Genotype–phenotype correlations and clinical diagnostic criteria in Wolf-Hirschhorn syndrome
Abstract
We report on a clinical-genetic study of 16 Wolf-Hirschhorn syndrome (WHS) patients. Hemizygosity of 4p16.3 was detected by conventional prometaphase chromosome analysis (11 patients) or by molecular probes on apparently normal chromosomes (4 patients). One patient had normal chromosomes without a detectable molecular deletion within the WHS “critical region.” In each deleted patient, the deletion was demonstrated to be terminal by fluorescence in situ hybridization (FISH). The proximal breakpoint of the rearrangement was established by prometaphase chromosome analysis in cases with a visible deletion. It was within the 4p16.1 band in six patients, apparently coincident with the distal half of this band in five patients. The extent of each of the four submicroscopic deletions was established by FISH analyses with a set of overlapping cosmid clones spanning the 4p16.3 region. We found ample variations in both the size of the deletions and the position of the respective breakpoints. The precise definition of the cytogenetic defect permitted an analysis of the genotype-phenotype correlations in WHS, leading to the proposal of a set of minimal diagnostic criteria, which in turn may facilitate the selection of critical patients in the search for the gene(s) responsible for this disorder. We observed that genotype-phenotype correlations in WHS mostly depend on the size of the deletion, a deletion of <3.5 Mb resulting in a mild phenotype, in which malformations are absent. The absence of a detectable molecular deletion is still consistent with a WHS diagnosis. Based on these observations a “minimal” WHS phenotype was inferred, the clinical manifestations of which are restricted to the typical facial appearance, mild mental and growth retardation, and congenital hypotonia. Am. J. Med. Genet. 94:254–261, 2000. © 2000 Wiley-Liss, Inc.
INTRODUCTION
Haploinsufficiency of the 4pter chromosome region results in the Wolf-Hirschhorn syndrome (WHS), a well-recognizable clinical entity comprising mental retardation, prenatal and postnatal growth retardation, congenital hypotonia, typical facial appearance with large and protruding eyes, hypertelorism, high nasal bridge and maxillary hypoplasia, congenital heart defects, midline defects, and seizures [Wilson, et al., 1981; Wolf; Hirschhorn; de Grouchy and Turleau, 1984]. Most of the reported patients carry a large deletion, several megabases in length, that can be detected easily by conventional chromosome analysis. A severe phenotypic expression is usually observed in these cases, with clinical manifestations including multiple malformations.
In other patients a 4p16.3 microdeletion can only be detected by molecular probes [Altherr et al., 1991; Roulston et al., 1991; Johnson et al., 1994; Zackai et al., 1994; El-Rifai et al., 1995]. Patients in this latter group tend to present with a milder phenotype, usually lacking malformations.
Most of the phenotypic manifestations in WHS satisfy a model of a contiguous gene syndrome, leading to the generation of a phenotypic map of chromosome 4p, as proposed by Estabrooks et al. [1995]. However, it is well known that similar genetic rearrangements in WHS may determine variable phenotypic effects, most likely as a consequence of allelic variation in the homologous 4p region.
By overlapping deletion analysis, the WHS “critical region” (WHSCR) recently was restricted to an interval of 165 kb in 4p16.3 between the loci D4S166 (defined by cosmid 174g8) and D4S3327 (defined by cosmid 190b4); and it was suggested that only a few pleiotropic genes, perhaps just one, may be responsible for the WHS phenotype, acting as transcriptional regulator(s) of other genes [Wright et al., 1997]. Two novel genes, WHSC1 [Stec et al., 1998], two thirds of which maps within the distal half of the WHSCR, and WHSC2 [Wright et al., 1999], which falls entirely within the WHSCR, were independently described as excellent candidate genes for WHS, although productive mutations have not been detected so far. It was recently demonstrated that the WHSCR is highly conserved between man and mouse, leading to the possibility of generating a WHS mouse model that will elucidate the pathogenesis of this condition [Endele et al., 2000].
We analyzed 16 WHS patients cytogenetically and molecularly. By comparing the extent of the deletions with the respective phenotypes, some significant phenotype-genotype correlations could be established. We observed one apparently nondeleted patient who is potentially important in the search for a WHS gene.
SUBJECTS AND METHODS
Patients
Sixteen WHS patients (11 males, 5 females), age 1 to 15 years, were analyzed for their clinical phenotypes and their respective genotypes (Fig. 1). They were subdivided into two groups, A and B (Table I).
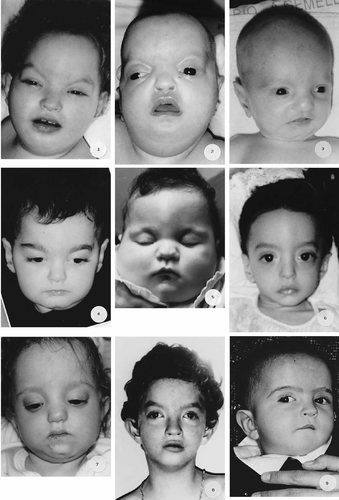
Facial appearance of the 16 WHS patients. This picture was arranged according to Table I, that is according to a decreasing extent of the deletion. Patients from 1 to 11 and from 12 to 16 belong to groups A and B, respectively.
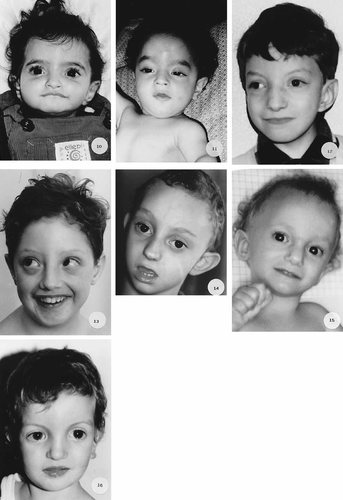
(Continued).
| Patient | Sex | Age at the last observation (yr) | Karyotype |
|---|---|---|---|
| Group A | |||
| 1 | F | 3 | 46,XX,del(4)(p15.1) |
| 2 | F | 3 | 46,XX,del(4)(p15.3) |
| 3 | M | 3, 6/12 | 46,XY,del(4)(p15.32) |
| 4 | M | 2, 4/12 | 46,XY,del(4)(p15.32) |
| 5 | F | 5 | 46,XX,del(4)(p15.32) |
| 6 | M | 2 | 46,XY,del(4)(p16.1) |
| 7 | M | 2, 6/12 | 46,XY,del(4)(p16.1) |
| 8 | M | 9 | 46,XY,del(4)(p16.1) |
| 9 | M | 1 | 46,XY,del(4)(p16.1) |
| 10 | F | 1, 4/12 | 46,XY,del(4)(p16.1) |
| 11 | M | 1, 2/12 | 46,XY,del(4)(p16.1) |
| Group B | |||
| Deletion size (Mb) | |||
| 12 | M | 11 | 4.4 |
| 13 | M | 8 | 3.4 |
| 14 | M | 6 | 3.2 [+dup(4)(p16.1-p16.3)] |
| 15 | F | 10 | 2.8 |
| 16 | M | 15 | Undetectable |
- * F, female; M, male.
Group A includes 11 patients in which a 4pter deletion was detected by conventional prometaphase chromosome analysis. Group B comprises five patients with apparently normal chromosomes. Two of these were reported previously [Zollino et al. 1996, 1999].
Clinical evaluation was performed by the same person (M.Z.) in 10 patients; the remaining patients were evaluated by other expert clinicians. Clinical signs were scored according to a consensus list of multiple manifestations, namely: degree of mental retardation; degree of prenatal and postnatal growth retardation; occurrence of preterm delivery; presence of congenital heart defects, renal abnormalities, and midline defects; frequency and severity of seizures; severity of congenital hypotonia; presence of microcephaly; age at the time of independent walking; age when language started to develop; interaction with the environment and personality. A relative score was assigned to each trait, varying from 10 (the most severe) to 2 (the mildest).
A summary of the clinical signs is provided in Table II.
| Case | Growth delay | Characteristic face | Hypotonia | Microcephaly | CHD | Midline defects | Renal defects | Seizures | MR | |
|---|---|---|---|---|---|---|---|---|---|---|
| Prenatal | Postnatal | |||||||||
| 1 | + | +++ | + | +++ | + | VSD, ASD, PDA | Cleft palate | — | ++ | ++++ |
| 2 | + | ++++ | + | +++ | + | VSD, ASD | Iris coloboma | Renal hypoplasia | ++ | ++++ |
| 3 | + | ++ | + | ++ | + | ASD | Cleft palate | — | + | +++ |
| 4 | + | ++ | + | ++ | + | ASD, PS, PDA, BAV | Hypospadias | Renal hypoplasia | + | +++ |
| 5 | + | ++++ | + | +++ | + | ASD, Pulm. sten. | — | Renal fusion | + | ++++ |
| 6 | + | +++ | + | +++ | + | VSD | Cleft palate | — | + | +++ |
| 7 | + | +++ | + | ++ | + | PS | Cleft palate | Renal hypoplasia | ++ | +++ |
| 8 | + | ++ | + | + | + | — | Hypospadias | Kidney pelvis dil. | + | +++ |
| 9 | + | ++ | + | ++ | + | ASD, PDA | Hypospadias | Kidney pelvis dil. | ++ | ++ |
| 10 | + | ++ | + | ++ | + | ASD | Cleft palate | Kidney pelvis dil. | − | +++ |
| 11 | + | + | + | ++ | + | — | Hypospadias | — | + | ++ |
| 12 | + | ++ | + | + | + | — | Cleft palate Hypospadias | — | + | ++ |
| 13a | +/− | + | + | + | + | — | — | — | +/− | + |
| 14 | − | + | + | + | + | — | — | — | + | ++ |
| 15 | − | + | + | ++ | − | — | — | — | + | + |
| 16a | − | − | + | + | − | — | — | — | − | + |
- * CHD, congenital heart defect; VSD, ventricular septal defect; ASD, atrial septal defect; MR, mental retardation; PDA, patent ductus arteriosus; PS, pulmonary stenosis; BAV, bicuspid aortic valve; +, present; −, absent.
- a Radio-ulnar synostosis.
Conventional and molecular chromosome analysis
Prometaphase chromosome analysis (600–800 bands) was performed on peripheral blood lymphocytes by R(RBG) banding. A minimum of 20 metaphases was analyzed for each patient.
Fluorescence in situ hybridization (FISH) analyses were performed on standard chromosomes from peripheral blood lymphocytes as described [Lichter et al., 1990], with a series of overlapping cosmid clones spanning the 4p16.1-p16.3 chromosome region. Briefly, 0.2 μg of a digoxigenin-labeled probe, previously denaturated in the presence of Cot-1 human DNA, was mixed with a chromosome 4-specific centromeric probe (Oncor, Gaithersburg, MD), which was used at a lower concentration (1:4) than recommended.
In each patient, FISH analyses were carried out with the subtelomeric probe pC847.351 and with probes 190b4 and 174g8, delimiting, distally and proximally, the WHSCR [Wright et al., 1997]. An average of 20 metaphases was scored for each experiment.
Group B patients were analyzed further by FISH with a series of cosmid probes, specifically selected from the following: CD2, IS92, c16Dp, A157.6, IS28, pC678, pC385.12, 19h1, 96a2, 141a8, 108f12, 10d12, 33c6, 161c2, 79f5, 212a9, 65c1, 139h8, 185e6, 241c2, 247f6, 2f10, 27f12, 21f12, and 228a7, all mapping in 4p16.3 [Baxendale et al., 1993; Wright et al., 1997], and MSX1, mapping in 4p16.1 [Hewitt et al., 1991].
Briefly, in a first round of FISH experiments, cosmids were chosen that mapped in 4p16.3 at an average distance of 500 kb. Following this preliminary analysis, which set the boundaries of the deletion, FISH tests were carried out with overlapping cosmids mapping within these boundaries. The breakpoint was established to occur within the probe that showed a weak fluorescent signal on one chromosome 4 in respect to the signal detected on the homologous chromosome, provided that the overlapping distal probe hybridized to one chromosome only, and the overlapping proximal probe hybridized to both chromosomes.
A relative score was assigned to each individual deletion, varying from 10 (the largest) to 0 (absent).
Parents' karyotype was evaluated in all cases on a minimum of 50 metaphases by conventional chromosome analysis or by FISH with selected probes. It must be specified that the resulting phenotypic and genotypic scores represent a relative value among the present patients, most of them having been evaluated by the same observer. Therefore, the reported scores are not proposed as means of objective evaluation of WHS patients.
RESULTS
Chromosomes
In group A, the proximal breakpoint of the deletion varied from 4p15.1 to 4p16.1, being apparently coincident at the distal half of band p16.1 in five cases. By FISH, the deletions were demonstrated to be terminal, and all included the WHSCR and telomere.
In group B, molecular deletions of 4.4, 3.4, 3.2, and 2.8 Mb, respectively, were ascertained in four cases, proximal breakpoints occurring within cosmid clones 228a7 (D4S81), 26e12 (about 500 kb proximal to D4S43), 79f5 (about 280 kb proximal to D4S43), and 108f12 (D4S132), respectively.
It is worth noting that a commercially available probe failed to identify a molecular deletion in three patients (013, 014, and 015). According to the manufacturer, this probe should map at locus D4S96. On the basis of our results, obtained with a considerable number of probes mapping at or near D4S96, its localization is probably different.
In patient 014 (Table I), the subtle 3.2-Mb deletion was detected in association with a 4p16.1-p16.3 “tandem” duplication, as described previously [Zollino et al., 1999]. This rearrangement resulted in a WHS phenotype only, clinical manifestations of partial 4p trisomy being absent.
One patient had normal chromosomes without a detectable deletion in the WHSCR. In this patient, FISH experiments were carried out with the following cosmid clones: pC678, 190b4, 19h1, 96a2, 174g8, 108f12, and 33c6.
A summary of the conventional and molecular chromosome analysis results is shown in Fig. 2.
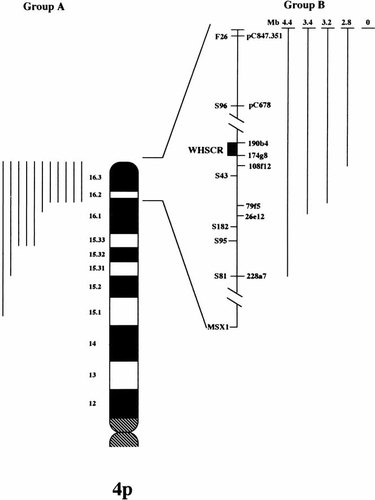
A summary of the observed genotypes. Deletions detected by conventional chromosome analysis (group A, 11 patients) are collected on the left. Deletions detected by molecular probes or absent (group B, 5 patients) are illustrated on the right. Corresponding to each deletion is a dark bar; bars on the different sides of the ideogram are not in scale.
Genotype-Phenotype Correlations
As shown in Table II, the phenotypic manifestations of WHS were quite variable in their severity, largely in accordance with the magnitude of the deletion.
It is also evident that the only clinical signs shared by all patients consisted of typical facial appearance, mental retardation, and congenital hypotonia.
With respect to the number of abnormal findings and to their degree of expression, on average the patients in group A presented with a far more severe phenotype than patients in group B. They suffered from severe psychomotor and growth retardation, congenital heart defects (9/11), midline defects (9/11), renal abnormalities (5/11), and seizures (10/11). None of these patients was able to walk, and language was absent.
Congenital heart defects included ventricular and atrial septal defects (VSD/ASD), patent ductus arteriosus, and pulmonary stenosis; midline defects occurred as cleft palate, hypospadias, and iris coloboma; and renal abnormalities consisted of unilateral renal hypoplasia and renal fusion (Table II).
Patients in this group usually were described as irritable babies, although they had a very warm relationship with their parents.
In group B, with the exception of patient 012 with the largest microdeletion and hypospadias and cleft palate, congenital malformations were absent. The phenotype of the patients in this group included not only fewer, but also less severe clinical signs. Walking age, like the age of language development, was usually around 24 to 30 months. In particular, growth retardation was mild in three of five patients (013, 014, and 016), and head circumference was normal in two (patients 015 and 016). A few seizure episodes did occur with fever in four patients (012, 013, 014 and 015). Patient 015, carrying the smallest microdeletion in this group (2.8 Mb), presented with a relatively pronounced psychomotor retardation (she walked unsupported and spoke words at age 6 years), but, more importantly, she lacked microcephaly and other malformations. Most of these patients had a very warm, sociable personality.
The comparative analysis between the extent of patients' deletions and their respective phenotypes is shown in Fig. 3. Apparently, no clear-cut genotype-phenotype correlations could be established in either group. However, the phenotypic score was largely in accordance with the size of the deletion.
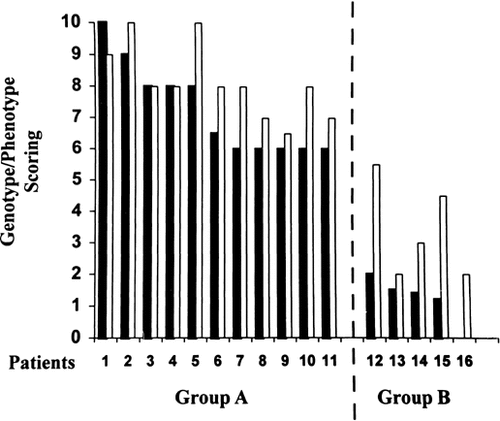
Comparison between the score of patients' deletion (dark bars) and that of their respective phenotype (gray bars).
We identified three clinical categories: (1) patients in the first category, all sharing a deletion detectable by conventional chromosome analysis, presented with a fully expressed phenotype; (2) patients with a microdeletion ranging from 4.4 to 3.2 Mb, presented with a milder phenotype, usually lacking malformations, with less severe developmental delay, but microcephaly still present; and (3) patients in the third category, carrying the smallest microdeletion (2.8 Mb) or no deletion, had, other than a mild phenotype without malformations, normal head circumference (Table III).
| Clinical signs | Class 1. large deletions: p15.1-p16.1→pter | Class 2. “large” molecular deletions: 4.4→3.2 Mb | Class 3: “small” molecular or no deletions: 2.8→0 Mb |
|---|---|---|---|
| Characteristic face | + | + | + |
| MR | + | + | + |
| Hypotonia | + | + | + |
| Growth retardation | + | + | + |
| Seizures | + | + | − |
| Microcephaly | + | + | − |
| Midline defects | + | − | − |
| CHD | + | − | − |
| Renal abnormalities | + | − | − |
- * MR, mental retardation; CHD, congenital heart defect; +, present; −, absent.
DISCUSSION
WHS is a segmental aneusomy syndrome defined by a deletion of a 4pter chromosome region. Large deletions, as have been detected in most patients reported so far by conventional chromosome analysis, result in a well-known clinical entity with well-established neurologic manifestations, and a pattern of minor anomalies and malformations. The introduction of FISH allowed the detection of an increasing number of submicroscopic deletions. The full clinical spectrum resulting from these smaller rearrangements is not entirely characterized yet, although patients undoubtedly present with a WHS “gestalt.” The matter is complicated by the fact that commercially available probes identify loci such as D4F26, D4S96, and D4S95 that lie outside the WHSCR. Therefore, it is likely that many WHS patients, in whom a deletion was apparently excluded by these probes, do in fact harbor a subtle molecular deletion, as demonstrated by Zackai et al. [1994] and by ourselves in the present report.
Sixteen WHS patients, age 1 to 15 years, were evaluated comparatively for their clinical phenotypes and their respective genotypes. On conventional cytogenetic or molecular-cytogenetic analysis, parental karyotype was normal in each case. Despite this, WHS was referred by clinicians, and was personally confirmed on clinical pictures, to recur in a brother, who died of pneumonia, of patient 012, most likely as a consequence of a parental gonadal mosaicism.
The 4pter deletion occurred as single segmental imbalance in 14 of 15 patients; a double segmental imbalance, consisting of a 3.2-Mb terminal deletion on 4p16.3 and a “tandem” duplication of 4p16.1-p16.3 was detected in one patient, as previously reported [Zollino et al., 1999].
The purposes of this study were, first, to look for genotype-phenotype correlations in WHS, making possible the delineation of a microdeletion-associated phenotype; and second, to verify, based on the suggestions offered by the genotype-phenotype correlation study, whether a WHS diagnosis is still tenable in the absence of a detectable deletion. Convincing clinical evidence that a subset of nondeleted WHS patients does exist will facilitate the search for a specific gene(s). In the meanwhile, the patients described here represent a useful resource for delineation of the minimal diagnostic criteria of WHS.
We observed that the severity of the phenotype in WHS mostly depends on the extent of the deletion, with 3.5 Mb representing some sort of discriminating size. Three patients with a deletion ranging in size from 3.4 to 2.8 Mb generally presented with a milder phenotype, with respect to the degree of mental and growth retardation, and of congenital hypotonia. They also lacked malformations. It is worth noting that the head circumference was normal (25th centile) in the patient with the smallest deletion (2.8 Mb).
One patient with a 1.9-Mb interstitial deletion reported by Fang et al. [1997] and two patients with a 2.8-Mb deletion described by Wright et al. [1997, 1998] had a mild phenotype, which is in agreement with our observations.
Some special considerations are warranted for the apparently nondeleted patient (016). He presented with a quite distinctive WHS facial appearance but with very mild congenital hypotonia (he walked unsupported at 24 months) and mental retardation. Growth retardation, affecting weight more than height, was noted in the first few years of life only, with subsequent improvement. More importantly, he never had seizures, his head circumference is normal, and malformations are absent. He also presented with a warm and sociable personality. These phenotypic manifestations overlapped to a large extent with those of the 3.4-Mb deletion patient (013) who presented, among the deleted patients, with the mildest phenotype. Unilateral radioulnar synostosis was an additional finding shared by patients 013 (deleted) and 016 (nondeleted).
The nondeleted patient may have a different genetic disorder, with WHS-like phenotype. Alternatively, he could carry a mutation in a single gene, perhaps in one of the recently described WHS candidate genes, WHSC1 [Stec et al., 1998] and WHSC2 [Wright et al., 1999]. We are inclined to support this second hypothesis and to perform in this patient a mutational analysis of these genes.
Based on the observed genotype-phenotype correlations, we identified three clinical classes of decreasing clinical severity (Table III). The first is a fully expressed phenotype due to a large deletion. The microdeletion-caused phenotype usually falls within the second category. If we look at WHS as a contiguous-gene syndrome, the clinical manifestations observed in patients in the third category are likely to represent the “basic” phenotype of this condition, consisting of typical facial appearance, mild mental retardation, mild congenital hypotonia, and mild growth retardation. We tentatively propose these signs as the minimal diagnostic criteria of WHS and suggest that mildly affected, nondeleted patients fulfilling these criteria are the ideal patients for mutational analysis of candidate genes.




