdominal ultrasonography revealed absence of the right kidney. At the age of 8 months she could roll over and transfer objects, and she pronounced her first syllables. She was not able to maintain a sitting position. She was late to achieve developmental milestones, but the loss or regression of her skills was not observed. Because of high vesicoureteral reflux, she was qualified for surgery.
Case 6 (A.F.)
This patient was born after a 37-week uncomplicated pregnancy. She was the first child of healthy and non-consanguineous parents. The mother was 26, and the father was 27 years old. The Apgar score was 8 at 1 min. Transient feeding difficulties were noted in the neonatal period. Growth delay was not observed during her early months of life. Developmental milestones were moderately retarded. She could maintain a sitting position at the age of 9 months and walked unassisted at 16 months. She pronounced her first words at the age of 2 years. The patient was referred to us because of short stature, mental retardation, and multiple dysmorphic features. Skeletal maturation was delayed: the bone age at 9 years was consistent with a chronological age of 7 years. Speech delay was observed and she mispronounced some sounds. At present she is in a classroom for the handicapped.
Case 7 (Z.A.)
A 10-year-old girl of young, healthy, non-consanguineous parents was referred to the Genetic Outpatient Clinic because of psychomotor retardation. The mother's uncle had a boy with hydrocephaly and spina bifida who died at the age of 13 months. The father's aunt has a boy with cerebral palsy. The patient was born to a 25-year-old father and a 23-year-old mother at 36 weeks of gestation. The Apgar score was 5 at 1 min. During the first 2 months the lack of sucking reflex and frequent vomiting were observed. She needed gastric tube feeding. Her psychomotor development was retarded. She was able to sit at 1 year, walk without support at 2 years, 7 months, and spoke simple words at 5 years. She had surgery for convergent strabismus. At the age of 9 years, 6 months her weight was 31 kg (50th centile); height, 130 cm (25th centile); and OFC, 53 cm (50th centile). Clinical examination revealed multiple minor anomalies (Fig. 1b).
Cytogenetic and FISH Analysis
Chromosome analysis was performed on peripheral blood lymphocyte cultures using GTG banding and FISH studies. Metaphases were analyzed under Axioscop and Axioplan microscopes equipped with the Cytoscan Ultra Vision (Applied Imaging, UK) (cases 1–3 and 5–6) and the ISIS (MetaSystems, Germany) (cases 4 and 7) softwares. The unique sequence probes were chosen using the databases of the Molecular Cytogenetics and Positional Cloning Center, Max-Planck-Institute (MPI) for Molecular Genetics, Berlin, Germany [Wirth et al., 1999], and the Resources for Human Molecular Cytogenetics, Istituto di Genetica, Università Degli Studi, Bari, Italy. DOP-PCR products of YACs 854g8, 937d3, 826c11, 803f9, 874b12, 747a6, 955c2, 781b4, 817b11, 890f12, 842d11, and 932b10 were obtained from MPI. Yeast stabs of YACs 881h1, 847b5, and 877b7 and DNA of chromosome 18 short-arm partial paint (pcp) were kindly provided by Dr. M. Rocchi. Isolated DNAs were amplified using inter-Alu-PCR. PCR products were labeled by nick translation reaction using biotin-labeled nucleotides (Life Technologies-GibcoBRL). FISH was essentially performed according to a modified procedure of Pinkel et al. [1988]. Biotin was visualized with two layers of FITC-avidin DCS (Vector Labs). Chromosomes were counterstained with DAPI and propidium iodide (PI) diluted in Vectashield antifade (Vector Labs). All r(18) chromosomes were analyzed using 18Z1 centromeric probe from Oncor, and in cases 1–3 and 6, subtelomeric probe D18S1390 (Cytocell). They were used according to manufacturers' instructions.
At least 20 metaphases were analyzed for the presence or absence of a signal on the abnormal chromosomes. The signal from the normal chromosome 18 was used as a control. Monochromatic images were captured using an Axioplan2 microscope equipped with appropriate filter combinations and a cooled CCD KAF 1400 S200 camera and were pseudocolored using IPLab Spectrum with Multiprobe extension software (Photometrics) for documentation of the results of FISH studies.
Molecular Analysis
Ten polymorphic microsatellites, D18S59, D18S453, D18S40, D18S1104, D18S66, DCC, D18S1147, D18S386, D18S61, and D18S488, for uniparental disomy UPD studies had been selected using the Genome Database (GDB) amplimer query and were amplified using routine methods with PCR primers obtained from InViTek (Berlin, Germany). The products were separated on 8% polyacrylamide gels and visualized by the silver staining method.
The loci order, locations, and the r(18) and dup(18) sizes were inferred from the Genetic Location Database (LDB). The assigning of YACs to chromosome 18 G-bands was done according to their Flpter values, determined from the MPI database.
RESULTS
The results of the molecular-cytogenetic analysis and microsatellite polymorphisms of abnormal chromosomes are summarized in Tables III and IV, respectively, and shown schematically in Figure 4. The parental karyotypes of all patients were normal. Abnormal chromosomes were positive with the chromosome 18 centromeric probe in all cases and, except case 6, double ring forms were observed in ∼2% of analyzed cells. In four cases, 1–3 and 6, the application of the 18q Cytocell subtelomeric probe with marker D18S1390 (< 270 Kb from 18qter, LDB [Brkanac et al., 1998]) showed that the 18q telomeres are not present on these abnormal chromosomes.
| Arm | Probe | Locus | Case 1 W.M. | Case 2 J.Z. | Case 3 L.S. | Case 4 M.M. | Case 5 A.T. | Case 6 A.F. | Case 7 Z.A. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| r(18) | dup(18) | r(18) | dup(18) | ||||||||
| Partial paint | 18p | + | + | – | – | + | + | – | 2+ | ||
| 854g8 | D18S476 | + | + | + | – | – | – | + | – | 2+ | |
| 18p | 881h1 | D18S63 | – | – | + | + | – | 2+ | |||
| 937d3 | D18S464 | + | + | – | |||||||
| 826c11 | D18S53 | – | – | + | + | – | 2+ | ||||
| Oncor | D18Z1 | + | + | + | + | + | + | + | + | + | |
| 803f9 | D18S44 | + | |||||||||
| 874b12 | D18S480/D18S1107 | + | + | + | + | ||||||
| 747a6 | D18S470 | + | + | + | |||||||
| 955c2 | D18S69 | + | + | – | + | + | + | + | + | ||
| 781b4 | D18S1144 | + | – | – | 2+ | – | 2+ | ||||
| 18q | 817b11 | D18S1155 | – | – | + | – | 2+ | – | 2+ | ||
| 890f12 | D18S1148/D18S1147 | – | – | – | + | – | 2+ | ||||
| 847b5 | D18S68 | + | + | – | 2+ | ||||||
| 842d11 | D18S465 | – | – | – | + | + | – | 2+ | |||
| 877b7 | D18S488 | – | – | ||||||||
| 932b10 | D18S462/D18S554 | – | – | – | – | – | – | 2+ | – | 2+ | |
| Cytocell | D18S1390 | – | – | – | – | 2+ | |||||
| Average ring/duplication size (Mb) | 58 | 55 | 50 | 56 | 56 | 54 | 30 | 35 | ∼20.0/∼30 | ||
- * Loci order, sizes of r(18) and dup(18) were done according to the Genetic Location Database.
- +, locus present; –, locus not present; 2+, locus present twice.
| Region | Locus | Microsatellite polymorphisms | |||
|---|---|---|---|---|---|
| F | M | P | Result | ||
| 18p11.32 | D18S59 | 2,4 | 1,3 | 1,2,2 | Bipar, pi |
| 18p11.2 | D18S453 | 2,4 | 1,3 | 1,2,2 | Bipar, pi |
| 18p11.2 | D18S40 | 1,3 | 2,4 | 3,3,4 | Bipar, pi |
| 18q11.2 | D18S1104 | 1,2 | 1,3 | 1,2 | ni |
| 18q12.1 | D18S66 | 2,3 | 1,2 | 2,3 | ni |
| 18q21.1 | DCC | 1,1 | 1,1 | 1,1 | ni |
| 18q22.1 | D18S1147 | 1,2 | 3,4 | 2,2,4 | Bipar, pi |
| 18q22.1 | D18S386 | 1,3 | 2,4 | 1,1,4 | Bipar, pi |
| 18q22.2 | D18S61 | 2,4 | 1,3 | 3,4,4 | Bipar, pi |
| 18q22.2 | D18S488 | 1,2 | 3,3 | 1,1,3 | Bipar, pi |
- The quantitative assessment of PCR products was not possible for all markers.
- ni, the result was not informative; bipar, biparental inheritance; pi, paternal isodisomy; P, proband; F, proband's father; M, proband's mother.
Case 1
A ring-shaped chromosome ∼21q in size was found in all analyzed metaphases. The application of the very distal subtelomeric YAC 854g8 (∼0.3 Mb from 18pter, LDB) showed no evidence of the deletion of the 18p material. The presence of the YAC probe 781b4 and the absence of 817b11 on r(18) enabled the assigning of its 18q breakpoint to 18q22.1. The patient's karyotype was assessed as 46,XX,r.ish r(18)(p11.32q22.1).
Case 2
A non-mosaic ring chromosome identified with the whole and partial chromosome paints 18 as r(18) was found. Similar to the ring from case 1, the lack of 18p material could not be demonstrated using YAC 854g8. The chromosome 18 long-arm breakpoint was found to map between YACs 955c2 and 781b4 on 18q21.3. Thus, the patient's karyotype was ascertained as 46,XX,r.ish r(18)(p11.32q21.3).
Case 3
Ring chromosome 18, similar in size to those from cases 1 and 2, was identified in all cells examined. The presence of 18q euchromatin was demonstrated using YAC 747a6. The karyotype was designated as 46,XY,r.ish r(18)(p11.32q21.2).
Cases 4 and 5
The same G banding pattern and FISH characteristics of two non-mosaic ring chromosomes were identified in both cases. No FISH signals were obtained using 18p-specific partial paint and several region-specific YAC probes (Table III). This indicated that no chromosome 18 short-arm sequences were present on both r(18) chromosomes. Thus, the karyotypes were described as 46,XY,r.ish r(18)(p11.1q22.3) and 46,XX,r.ish r(18)(p11.1q22.3), respectively.
Case 6
Routine cytogenetic studies revealed additional material on the short arm of chromosome 18 in all analyzed cells. Additionally, in ∼30% of cells the ring chromosome was identified. The G banding pattern of the add(18p) suggested that it may be a part of 18q. This assumption was confirmed by the hybridization with whole chromosome 18 paint. The ring chromosome was also completely painted by this probe. Systematic studies with the use of chromosome 18 YAC panel revealed the size and the inverted nature of the identified dup(18q). At the same time the r(18) could be precisely characterized (Fig. 2a,b). Unexpectedly, the loss of the small 18p11.32 fragment (< 1.6 Mb) on r(18) was found. The karyotype was finally established as 46,XX,add(18)(p11.2)[11]/47,XX,add(18)(p11.2),+r[5].ish der(18)inv dup(18)(qter→p11.32::q21.3→qter),r(18)(p11.32q21.3).
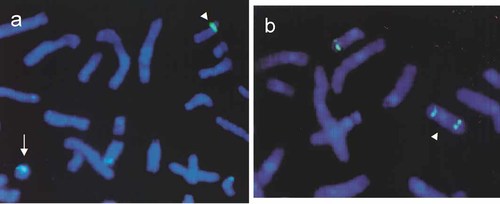
Abnormal chromosomes from case 6 identified as r(18) and dup(18)(q21.3qter) using FISH with short-arm partial chromosome 18 paint (a) and YAC 890f12 probes (b). The r(18) chromosome is indicated by an arrow and dup(18) by an arrowhead.
Case 7
A small mosaic ring chromosome (size ∼ ½ 21q) found in 40% of G-banded metaphases was associated with a nonmosaic add(18p). The G banding pattern did not unequivocally suggest their origin (Fig. 3a). FISH with the whole chromosome 18 paint documented that an extra material on 18p, as well as the ring, was in fact derived from chromosome 18. The application of the 18p partial paint (Fig. 3b) and the YAC probes (Fig. 3c–f) showed that dup(18) encompasses the genetic material from the whole short-arm 18p and the 18q21.3qter fragment. The inverted nature of dup(18p) was shown with chromosome 18 short-arm–specific YAC probes (Fig. 3d–f). The presence of r(18) euchromatin was further confirmed using a region-specific probe with marker D18S69, indicating that it consists of 18p11.32q21.3 material. The α-satellite D18Z1 probe revealed a single hybridization signal on both r(18) and dup(18) chromosomes. Microsatellite polymorphism analysis was informative for seven loci: D18S59, D18S453, D18S40, D18S1147, D18S386, D18S61, and D18S488. It indicated that dup(18) arose from paternal chromosome 18 (Table IV). The karyotype of the patient was finally designated as 46,XX,add(18)(p11.32)[60]/47,XX,add(18)(p11.32),+r[40].ish der(18)invdup(18)(qter→p11.32::p11.32→p11.1::q21.3→qter),r(18)(p11.1q21.3)pat.
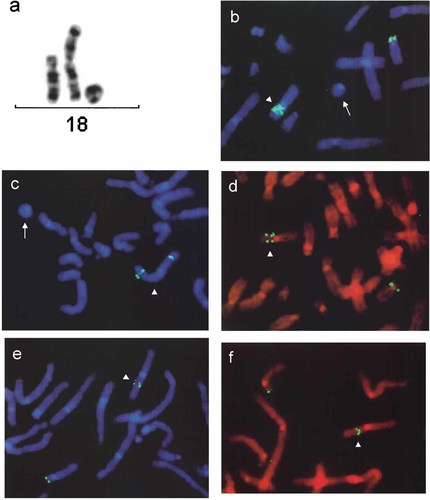
dup(18p) and dup(18)(q21.3qter) on abnormal der(18) chromosome in case 7 revealed by G banding (a) and the application of partial paint (b) and YACs probes: 847b5 (c), 826c11 (d), 881h1 (e), and 854g8 (f). Note the decreasing distances between signals of YAC probes 826c11, 881h1, and 854g8 (d–f), indicating the inverted nature of the 18p duplication. The r(18) chromosome is indicated by an arrow and the dup(18) chromosome by an arrowhead.
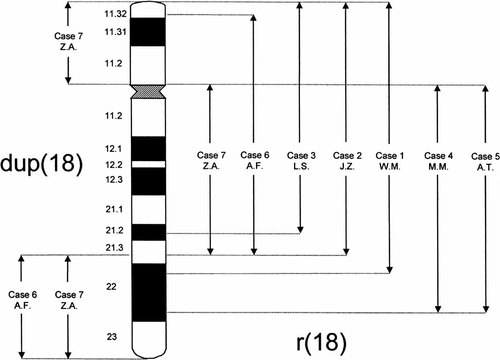
Results of molecular-cytogenetic analysis of seven patients with the r(18) chromosome. The assigning of YACs to chromosome 18 G-bands was done according to their Flpter values, determined from the database of MIP for Molecular Genetics.
DISCUSSION
Patients with ring chromosome 18 often share typical features with 18q- syndrome, 18p- syndrome, or a combination of both. Most of the characteristic traits of 18q- syndrome were found in cases 1–3, and of 18p- syndrome in cases 4 and 5. So far, several haploinsufficient genes have been postulated to play a role in the 18q- and 18p- syndrome phenotypes: HPE4 (18p11.3), holoprosencephaly [Overhauser et al., 1995]; GALNR1 (18q23), growth hormone deficiency [Cody et al., 1997a]; and MBP (18q23), hypomyelination [Gay et al., 1997]. Recently, Leach et al. [1999] hypothesized that the DCC gene (deleted in colon cancer, 18q21.2) may be associated with the severity of the neurological development in the 18q- syndrome phenotype in patients with large deletions of chromosome 18. The presence of this gene on r(18) in case 3 is indicated by only moderate mental retardation. The absence of IgA deficiency in all patients, except case 2, may be explained by the dosage compensation of the NFATC1 gene proposed recently by Wang et al. [1999]. A similar mechanism may concern the MBP gene, which was deleted in all nonmosaic r(18) cases, but the abnormal myelination features were found only in three patients.
The precise characterization of r(18) chromosomes can provide better predictive information of the phenotypes. The easiest method of their characterization is the application of a combination of both whole and partial chromosome 18 paints. Our results indicate that there are two main types of r(18)—one with a breakpoint at the centromere, or adjacent to it, and deletion of the whole 18p; and the second with only the deletion of distal 18q. We suggest that the association of a short neck with the absence of congenital cardiac anomalies may indicate the deletion of the 18p material on r(18) chromosome. Depending whether the 18p or 18q deletion is identified, the patients can then be followed by the appropriate dental or ophtalmological, otolaryngological, and endocrinological care (The Chromosome 18 Registry & Research Society).
In addition to the 18q- and 18p- syndromes, the presence of dosage-sensitive genes on chromosome 18 is further confirmed by patients with partial duplications of 18q presenting with Edwards syndrome phenotype, as well as with dup(18p). However, the last abnormality is usually not associated with major malformations [Johansson et al., 1988; Wolff et al., 1991; Moog et al., 1994; Li et al., 1998]. Interestingly, no typical Edwards syndrome features were observed in cases 6 and 7, although according to the duplicated regions one could expect them [Mewar et al., 1993; Boghosian-Sell et al., 1994]. Besides several symptoms characteristic for dup(18)(q22qter), the following combination of features observed in both cases may be typical for the described association of r(18) and dup(18): long face, broad nares, malocclusion, winged scapulae, wide finger tips, and speech delay.
We propose a novel mechanism of the formation of duplications and ring chromosomes found in cases 6 and 7. From our FISH and microsatellite polymorphism data in case 7, we suggest that the aberration could arise in paternal meiosis II during interchromatid junction and simultaneous or subsequent loop excision as demonstrated in Figure 5. Alternatively, as no two distinct paternal alleles were found for any analyzed markers, an early postzygotic error cannot be excluded. Interestingly, the vast majority of trisomy 18 and i(18p) chromosomes arises in maternal meiosis II [Bugge et al., 1996; Kotzot et al., 1996].
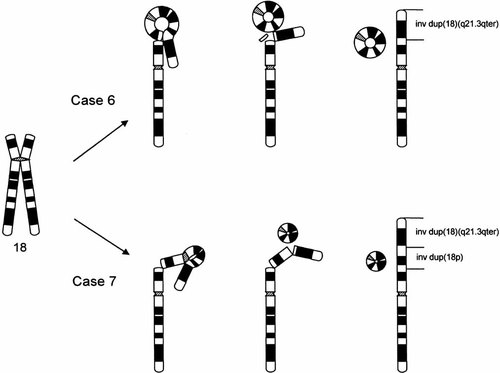
Schematic representation of suggested simultaneous inter-intrachromatid rearrangement mechanisms in cases 6 and 7. Note the small 18p11.32 “tearing” acentric fragment from r(18) in case 6.
The same mechanism can easily explain the identified abnormality in case 4, in which at the same time a very small acentric fragment 18p11.32 must have been lost (Fig. 5). A similar intrachromatid two-loop rearrangement in maternal meiosis II with two breakpoint pairs, 18p11.1::q21.1 and 18q11.1::q12.3, and a loop excision could have lead to the formation of the supernumerary marker chromosome der(18)(:p11.1→q11.1::q12.3→q21.1:) described recently by Röthlisberger et al. [2000].
No evidence of breakpoint clustering on chromosome 18 in individuals with 18q- syndrome have been reported [Strathdee et al., 1995; Cody et al., 1997b]. However, Röthlisberger et al. [2000] claimed the presence of a hot spot on 18q21 and five of nine 18q breakpoints identified in our cases map to the ∼3.5 Mb region between D18S69 and D18S1144 on 18q21.3.
The parental origin of identified r(18) could be established only in case 7. Although the preferential loss of the paternal alleles was demonstrated in the 18q- syndrome [Strathdee et al., 1995; Cody et al., 1997a], the authors could not find evidence for the genomic imprinting effect [Cody et al., 1997b]. These data have been recently confirmed by Bowen et al. [1999] and Maiwald et al. [2000]. However, the fact that no UPD18 cases have been described yet [reviewed by Kotzot, 1999] may be associated with the presence of such genes on this chromosome and early miscarriages [Kotzot et al., 1996].
ELECTRONIC-DATABASE INFORMATION
Molecular Cytogenetics and Positional Cloning Center, Max-Planck-Institute for Molecular Genetics, http://www.molgen.mpg.de/∼cytogen/CHRM18.HTM#CHROMOSOME 18 Resources for Human Molecular Cytogenetics, http://bioserver.uniba.it/fish/Cytogenetics/webbari/2_YAC/YAChromosome/YAC-18.html. The Genetic Location Database LDB, http://cedar.genetics.soton.ac.uk/pub/chrom18/map.html. The Genome Database GDB amplimer query, http://www.hgmp.mrc.ac.uk/gdb-bin/genera/genera/hgd/Amplimer?!action = queryform. The Chromosome 18 Registry & Research Society. San Antonio, Texas, http://www.chromosome18.org/syndrome.htm
Acknowledgements
We thank Dr. M. Rocchi from Istituto di Genetica, Università Degli Studi, Bari in Italy for kindly providing us with the pcp18p and YAC 882A10, 772B9, and 819C1 probes. We are very grateful to Drs. J.D. Cody and E. Obersztyn for critical reading of the manuscript and Dr. L. Shaffer for helpful discussion.




