Clinical findings in a patient with FGFR1 P252R mutation and comparison with the literature
Abstract
We report on a patient with the skeletal findings of Jackson-Weiss syndrome, who manifests only mild craniofacial anomalies. Molecular analysis of her fibroblast growth factor receptor 1 gene (FGFR1) identified a heterozygous P252R missense mutation, previously only reported with FGFR1-Pfeiffer syndrome like manifestations. Mutations in the immunoglobulin-like, II–III (IgII–III) linker region of FGFR1 and FGFR3 molecules may present as a skeletal dysplasia affecting the appendicular skeleton including, brachydactyly, short broad middle phalanges, phalangeal epiphyseal coning and broad halluces. This communication is a further example of the phenomenon of an activated FGFR molecule resulting in overlapping manifestations in FGFR syndromes. Am. J. Med. Genet. 93:22–28, 2000. © 2000 Wiley-Liss, Inc.
INTRODUCTION
The discovery that a substantial proportion of craniosynostosis syndromes was due to mutations in the genes encoding three of the FGFRs, has enabled molecular classification of these hitherto clinically defined syndromes. FGFR mutations that result in craniosynostosis also lead to extracranial manifestations, notably involving the skeleton and soft tissues of the limbs [Wilkie, 1997; Jabs, 1998]. The appendicular manifestations of the various craniosynostoses were utilized in the pre-molecular era for purposes of clinical classifications and syndrome delineation. The documentation of the Jackson-Weiss syndrome (JWS) in an Amish pedigree, however, in which the clinical spectrum in affected relatives apparently encompassed several craniosynostoses, suggested that these syndromes were allelic disorders [Jackson et al., 1976].
The molecular basis of these overlapping findings were clarified by the identification of FGFR2 missense mutations located in the third Ig-like domain (IgIII) for JWS, Crouzon and Pfeiffer (FGFR2) syndromes [Reardon et al., 1994, Rutland et al., 1995, Tartaglia et al., 1997], including the original JWS pedigree [Jabs et al., 1994]. Conversely, the wide spectrum of variability observed in the Pfeiffer phenotype seemed to have a molecular basis as FGFR1-Pfeiffer syndrome patients had less severe craniofacial derangement than FGFR2-Pfeiffer syndrome patients [Schell et al., 1995; Rutland et al., 1995; Gripp et al., 1998].
In this communication, we report on a patient with extra-cranial skeletal changes consistent with JWS, but who was discovered to harbor an FGFR1 P252R missense mutation.
MATERIALS AND METHODS
DNA Analysis
DNA was isolated from peripheral lymphocytes by standard methods [Miller et al., 1988]. A 216 base pair (bp) fragment of the FGFR1 Ig-like II–III linker region was amplified by PCR. The primers were; sense, 5′ GGA ATT CCA TCT TCC ACA GAG CGG and antisense, 5′ CTG CCT TAT GTC CAG ATC TTG AGG AAT TCC. PCR was carried out by 10 cycles of denaturation at 94°C for 60 sec, annealing at 65°C for 30 sec (−1°C per cycle) and extension at 72°C for 30 sec; followed by 25 cycles of 94°C for 60 sec, 51°C for 30 sec and 72°C for 30 sec [Muenke et al., 1994]. Thereafter, restriction analysis was carried out by incubation with MnlI (New England Biolabs).
RESULTS
Clinical Findings
Frontal bossing and broad medially deviated toes were observed in the neonatal period in the proposita (birth weight, 3.4 kg), but it was not until age 8 years that the patient was reviewed for consideration of an undiagnosed skeletal dysplasia. On examination, she was noted to be overweight (weight >99th centile; height, 75th centile; head circumference, 50th centile). Apart from frontal bossing and mild midface hypoplasia, there were no abnormalities of the skull. She had relative hypertelorism (interpupillary distance, 90th centile) and telecanthus (inner canthal distance, 97th centile). A broad nasal tip, a thin upper lip and folded and fused helices were present (Fig. 1A,B). The only abnormalities of her hands were a relative brachydactyly (midfinger length, 10th centile) and mild cutaneous 2/3 finger syndactyly. The thumbs were not enlarged. The great toes were broad, medially deviated and foreshortened (more marked on the right), as illustrated in Figure 2. A normal range of movement was present in all joints. There was no immediate family history of craniosynostosis or skeletal anomalies in the proposita's two half sisters or her mother (the father was unavailable).
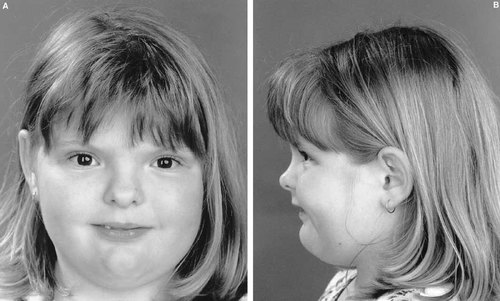
(A) Anterior face showing telecanthus, a broad nasal tip and a thin upper lip. (B) Lateral face showing frontal bossing, mild midfacial hypoplasia and folded and fused helices.
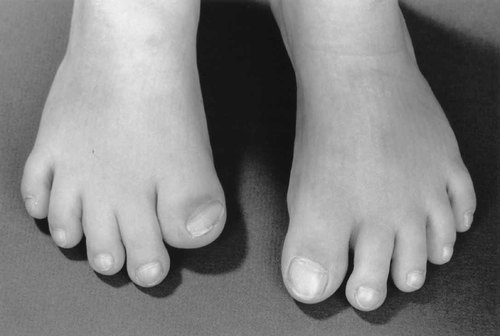
Proposita's feet showing broad, medially deviated and foreshortened halluces.
Radiological Abnormalities
Radiographs of the skull as shown in Figure 3, confirmed hypertelorism but there were no signs of a premature sutural synostosis. Radiographs of the proposita's hands depicted in Figure 4, showed cone epiphyses of the index fingers and short middle phalanges of fingers 1 and 5. Most strikingly as shown in Figure 5, radiographs of the feet demonstrated bilateral calcaneo-cuboid fusion, phalangeal epiphyseal coning, a short proximal phalanx of the right hallux and bilateral phalangeal fusion of the halluces. Radiographic findings of the elbows were normal.
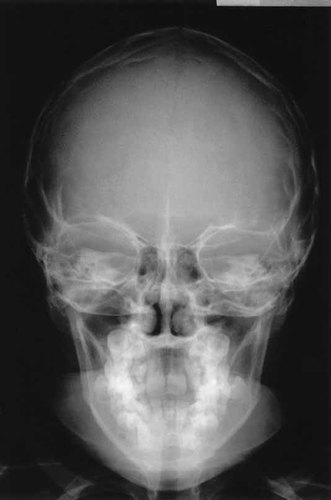
Skull radiograph showing hypertelorism but no abnormally fused sutures.
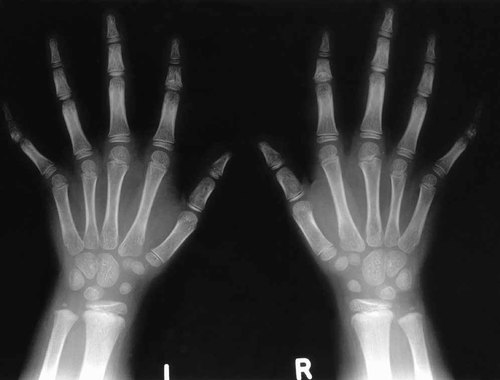
Radiographs of the proposita's hands showing coning of the epiphyses of the index fingers and short middle phalanges of digits 1 and 5.
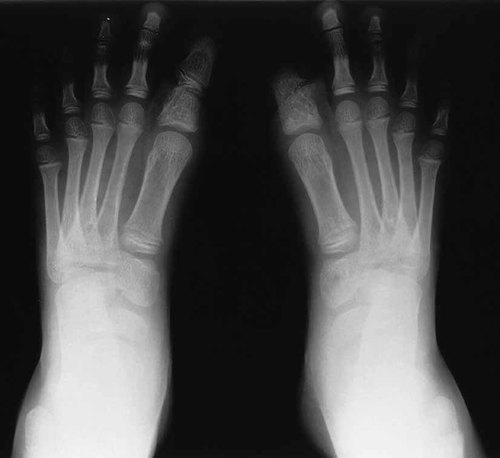
Radiographs of the feet demonstrating bilateral calcaneo-cuboid coalition, phalangeal epiphyseal coning, a short proximal phalanx of the great toe and phalangeal fusion in the halluces.
Restriction Analysis
Restriction analysis as shown in Figure 6, demonstrated heterozygous loss of the Mnl1 site in the PCR amplicon, indicating the P252R missense mutation was present in one allele of FGFR1 of the proposita Muenke et al., 1994. The P252R mutation was not present in the mother.
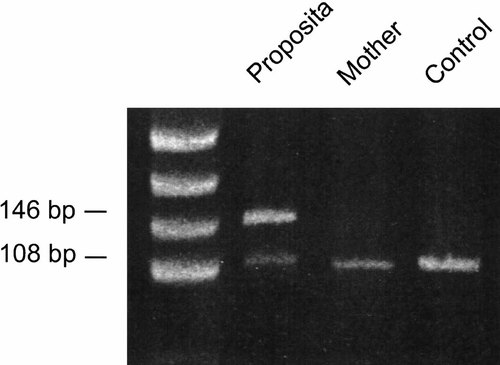
Restriction analysis of the amplified region of FGFR1, demonstrating heterozygous loss of an Mnl1 site due to the C to G transversion at nucleotide 756. This results in the P252R substitution. Loss of the Mnl1 site leads to a 146 bp band in our proposita that is not present in the wild type allele, that exhibits a 108 bp fragment (digestion products of 53, 38 and 17 bp, also present in the wild type allele are not visible). The marker in lane 1 is pUC19 digested with HpaII.
DISCUSSION
The JWS phenotype comprises a variable craniosynostosis, mid-facial hypoplasia and broad great toes with medial deviation. When JWS cases have been investigated by radiographic means, skeletal anomalies including short and broad first metatarsals, fused metatarsals, navicular-cuneiform coalition and calcaneo-cuboid coalition are detected [Jackson et al., 1976; Gorlin et al., 1990]. Despite the absence of craniosynostosis, our patient was assigned to JWS because calcaneo-cuboid coalition seemed to be specific for JWS [Jackson et al., 1976; Gorlin et al., 1990]. Moreover, the presence of normal thumbs seemed to exclude Pfeiffer syndrome [Muenke et al., 1994]. Thus, it was anticipated that she would harbor an exon 9 FGFR2 missense mutation, as described in JWS [Jabs et al., 1994; Tartaglia et al., 1997]. Unexpectedly, analysis of her FGFR1 gene demonstrated the presence of a heterozygous C to G transversion, leading to a proline substitution for arginine at residue 252 (P252R) [Muenke et al., 1994].
Clinical and radiological documentation of patients identified with heterozygous proline substitution for arginine, at residues 252, 253 and 250 in FGFR1, 2 and 3 has generated phenotype-genotype correlations. Table I lists the skeletal changes documented in such cases of IgII–III linker domain missense mutations of FGFR1-3. Although calcaneo-cuboid coalition has been considered JWS-specific, Pflanzer [1978] reported this finding in two patients with Apert syndrome. Furthermore, tarsal coalition is seen in about one third of patients with FGFR3-coronal craniosynostosis [Muenke et al., 1997; Reardon et al., 1997; Graham et al., 1998] (Table I) and is also present in some Crouzon patients with FGFR2 Ig III mutations [Anderson et al., 1997]. Thus, tarsal coalition is not exclusive to JWS but to our knowledge, this is the first report of a calcaneo-cuboid coalition in a case with a documented FGFR1 P252R mutation (Pfeiffer syndrome).
| Gene missense mutation syndrome/phenotype | FGFR1 P252R Pfeiffer | FGFR2 P253R/S252W Apert | FGFR3 P250R Coronal | |||
| Findings | % | n | % | n | % | n |
| Upper limb | ||||||
| broad thumbs | 44 | 9 | 40 | 5 | 1 | 76 |
| medially deviated thumbs | 22 | 9 | 20 | 5 | 0 | 76 |
| clinodactyly | 0 | 9 | 0 | 5 | 30 | 47 |
| osseous/soft tissue syndactyly | 11 | 9a | 100 | 41 | 0 | 77 |
| short/broad middle phalanges | 86 | 7a | 20 | 5 | 46 | 35 |
| distal phalangeal hypoplasia | 0 | 9 | 20 | 5 | 13 | 15 |
| fused phalanges | 57 | 7 | 60 | 5 | 6 | 34 |
| phalangeal epiphyseal coning | 14 | 7a | 0 | 5 | 35 | 23 |
| short metacarpal/s | 29 | 7a | 0 | 5 | 40 | 15 |
| carpal coalition | 0 | 7 | 0 | 5 | 26 | 31a |
| elbow synostosis/hypoplasia | 66 | 3 | 39 | 41 | 0 | 19 |
| rhizomelia | 0 | 9 | 49 | 41 | 0 | 19 |
| shoulder abnormalities | 0 | 9 | 51 | 41 | 0 | 19 |
| Lower limb | ||||||
| broad halluces | 100 | 11a | 40 | 5 | 29 | 42a |
| deviated halluces | 100 | 8a | 40 | 5 | 0 | 19 |
| syndactyly | 54 | 11 | 100 | 41 | 1 | 80 |
| short/broad middle phalanges | 33 | 6 | 20 | 5 | 17 | 18a |
| short prox. phalanx first toe | 83 | 6a | 20 | 5 | 50 | 2a |
| fused phalanges | 100 | 6a | 60 | 41 | 0 | 2 |
| phalangeal epiphyseal coning | 33 | 6a | 0 | 5 | 67 | 9 |
| short/broad metatarsals | 33 | 6 | 20 | 5 | 0 | 3 |
| tarsal coalition | 50 | 6a | 0 | 5 | 29 | 21 |
- * References for this table [Saldino et al., 1972, Baraitser et al., 1980, Muenke et al., 1994, Park et al., 1995, Rutland et al., 1995, Schell et al., 1995, Hollway et al., 1997, Muenke et al., 1997, Reardon et al., 1997, Graham et al., 1998, Passos-Bueno et al., 1998]. n = number of patients.
- a Present in our patients (includes two unpublished FGFR3 P250R cases).
Pfeiffer syndrome patients have been reported with a variety of limb anomalies including radio-ulnar fusion, broad/medially deviated thumbs, brachymesophalangy, broad first toe distal phalanx and broad/duplicated first metatarsal [Gorlin et al., 1990] and fusion of carpals and tarsals [Cohen, 1975]. Some of these skeletal anomalies have been confirmed in FGFR1 and FGFR2 Pfeiffer syndrome patients after molecular diagnosis. To date, minimal differences in the appendicular findings seem to exist between these syndromes [Schell et al., 1995; Rutland et al., 1995; Gripp et al., 1998].
The recently delineated entity of FGFR3-related craniosynostosis also has skeletal manifestations, as outlined in Table I [Glass et al., 1994; Moloney et al., 1997; Muenke et al., 1997; Graham et al., 1998]. Interestingly, phalangeal epiphyseal coning, previously thought to be a distinctive finding in FGFR3-coronal craniosynostosis [Muenke et al., 1997], is also present in FGFR1 P252R cases (Table I). As Table I suggests, the appendicular findings of broad thumbs, fused phalanges, carpal coalition, elbow anomalies and broad/deviated halluces may assist in distinguishing between FGFR1-Pfeiffer and FGFR3-coronal craniosynostosis, before molecular analysis.
Thus, common skeletal outcomes result from mutations in the FGFR Ig II–III linker domain. Short broad middle phalanges and broad halluces are common outcomes for all three receptors. Overlap between the FGFR1/3 IgII–III linker includes a short proximal phalanx of the hallux, phalangeal epiphyseal coning and tarsal coalition, with mutations in FGFR1 or 3 producing greater overlap in skeletal abnormalities than mutations within the FGFR2 IgII–III linker in Apert syndrome (Table I). Overlap between FGFR1/3 IgII–III linker mutations and FGFR2 IgIII mutations (JWS/Pfeiffer/Crouzon syndromes) includes short/broad middle phalanges, tarsal coalition as well as lacking the syndactyly, upper limb rhizomelia and shoulder abnormalities typical of Aperts syndrome (Table I) [Anderson et al., 1997; Gorlin et al., 1990].
Differences between FGFR receptor effects may relate more to the strength of tyrosine kinase activity rather than qualitative differences between the homologous receptors [Raffioni et al., 1999]. This suggests activated receptors have common downstream effects, independent of ligand binding. Thus, FGFR mutations would be anticipated to result in an overlap of radiological and clinical findings. It may be speculated, that quantitative tyrosine kinase differences in signaling may be more closely matched in vivo for FGFR1/3 IgII–III domain substitutions and the FGFR2 IgIII mutations versus the FGFR2 IgII–III linker (Apert) mutations, consistent with clinical comparisons. The variability of clinical findings, however, suggests that other genes involved in skeletal development are important in determining the final phenotype [Raffioni et al., 1999; Zhang et al., 1999]. It is probable that there are further common and perhaps distinctive clinical and radiological findings resulting from mutations in the three FGFR IgII–III linker domains. Identification of such findings, accompanied by their concomitant insights into skeletal developmental processes, will be facilitated by extended correlations of clinical, radiological and molecular data.
Acknowledgements
The Royal Children's Hospital Foundation, Brisbane, is acknowledged for the provision of funds to undertake this research (Grant 724). We are also very grateful to Dr. C.J. Epstein for providing original photos of X-rays.




