Atherosclerosis: Cholesterol Management
Abstract
Cardiovascular disease (CVD) is the most common cause of death in the United States and the industrialized world. The principal components of CVD are coronary heart disease (CHD), stroke, heart failure, and congenital heart disorders. Atherosclerosis, the chronic inflammatory arterial wall disease associated with the deposition of cholesterol into arterial plaques and hardening of the arteries, is a major contributor to CHD and is the principal cause of heart attacks and stroke. The risk factor most closely associated with a higher incidence of CHD is serum cholesterol levels. This report summarizes efforts in the pharmaceutical industry to manage cholesterol levels to reduce risk for CHD due to atherosclerosis.
1 Introduction
Cardiovascular disease (CVD) in all its forms remains the single most common cause of death in the United States and the industrialized world (1, 2). In the United States, CVD was responsible for over 840 000 deaths in 2016 and the associated direct and indirect costs to the US economy were estimated to be $351.1 billion in 2014–2015 (3). The principal components of CVD included in these numbers are coronary heart disease (CHD), stroke, heart failure, and congenital heart disorders. Atherosclerosis, the chronic inflammatory arterial wall disease associated with the deposition of cholesterol into arterial plaques and hardening of the arteries, is a major contributor to CHD and is the principal cause of heart attacks and stroke. Although there are multiple risk factors for CHD including age, family history, obesity, sedentary lifestyle, smoking, diabetes, and high blood pressure, the risk factor most closely associated with a higher incidence of CHD is serum cholesterol levels.
Cholesterol is carried in vivo in lipoprotein particles of varying density and with differing surface proteins. Low-density lipoproteins (LDLs) have been shown to cause atherosclerosis, and they have a cumulative effect (4). High-density lipoproteins (HDLs) have also been hypothesized to be cardioprotective. This article focuses mainly on therapeutic agents that lower low-density lipoprotein cholesterol (LDL-C) levels as these agents have progressed to market for the treatment and/or prevention of atherosclerosis.
Lipoproteins are lipid–protein complexes consisting of surface apoproteins, a phospholipid monolayer, and free cholesterol, with cores containing neutral lipids including triglycerides (TGs) and cholesteryl esters (CEs). As shown in Figure 1, LDL are derived from very-low-density lipoproteins (VLDLs) synthesized by the liver (5). Hepatocytes package TGs and cholesterol along with apoprotein B (isoform B100) and phospholipids to form the TG-rich lipoprotein VLDL for secretion into plasma. VLDL TGs are hydrolyzed by lipoprotein lipase (LPL), releasing free fatty acids, which reduces the size of VLDL to intermediate-density lipoproteins (IDLs). IDL TGs are hydrolyzed by hepatic lipase, forming LDL that contains one molecule of apoB and primarily esterified cholesterol in its core. VLDL, IDL, and LDL obtain CEs through the exchange of TGs and CEs from HDLs by the activity of cholesteryl ester transfer protein (CETP). LDL transports cholesterol to peripheral tissues where it is taken up by the low-density lipoprotein receptor (LDL-R) (6). LDL is cleared from the circulation primarily by hepatic LDL-Rs. LDL-Rs bind apoB and apoE enriched lipoproteins like LDL, internalize LDL through clathrin-coated pits, release the LDL into acidified endosomes, and the LDL-R recycles to the cell surface to bind additional LDL particles. Proprotein convertase subtilisin-like/kexin type 9 (PCSK9) binds to the LDL-R and directs it to be degraded, interrupting the recycling LDL-R pathway (described in detail in Section 3.1 (7)).
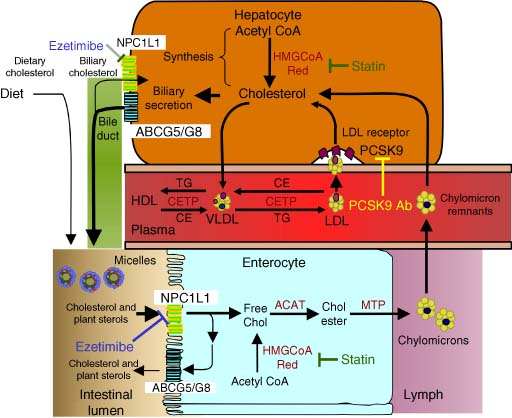
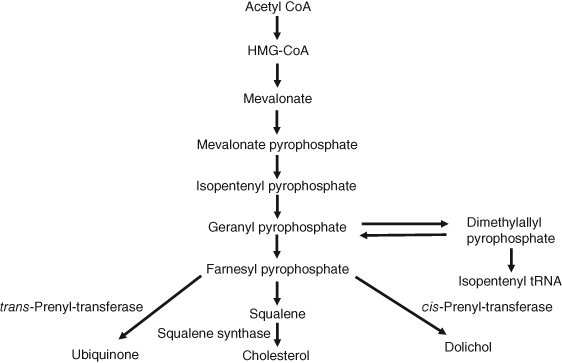
Since cholesterol is the principal component of the atherogenic lipoproteins, the two sources of this important biochemical merit mention. The majority of endogenous cholesterol comes from a complex biosynthetic pathway with over 25 dedicated synthetic steps beginning with acetate as depicted in Figure 2 (8). The slowest or rate-determining step in this pathway is the synthesis of mevalonate via the enzyme 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase. The antihypercholesterolemic agents called statins inhibit this step in the biosynthetic pathway. It is estimated that the proportion of cholesterol from this source in the human population is approximately two-thirds (9). The second source of cholesterol comes from dietary intake. These two pools of cholesterol are intermingled in the gut where cholesterol that has been cleared from the periphery via the liver is mixed with dietary sources. A large percentage of this intestinal cholesterol is reabsorbed in a transporter-mediated reuptake process (10). A more detailed discussion of cholesterol metabolism is presented later in this article. Nevertheless, it is clear that the complex nature of the synthesis, clearance, and absorption of cholesterol offers multiple possible avenues for pharmacological intervention to regulate cholesterol homeostasis to a level that is consistent with positive effects on CHD.
A precise definition of the desired LDL-C level in humans is routinely reviewed and epidemiological data have driven recommendations to ever lower levels over the past few decades. The American College of Cardiology/American Heart Association Task Force suggests that in very high-risk atherosclerosis cardiovascular disease (ASCVD) patients, physicians should aim to reduce LDL-C levels to below 70 mg dL−1 (11). Clinical results from the PROVE-IT trial suggest that LDL-C levels below 70 mg dL−1 may have further advantage in lowering the risk of CHD (12). In 2019, the European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) recommended to reduce LDL-C levels to below 55 mg dL−1 from their previous guidance of 70 mg dL−1 for primary and secondary prevention (13). The “lower is better” concept appears to be holding strong when it comes to LDL-C (14). Lowering LDL-C by any means appears to have a direct correlation to lowering incidence of cardiovascular events (13). This correlation has been used to argue against the so-called pleiotropic effects of statins, benefits beyond those expected by LDL-C lowering alone (15, 16).
Consequently, LDL-C lowering has been one of the largest sectors in pharmaceutical sales worldwide, and the sector is expected to continue to increase due to an expanding patient population. The value of worldwide sales is expected to remain high with continued growth in the sector due to increases in world population and increased prevalence of hypercholesterolemia (17). For patients, the major outcome of the intense effort to treat CHD in this manner has been the continuous identification of more effective agents to lower LDL-C, increased competition for their business, and improved outcomes.
2 Therapeutic Agents
A great effort has been made to identify pharmacological agents to modify lipid profiles by reducing atherogenic lipoproteins and harmful lipids or increasing beneficial lipoproteins. A list of these marketed LDL-C lowering agents is shown in Table 1. This article will focus on the options that exist for reducing LDL lipoproteins, specifically HMG-CoA reductase inhibitors, cholesterol absorption inhibitors, bile acid sequestrants, and PCSK9 inhibitors. It will also discuss some of the newer drugs and novel approaches to lower LDL-C.
| Compound | Generic name | Marketed name | Company | Doses (mg d−1) | LDL loweringa |
|---|---|---|---|---|---|
| 2 | Lovastatin | Mevacor | Merck | 20–80 | + |
| 3 | Pravastatin | Pravacol | BMS Co./Sankyo | 40 | + |
| 4 | Simvastatin | Zocor | Merck | 10–80 | ++ |
| 5 | Fluvastatin | Lescol | Novartis AG | 40–80 | ++ |
| 6 | Atorvastatin | Lipitor | Pfizer, Inc. | 10–80 | +++ |
| 7 | Rosuvastatin | Crestor | AstraZeneca/Shionogi | 5–40 | +++ |
| 9 | Pitavastatin | Livalo | Kowa/Sankyo | 1–4 | +++ |
| 10 | Ezetimibe | Zetia | Merck | 10 | + |
| 11 | Ezetimibe/simvastatin | Vytorin | Merck | 10/10–10/80 | +++ |
| 20 | Cholestyramine | Questran | Bristol-Myers Squibb | 4 000–24 000 | + |
| 21 | Colestipol | Colestid | Pfizer, Inc. | 2 000–16 000 | + |
| 22 | Colesevelam | Welchol | Genzyme/Daichi Sankyo | 2 300–4 500 | + |
| 23 | Alirocumab | Praluent | Sanofi | 75 mg SC per 2 wk | +++ |
| 24 | Evolocumab | Repatha | Amgen | 420 mg SC per mo | +++ |
| 25 | Mipomersen | Kynamro | Ionis/Genzyme | 200 mg SC per wk | ++ |
| 26 | Lomitapide | Juxtapid | Aegerion | 30–40 | +++ |
- a +, ∼≤30% reduction; ++, 30–45% reduction; +++, up to 61% reduction.
2.1 HMG-CoA Reductase Inhibitors (Statins)
Statins have a primary role in the pharmacological treatment of CHD (18). Their direct mechanism of action is to block the key step in the cholesterol biosynthetic pathway, HMG-CoA reductase. There are several downstream consequences of this inhibition. First, the machinery for the biosynthesis of cholesterol is upregulated, the body's attempt to compensate. This compensation is effectively blocked by the inhibitory action of the statins. The second, and perhaps most important consequence, is the upregulation of the LDL-R in the liver (19). Increasing LDL-R numbers act to promote clearance of this atherogenic lipoprotein from the periphery. In this two-pronged manner, statins effectively reduce the LDL-C in humans and have proven to be an effective treatment to reduce the incidence of CHD (20, 21).
2.1.1 Statins Derived from Natural Products
The interest in statins as a drug class began with the systematic search for inhibitors of HMG-CoA reductase by Endo and coworkers in 1971 (22-24). This broad screening of microbial broths focused initially on the inhibition of the incorporation of [14C]acetate into nonsaponifiable lipids, followed by interrogating hits for their ability to inhibit [3H]mevalonate incorporation into lipids (25). Compounds active in the first screen, but inactive in the second (thus not likely to inhibit an early stage of cholesterol biosynthesis) were then tested in a rat liver enzyme assay (26). Their efforts led first to the identification of mevastatin (compactin, 1) as a natural product that is a nanomolar inhibitor of HMG-CoA reductase (27, 28). Structurally, compactin contains a β-hydroxy-δ-lactone moiety that can open in basic media to give the corresponding water soluble dihydroxy acid form. This β,δ-dihydroxy carboxylic acid moiety closely mimics HMG-CoA in structure, and either this acid or the corresponding lactone prodrug is the common pharmacophore found in all the statins. Completing the structural analysis, this HMG-CoA mimetic region is connected to a lipophilic anchor region via a short linker moiety. A variety of lipophilic anchor regions have been designed to optimize overall pharmacological and physicochemical properties in second- and third-generation statins.
Numerous statins have been subsequently discovered and brought to market. Their structures are shown in Figure 3. The first statin approved by the US FDA to lower LDL-C in humans was lovastatin 2 in 1987 (29), on the basis of its safety (30) and efficacy in clinical trials (31-37). Lovastatin was shown to lower LDL-C in clinical trials in heterozygous familial hypercholesterolemia (FH) patients (31-33) as well as in non-FH patients (34-36). It was also effective at lowering β-VLDL in familial dysbetalipoproteinemia patients (38, 39) and both VLDL cholesterol and LDL-C in patients with diabetes (40) or nephrotic dyslipidemia (41). The ultimate desire of these pharmacological interventions is the reduction of clinical events and death. Lovastatin was reported to have just such an effect, lowering the risk of the first acute major coronary event in men and women of average cholesterol levels in a primary prevention trial (42). This result underscored the importance of lowering LDL-C to a target goal as a viable method for lowering the morbidity and mortality associated with CHD.
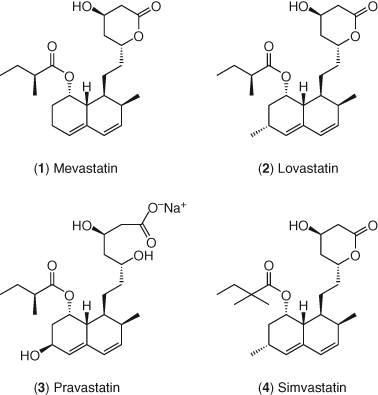
Prompted by the success of these efforts, other naturally occurring statins were discovered and developed (43). Pravastatin 3 was discovered as a bioactive hydroxylated open acid metabolite of mevastatin in dogs and in culture by scientists at Sankyo (44-47). The enzymatic production of pravastatin from mevastatin has been studied extensively by Sankyo scientists (48). Subsequently, it was developed in collaboration with scientists at Squibb (now Bristol–Myers Squibb) and approved for sale in Japan in October 1989 and in the United States by the FDA in October 1991. Pravastatin was effective in reducing LDL-C in hypercholesterolemic patients and in diabetic patients (49, 50). Pravastatin has further been shown to reduce cardiovascular morbidity and mortality in one primary and two secondary prevention trials in patients with a wide range of cholesterol levels (51-54).
The final semisynthetic statin derived from a natural product starting point is simvastatin 4, developed by scientists at Merck (55, 56). Extensive structure activity relationships (SAR) studies led to a full understanding of the lipophilic requirements at the ester side chain, which was optimized in simvastatin (55). Like lovastatin, simvastatin is administered as the less active hydroxy lactone form, which readily opens to the dihydroxy acid form in vivo (57). The in vivo potency of simvastatin served to distinguish it from its early competitors. Simvastatin was first approved for use in humans in 1991 to reduce elevated cholesterol levels and subsequently to reduce cardiovascular events and mortality associated with CHD (58, 59).
2.1.2 Designed, Fully Synthetic Statins
The search for more potent inhibitors of HMG-CoA reductase, encouraged by positive clinical outcomes as well as the developing understanding of the SAR requirements prompted the development of fully synthetic statins. These compounds maintain the hydroxyglutaric acid moiety (or lactone equivalent) but have fundamentally distinct lipophilic moieties in the anchor region as shown in Figure 4. The first such synthetic statin was fluvastatin 5, developed by Novartis (Sandoz), and marketed as Lescol (60, 61). Fluvastatin utilizes a substituted indole core for the lipophilic moiety, a trans double bond as the two-carbon linker and displays the dihydroxyglutaric acid moiety in the open form. Fluvastatin is similar in efficacy at lowering LDL-C to pravastatin and lovastatin in clinical trials (62-64).
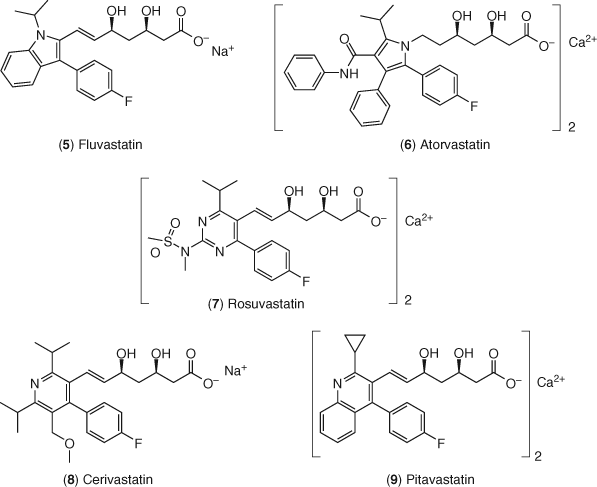
The next statin to make it to market was atorvastatin (6) developed by Parke-Davis (now Pfizer), approved in late 1996, and marketed as Lipitor (65). Extensive SAR studies based on a timely development of enabling synthetic methodology allowed for optimization of the desired biological profile (66-70). Atorvastatin uses a substituted pyrrole in the lipophilic anchor region, a saturated two-carbon linker and the dihydroxyglutaric acid in the open form formulated as a calcium salt. Supported by some initial preclinical evidence that atorvastatin had a desirable in vivo activity profile relative to competitors' compounds, the compound was tested in clinical trials for LDL-C lowering (71, 72). Atorvastatin showed a significant advantage over its competition, lowering LDL-C levels as much as 60% at the highest dose of 80 mg once per day (65). Before long, it was considered by many to be the best-in-class HMG-CoA reductase inhibitor on the market and subsequently became the number one selling medication in the world for many years.
In the Anglo-Scandinavian Cardiac Outcomes Trial – lipid lowering arm (ASCOT-LLA), atorvastatin was shown to reduce incidence of fatal and nonfatal CHD in hypertensive patients without prior incidence of myocardial infarctions (MIs). CHD risk was reduced across the board for this patient population without a clear correlation to baseline LDL-C levels, age, smoking, obesity, or renal function (73), and the CHD effects were long lasting (74). Atorvastatin had similar effects in diabetic patients, reducing incidence of major CHD events including MI, acute CHD death, unstable angina, coronary revascularization, or stroke (75). In the Treating to New Targets (TNT) trial, investigators showed that aggressive lipid-lowering therapy with atorvastatin provided higher levels of risk reduction against major CHD events adding evidence to the “lower is better” hypothesis (76). In the secondary prevention IDEAL trial, comparing high dose of atorvastatin versus the nominal dose of simvastatin in patients with previous MIs, high-dose atorvastatin resulted in a higher level of LDL-C lowering but did not further reduce the risk of major coronary events (77). It did, however, show that aggressive LDL-C lowering with atorvastatin provided patient benefit by further reducing the risk of nonfatal acute MI as well as secondary CHD events.
The next generation of statins became known as the “superstatins” due to their increased potency in vitro and in vivo. Rosuvastatin 7 has become the most important member of this new class and was developed by AstraZeneca and approved for use in hypercholesterolemic patients in 2003 (78). It was developed as the calcium salt of the open dihydroxy acid with a methanesulfonamide substituent on the pyrimidine moiety that occupies the lipophilic binding pocket of the enzyme (79). Rosuvastatin is approximately fourfold more potent at inhibiting HMG-CoA reductase than lovastatin and increased LDL-R mRNA more than ten times than seen with pravastatin. Rosuvastatin was designed to have a very low LogP value in an effort to increase its selectivity for liver as its primary site of action thereby reducing its potential for peripheral side effects (see below). The in vitro potency of rosuvastatin has translated well in the clinic. On a dosage basis, rosuvastatin is more efficacious at lowering LDL-C as well as raising high-density lipoprotein cholesterol (HDL-C) while maintaining a similar side effect profile to atorvastatin, pravastatin, and simvastatin (80-82). Similar efficacy advantages of rosuvastatin as compared with atorvastatin were also seen in patients with type 2 diabetes, allowing a higher percentage of patients to reach their target LDL-C goals (83). In the JUPITER clinical trial, patients with LDL-C levels less than 130 mg dL−1, but with hs-CRP levels greater than 2 mg L−1 were treated with rosuvastatin (84). C-reactive protein is a biomarker of inflammation and has been positively correlated with incidence of CHD (85-91). In JUPITER, rosuvastatin reduced the rate of the first incidence of CHD and mortality from all causes as compared to placebo, providing an intriguing suggestion of the importance in lowering inflammation in treating CHD as well as lowering cholesterol. It should be noted that this pharmacological effect has been seen with other statins and particularly with statins in combination with cholesterol absorption inhibitors (92-97).
The last two fully synthetic statins to be discussed are cerivastatin (8) and pitavastatin (9). Cerivastatin is a dihydroxy carboxylic acid sodium salt first approved for treating hypercholesterolemia in 1999 and marketed as Baycol by Bayer (98). It is about 100 times more potent than lovastatin in vitro and this potency translated well in vivo in rats, rabbits, dogs, and ultimately humans (99, 100). In humans, this compound was extremely potent at lowering LDL-C, demonstrating high efficacy at submilligram doses (101, 102). Unfortunately, cerivastatin was plagued with a relatively high incidence of a dangerous muscle reaction, rhabdomyolysis, which appeared when used in high doses in the elderly or when taken along with gemfibrozil. In these patient populations, use of the drug was associated with a significant number of patient deaths and the drug was subsequently taken off the market (103). Pitavastatin is also a highly potent synthetic statin effectively reducing LDL-C levels in clinical trials (104, 105). Its use thus far has been limited to Japan and a few countries in the Asian market.
2.1.3 Tissue Selectivity and Safety of Statins
The safety of such widely used drugs as statins has been extensively studied (106). Although rare, the most feared principal adverse side effect associated with statin use has been muscle toxicity and in the most severe cases, rhabdomyolysis. These muscle issues often present themselves as muscle pain, tenderness, or weakness. In cases of rhabdomyolysis, muscle injury can induce irrevocable weakness, renal failure, and can even result in death. Another early concern of statin use was a lenticular opacity that was seen in animal models and carefully monitored in human use. It has been hypothesized that inhibition of HMG-CoA reductase and hence cholesterol synthesis in specific tissues is at the root of these problems, and the design of new HMG-CoA reductase inhibitors has subsequently focused on inhibitors whose site of action is predominantly the liver (107, 108). For the muscle issues, inhibition of cholesterol synthesis in myocytic sites is the primary concern; for the lens issue, prolonged inhibition at the level of the lens epithelial cells is the major risk factor. A thoughtful assessment of the toxicology seen in animal models for statins and its predicted risk in humans allowed the careful introduction of new statins into the market (109, 110). In vitro experiments looking for evidence of tissue-specific statin toxicity are often misleading of the actual in vivo results. Even with the proper experiments, a careful assessment of risk and benefit is important as evidence of the overall benefit of this drug class is overwhelming.
The tissue selectivity of a drug can be influenced by many factors, including lipophilicity/hydrophilicity, metabolism, and transport systems within the body. The first pass metabolic hydrolysis of the lactone containing statins both improves their pharmacological activity at the receptor as well as increases their hydrophilicity. This change, however, is insufficient to effectively minimize exposure to peripheral tissues. Consequently, much care has been taken to evaluate statins in different tissue types to determine their relative ability to inhibit cholesterol synthesis in the liver, but less so in target organ toxicity tissues. In general, more hydrophilic statins have faired well in this comparison. For example, pravastatin is about 100-fold more selective at inhibiting cholesterol synthesis in rat hepatocytes as compared to rat lens tissues and this selectivity translated to a 10-fold selectivity in vivo (109, 111). A study using tissue homogenates and evaluating exposure to simvastatin and lovastatin versus pravastatin concluded that the more hydrophobic drugs simvastatin and lovastatin had superior liver exposures (112). When cholesterol synthesis was monitored by incorporation of [14C]acetate and compound distribution carefully assessed, Bocan et al. (113) determined that a spectrum of statins can be classified as liver selective based on metabolic fate, selective uptake by the liver relative to other tissues, or intrinsic activity. The percentage of the dose taken up by the liver (hepatic extraction) is high for many of the statins: ≥70% for lovastatin and fluvastatin, >80% for simvastatin, and 46% for pravastatin (17, 114). High hepatic extraction and high protein binding have been postulated to contribute to the liver selectivity of these drugs, although none have been absent side effects in humans.
The lenticular opacity issue reported with early statins is believed to be due to inhibition of HMG-CoA in the lens itself (115-117). This problem has largely become a less significant concern as the design of inhibitors has evolved to those with liver selectivity and in the light of clinical experience.
Another potential safety risk associated with statin use is hepatotoxicity. Since the liver is a primary site of action for these molecules, liver function has been a primary focus of safety monitoring. Lovastatin, the first approved statin for clinical use, carried warning labels for liver toxicity and routine monitoring of serum transaminase levels was recommended. Liver function testing is routinely recommended for patients on HMG-CoA reductase inhibitor therapy (118). Clinically it has been observed that there is a small percentage of patients on a variety of statins that experience elevated ALT levels, primarily at the highest doses of the specific statin (119). These elevated ALT levels are usually without overt symptoms and are reversible upon discontinuation of the treatment. It has been observed that slight elevation of two to three times the upper limit of normal of these liver enzyme biomarkers is common to all lipid-lowering agents, including nonabsorbed bile acid sequestrant resins (109). These effects do not present a consistent liver cellular pathology and may be due to alterations in the hepatic cellular membrane permeability. It should also be noted that many of the HMG-CoA reductase inhibitors (i.e. statins) discussed herein are metabolized primarily by cytochrome P450 isoform 3A4. Concomitant treatment with a CYP 3A4 inhibitor may elevate the levels of these agents in patients and increase their risk for these associated adverse liver effects and myopathies.
2.2 Cholesterol Absorption Inhibitors
Cholesterol cleared from circulation by LDL-R-mediated uptake in the liver is processed and introduced to the intestinal tract via the bile duct from the gall bladder (see Figure 1). Once in the intestinal tract, this cholesterol becomes indistinguishable from cholesterol from dietary sources. Importantly, a large percentage of intestinal cholesterol is reabsorbed by the body. The intestinal site of absorption is another potential avenue for affecting endogenous overall cholesterol levels. The free cholesterol absorbed in the intestinal lumen is acylated with fatty acids in the endoplasmic reticulum by the enzyme acyl CoA:cholesterol acyltransferase (ACAT) for storage purposes and for packaging into chylomicrons for export to the portal plasma system. Many ACAT inhibitors have been prepared as possible LDL-C-lowering agents over the years, but none have proven effective in humans and therefore will not be discussed in detail in this article (120). A specific inhibitor of cholesterol absorption efficacious in man has, therefore, been a desirable therapeutic goal for some time.
2.2.1 Ezetimibe
In 2002, this goal was realized with the introduction of ezetimibe 10 as the first therapy to treat elevated LDL-C levels in patients by inhibiting cholesterol absorption (Figure 5). Ezetimibe (Zetia) was shown to inhibit cholesterol absorption in mildly hypercholesterolemic individuals at a dosage of 10 mg day−1, dropping fractional cholesterol absorption rates by 54% (121, 122). LDL and total cholesterol levels following ezetimibe treatment were reduced 20% and 15%, respectively, whereas campesterol and sitosterol were decreased by 48% and 41%, respectively (121). The reduction of plasma concentrations of other exogenously derived neutral sterols, sitosterol, and campesterol, indicate a direct effect on the absorption of these sterols by ezetimibe. Fractional cholesterol absorption rates in vegetarians on ezetimibe were reduced an average of 58% relative to patients on placebo. Although these individuals were consuming less than 30 mg of dietary cholesterol, LDL-C levels following ezetimibe treatment were still reduced 17% (122). These results indicate that ezetimibe significantly reduces LDL-C by inhibiting the reabsorption of biliary cholesterol in individuals consuming very little dietary cholesterol, and ezetimibe's LDL-C lowering activity is not diet dependent. Therefore, all sources of cholesterol are affected by ezetimibe treatment and this inhibition directly leads to reductions in LDL-C levels.
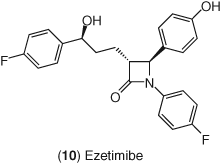
2.2.2 Ezetimibe as Monotherapy
The efficacy and safety of ezetimibe in patients with primary hypercholesterolemia were evaluated in two multicenter, randomized, double-blind placebo-controlled studies (123, 124). Following dietary stabilization, a washout period, and a four-week placebo lead-in period, patients were randomized to ezetimibe 10 mg or placebo once daily for 12 weeks. In a pooled analysis of the 1719 patients from both studies (125), ezetimibe significantly reduced mean LDL-C by 18% (vs a 0.9% increase with placebo, p < 0.01). These patients also showed a small, but statistically significant increase in HDL-C, and statistically significant reductions in TGs and apolipoprotein B. The response to ezetimibe was consistent across all subgroups analyzed. Ezetimibe was well tolerated and exhibited a safety profile similar to placebo.
2.2.3 Ezetimibe in Combination with Statins
The complementary nature of the mechanisms of action of statins and ezetimibe suggests that in combination, these agents should be very effective at reducing LDL-C. As the biosynthesis inhibitor blocks synthesis and increases the expression of the LDL-R, promoting cholesterol efflux, ezetimibe blocks the reabsorption of this biliary cholesterol. The LDL-C-lowering efficacy of inhibiting both cholesterol absorption and synthesis has been evaluated in several large clinical trials. These trials have evaluated the efficacy of adding ezetimibe to ongoing statin therapy as well as the efficacy of coadministration of ezetimibe and a statin.
In the Ezetimibe Add on to Statin Effectiveness (EASE) trial, the benefit of adding ezetimibe to ongoing statin therapy was evaluated for patients who had not achieved NCEP Adult Treatment Panel III LDL-C goals after six weeks of statin therapy (10, 126). Addition of ezetimibe to ongoing statin therapy resulted in a mean decrease in LDL-C of 26% compared to 3% with placebo after an additional six weeks. Importantly, addition of ezetimibe resulted in 71% of patients attaining their LDL-C goal compared with 21% for placebo. Addition of ezetimibe was also more efficacious in lowering other lipid parameters, including TGs, non-HDL-C, and apo B. The two treatment regimens had similar safety and tolerability profiles (126).
The addition of ezetimibe to either atorvastatin or simvastatin was evaluated in homozygous familial hypercholesterolemic (HoFH) patients who cannot express functional LDL-Rs (127). Patients on 40 mg of statin (either atorvastatin or simvastatin) were given ezetimibe (10 mg) for 12 weeks resulting in an additional 14% LDL-C reduction. The effect of ezetimibe was even more pronounced in a group receiving ezetimibe plus 80 mg of the statin, lowering LDL-C an additional 21% as compared to the statin alone. LDL-C lowering through the inhibition of cholesterol absorption by ezetimibe does not, therefore, require the expression of highly functional LDL-Rs and offers an additional treatment option for patients with HoFH.
Ezetimibe has been studied in combination with all marketed statins resulting in LDL-C reductions ranging from 44% to 57% for simvastatin (10–80 mg), 50% to 60% for atorvastatin (10–80 mg), 34% to 41% for pravastatin (10–40 mg), and 33% to 45% for lovastatin (10–40 mg) in parallel 12-week studies (128-131). With every statin, the combination of ezetimibe with the lowest statin dose (10 mg) resulted in similar reductions in LDL-C as the highest statin alone dose (80 mg for atorvastatin and simvastatin, 40 mg for pravastatin and lovastatin). These trials demonstrate a consistent, significant additional reduction in LDL-C with coadministration of ezetimibe plus statin compared to statin alone regardless of the statin or dose. In addition, C-reactive protein was found to be reduced nearly twofold greater in the ezetimibe plus simvastatin pooled groups when compared to the simvastatin alone pooled groups (97).
Further study of the combination of ezetimibe and simvastatin in patients with primary hypercholesterolemia demonstrated reductions of LDL-C by 46–61% (10–80 mg dose of simvastatin) compared to 31–46% with the statin alone (132). In this study, more than 82% of patients reached their targeted LDL-C goals versus 43% of patients treated with simvastatin alone. The positive results of these studies led to the development of a fixed combination of ezetimibe 10 and simvastatin 4 (Vytorin, 11) that proved to have LDL-C lowering and C-reactive protein lowering efficacy in a single tablet consistent with previous studies (133). The combination of ezetimibe and simvastatin demonstrated a safety profile similar to simvastatin monotherapy, including liver transaminase and creatine kinase levels.
Two large clinical outcome trials were performed to determine the benefit of ezetimibe in patients on statin therapy – IMPROVE-IT and SHARP. The IMPROVE-IT trial was an 18 144 patient outcome trial that studied ezetimibe in patients with acute coronary syndrome who were on a background of simvastatin (134). Patients on simvastatin alone achieved a median time-weighted average LDL-C level of 69.5 mg dL−1 while patients on ezetimibe and simvastatin achieved 53.7 mg dL−1 – a 23% further decrease of LDL-C due to the addition of ezetimibe. The primary endpoint for the trial was a composite of death from CVD. Patients taking ezetimibe and simvastatin exhibited a primary endpoint event rate of 32.7% compared to an event rate of 34.7% for patients on simvastatin alone. The study concluded that ezetimibe improved overall cardiovascular outcomes when added to statin therapy. The SHARP trial studied ezetimibe (10 mg daily) and simvastatin (20 mg daily) in 9270 patients with chronic kidney disease (135). Patients taking ezetimibe/simvastatin benefited from a 17% proportional reduction in their first major atherosclerotic event (primary endpoint) compared to patients on placebo. Surrogate markers such as intima medial thickness (IMT) have also been investigated in clinical trials (136, 137), but results from IMT trials of ezetimibe/simvastatin were mixed compared to the long term outcome trials such as IMPROVE-IT and SHARP.
2.2.4 Discovery of Ezetimibe
Ezetimibe was discovered through a combination of serendipity and careful scientific inquiry (138). In the late 1980s, scientists at Schering-Plough started an atherosclerosis program looking for novel ACAT inhibitors to block cholesterol absorption. In addition to an in vitro ACAT assay, they established an in vivo assay of cholesterol absorption in hamsters (139). One course of their investigations into the known 1,2-diphenylethyl amide class of ACAT inhibitors like SA58035 12 was vested in trying to introduce conformational constraints that would more closely define the bioactive conformer of the series (Figure 6). To this end, they attempted to prepare 2-azetidinone analogs as conformationally restricted targets. A minor by-product 13 in one attempt was fully characterized against ACAT in vitro and also in the in vivo cholesterol absorption assay (140). Although this compound was a poor ACAT inhibitor, it showed modest activity in vivo and ultimately became a new lead for the program. As the SAR evolved, they discovered that although their compounds were only modestly active at ACAT, they inhibited cholesterol absorption in vivo, and further, the SAR of the two assays were unrelated (141). Additional evidence of a mechanistic disconnect was established by resolving racemates into their enantiomeric components where the enantiomer with the weaker ACAT activity maintained the full measure of in vivo activity, in contrast to its mirror image. SAR development around this four-membered ring core, relying on seven-day cholesterol-fed hamster in vivo data, serendipitously led to their first clinical candidate, SCH 48461, 14. This first clinical candidate was found to be safe and efficacious in small, preliminary human clinical trials, suggesting activity for this class of compounds in man.
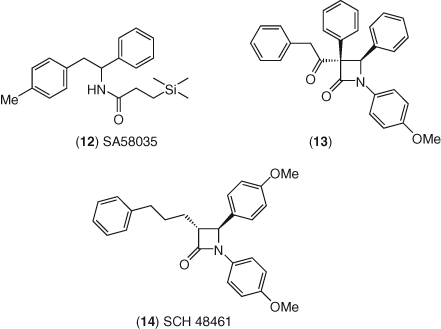
The metabolic fate of 14 is complex and isolation of a biliary metabolite in a bile duct-cannulated rat model showed that a specific fraction possessed excellent in vivo activity (142). An SAR study of C-3 side-chain analogs, targeting stereochemical isomers and concurrently exploring aryl modifications relying on activity results from their seven-day cholesterol-fed hamster model led, after much work and some luck, to the discovery of ezetimibe (143). In vivo potency in animal models for ezetimibe was remarkable as compared to its predecessor, improving 400-fold in a cholesterol-fed rhesus monkey assay (ED50 = 0.0005 mg kg−1 versus 0.2 mg kg−1 for SCH 48461).
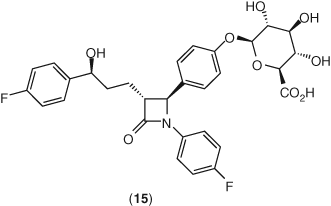
Ezetimibe is rapidly metabolized in the intestine to its phenolic glucuronide 15 (Figure 7) by uridine 5-diphosphate (UDP)-glucuronosyl-transferase 1A1, 1A3, and 2B15, with little oxidative cytochrome P450-mediated metabolism (144, 145). Once ezetimibe is glucuronidated, it is excreted in the bile, thereby delivering the drug back to the intestinal site of action (146). Glucuronidated ezetimibe localizes readily to its primary site of action, the intestine, consistent with its improved potency in cholesterol absorption studies. Autoradiographic analysis demonstrated that radiolabeled drug-related material was found in the villi of the small intestine and concentrated at the enterocyte brush border (146, 147). Ezetimibe and its glucuronide undergo enterohepatic recycling and have a plasma half-life of approximately 22 h. Ezetimibe and/or the glucuronide metabolite are excreted in the feces (90%) and urine (10%). Since ezetimibe does not influence the activities of cytochrome P450 enzymes, no significant pharmacokinetic interactions occur with most medications. Pharmacokinetic interaction studies with ezetimibe in humans have found no significant changes in the plasma levels of many medications, including statins (atorvastatin, simvastatin, pravastatin, rosuvastatin, lovastatin, and fluvastatin), fibrates (gemfibrozil and fenofibrate), digoxin, glipizide, warfarin, and oral contraceptives (ethinyl estradiol and levonorgestrel) (127, 144, 145). Preclinical safety studies did not demonstrate any target organ toxicities or carcinogenic potential with ezetimibe (148).
2.2.5 Discovery of the Mechanism of Action of Ezetimibe (NPC1L1)
Ezetimibe was discovered without knowing its molecular mechanism of action. Unlike ACAT inhibitors, ezetimibe selectively inhibits the transport of free cholesterol across the intestinal wall. Ezetimibe does not affect the absorption of TG, ethinyl estradiol, progesterone, fat-soluble vitamins A and D, or taurocholic acid (127, 149). Ezetimibe does not affect pancreatic lipase, and therefore shares no properties with orlistat (Xenical). Ezetimibe also does not sequester bile acids or block their absorption and thus differs from cholestyramine. Ezetimibe potently and selectively inhibits biliary and dietary cholesterol absorption in the intestine. The SAR of the cholesterol absorption activity suggests a pharmacophore presentation that is exquisitely well defined in three dimensions for this class of molecules (138, 141). These data all suggest that the mechanism of action of ezetimibe is via inhibition of a specific mediated uptake in the intestines (121, 139, 146, 149, 150).
The suspected intestinal cholesterol transporter was predicted to have certain properties based on the behavior of ezetimibe in animal models of cholesterol uptake. This putative transporter should be expressed in the proximal intestines where cholesterol is absorbed and localized to the brush border membrane of jejunal enterocytes. After years of unsuccessful biochemical and molecular biological approaches aimed to discover the target of ezetimibe, a genomics/bioinformatics technique was used to identify genes involved in cholesterol uptake (151). It was hypothesized that a cholesterol transporter should possess several critical features. In addition to being expressed in intestinal jejunal enterocytes, the protein should also be expressed on the cell surface at the brush border membrane to have direct access to the luminal contents. Finally, considering its function, the gene should contain sequence motifs known to interact with sterols (151). A rat intestinal cDNA library was generated and ∼16 500 genes were sequenced and annotated by cross-referencing the rat sequences with both mouse and human data. Filtering this database for all transcripts with the anticipated properties afforded a single candidate gene, Niemann-Pick C1 like 1 (NPC1L1). NPC1L1 has all the predicted features of a plasma membrane expressed transporter including a secretion signal, 13 predicted transmembrane domains and extensive N-linked glycosylation sites located within the extracellular loops, and it contains a sterol sensing domain (151).
Whole animal studies established that NPC1L1 is a central pathway for cholesterol and phytosterol uptake into enterocytes and is sensitive to ezetimibe. NPC1L1 was found to be highly expressed in the jejunum and localized on the surface of the absorptive jejunal enterocytes (151, 152). Importantly, NPC1L1 knockout mice displayed a reduced uptake and absorption of cholesterol into the enterocytes of the jejunum, indicating that NPC1L1 plays an essential role in the uptake of cholesterol from the lumen of the intestine to the brush border membrane of the enterocyte. NPC1L1 null mice also demonstrated insensitivity to further cholesterol reductions by ezetimibe. Ezetimibe is also reported to reduce plasma phytosterol levels in hypercholesterolemic patients and in patients with sitosterolemia, which is caused by a mutation in the ATP-binding cassette (ABC) cotransporters, either ABCG5 or ABCG8 (121, 153, 154). Plasma levels of the plant sterols campesterol and sitosterol were found to be nearly undetectable in the NPC1L1 null mice (155). These results indicated that NPC1L1 is the intestinal transporter for the uptake of both cholesterol and structurally related phytosterols and that ezetimibe acts through inhibition of NPC1L1 (155, 156).
The development of a binding assay of labeled ezetimibe to human NPC1L1 expressing cells made clear a specific molecular role for this protein by demonstrating a specific, single site, saturable binding profile (157). Ezetimibe bound to the cell surface membrane of the NPC1L1-expressing cells and the binding was completely abolished in the presence of excess unlabeled ezetimibe. No such binding occurred to enterocyte brush border membranes prepared from NPC1L1 null mice. These studies were extended to include binding to mouse, rat, hamster, rabbit, monkey, and canine NPC1L1, the results of which correlated to the in vivo activity of ezetimibe in the different species (158). The observation that ezetimibe binding affinity to dog NPC1L1 was 50-fold higher than that for mouse NPC1L1 was used to determine the specific ezetimibe binding site on NPC1L1. Chimeras of dog and mouse NPC1L1 were generated and binding of an ezetimibe analog was evaluated. These studies identified a 61 amino acid region of the middle extracellular domain of NPC1L1 as the ezetimibe binding site (159). Further evidence shows that ezetimibe prevents the internalization of NPC1L1 and thus prevents the receptor from transporting sterols (160, 161). In light of the discovery that NPC1L1 knockout mice are defective in intestinal cholesterol and phytosterol uptake, and are no longer responsive to ezetimibe, these binding studies definitively established NPC1L1 as the direct molecular target of ezetimibe.
2.2.6 Additional Cholesterol Absorption Inhibitors
The clinical and commercial success of ezetimibe has attracted competitors, although, to date, none have progressed to market (162). Since ezetimibe was discovered through an in vivo screening paradigm without knowing the mechanism of action, competitors lacked the ability to rapidly screen their collections and faced discovery programs committed largely to in vivo screens as the exclusive pivotal drivers of the research, not the usual course in modern drug discovery. Thus, ezetimibe itself has been the key starting point for most competitive efforts with an overall emphasis on lead hopping strategies.
Sanofi-Aventis took a nonabsorbable cholesterol absorption inhibitor, AVE 5530 (16), through Phase 3 clinical trials (Figure 8) but subsequently discontinued its development due to weak efficacy in humans (163). This compound was a scaffold-hopping effort based on ezetimibe and its reported SAR. It was designed to be poorly absorbed with low systemic bioavailability thus limiting peripheral exposure while targeting the intestinal site of action (164).
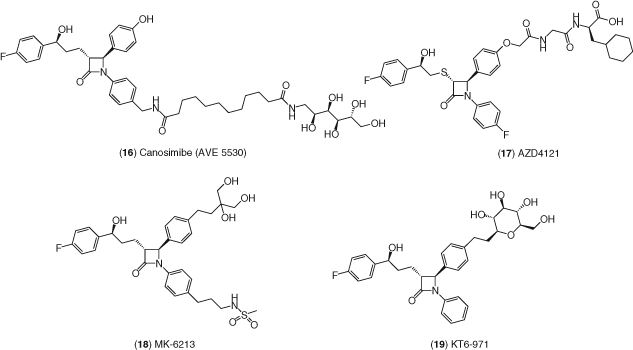
Another 2-azetidinone cholesterol absorption inhibitor based on modification of ezetimibe's structure is AZD4121 17 from AstraZeneca (for a lead reference, see (165)). This compound is also reported to have low systemic exposure, an excellent safety and tolerability profile and high efficacy at preventing cholesterol absorption in mouse models. The compound was discontinued in advanced clinical trials.
Merck's own efforts on a back-up program led to the identification of MK-6213 18 which was also a potent cholesterol absorption inhibitor with low systemic exposure in preclinical species. MK-6213 was progressed into Phase 2 clinical trials but was discontinued due to lower-than-expected efficacy at LDL-C reduction (162). Similarly, Ironwood Pharmaceuticals expanded the SAR of ezetimibe's C4 aryl moiety to cover new biaryl motifs and their lead clinical candidate, MD-0727 (structure not reported) progressed to Phase 2 clinical trials (166), before being discontinued (162). It was reported to have efficacy similar to ezetimibe, with significantly lower plasma levels (167). Finally, Kotobuki Pharmaceuticals identified KT6-971 19 as a non-absorbed cholesterol absorption inhibitor based off the ezetimibe glucuronide metabolite 15, and this compound was also ultimately discontinued (162).
2.3 Bile Acid Sequestrants
Bile acid sequestrants have had a long history of lowering LDL-C (168). These compounds exploit the biology that bile acids are synthesized in the body by oxidative metabolism of cholesterol. Bile acids are important for the solubilization of dietary fat and impart their function through their amphiphilic nature. Although they are derived from a very lipophilic sterol core, the introduction of an acidic residue via oxidative metabolism allows bile acids to emulsify dietary fats, critical for effective fat absorption. Bile acid sequestrants are essentially polymeric resins bearing basic functionality designed to bind to the acidic residues of bile acids and inhibit the mediated reabsorption of these bile acids in the ileum of the intestines. Deprived of these essential emulsifying agents the body increases their production in the liver, effectively catabolizing cholesterol in the process. The reduction of hepatic cholesterol causes an upregulation of LDL-Rs, which in turn increases cholesterol clearance from the periphery. Overall, this class of compounds has a beneficial effect on LDL-C and has proven effective in humans (168, 169).
Cholestyramine 20, the first LDL-C lowering agent in this class, and colestipol 21 shown in Figure 9 are modestly effective at lowering LDL-C in humans particularly in combination with statins, but these agents have received less acceptance in the treatment of hypercholesterolemia due to a difficult dosing regimen, poor palatability, and significant gastrointestinal side effects (170-172). As might be expected for a single pass use of a polymeric resin, timing of the dose just prior to meals as well as quantity and effective loading of the resin is crucial to its successful application. For cholestyramine, the typical dosage is 4 or 8 g twice daily with a maximum dosage of 24 g day−1. In a small group of patients with primary hypercholesterolemia, lovastatin (20 mg twice daily), and colestipol 21 (10 g twice daily) reduced plasma cholesterol levels 36% while reducing LDL-C levels 48%.
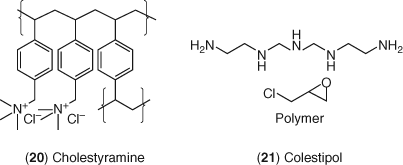
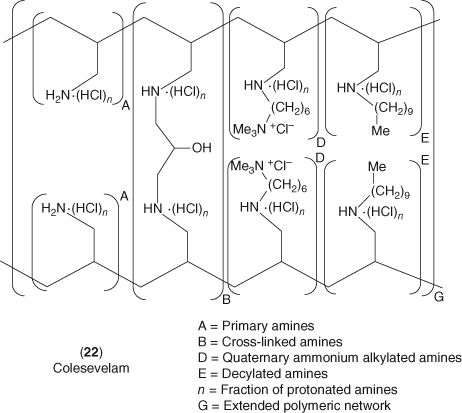
A major improvement in bile acid sequestrant therapy came with the development of colesevelam 22 (173). This compound is a polymer of allylamine, cross-linked with epichlorohydrin and alkylated with decyl bromide and 6-bromohexyltrimethylammonium chloride as shown in Figure 10. The structural improvements include the incorporation of primary amine sites, lipophilic side chains, and quaternary ammonium sites into the polymer that not only increase binding affinity for bile acids but also improve the polymer's physical properties and tolerability. In patients with primary hypercholesterolemia, colesevelam (3.75 g day−1) reduced LDL-C approximately 15% along with a small 3–4% increase in HDL-C (174). This dose-ranging study demonstrated a 9–18% reduction of LDL-C using doses from 2.3 to 4.5 g of colesevelam once per day. Colesevelam also provided additive LDL-C lowering when added on to statin therapy even showing positive lowering effects on C-reactive protein (173-175). Colesevelam (2.3–3.5 g day−1) provided additional LDL-C lowering of about 8–16% when added on to 10 or 20 mg doses of simvastatin in patients with primary hypercholesterolemia (176).
2.4 PCSK9 Inhibitors
A relatively new mechanism to regulate LDL-C levels was discovered through mendelian analysis and characterization of individuals with genetic hypercholesterolemia. PCSK9 was found to cause autosomal dominant hypercholesterolemia when mutations caused a gain-of-function (177). PCSK9 is secreted after autocatalytic cleavage of its zymogen form, with the prodomain noncovalently associated with the catalytic domain that inactivates its protease activity (6). Secreted primarily from the liver, circulating PCSK9 binds to LDL-R resulting in their degradation. Gain-of-function mutations in PCSK9 result in low LDL-R and increased plasma LDL-C levels while loss-of-function mutations reduce LDL-C levels and result in low levels of CVD (6, 178). PCSK9 is regulated through sterol regulatory element-binding protein-2 (SREBP-2) like the LDL-R (179). Statin and/or ezetimibe treatment results in hepatic cholesterol reduction and an upregulation of the LDL-R to reduce plasma LDL-C levels, but PCSK9 levels are also increased (180, 181). By blocking the production of PCSK9 or its binding to LDL-R, LDL-R levels will increase, plasma LDL-C will decrease, and these effects are additive and/or synergistic with statins (182, 183).
Multiple approaches have been taken to reduce PCSK9 but to date, no small-molecule inhibitors have progressed into the clinic (184, 185). A recent novel small-molecule approach has been reported that inhibits PCSK mRNA translation (186). Large molecule approaches have progressed further than small molecules and inhibition of the production of PCSK9 through antisense oligonucleotides (ASOs) or small interfering ribonucleic acids (siRNA) such as inclisiran have been shown to reduce plasma levels of PCSK9 resulting in an increase in hepatic LDL-R and reduced plasma LDL-C levels (187, 188). The siRNA agent inclisiran is reported to have a relatively long duration of action at reduction of both PCSK9 and LDL-C (189).
The most successful approach to inhibiting PCSK9 has been through the use of monoclonal antibodies (188, 190). To date, two fully human monoclonal antibodies for PCSK9 have emerged as approved drugs – alirocumab (Praluent) 23 from Sanofi and evolocumab (Repatha) 24 from Amgen. Both drugs are approved for the treatment of ASCVD and FH. The lipid-lowering effects of these antibodies occur within one-day postinjection, with 50–60% LDL-C reduction, 25–30% lipoprotein (a) reduction, and 10–20% TG reductions noted (191, 192). The large cardiovascular outcome trial (CVOT) FOURIER evaluated 27 564 patients on either maximally tolerated statin therapy or evolocumab (193). Evolocumab performed well in the study achieving a 60% reduction in LDL-C which in turn induced a 15% relative risk reduction (RRR) in the primary 5-point MACE endpoint (cardiovascular death, MI, stroke, hospitalization, for unstable angina, or coronary revascularization). Alirocumab performed similarly well in the large ODYSSEY CVOT with 18 924 subjects with recent acute coronary syndrome on a background of high-intensity statin therapy (194). Bococizumab was another PCSK9 antibody in late-stage clinical trials, but this compound was discontinued due to a high incidence of antidrug antibodies attributed to a small (3%) mouse sequence in the IgG (195). As both alirocumab and evolocumab are both fully human antibodies, they were both only minimally antigenic. Despite their excellent lipid lowing profiles, the clinical uptake of these drugs has been modest, and this has been attributed to both the cost and the route of administration of these therapeutics (10, 196). When they came to market in 2015, their price was roughly $14 000 per year but recently the price has dropped to roughly $5500 per year. Nevertheless, the “lower is better” hypothesis continues to be supported by the CVOT results from both FOURIER and ODYSSEY.
2.5 Antisense Oligonucleotide for ApoB100
ApoB100 is the apoprotein required for VLDL and LDL synthesis by the liver. The inhibition of the production of hepatic apoB100 is an additional mechanism to reduce LDL-C levels. This approach to reduce VLDL and LDL-C, as well as apoB100, was realized by Ionis Pharmaceuticals using an ASO, mipomersen 25, which was approved 2013 (162, 197). In various patient populations, mipomersen reduces LDL-C in ranges of 25–36% (198-201). Side effects included injection site issues, elevations in transaminases, and hepatic steatosis (162, 197, 202). As a result of its tolerability profile, mipomersen is currently restricted for use in HoFH (203).
2.6 Microsomal Triglyceride Transfer Protein Inhibitors
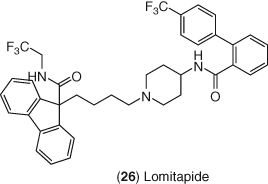
Microsomal triglyceride transfer protein (MTP) is involved it transporting lipids, including TGs and CEs, into chylomicrons in the intestine (Figure 1) and VLDL particles in the liver. MTP inhibitors reduce the assembly and production of VLDL particles from hepatocytes, resulting in decreased apolipoprotein B100 production. Consequently, inhibition of MTP decreases plasma VLDL cholesterol and LDL-C levels. MTP inhibition also reduces the absorption of TGs and cholesterol in the intestines by inhibiting chylomicron production in the enterocytes. The MTP inhibitor lomitapide 26, shown in Figure 11, is a potent inhibitor of MTP with a Ki of 0.5 nM and was reported to decrease plasma cholesterol levels by 90% and TGs more than 49% in Watanabe rabbits, which lack functional LDL-Rs (204). Clinical trials with lomitapide reported LDL-C reductions of about 50% with a dose of 40 mg daily in patients with HoFH (205). The development of many MTP inhibitors was stopped due to intestinal nutrient malabsorption and the development of fatty liver. Early clinical trials with lomitapide revealed a high prevalence of GI distress, fatty liver, and transaminitis; however, the benefits of reduced ASCVD risk and quality of life due to reduced need for lipid apheresis treatments outweighed these risks for patients with HoFH (206). Moreover, GI side effects can be mitigated by a gradual dose escalation in patient populations (205). Lomitapide was approved in 2012 as an adjunctive treatment for patients with HoFH (207).
2.7 Combination Products
The quest for the optimal lipid-modifying therapy has logically led to the consideration of combinations of products directed at complementary biological targets. From a pharmacological perspective, this approach offers significant benefits to the patient, directly affecting multiple lipid (or cardiovascular) parameters in a single dosing regimen (208). The key to success in this strategy is threefold. First, the individual agents in the combination must not affect the absorption, metabolism, or excretion of the other agents. Consequently, attention must be closely paid to the drugs' effects on cytochrome P450 isozymes, both induction and inhibition. Secondly, the dosing regimens of the individual agents need to be the same. A once-daily drug should be combined with other drugs dosed once daily, a requirement that prompts consideration of extended-release formulations to reconcile differences. Simplifying dosing regimens may also improve patient compliance, which in turn should be significantly beneficial to the patient's health. Finally, the safety of the combination must comparable or better than the individual agents. Often, these combinations are driven by doctors' usage of the individual component drugs to treat patients, reflecting clinical practice. These combinations can also provide a very practical economic benefit to the patients as well, reducing overall cost of the therapy, especially if insurance copayments are involved.
The first combination agent introduced to treat hyperlipidemia is Vytorin, introduced in 2004 and marketed by Merck/Schering-Plough. This product is a combination of simvastatin and ezetimibe and has been discussed already in this article. For this agent, the complementary nature of the combination results directly from the biological mechanisms of LDL-C synthesis, absorption, and excretion.
The second combination product for affecting lipid parameters is Advicor.1 Advicor is a combination of lovastatin and extended-release niacin. Niacin and its extended-release formulation have been shown to raise HDL-C levels and lower LDL-C, albeit with significant side effects, such as flushing. Niacin has been shown to have beneficial effects on cardiovascular outcomes in the clinic. Further discussion on niacin can be found in the article of this text concerning HDL-C raising. The lovastatin component also is effective at lowering LDL-C and brings along its associated side effects of muscle toxicity and sensitivity to CYP 3A4 inhibitors. Thus, this agent is intended to both lower LDL-C and increase HDL-C. A follow-up to this combination is Simcor from AbbVie. Simcor is a combination of simvastatin and extended-release niacin, intended to both lower LDL-C and raise HDL-C, similar to Advicor (see Endnote 1). In this particular combination, the more potent simvastatin takes the role of the HMG-CoA reductase inhibitor. As with Advicor, the combination is designed to emulate dosing the agents individually in terms of PK and biological effects, and as such brings along warnings associated with each, including potential muscle toxicity and flushing. Atozet is another cholesterol lowering fixed-dose combination drug approved in the United States. This drug is a combination of ezetimibe and atorvastatin.
Finally, Pfizer has brought a combination of atorvastatin (Lipitor) and amlodipine besylate (Norvasc), a long-acting calcium channel antagonist, to the market as a single combination product named Caduet to both lower LDL-C and treat hypertension simultaneously (see Endnote 1). It is also approved to treat constant stable or variant angina in patients needing LDL-C modulation. As with the other combination products, Caduet offers the promise of simplified dosing regimens and potential cost savings to patients.
In future years, doctors will undoubtedly have additional new combination products to use in the treatment of their patients, designed to simplify dosing and to treat multiple conditions associated with their disease. These medications will provide pharmacological and economic value to the patient. With improved patient compliance, outcomes should also be improved.
3 Other Approaches to Treat Dyslipidemia
3.1 LDL-C Lowering
ATP citrate lyase (ACL) is the enzyme that catalyzes the conversion of citrate to acetyl CoA prior to the beginning of the cholesterol biosynthesis pathway. This target is upstream of HMG CoA reductase and, consequently, inhibitors of this target can inhibit the biosynthesis of cholesterol. Bempedoic acid 27 (Figure 12) is a small molecule that reduces LDL-C through inhibition of ACL (209-211). The compound itself is a prodrug that gets converted to bempedoic acid-CoA in vivo and it is this active drug that inhibits ACL in the liver (212). Bempedoic acid as monotherapy reduced LDL-C by 13–43% and when administered on a background of statin therapy it reduced LDL-C by 24% (213-215). When co-administered with ezetimibe, bempedoic acid reduced LDL-C by 48% and this combination was effective at reducing not only LDL-C but also non-HDL-C, apoB, and C-reactive protein (216, 217). Progression of bempedoic acid to market remains on the horizon.
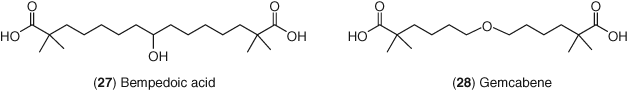
Although its precise mechanism-of-action is not elucidated, gemcabene 28 reduced LDL-C by a further 23–28% when administered on a background of statin therapy (218). Additionally, the drug reduced high-sensitivity C-reactive protein by up to 54%. Currently, gemcabene continues to undergo trials with potential to be an add-on therapy to current LDL-C lowering agents and for the treatment of other dyslipidemias.
3.2 HDL Raising Agents
In recent years, the role of HDL-C in CVD has undergone a revision (197, 219). Early research suggested that elevations of HDL-C could protect against CHD and consequently drugs that increase HDL-C were expected to protect patients against CHD [Framingham study]. Epidemiological studies suggest that low HDL-C is associated with increased cardiovascular risk (220, 221). On the other hand, high levels of HDL-C are not necessarily indicative of low cardiovascular risk, especially when combined with high LDL-C levels. Nevertheless, HDL plays a key role in shuttling excess cholesterol from the periphery to the liver in a process called reverse cholesterol transport (RCT), and LDL-C also plays a role in this process (222-225). A few epidemiological studies demonstrated increased mortality with elevated HDL-C (226-228). Some research suggests that HDL can become dysfunctional and subsequently detrimental to vascular health (229). Nevertheless, HDL-C has also been documented to play a positive role in vascular protection and consequently therapies that increase HDL-C may offer a different mechanism to treat dyslipidemias compared to LCL-C reduction therapies (197, 219).
3.2.1 Cholesterol Ester Transfer Protein Inhibition
CETP is responsible for shuttling lipids between circulating lipoprotein particles and loss of CETP has been linked to increased HDL-C levels together with protection from ASCVD (230). Consequently, inhibition of CETP became a strategy of intense interest in the pharmaceutical industry for several years (231-233). The first CETP inhibitor to advance to late-stage clinical trials was Pfizer's torcetrapib 29 in Figure 13. Although torcetrapib was able to induce a 72% increase in HDL-C and −25% LDL-C in a large-scale CV outcomes trial, there was a significant rise in the incidence of cardiovascular endpoints and in all-cause mortality in patients taking the drug (234). This clinical trial was stopped early due to these findings – and a number of factors may have contributed to the increased mortality including an increase in systolic blood pressure (+5.4% mmHg) observed in the drug-treated group (232-234).
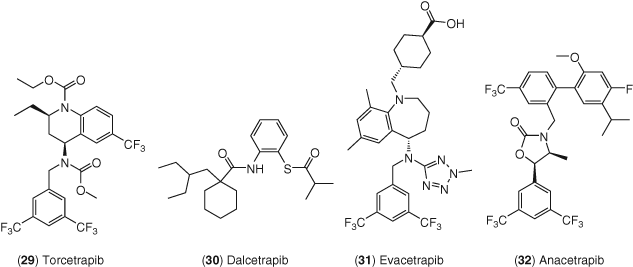
Dalcetrapib 30 from Roche was the second CETP modulator to enter clinical trials. This drug displays time-dependent inhibition of CETP neutral lipid transfer activity and is a weaker blocker of CE-TG transfer than torcetrapib. A large-scale CV outcomes trial called dal-OUTCOMES did not achieve any significant effects on CV mortality and morbidity although it did increase HDL-C by 40% with no changes in LDL-C and only a small increase in systolic blood pressure of 0.6 mmHg (235).
Lilly's Evacetrapib 31 was evaluated in the 12 092 patient ACCELERATE phase 3 trial to evaluate whether the addition of this drug to standard therapy reduced CV morbidity and mortality (236). After 26 months of dosing, there was no significant difference between the evacetrapib treated patients and patients treated in the placebo arms, despite the demonstration that evacetrapib decreased LCL-C by 37%, increased HDL-C by 132%, and reduced TG (6%), apoB (12%), and Lp(a) (22%). A small increase in systolic blood pressure of 1.2 mmHg was observed in the ACCELERATE trial. This trial was stopped along with three other Phase 3 studies that were ongoing with evacetrapib.
Anacetrapib 32 from Merck was evaluated in a 30 449 patient study called REVEAL to evaluate its effects on patients with a background of atorvastatin (237). Anacetrapib raised HDL-C by 104% and reduced LDL-C by 17%. Additionally, the primary outcome of first major coronary event was decreased by 9% in the patients receiving anacetrapib thus marking the first trial where a CETP inhibitor achieved its primary endpoint. A small increase in systolic blood pressure was observed in REVEAL of 0.74 mmHg. Merck announced it will not seek regulatory approval of anacetrapib as they did not anticipate that the clinical profile would support regulatory filing.
Deeper analysis of the data from the dal-OUTCOMES study revealed significant genotype-dependent effects of the drug on patients with a specific adenosine cyclase 9 (ADCY9) rs1967309 SNP (238, 239) and so a new clinical study is underway to evaluate this drug in this more defined patient population. The question of whether the ADCY9 genotype affects outcomes with CETP inhibitors was recently investigated in two analyses of patients treated with evacetrapib (240) and anacetrapib (241), and neither of these studies showed any significant association between the ADCY9 genotype and cardiovascular outcomes of these two drugs.
3.2.2 Niacin Approaches
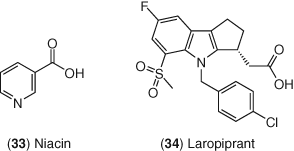
The lack of success of CETP inhibitors underscores the challenges associated with drugs to treat dyslipidemia and in particular the challenge associated with deriving a clinical benefit to accompany increases in HDL-C. One of the first drugs approved to treat dyslipidemia was niacin 33 (Figure 14), which has been known since the 1950s to decrease LDL-C, TGs, and increase HDL-C in humans (242). Niacin also induces flushing as a side effect, which hinders patient compliance. In recent years, an extended-release formulation of niacin is available called Niaspan and this formulation helps mitigate flushing and improve overall patient compliance. Additionally, researchers at Merck discovered laropiprant 34 which is an agent that can block the binding of PGD2 to the DP1 receptor which is believed to be responsible for niacin-induced flushing. By co-dosing niacin with laropiprant, the flushing side-effect is significantly blocked thus resulting in improved patient compliance (243). Although multiple clinical trials with niacin indicated a benefit of this drug to reduce mortality and slow the progression of atherosclerosis (244-246), more recent studies suggest no little benefit for niacin in the treatment of dyslipidemia either alone or with a background of a statin (247-249).
The benefits of raising HDL-C have come under question recently, and clearly more basic science is needed to better understand their impact on cardiovascular risk (229, 233, 250). It appears, however, that increased HDL-C alone is insufficient to afford a clear benefit to patients. Unfortunately, large and expensive cardiovascular outcome clinical trials are necessary to better understand the risks and benefits of these agents. A significant improvement in the ability to link pharmacodynamic (PD) biomarkers to cardiovascular outcomes is needed to further facilitate progress in this important area of human health.
4 Conclusion
Over the past 50 years, improvements in drugs used to treat CVD have contributed dramatically to an overall improvement in human health. Although prevalence of obesity and diabetes has increased in the past few decades, the rates of death due to CHD in the United States have actually decreased. One study attributed much this decrease to the success of drugs such as statins (251), as well as other drug classes including ACE inhibitors, β-blockers, and antiplatelet agents (252). Newer medicines such as PCSK9 inhibitors are emerging through successful discovery efforts with modalities outside of traditional small-molecule space. The range of modalities continues to increase (253), and newer drugs will undoubtedly emerge that will further enable doctors to further reduce risk of CVD and consequently human longevity.
Failures in the past decade to connect PD biomarker changes with cardiovascular outcomes (250) have contributed to a need for increased investment in newer drugs that can provide benefits beyond current therapies for lipid modification. Additionally, species differences in lipid metabolism add yet another layer of complexity to our ability to effectively translate preclinical efficacy/endpoint readouts to human outcomes (254). Researchers who study species lipidome differences are beginning to find links between different species longevities and their respective lipid determinants (255), thus paving the pathway to connect lipid metabolism with aging (256). Despite species differences and PD biomarker challenges, the success of the past 50 years at increasing longevity through reduction of cardiovascular risk is a strong indicator that future decades will continue to bring additional benefits to human cardiovascular health.
Additional Sources of Information
- American College of Cardiology: www.acc.org
- American Heart Association: www.americanheart.org
- World Health Organization: www.who.int
- Center for Disease Control: www.cdc.gov
- National Heart, Lung, and Blood Institute: www.nhlbi.nih.gov
- NIH Clinical Trials Registry: www.clinicaltrials.gov
- National Library of Medicine: www.nlm.nih.gov
- PubMed: www.pubmed.gov
- American Diabetes Association: www.diabetes.org
- Mayo Clinic: www.mayoclinic.com
- American Stroke Association: www.strokeassociation.org



