Case with autistic syndrome and chromosome 22q13.3 deletion detected by FISH
Abstract
Autism is a rare neurodevelopmental disorder with a strong genetic component. Co-occurrence of autism and chromosomal abnormalities is useful to localize candidate regions that may include gene(s) implicated in autism determinism. Several candidate chromosomal regions are known, but association of chromosome 22 abnormalities with autism is unusual. We report a child with autistic syndrome and a de novo 22q13.3 cryptic deletion detected by FISH. Previously described cases with 22q13.3 deletions shared characteristic developmental and speech delay, but autism was not specifically reported. This case emphasizes a new candidate region that may bear a gene involved in autism etiopathogenesis. Am. J. Med. Genet. (Neuropsychiatr. Genet.) 96:839–844, 2000. © 2000 Wiley-Liss, Inc.
INTRODUCTION
Autism is a relatively rare neurodevelopmental disorder resulting in abnormal or delayed development affecting social, communicative, and behavior skills [Rapin, 1997]. Its differential diagnosis from mental retardation is related to the qualitative impairment in reciprocal social interactions and social communication abilities [Gillberg, 1990]. Age of onset is before 3 years. A strong genetic component in pathogenesis of autism is supported by results of family and twin studies [Bolton et al., 1994; Bailey et al., 1995] and co-occurrence between autism and chromosomal abnormalities [for review, see Gillberg, 1998; Lauritsen et al., 1999]. In the latter cases, candidate genes for autism could be localized close to chromosomal breakpoints or in deleted or duplicated regions, and many candidate regions have been localized. However, association of chromosome 22 abnormalities with autism appears unusual in regard to their association with other psychiatric disorders, especially with schizophrenia.
We describe a child with autistic syndrome and a de novo 22q13.3 cryptic deletion unexpectedly detected by FISH after routine cytogenetics failed to discover any visible anomaly. Using the commercially available Oncor (Gaithersburg, MD) probe for DiGeorge syndrome to exclude a 22q11.2 deletion in the child, we identified a deletion of the control probe, located distally in 22q13.3. This is the first report of 22q subtelomeric deletion associated with autism, suggesting a new candidate region which could be screened by FISH in autistic patients.
CASE REPORT
Medical History
The proband, a 14-year-old girl, is the second child of four, born to non-consanguineous, healthy parents. The proband's mother had one spontaneous abortion before four term-delivered pregnancies. Apart from this episode, family history was unremarkable. Pregnancy was marked during the fourth month by uterine contractions, secondary to a viral respiratory infection, successfully treated with progesterone and rest. At term, delivery was uneventful (Apgar scores at 10). At birth, weight was 3,400 g, length 50 cm, and occipito-frontal circumference (OFC) 34 cm. In early infancy, moderate and fluctuant hypotonia was noted with frequent opisthotonos fits. Recurrent otitis led to an audiogram showing no hearing impairment. Abdominal ultrasound, performed at age 5 years after a urinary infection, revealed a duplicate right kidney. She was successfully treated with valproate after partial seizures at age 8.
Physical Examination
At age 14, her weight was 46 kg (M), height was 154 cm (−1 SD) and OFC was 54 cm (−0.5 SD). She had subtle facial dysmorphic features including moderate hypertrophic nasal root, upslanting palpebral fissures, and thick lips (Fig. 1). Oral cavity was normal. Fingers were thin and moderately tapering. There was a unilateral unique transverse palmar crease. Neurological examination was normal. She had a moderate scoliotic deviation. Constipation was present. Metabolic screening and neurological investigations, including electroencephalogram, brain CT scan, and MRI, were normal.

Frontal view of the patient at age 14 years. Note moderate hypertrophic nasal root, upslanting palpebral fissures, thick lips, and relatively normal appearance.
Psychiatric Examination
Early development from birth to age 5 years
Parent worries began when the patient was 6 months because they observed marked differences compared to their first child. She was diagnosed as having autistic disorder at age 18 months and treated for more than 10 years. She displayed early signs of deviant psychomotor development and all developmental milestones were delayed. She sat without support at 8 months and walked alone at 22 months. After an evident lack of babbling, speech began at 7 months with monosyllabic repetitions, but she stopped talking 1 month later and did not say a word for 2 years. Since age 6 months and for a period longer than 3 years, she had feeding problems with oppositional behavior and vomiting, and sleep troubles with nightmares and difficulty falling asleep. She was potty-trained at 4 years.
The patient failed to demonstrate anticipatory social responses. She avoided eye contact and did not look even when she was called. Lack of social smiling and poor facial expression usually made her look anxious. She seldom tried to catch objects and showed lack of interest and joint attention for anyone. She could not calm down when her mother held her and she demonstrated aggressive attitudes such as biting her bed or herself. She also had stereotypical movements such as rocking behaviors. Later, she avoided physical contact and often withdrew. She frequently used other people's bodies as a tool, for example, directing an adult's hand toward a desired object. Changing daily routine led her to demonstrations of anger. Before age 5 years, she did not play with toys in an appropriate manner and had repetitive attitudes such as smelling objects or putting them in her mouth. There was an absence of pretend play until she was 8 years old.
Psychiatric Assessment
The patient, age 14, met the DSM IV [APA, 1994] criteria for autistic disorder and attended a special psychoeducational establishment for 10 years. Delayed and abnormal functioning was noticed in social interaction, language, and symbolic play before her third birthday.
-
Lack of reciprocal gaze interaction: sometimes she had eye contact but generally she had peripheral gaze and avoidance of eye contact, particularly with strangers.
-
Failure to develop peer relationships: she could play imaginative games with toys but never played with other children; she never interacted with nonfamiliar peers and could only stand by and remain passive.
-
Lack of emotional reciprocity: she was able to share emotions, but she could laugh when someone was hurt or be serious when everyone else was laughing.
-
Lack of social reciprocity: she could look for shared interests or enjoyment with her family but she could also refuse to communicate, avoid eye contact, and withdraw; she never initiated interaction with others and avoided crowded situations.
She had qualitative impairments in communication, with important delay of spoken language and lack of communicative gestures. She had make-believe games with dolls but no social imitative play.
-
She showed temper tantrums when established routines were altered; for example, she wanted to drink each time from the same glass, had to arrange her dolls in a special way before going to sleep, and always wanted to empty the garbage can.
-
She had stereotyped and repetitive motor mannerisms: she showed stereotypical movements of hands and inappropriate shouts when enjoying something; in supermarkets she always walked with her legs apart; she otherwise moved stiffly, turning her knees inwards with a scoliotic attitude, but she could run more easily than she walked; fine motor skills were limited because she only used her thumbs and forefingers and kept the other three fingers clenched.
Furthermore, the child showed a high threshold of pain sensitivity and absent or delayed defensive reaction, frequently found in autism.
At age 14 years, 6 months, a psychiatric evaluation was undertaken. PEP-R (Psycho Educational Profile-Revised) [Schopler et al., 1994] and WISC-III (Wechsler Intelligence Scale for Children) [Wechsler, 1991] showed that all developmental milestones were delayed. Her global IQ score was 32 (4.5-year level), verbal skills were 14 (2-year level) and nonverbal skills were 51 (7-year, 3-month level). Imitation, perception, and gross motor skills were less delayed than verbal abilities, fine motor skills, and oculo-manual coordination. Severe expressive speech delay contrasted with comprehension abilities. She could speak a few words, albeit some mispronounced, and straightforward sentences with intent to communicate or not. She had cognitive difficulties in spatial perception, temporal conceptualization, flexibility, and mental representation. We assessed autistic disorder using the Vineland Adaptative Behavior Scale (VABS) [Sparrow et al., 1984] and the Children Autistic Rating Scale (CARS) [Schopler et al., 1986] with both parents. Specific symptoms of autism were obtained on CARS. Her score was 31, corresponding to a moderate autistic disorder. On the VABS, she displayed a communication coefficient corresponding to that of a person with a severe mental deficiency (SC = 41, 1-year, 9-month level). Receptive language skills (2-year, 11-month level) were better than expressive skills (1-year, 8-month level). Socialization coefficient was 78 (5-year, 3-month level) and Daily Living Skills score was 89 (4-year, 10-month level). Daily Living Skills domain placed her Domestic Skills at the 8-year level, while it was only at the 4-year, 1-month level for Personal Performance and 3-year, 8-month level for Community Behaviors. Socialization domain assessed Coping Skills at the 5-year, 10-month level, Play and Leisure at the 4-year, 11-month level, and Interactions with Others at only the 4-year, 5-month level.
Genetics and FISH Studies
Chromosomes were prepared from peripheral blood lymphocyte cultures from the patient and her parents. Karyotyping with RBG banding was performed at the 550 band level according to standard methods. FISH was performed twice with D22S75 DiGeorge chromosome region probes, using either D22S39 or STS WI-941 chromosome 22 probes as a control (Oncor digoxigeninylated probe and dual color probe, respectively), according to the manufacturer's instructions. D22S39 locus is more distal than STS WI-941 locus. Hybridization to the previously denatured chromosomes was done overnight at 37°C. Posthybridization wash was carried out at 72°C in 2× SSC (5 min). Digoxigeninylated probe detection was performed with mouse antidigoxin-conjugated fluorescein isothiocyanate (FITC; Sigma, St. Louis, MO). Slides were analyzed on a Zeiss Axioskop microscope equipped with a Pinkel filter set for visualization of FITC, Cy 3, and DAPI fluorescence. Digital images were recorded with a Photometrics cooled CCD camera. Pseudocoloring and merging of images were done with the Mac Probe 3.3 software (PSI, League City, TX). Metaphases were karyotyped by direct identification of chromosomes on the digital image of the DAPI and Vectashield double stain according to the reverse band standard karyotype [Korenberg et al., 1992]. Symmetrical labeling of sister chromatids on both chromosome homologs was evaluated on more than 20 metaphase spreads.
Karyotype of the patient was normal. Although no deletion of the D22S75 probes was evidenced, a cryptic heterozygous microdeletion for D22S39, the more subtelomeric control locus, was observed (Fig. 2). The presence of STS WI-941 signals in appropriate localization allowed us to rule out the existence of a paracentromeric inversion with a breakpoint in 22q11.2 (data not shown). The normality of both parents' karyotypes and FISH analysis, the latter technique showing no asymmetric or ectopic signal on any other chromosome (data not shown), excluded a parental balanced translocation. This microdeletion 22q13.3 must, therefore, have occurred de novo.
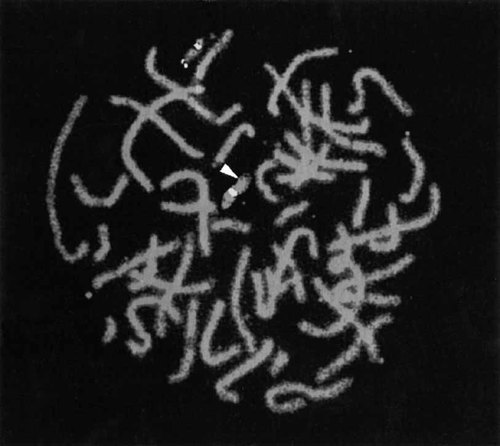
FISH analysis of representative chromosomes 22 from the proband showing deletion of D22S39. Proximal signals indicate hybridization of D22S75 and distal signal indicates hybridization of D22S39. Arrow indicates absence of signal on distal end of one chromosome 22.
DISCUSSION
We identified a de novo 22q13.3 deletion by FISH in a patient displaying developmental delay with autistic syndrome, mental retardation, subtle dysmorphic traits, and kidney malformation. The autistic disorder was moderate but improved after 10 years of psychiatric, educational, and speech therapy. The patient had nonspecific autistic signs, such as lack of flexibility, stereotyped movements, and nonadapted responses to emotional situations. However, there is actually no doubt that she also had specific symptoms of autism, such as social and communicative impairments and very precocious signs of deviant development [Vig and Jedrysek, 1999]. Thus, this is a case of precocious pervasive developmental disorder with autistic disorder and mental retardation. Although the pattern of VABS is not characteristic of autistic disorder, it doesn't exclude it. For instance, the Sociability quotient was higher than the Daily Living Skills sum score (difference of 5 months) and much better than the Communication score. Daily Living Skills subscale scores are therefore heterogeneous, with a Domestic performance at the 8-year level, much higher than Personal or Community skills (around 4 years). Socialization subscale results were more homogeneous; Interaction with Others being the most delayed (4-year, 5-month level). Thus, all subscales scores of the socialization domain were much more delayed compared to domestic performance.
Chromosome 22 alterations have been rarely described in autistic patients [Gillberg, 1998; Lauritsen et al., 1999]. Recently, three additional cases were reported. A translocation (20;22) (q13.3;q11.2) was found in an autistic 3-year-old boy with mildly dysmorphic face and no speech [Carratala et al., 1998]. A ring 22 chromosome was identified in a 13-year-old boy with autism but no dysmorphic trait [Assumpcao, 1998]. A girl with velocardiofacial syndrome (VCFS), profound mental retardation (IQ = 13), and autism was demonstrated to carry a 22q11.2 microdeletion using dosage analysis of two markers [Kozma, 1998]. Other psychiatric disorders have been frequently linked to chromosome 22q11.2 deletion, particularly in association with VCFS [Carlson et al., 1997]. FISH studies and molecular analysis documented 22q11.2 deletion in 20% of 15 schizophrenic patients with abnormalities characteristic of VCFS, such as cardiac anomalies or cleft palate [Gothelf et al., 1997]. The case reported by Kozma [1998] is therefore remarkable since it represents the only case of autism associated with a 22q11.2 deletion.
More interesting, 19 cases of 22q13.3 deletion have been reported to be associated with constant developmental delay and other occasional traits, depending on the size of the deletion, but no autistic syndrome. A summary of the clinical findings in these patients is provided in Table I. Thirteen deletions were cytogenetically apparent [Watt et al., 1985; Herman et al., 1988; Kirschenbaum et al., 1988; Romain et al., 1990; Zwaigenbaum et al., 1990; Narahara et al., 1992; Phelan et al., 1992; Nesslinger et al., 1994]. Two were revealed by Southern blot analysis in a screening of a population of mentally retarded subjects [Flint et al., 1995], while only four were identified by FISH analysis [Doheny et al., 1997; Precht et al., 1998]. In Doheny's report [1997], FISH was used to confirm a suspicion of a subtle subtelomeric deletion after routine cytogenetic analysis. In the report of Precht et al. [1998], deletions were serendipitously detected by FISH since, as in our case, studies were directed to rule out DiGeorge syndrome. Although the severity of the phenotype may vary among patients depending on the size of the deletion, clinical presentation of these patients is remarkably similar, with a common phenotype including hypotonia, as well as developmental and speech delays. Dysmorphic traits are frequently associated but can be lacking, especially in patients with an infra-cytogenetic deletion. Epicanthic folds, dysplastic ears, and dolichocephaly are frequently observed with a 22q13.3 cytogenetically apparent deletion, but facial dysmorphism is absent or subtle in the other cases. Hypotonia and developmental delay are features commonly observed in many syndromes; however, in association with severe speech delay these phenotypes may be a significant indication to test for a 22q13.3 deletion [Precht et al., 1998]. It has been proposed that these features constitute a recognizable dysmorphic and developmental syndrome [Zwaigenbaum et al., 1990].
| Features | Cases with cytogenetically apparent 22q13 deletiona | Cases with cytogenetically invisible 22q13 deletion | |||
|---|---|---|---|---|---|
| Total |
Flint et al., 1995 |
Precht et al., 1998 |
Our case | Total | |
| Birth measurements appropriate for | |||||
| gestation age | 13/13 | ? | 1/2 | Yes | 2/3 |
| Neurological anomalies | |||||
| Hypotonia | 12/15 | ? | 2/2 | Yes | 3/3 |
| Developmental delay | 15/15 | 2/2 | 2/2 | Yes | 5/5 |
| Delay of gross motor milestones | 15/15 | 0/2 | 2/2 | Yes | 3/5 |
| Delays or absence of expressive speech | 13/13b | 2/2 | 2/2 | Yes | 5/5 |
| Mild dilatation of cerebral ventricles | 5/15 | 0/2 | 1/2 | No | 1/5 |
| Seizures | 3/15 | ? | 0/2 | Yes | 1/3 |
| Dysmorphic features | |||||
| Dolichocephaly | 8/15 | 0/2 | 0/2 | No | 0/5 |
| Ptosis | 5/15 | 0/2 | 0/2 | No | 0/5 |
| Epicanthic folds | 9/15 | 0/2 | 1/2 | No | 1/5 |
| Dysplastic ears | 9/15 | 1/2 | 0/2 | No | 1/5 |
| Hypertrophic nasal root or prominent | |||||
| nose | 2/2 | 1/2 | 1/2 | Yes | 3/5 |
| Kidney anomalies | 2/15c | ? | 0/2 | Yes | 1/5 |
- a Cases described in Watt et al., 1985; Herman et al., 1988; Kirschenbaum et al., 1988; Romain et al., 1990; Zwaigenbaum et al., 1990; Narahara et al., 1992; Phelan et al., 1992; Nesslinger et al., 1994; and Doheny et al., 1997 reports. Number in denominator reflects whether the feature was mentioned in the report. Note that the patient in Phelan et al. and patient A in Zwaigenbaum et al. are the same as FB and QM, respectively, in Nesslinger et al.
- b Two patients were too young to ascertain.
- c Two cases with vesicoureteral reflux, one with additional bilateral hydronephrosis; ? = information not provided.
Our case adds to the list of patients with the 22q13.3 deletion syndrome and emphasizes that autistic syndrome can be part of the syndrome. None of the previously reported cases had been described as autistic, but their precise psychiatric and psychometric assessment are often lacking. The particular pattern of the VABS observed in our patient could provide a clue to the subgrouping of this pervasive developmental syndrome as it relates to autism. In the light of our observations, the chromosome 22q13.3 region could be a candidate region to contain gene(s) implicated in autism determinism. After the molecular characterization of a terminal deletion found in one patient [Wong et al., 1997], it has been postulated that a gene(s) involved in expressive speech maps within 130 kb of the telomere [Precht et al., 1998]. It will be of great interest to investigate the role of this potential gene in the etiopathogenesis of autism.
Finally, subtle telomeric rearrangements have been demonstrated as the commonest cause of mental retardation in children with undiagnosed moderate-to-severe mental retardation [Knight et al., 1999]. In autism, telomeric rearrangements involving other chromosomes have also been described [Gillberg, 1998; Lauritsen et al., 1999]. Sensitivity of a more general screen of the chromosomes ends by FISH, rather than a single assay for 22q13.3 deletion, could be evaluated in an autistic and mentally retarded population in further studies.
Acknowledgements
The authors thank the family for their collaboration and the reviewers for valuable comments.




