Abstract
[7646-69-7] HNa (MW 24.00)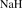
InChI = 1S/Na.H
InChIKey = MPMYQQHEHYDOCL-UHFFFAOYSA-N
(used as a base for the deprotonation of alcohols, phenols, amides (NH), ketones, esters, and stannanes; used as a reducing agent for disulfides, disilanes, azides, and isoquinolines)
Physical Data: mp 800 °C (dec); d 1.396 g cm−3.
Solubility: decomposes in water; insol all organic solvents; insol liq NH3; sol molten sodium.
Form Supplied in: free-flowing gray powder (95% dry hydride); gray powder dispersed in mineral oil.
Handling, Storage, and Precautions: the dispersion is a solid and may be handled in the air. The mineral oil may be removed from the dispersion by stirring with pentane, then allowing the hydride to settle. The pentane/mineral oil supernatant may be pipetted off, but care should be exercised to quench carefully any hydride in the supernatant with a small amount of an alcohol before disposal. The dry powder should only be handled in an inert atmosphere.
Sodium hydride dust is a severe irritant and all operations should be done in a fume hood, under a dry atmosphere. Sodium hydride is stable in dry air at temperatures of up to 230 °C before ignition occurs; in moist air, however, the hydride rapidly decomposes, and if the material is a very fine powder, spontaneous ignition can occur as a result of the heat evolved from the hydrolysis reaction. Sodium hydride reacts more violently with water than sodium metal (eq 1); the heat of reaction usually causes hydrogen ignition.
Bibliography
- 1 (a) Mackay, K. M. Hydrogen Compounds of the Metallic Elements; Spon: London, 1966. (b) Hurd, D. T. An Introduction to the Chemistry of the Hydrides; Wiley: New York, 1952. (c) Wiberg, E.; Amberger, E. Hydrides of the Elements of Main Groups I–IV; Elsevier: New York, 1971.
- 2 Tate, M. E.; Bishop, C. T., Can. J. Chem. 1963, 41, 1801.
- 3
Doornbos, T.;
Strating, J.,
Synth. Commun.
1971,
1,
175.
10.1080/00397917108081744 Google Scholar
- 4 (a) Stoochnoff, B. A.; Benoiton, N. L., Tetrahedron Lett. 1973, 21. (b) Brown, C. A.; Barton, D.; Sivaram, S., Synthesis 1974, 434.
- 5 Hajos, Z. G.; Duncan, G. R., Can. J. Chem. 1975, 53, 2971.
- 6 (a) Brimacombe, J. S.; Jones, B. D.; Stacey, M.; Willard, J. J., Carbohydr. Res. 1966, 2, 167. (b) Brimacombe, J. S.; Ching, O. A.; Stacey, M., J. Chem. Soc. (C) 1969, 197.
- 7 Kochi, J. K.; Hammond, G. S., J. Am. Chem. Soc. 1953, 75, 3443.
- 8 Groenen, L. C.; Ruël, B. H. M.; Casnati, A.; Timmerman, P.; Verboom, W.; Harkema, S.; Pochini, A.; Ungaro, R.; Reinhoudt, D. N., Tetrahedron Lett. 1991, 32, 2675.
- 9 Sato, F.; Tanaka, Y.; Sato, M., Chem. Commun./J. Chem. Soc. 1983, 165.
- 10 Singh, B., Tetrahedron Lett. 1971, 321.
- 11 Coggins, J. R.; Benoiton, N. L., Can. J. Chem. 1971, 49, 1968.
- 12 McDermott, J. R.; Benoiton, N. L., Can. J. Chem. 1973, 51, 1915.
- 13 (a) Baldwin, J. E.; Christie, M. A.; Haber, S. B.; Kruse, L. I., J. Am. Chem. Soc. 1976, 98, 3045. (b) Wasserman, H. H.; Hlasta, D. J.; Tremper, A. W.; Wu, J. S., Tetrahedron Lett. 1979, 549. (c) Wasserman, H. H.; Hlasta, D. J., J. Am. Chem. Soc. 1978, 100, 6780.
- 14 Corriu, R. J. P.; Guerin, C., J. Organomet. Chem. 1980, 197, C19.
- 15 Zaugg, H. E.; Dunnigan, D. A.; Michaels, R. J.; Swett, L. R.; Wang, T. S.; Sommers, A. H.; DeNet, R. W., J. Org. Chem. 1961, 26, 644.
- 16 (a) Kershaw, J. R.; Uff, B. C., Chem. Commun./J. Chem. Soc. 1966, 331. (b) Uff, B. C.; Kershaw, J. R., J. Chem. Soc. (C) 1969, 666.
- 17 Pi, R.; Friedl, T.; Schleyer, P. v. R.; Klusener, P.; Brandsma, L., J. Org. Chem. 1987, 52, 4299.
- 18 Hinckley, A. A. Sodium Hydride Dispersions; Metal Hydrides, Inc., 1964 (quoted in: Fieser & Fieser 1967, 1, 1075).
- 19 Swamer, F. W.; Hauser, C. R., J. Am. Chem. Soc. 1946, 68, 2647.
- 20 Ainsworth, C., Org. Synth., Coll. Vol. 1963, 4, 536.
- 21 (a) Bloomfield, J. J., J. Org. Chem. 1961, 26, 4112. (b) Bloomfield, J. J., J. Org. Chem. 1962, 27, 2742. (c) Anselme, J. P., J. Org. Chem. 1967, 32, 3716.
- 22 Miles, M. L.; Harris, T. M.; Hauser, C. R., J. Org. Chem. 1965, 30, 1007.
- 23 Gruen, H.; Norcross, B. E., J. Chem. Educ. 1965, 42, 268.
- 24 (a) Ref. 18. (b) Daub, G. H.; Johnson, W. S., J. Am. Chem. Soc. 1948, 70, 418. (c) Daub, G. H.; Johnson, W. S., J. Am. Chem. Soc. 1950, 72, 501.
- 25 (a) Ref. 18. (b) Della Pergola, R.; DiBattista, P., Synth. Commun. 1984, 14, 121.
- 26 Stork, G.; Winkler, J. D.; Saccomano, N. A., Tetrahedron Lett. 1983, 24, 465.
- 27 Pinkney, P. S., Org. Synth., Coll. Vol. 1943, 2, 116.
- 28 Liu, H.-J.; Lai, H. K., Tetrahedron Lett. 1979, 1193.
- 29 Powers, J. C.; Seidner, R.; Parsons, T. G., Tetrahedron Lett. 1965, 1713.
- 30 Carbon, J. A.; Martin, W. B.; Swett, L. R., J. Am. Chem. Soc. 1958, 80, 1002.
- 31 Tamura, Y.; Sasho, M.; Nakagawa, K.; Tsugoshi, T.; Kita, Y., J. Org. Chem. 1984, 49, 473.
- 32 Bortolussi, M.; Bloch, R.; Conia, J. M., Tetrahedron Lett. 1977, 2289.
- 33 Corriu, R. J. P.; Guérin, C., Chem. Commun./J. Chem. Soc., 1980, 168.
- 34 Krull, L. H.; Friedman, M., Biochem. Biophys. Res. Commun. 1967, 29, 373.
- 35 Lee, Y.-J.; Closson, W. D., Tetrahedron Lett. 1974, 381.
- 36 Natsume, M.; Kumadaki, S.; Kanda, Y.; Kiuchi, K., Tetrahedron Lett. 1973, 2335.
- 37 Liu, W.; Szewczyk, J.; Waykole, L.; Repic, O.; Blacklock, T. J., Synth. Commun. 2003, 33, 2719.
- 38 Roussel, F.; Knerr, L.; Grathwohl, M.; Schmidt, R. R., Org. Lett. 2000, 2, 3043.
- 39 Di Nunno, L.; Franchini, C.; Nacci, A.; Scilimati, A.; Sinicropi, M. S., Tetrahedron: Asymmetry 1999, 10, 1913.
- 40 Besson, T.; Rees, C. W., J. Chem. Soc., Perkin Trans. 1 1996, 2857–2860.
- 41 Buttero, P. D.; Molteni, G.; Papagni, A.; Pilati, T., Tetrahedron 2003, 59, 5259.
- 42 Sureshan, K. M.; Shashidhar, M. S.; Praveen, T.; Gonnade, R. G.; Bhadbhade, M. M., Carbohydr. Res. 2002, 337, 2399.
- 43 Peng, X.; Bondar, D.; Paquette, L. A., Tetrahedron 2004, 60, 9589.
- 44 Takahashi, S.; Souma, K.; Hashimoto, R.; Koshino, H.; Nakata, T., J. Org. Chem. 2004, 69, 4509.
- 45 Chaudhary, A. G.; Chordia, M. D.; Kingston, D. G. I., J. Org. Chem. 1995, 60, 3260.
- 46 Chaudhary, A. G.; Rimoldi, J. M.; Kingston, D. G. I., J. Org. Chem. 1993, 58, 3798.
- 47
Albanese, D.;
Landini, D.;
Penso, M.;
Spano, G.;
Trebicka, A.,
Tetrahedron
1995,
51,
5691.
10.1016/0040-4020(95)00232-W Google Scholar
- 48 Subrayan, R. P.; Rasmussen, P. G., Tetrahedron 1995, 51, 6167.
- 49
Mettery, Y.;
Michaud, S.;
Vierfond, J. M.,
Heterocycles
1994,
38,
1001.
10.3987/COM-93-6636 Google Scholar
- 50 Cesare, V.; Lyons, T. M.; Lengyel, I., Synthesis 2002, 1716.
- 51 Ohno, H.; Toda, A.; Fujii, N.; Miwa, Y.; Taga, T.; Yamaoka, Y.; Osawa, E.; Ibuka, T., Tetrahedron Lett. 1999, 40, 1331.
- 52 Ohno, H.; Hamaguchi, H.; Tanaka, T., Org. Lett. 2001, 3, 2269.
- 53 Xu, J.; Jiao, P., J. Chem. Soc., Perkin Trans. 1 2002, 1491.
- 54 Hirai, Y.; Watanabe, J.; Nozaki, T.; Yokoyama, H.; Yamaguchi, S., J. Org. Chem. 1997, 62, 776.
- 55 Azoulay, S.; Monteiro, N.; Balme, G., Tetrahedron Lett. 2002, 43, 9311.
- 56 Garcon, S.; Vassiliou, S.; Cavicchioli, M.; Hartmann, B.; Monteiro, N.; Balme, G., J. Org. Chem. 2001, 66, 4069.
- 57 Shen, Y.; Friestad, G. K., J. Org. Chem. 2002, 67, 6236.
- 58 Lalot, J.; Manier, G.; Stasik, I.; Demailly, G.; Bequpere, D., Carbohydr. Res. 2001, 335, 55.
- 59 Harrison, P. A.; Murtagh, L.; Willis, C. L., J. Chem. Soc., Perkin Trans. 1 1993, 3047.
- 60 Taggi, A. E.; Wack, H.; Hafez, A. M.; France, S.; Lectka, T., Org. Lett. 2002, 4, 627.
- 61 Vales, M.; Lokshin, V.; Pepe, G.; Guglielmetti, R.; Samat, A., Tetrahedron 2002, 58, 8543.
- 62 Van de Poel, H.; Guillaumet, F.; Viaud-Massuard, M. C., Tetrahedron Lett. 2002, 43, 1205.
- 63 Kim, B. S.; Kim, K., Tetrahedron Lett. 2001, 42, 4637.
- 64 Di Santo, R.; Costi, R.; Massa, S.; Artico, M., Synth. Commun. 1995, 25, 795.
- 65 Frieman, B. A.; Bock, C. W.; Bhat, K. L., Heterocycles 2001, 55, 2099.
- 66 Song, Z.; Reiner, J.; Zhao, K., Tetrahedron Lett. 2004, 45, 3959.
- 67 Dejaegher, Y.; Mangelinckx, S.; De Kimpe, N., J. Org. Chem. 2002, 67, 2075.
- 68 Stefanic, P.; Breznik, M.; Lah, N.; Leban, I.; Plavec, J.; Kikelj, D., Tetrahedron Lett. 2001, 42, 5295.
- 69 Bois-Choussy, M.; De Palois, M.; Zhu, J., Tetrahedron Lett. 2001, 42, 3427.
- 70 Jiang, N.; Ma, Z.; Qu, Z.; Xing, X.; Xie, L.; Wang, J., J. Org. Chem. 2003, 68, 893.
- 71 Suzuki, Y.; Toyota, T.; Imada, F.; Sato, M.; Miyashita, A., Chem. Commun. 2003, 1314.
- 72 Ding, Y.; Huang, X., Synth. Commun. 2001, 31, 449.
- 73 Krief, A.; Derock, M., Tetrahedron Lett. 2002, 43, 3083.
- 74 Okuma, K.; Kuge, S.; Koga, Y.; Shioji, K.; Wakita, H.; Machiguchi, T., Heterocycles 1998, 48, 1519.
- 75 Silveira, C. C.; Lenardao, E. J.; Comasseto, J. V., Synth. Commun. 1994, 24, 575.
- 76 Suzuki, H.; Nakamura, T., J. Org. Chem. 1993, 58, 241.
- 77 Matteson, D. S.; Soundararajan, R.; Ho, O. C.; Gatzweiler, W., Organometallics 1996, 15, 152.
- 78 Zhang, Y.; Liao, S.; Xu, Y.; Yu, D., Synth. Commun. 1997, 27, 4327.
- 79 Uchiyama, M.; Furumoto, S.; Saito, M.; Kondo, Y.; Sakamoto, T., J. Am. Chem. Soc. 1997, 119, 11425.
- 80 Massicot, F.; Schneider, R.; Fort, Y.; Illy-Cherry, S.; Tillement, O., Tetrahedron 2001, 57, 531.
- 81 Lin, G.; Hong, R., J. Org. Chem. 2001, 66, 2877.
- 82 Serrano-Wu, M. H.; Regueiro-Ren, A.; St. Laurent, D. R.; Carroll, T. M.; Balasubramanian, B. N., Tetrahedron Lett. 2001, 42, 8593.
- 83 Al Dulayymi, A. R.; Baird, M. S., J. Chem. Soc., Perkin Trans. 1 1994, 1547 and 3047.




