Variegated aneuploidy related to premature centromere division (PCD) is expressed in vivo and is a cancer-prone disease
Abstract
We present three patients with variegated aneuploidy and premature centromere division (PCD), a rare chromosomal abnormality in humans. Comparison of these three and eight other patients with variegated aneuploidy related to PCD demonstrates a phenotype comprising most frequently micro- cephaly, CNS anomalies (with cerebellar affection and migration defects), mental retardation, pre-and postnatal growth re-tardation, flat and broad nasal bridge, apparently low-set ears, eye and skin abnormalities, and ambiguous genitalia in male patients. The occurrence of Wilms tumor in three patients, rhabdomyosarcoma in two others and acute leukemia in a fifth characterizes this condition as a chromosome or genome instability disorder with a high risk of malignancy. FISH studies in uncultured blood and buccal smear cells demonstrate that the random aneuploidies are not limited to cultured cells, but also occur in vivo. © 2001 Wiley-Liss, Inc.
INTRODUCTION
-
Premature centromere division (PCD)-related variegated aneuploidy [Scheres et al., 1986; Miller et al., 1990; Warburton et al., 1991; Kajii et al., 1998; Kawame et al., 1999; Limwongse et al., 1999; D'Agostino et al., 2000; Matsuura et al., 2000];
-
Isolated variegated aneuploidy [Tolmie et al., 1988; Papi et al., 1989; Flejter et al., 1998; Rosensaft et al., 1999]; and
-
Roberts syndrome related variegated aneuploidy [Petrinelli et al., 1984; Jabs et al., 1991].
Chamla et al. [1980] described C-anaphases as cells in division that have overcome a colchicine-induced metaphase block. The resulting mitotic configuration shows split centromeres and splayed chromatids in all or most of the chromosomes. The terms PCD [Rudd et al., 1983; Gabarron et al., 1986; Scheres et al., 1986; Madan et al., 1987; Murthy and Prabhakara, 1990; Keser et al., 1996], premature anaphase [Bajnóczky and Gardó, 1993], premature chromatid separation [Kajii et al., 1998; Matsuura et al., 2000] and asynchrony of mitotic stages [Limwongse et al., 1999] have also been applied to describe the same phenomenon. This study will follow the most currently used term PCD.
Colchicine-treated lymphocyte cultures from normal individuals generally show low frequencies of PCD (up to 3% of the mitoses), that seems to have no pathological relevance or recognizable pattern of inheritance [Dominguez and Rivera, 1992]. An increase of the incidence of this baseline PCD has been reported under culture conditions of fragile site expression [Fuster et al., 1992] and in cultures exposed to diepoxybutane (DEB) from patients with multiple endocrine neoplasia type 1 [Sakurai et al., 1999]. An increase of PCD has also been found in standard lymphocyte cultures from unaffected and affected individuals from families with familial breast cancer [Rao et al., 1996], individuals with Fanconi anemia and ataxia-teleangiectasia [Méhes and Bühler, 1995] and in normal individuals after exposure to genotoxic chemicals [Dolara et al., 1994; Major et al., 1999].
In some individuals, however, a PCD frequency of 5% or more is found in colchicine exposed lymphocyte cultures, that has been estimated to occur in a frequency of 0.1% of the population [Chamla, 1988]. This type of PCD has autosomal dominant inheritance (MIM 176430) and has not only been seen in blood lymphocytes but also in skin fibroblasts, hair-root [Chamla, 1988], bone marrow [Kawame et al., 1999] and trophoblastic cells of chorionic villi [Mediano et al., 1992]. Association of this PCD trait with subfertility and repeated abortion has been noted [Rudd et al., 1983; Gabarron et al., 1986] but other authors consider this as an ascertainment bias and suggest the trait to be harmless [Madan et al., 1987; Chamla, 1988; Dominguez and Rivera, 1992].
In few patients an increased frequency of PCD was found in combination with an increased number of cells with mosaic aneuploidies (variegated aneuploidy). These patients may have microcephaly, mental retardation and a variety of malformations (Table IV).
We report clinical and cytogenetic data on three such patients with PCD and variegated aneuploidy. One of them is the first patient in whom this condition was ever recognized; however, until now only partial and experimental cytogenetic data were published on this index-patient [Scheres et al., 1986; Unteregger et al., 1987] who died of leukemia. The other two cases were detected prenatally because of intrauterine growth retardation; one of them developed a rhabdomyosarcoma at an early age.
CLINICAL REPORTS
Case 1
The proposita was a mentally retarded girl with microcephaly, small stature (147 cm at the age of 29) and primary amenorrhea. She was the second child of a healthy and non-consanguineous mother and father who at the time of her birth were 26 and 32 years old, respectively. Her brother was also mentally retarded. The pregnancy had been uneventful. At 3 months she experienced a period of vomiting and from that time growth declined. At that time “microcephaly” was noted but not documented. When she became sexually active in adulthood her mother asked for contraceptives. Although she had normal secondary characteristics an Ulrich-Turner syndrome variant was considered because of small stature and primary amenorrhea. At 29 her chromosomes were investigated and premature centromere division was found (for results see below). To exclude Roberts syndrome X-ray examination of the upper limbs was performed that showed a normal skeleton. At 42 the patient died of acute nonlymphocytic leukemia (M4 subtype); with the exception of a single bone marrow biopsy the patient's mother had refused all further diagnostic procedures. Partial descriptions of clinical and experimental data from this patient were presented by Scheres et al. [1986] and Unteregger et al. [1987].
Case 2
The patient, a girl, was the second child of healthy, non-consanguineous parents. At her birth, the mother was 30 and the father 35 years old. The patient's brother is well and the pedigree is unremarkable. At the 35th week of pregnancy growth retardation was noted by ultrasonography and it was decided to perform a Caesarean section. Apgar scores were 8/9/1. Birth weight was 1,700 g (−2 SD), length 44 (−0.8 SD) cm and occipito-frontal head circumference (OFC) 27.5 cm (−2.6 SD). Anomalies identified at birth included severe micro-cephaly and single umbilical artery. Cerebral ultrasound, EEG and funduscopic findings and results of viral serological studies were normal. At 4 months, horizontal nystagmus was noted and at 5½ months, mildly delayed motor development. At this time visual evoked potentials (VEPS) were immature and CAT scan documented cerebellar vermis hypoplasia, ventriculomegaly and colpocephaly. At 9 months weight was 6,700 g (−1.7 SD), length 65 cm (−1.8 SD) and OFC 39 cm (−4 SD).
Case 3
The patient, a girl, was the second child of healthy, non-consanguineous parents of Chinese origin. At birth, the mother was 33 and the father 32 years old. Her brother is well. The pregnancy had been uneventful until the 23rd week, when ultrasonographic exploration showed a fetal growth retardation of 2 weeks. At the 38th week, a severe growth retardation of −9 weeks and oligohydramnios were noted. Apgar scores were 8/9/9. Birth weight was 1,600 g (−2.9 SD), length 41 (−2.8 SD) cm and OFC 26 cm (−4.5 SD). Anomalies identified at birth included convergent strabismus, lack of ocular movements, permanent thumb adduction, capillaris hemangioma simplex on the glabella, severe microcephaly, thin upper lip and square-shaped ears. Cerebral ultrasonography showed a dilated cisterna magna without lateral ventricle involvement. Nuclear magnetic resonance demonstrated a cyst in the posterior fossa and normal cerebellum. EEG and funduscopic examinations were normal. Results of viral serological studies were normal. At the age of 5 months a vaginal botryoid rhabdomyosarcoma was diagnosed.
CYTOGENETIC AND FISH STUDIES
Case 1
Chromosome examination of T and B lymphocytes (standard PHA and Pokeweed stimulated short-term blood lymphocyte cultures), cultured skin and ovary fibroblasts cells [Verma and Babu, 1995] showed PCD (Fig. 1a) in about 60% of the cells (Table I). Almost 15% of the cells without the PCD phenotype showed gain and loss of chromosomes (12% hyperploidy and 2.6% hypoploidy). Trisomy X, 8 and 18 were the most frequent aneuploidies (Fig. 2), but polysomy X and double trisomies also occurred. At that time (1984) interphase FISH-studies were not available in a clinical laboratory. In three of 842 interphase nuclei of buccal smears (0.4%), however, two distinct X-chromatin bodies could be seen. Increased frequencies of cells with PCD were also detected in the standard lymphocyte cultures of the patient's parents: 38/300 (12.6%) of the father's cells and 6/103 (5.8%) of the mother's cells. The frequency of PCD in cultured lymphocytes from the patient's mentally retarded brother was 3/110 (2.7%). No hyperploidies were found in the parents and brother of the patient.
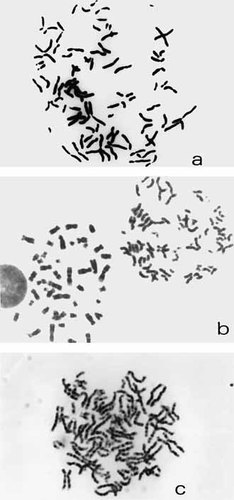
Cell of patient 1 showing PCD (a). Cells of Patient 2 showing PCD (left) and a normal metaphase (right) (b) and cell of Patient 3 showing PCD (c).
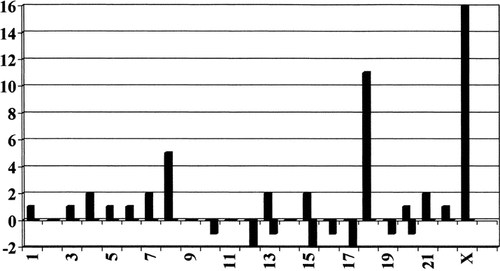
Involvement of the different chromosomes in the aneuploidies of 47 cells with chromosome gains and 10 cells with chromosome loss from a lymphocyte culture of Patient 1.
| Cell type | % cells with PCD | % normal cells | Number of cells |
|---|---|---|---|
| T-cells | 51 | 49 | 565 |
| B-cells | 44 | 56 | 106 |
| Fibroblasts | |||
| (ovary) | 65 | 35 | 20 |
| (skin) | 80 | 20 | 20 |
Case 2
Chromosome examination of two independent standard PHA-stimulated short-term blood lymphocyte cultures [Verma and Babu, 1995] (RPMI-1640 medium with 20% fetal calf serum, 3 days, 2 hr Colcemid) showed PCD (Fig. 1b) in 81% of the metaphases of the propositus (243/300). Cells not showing PCD phenotype had a high frequency of gain and loss of chromosomes (Table II). Chromosome examination showed PCD in 16.5% of the cultured lymphocytes of the father (19/115), in 1% (4/406) and 3% (16/517) of those of the mother (two different examinations), and in 4.9% (10/205) of the brother's lymphocytes. Interphase FISH studies were always carried out using a mixture of two probes to differentiate tri/tetraploid chromosome constitutions from aneuploidy. In this conditions, no artefactual trisomies were found in five control buccal mucosa smears [Català et al., 1999], five cultured and five uncultured control blood samples (unpublished results). Interphase FISH studies on cultured and uncultured blood from patient and his parents and on buccal mucosa of the patient were carried out using a mixture of directly labeled alphoid probes of chromosomes X and 18 (Vysis DXZ1 and D18Z1). An additional FISH study was carried out using a mixture of alphoid probes of chromosomes 19 and 22 (Oncor 19q13.1 and Vysis 22q13.1-bcr) in cultured and uncultured blood of the patient. In all cases hybridization were carried out according protocols provided by the manufacturer. In more than 500 nuclei of the parents no aneuploidies of chromosomes 18, 19, 22 or X were seen. Up to 6% aneuploid nuclei, however, were noted in the patient's tissue samples. Results of FISH-studies are summarized in Table III.
| Patient 2 | Father of patient 2 | Mother of patient 2 | Brother of patient 2 | Patient 3 | Father of patient 3 | Mother of patient 3 |
|---|---|---|---|---|---|---|
| (46 cells) | (87 cells) | (50 cells) | (50 cells)a | (50 cells) | (50 cells) | (50 cells) |
| 2 cells + 19 | 1 cell + 6 + 12 + 21 + 22 | 1 cell −4 −5 | 4 cells + 8 | 1 cell + 7 | 2 cells −19 | |
| 1 cell + 8 | 2 cells + 18 | |||||
| 1 cell + 13 | 1 cell −10 | |||||
| 3 cell + 21 | ||||||
| 1 cell + X | ||||||
| 1 cell + 15 | ||||||
| 1 cell + 17 | ||||||
| 1 cell + 14 + 19 | ||||||
| 1 cell + 5 + X | ||||||
| 1 cell + 14 + 19 + 21 |
- a No random gain or loss of chromosomes detected.
| Chromosomes tested | Tissue tested | Patient 2 | Father of patient 2 | Mother of patient 2 | Patient 3 |
|---|---|---|---|---|---|
| 18/X | Uncultured blood | 770 cells (5 cells +X, 2 cells +18) | 560 cells (all normal) | 550 cells (all normal) | 146 cells (20 cells −X, 6 cells +X, 7 cells −18, 4 cells +18) |
| Cultured blood | 503 cells (13 cells +X+X +18+18 and 17 cell +X +18) | ||||
| Buccal mucosa | 150 cells (4 cells +X) | 120 cells (5 cells +X, 3 cells −18, 3 cells +18) | |||
| 19/22 | Uncultured blood | 160 cells (all normal) | |||
| Cultured blood | 150 cells (3 cells +22 +22, 2 cells +22, 5 cells +19 +19) |
Case 3
Chromosome examination of a PHA-stimulated short-term blood lymphocyte culture of Patient 3 (RPMI-1640 medium with 20% fetal calf serum, 3 days, 2 hr Colcemid) [Verma and Babu, 1995] showed PCD (Fig. 1c) in 47% of the metaphases (38/80). Twelve percent of the cells without the PCD phenotype showed gain and loss of chromosomes (Table II). Chromosome examination of the parents showed PCD in 17% of the father's cells (35/165) and in 3.6% of those of the mother (22/610). Interphase FISH studies were carried out on uncultured blood and buccal mucosa cells from Patient 3 using a mixture of directly labeled alphoid probes of chromosomes X and 18 (Vysis DXZ1 and D18Z1) according to protocols provided by the manufacturer. Results are summarized in Table III; in about every fourth nucleus an aneuploidy for X or 18 was found.
DISCUSSION
We have presented three patients with the same, rare and peculiar cytogenetic abnormality, namely abnormal mitosis resulting in a high frequency of PCD and an unstable karyotype with variegated aneuploidy.
A comparison of these three patients with eight other cases from literature apparently having the same condition (Table IV) documents a clinical phenotype that most frequently includes microcephaly, CNS anomalies with cerebellar defects and migration defects, mental retardation, pre and postnatal growth retardation, flat and broad nasal bridge, apparently low-set ears, eye and skin abnormalities and ambiguous genitalia in male patients. The microcephaly, mental retardation and growth deficiency shared by most of these patients might well be the result of an increased cellular mortality induced by the aneuploidies during fetal and early postnatal development. Similar clinical findings have been found in a few other patients with variegated aneuploidy, but apparently not-related to PCD [Tolmie et al., 1988; Papi et al., 1989; Flejter et al., 1998] and in the “ring syndrome” described by Kosztolányi [1987], conditions all expected to have a high cellular mortality.
| Main clinical findings | Miller et al. [1990] | Warburton et al., [1991] | Kajii et al. [1998], Patient 1 | Kajii et al. [1998], Patient 2 | Limwongse et al. [1999] | Kawame et al. [1999] | D’ Agostino et al. [2000] | Matsuura et al. [2000], Patient 1 | Present report, Patient 1 | Present report, Patient 2 | Present report, Patient 3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | M | F | F | M | M | M | M | M | F | F | F |
| Age | 8 months | 17 year | 13 months | 7 months | 7 years | 19 months | ? | 29 years | 9 months | 5 months | |
| Weeks of gestation | 37 | ? | 37 | 40 | 33 | 37 | 34 | ? | ? | 35 | 38 |
| Birth weight (gm)a | 1300 (−3 SD) | ? | 1540 (−2.4 SD) | 1330 (−4.1 SD) | 1450 (−1.3 SD) | 1748 (−2.2 SD) | 1000 (−2.4 SD) | ? | ? | 1700 (−2 SD) | 1600(−2.9 SD) |
| Length (cm)a | 39 (−3.1 SD) (birth) | 44 (−2.7 SD) (17 years) | 40 (−2.8 SD) (birth) | 36.4 (−4.7 SD) (birth) | 30(−5.4 SD) (birth) | 39.5 (−2.5 SD) (birth) | ? | ? | 147(−2.2 SD) (29 years) | 44(−0.8 SD) (birth) | 41(−2.8 SD) (birth) |
| OFC (cm)a | 27 (−3.7 SD) (birth) | 43 (−9.8 SD)17 years) | 25.8 (−4.4 SD) (birth) | 25.0 (−5.8 SD) (birth) | 47 (−3.3 SD) 7 (years) | 27.8 (−3.3 SD ) birth | ? | ? | ? | 27.5(−2.6 SD) (birth) | 26 (−4.5 SD) (birth) |
| Microcephaly | + | + | + | + | + | + | + | + | + | + | + |
| Mental retardation | + | + | + | + | − | ? | ? | + | + | + | + |
| Growth retardation (fetal detection) | 34 weeks | + | 32 weeks | 28 weeks | 27 weeks | 20 weeks | ? | + | + | 35 weeks | 23 weeks |
| CNS anomalies | ? | ? | + | + | − | + | ? | + | ? | + | + |
| Flat, broad nasal bridge | + | + | + | ? | ? | + | ? | ? | − | − | − |
| Low set ears | + | + | + | + | − | + | ? | ? | ? | − | − |
| Eye abnormalities | Nystagmus | Myopia, esotripia | Bilateral cataract | Bilateral cataract | − | Coloboma, cataracts | ? | − | +b | Nystagmus | Strabismus |
| Skin abnormalities | Hemangiomata | Atopic dermatitis | − | − | − | − | ? | ? | − | − | Hemangiomata |
| Seizures | + | + | − | + | − | + | ? | + | − | − | − |
| Ambiguous genitalia | + | + | − | + | + | − | |||||
| Malignancys | − | − | Wilms tumor | − | Rhabdomyosarcoma | Wilms tumor | ? | Wilms tumor | Acute nonlymphocytic leukemia (M4 subtype) | − | Rhabdomyosarcoma |
| Patient % PCDc | 39–51% | + | 67–86.5% | 84–87.5 | + | 82% | < 25% | < 48% | 60% | 81% | 47% |
| % aneuploid cellsc | 78% | 6–20% | 14.5–20% | 7–15% | 7.7–34% | 17–35% | 40% | + | 15% | 28% | 12% |
| Parents % PCDc | − | ? | Mother 2.5–13.5% Father 15.5% | Mother 32.5% Father 11% | ? | Mother 42.5% Father 5% | ? | ? | Mother 5.8% Father 12.6% | Mother 3%d Father 16.5% | Mother 3.6% Father 17% |
| Parents age at delivery | Mother 24 Father 29 | Mother 28 Father 28 | Mother 26 Father 26 | Mother 30 Father 37 | Mother 20 Father ? | Mother 27 Father 25 | ? | ? | Mother 26 Father 32 | Mother 30 Father 35 | Mother 33 Father 32 |
- a SD was recalculated in all cases to allow comparisons.
- c On first visual contact, patient usually turns eyes extremely upwards showing only the white of her eyes.
- b % PCD were calculated on lymphocyte cultures.
- d Only 1% in a repeated culture.
Cancer seems to be a major concern in the clinical management of these patients. The occurrence of malignancy in five of the 11 patients (Wilms tumor in three patients, rhabdomyosarcoma in two others and acute leukemia in the fifth, Table IV) clearly confirms the high cancer-risk of this disorder, as was previously also suggested by Kajii et al. [1998] and Kawame et al. [1999]. Most of the non-random trisomies reported in embryonal rhabdomyosarcoma [Weber-Hall et al., 1996] and Wilms tumor [Soukup et al., 1997] have also been reported to be continuously produced in patients with variegated aneuploidy. Preferential loss of the maternal 11p15.5 chromosome region has been reported repeatedly in Wilms tumor [Mannens et al., 1988] and in rhabdomyosarcoma [Scrable et al., 1989]. It seems quite acceptable that in patients with PCD-related variegated aneuploidy unstable mitosis can lead to cells with various chromosome 11 constitutions, such as uniparental disomy or monosomy, and give rise to Wilms tumor or rhabdomyosarcoma. Preferential loss of a particular parental allele has also been observed in at least one type of leukemia [Katz et al., 1992].
The previous discussion has assumed that the variegated aneuploidy occurs in vivo. Case 1 was diagnosed years before the general introduction of FISH techniques, when reliable interphase studies were only possible with the X and Y chromatin [Scheres et al., 1986]. The presence of two distinct X-chromatin bodies in three of 842 interphase nuclei of buccal smears suggests, but does not prove, the occurrence of aneuploidy of chromosome X in vivo. Bone marrow studies in two other patients [Warburton et al., 1991; Kawame et al., 1999] strongly suggest the occurrence of aneuploidy of several chromosomes in vivo. FISH studies in uncultured blood and buccal smear of our Patients 2 and 3 confirm that random aneuploidies, but not tetra/triploidies, occur in vivo. A substantial number of cells with trisomy or tetrasomy 19 were present in the blood cultures of Patient 2 but they could not be found in vivo. This might suggest that in this disorder aneuploidy of every chromosome might be the result of the disturbed mitosis, but only a subset of aneuploidies is tolerated in vitro and a most limited subset in vivo. Tissue specific selective factors seem to operate in the presence of random aneuploidies. Trisomies 8, 18 and X predominate in lymphocyte cultures of patients with variegated aneuploidy whereas trisomy 2, 7, 12 and 20 predominates in fibroblasts [Warburton et al., 1991; Kajii et al., 1998; Kawame et al., 1999]. The latter chromosomes are also frequently involved in mosaicism diagnosed in amniocyte cultures for prenatal diagnosis [Hsu et al., 1992, 1997].
Kajii et al. [1998] suggested that patients with PCD and variegated aneuploidy would be homozygous, that would have important consequences for the genetic counseling of a not totally infrequent trait (possibly 0.1% of the population) [Chamla, 1988]. The finding of slightly or even highly increased rates of PCD in both parents of at least four patients (Table IV) seems to confirm this interpretation. We found a maximum of only 3% and 3.6% cells with PCD in the mothers of Patients 2 and 3. Both parents of Miller's patient also were within normal limits (although no paternity testing was performed). Kajii et al. [1998] reported the mother of one patient to have only 2.5% positive cells on first examination but she had 13.5% positive cells in a repeat examination. Therefore, we also performed a repeated examination of the mother of our Patient 2 but found even a lower frequency of positive cells than in her first culture (only 1%). If in this disorder the parents indeed are heterozygous, the mother of our Patient 2 would reduce the lower heterozygote limit to 1%, a figure frequently seen in normal individuals [Dominguez and Rivera, 1992]. It is possible that other factors, perhaps hormonal, influence the expression of PCD in a carrier, especially in females. An alternative explanation might be that this disorder concerns different genes, or different mutations of the same gene mutated in the families with PCD and variegated aneuploidy. Patients are known with random aneuploidy but without PCD [Tolmie et al., 1988; Papi et al., 1989; Flejter et al., 1998; Rosensaft et al., 1999]. Finally, several mechanisms are known that can lead to a homo-or hemizygous state in a patient even when one of the parents is not heterozygous, such as isodisomy of one chromosome [Voss et al., 1989] and loss of heterozygosity [Cavenee et al., 1983].
It is clear that more studies are necessary to obtain more insight into PCD with or without variegated aneuploidy, its inheritance and its effects. This is especially needed to enable us to discern normal individuals with low-grade PCD frequencies from those individuals who have similarly low frequencies of PCD but in fact are carriers of a gene that increases their risk of abnormal offspring, of cancer in their offspring and maybe also in themselves. Very recent data suggest those patients having a defective mitotic-spindle checkpoint [Matsuura et al., 2000]. The elucidation of the molecular defect in patients with such aberrant mitosis will probably yield new clues to the complex tuning and checkpoint mechanisms that regulate the duplication and correct separation of chromosomes, and that are of fundamental significance for the propagation of cells, for the health of the organism and for the conservation of its species.




