Abstract
[591-51-5] C6H5Li (MW 84.05)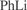
InChI = 1S/C6H5.Li/c1-2-4-6-5-3-1;/h1-5H;
InChIKey = NHKJPPKXDNZFBJ-UHFFFAOYSA-N
(organometallic agent useful as nucleophile for addition and substitution reactions,1, 3-20 and in the preparation of organolithium building blocks via metalation32 and lithium–metalloid exchange reaction1)
Physical Data: X-ray structures of the PMDTA-complexed monomer,2i the TMEDA-complexed dimer,2a the diethyl ether-complexed tetramer,2b and the PhLi∶LiBr (3∶1) mixed tetramer2b have been reported. Cryoscopic2h and NMR2c–e studies have shown that a monomer–dimer equilibrium exists in THF solution and that monomers exist in THF/HMPA2f and THF/PMDTA.2c PhLi is tetrameric in ether.
Solubility: sol ether solvents; sol hydrocarbon solvents only by the addition of donor solvents/additives such as ether or TMEDA.2a
Form Supplied in: commercially available in cyclohexane/ether solution.
Analysis of Reagent Purity: standard titration methods should be used.
Preparative Methods: prepared from phenyl halide (PhCl,2j PhBr,2k,l PhI2m) and lithium metal in ether. Several procedures have been used to prepare solid, salt-free PhLi.2n–r The synthesis of 13C-labeled PhLi (ipso carbon only) has been described.2h
Handling, Storage, and Precautions: moisture and oxygen sensitive. Concentrated solutions are pyrophoric. Ether solutions slowly undergo proton abstraction to generate benzene and should be stored in the freezer.
Bibliography
- 1
(a) Wakefield, B. J.
The Chemistry of Organolithium Compounds;
Pergamon:
Oxford,
1974.
(b) Wakefield, B. J. In
Comprehensive Organometallic Chemistry;
G. Wilkinson;
F. G. A. Stone;
E. W. Abel, Eds.;
Pergamon:
Oxford,
1982;
Vol. 7,
Chapter 44.
10.1016/B978-008046518-0.00090-8 Google Scholar(c) Wardell, J. L. In The Chemistry of the Metal–Carbon Bond; Wiley: New York, 1987; Vol. 4, Chapter 1. (d) Bailey, W. F.; Patricia, J. J., J. Organomet. Chem. 1988, 352, 1. (e) Reich, H. J.; Green, D. P.; Phillips, N. H.; Borst, J. P.; Reich, I. L., Phosphorus Sulfur/Phosphorus Sulfur Silicon 1992, 67, 83.
- 2 (a) Weiss, E.; Thoennes, D., Ber. Dtsch. Chem. Ges./Chem. Ber. 1978, 111, 3157. (b) Hope, H.; Power, P. P., J. Am. Chem. Soc. 1983, 105, 5320. (c) Bauer, W.; Winchester, W. R.; Schleyer, P. v. R., Organometallics 1987, 6, 2371. (d) Reich, H. J.; Green, D. P.; Phillips, N. H., J. Am. Chem. Soc. 1991, 113, 1414. (e) Jackman, L. M.; Scarmoutzos, L. M., J. Am. Chem. Soc. 1984, 106, 4627. (f) Reich, H. J.; Green, D. P., J. Am. Chem. Soc. 1989, 111, 8729. (g) Bauer, W.; Seebach, D., Helv. Chim. Acta 1984, 67, 1972. (h) Seebach, D.; Hässig, R.; Gabriel, J., Helv. Chim. Acta 1983, 66, 308. (i) Weiss, E.; Schümann, U.; Kopf, J., Angew. Chem., Int. Ed. Engl. 1985, 24, 215. (j) Esmay, D. I., Adv. Chem. Ser. 1959, 23, 47. (k) Gilman, H.; Morton, J. W., Org. React. 1954, 8, 286. (l) Gilman, H.; Caj, B. J., J. Org. Chem. 1957, 22, 1165. (m) Müller, E.; Ludsteck, D., Ber. Dtsch. Chem. Ges./Chem. Ber. 1954, 87, 1887. (n) Wittig, G.; Meyer, F. J.; Lange, G., Justus Liebigs Ann. Chem./Liebigs Ann. Chem. 1951, 571, 167. (o) Waack, R.; Doran, M. A., J. Am. Chem. Soc. 1963, 85, 1651. (p) Fraenkel, W. E.; Dagagi, S.; Kobayashi, S., J. Phys. Chem. 1961, 83, 3585. (q) Schlosser, M., J. Organomet. Chem. 1967, 8, 193. (r) Wehman, E.; Jastrzebski, J. T. B. H.; Ernsting, J.; Grove, D. M.; van Koten, G., J. Organomet. Chem. 1988, 353, 133.
- 3
(a) Alonso, F.;
Yus, M.,
Tetrahedron
1991,
47,
7471.
(b) Alonso, F.;
Yus, M.,
Tetrahedron Lett.
1992,
48,
2709.
(c) Liebeskind, L. S.;
Wirtz, K. R.,
J. Org. Chem.
1990,
55,
5350.
(d) Reetz, M. T.;
Wang, F.;
Harms, K.,
J. Chem. Soc., Chem. Commun.
1991,
1309.
(e) Braun, L. L.;
Torian, B. E.,
J. Heterocycl. Chem.
1984,
21,
293.
(f) Franck-Neumann, M.;
Chemla, P.;
Martina, D.,
Synlett
1990,
641.
10.1055/s-1990-21197 Google Scholar
- 4 (a) Ruden, R. A.; Gaffney, B. L., Synth. Commun. 1975, 5, 15. (b) Barrett, A. G. M.; Flygare, J. A., J. Org. Chem. 1991, 56, 638.
- 5 Kano, S.; Ebata, T.; Shibuya, S., Chem. Pharm. Bull. 1979, 27, 2450.
- 6 (a) Beel, J. A.; Vejvoda, E., J. Am. Chem. Soc. 1954, 76, 905. (b) Baigrie, L. M.; Seiklay, H. R.; Tidwell, T., J. Am. Chem. Soc. 1985, 107, 5391. (c) Seebach, D.; Laube, T.; Häner, R., J. Am. Chem. Soc. 1985, 107, 5396.
- 7 (a) Levine, R.; Karten, M. J.; Kadunce, W. M., J. Org. Chem. 1975, 40, 1770. (b) Breitmaier, E.; Zadel, G., Angew. Chem., Int. Ed. Engl. 1992, 31, 1035.
- 8 Fuchs, P. L.; Conrad, P. C., J. Am. Chem. Soc. 1978, 100, 346.
- 9 Cohen, T.; Abraham, W. D.; Myers, M., J. Am. Chem. Soc. 1987, 109, 7923.
- 10 (a) Tanaka, J.; Kanemasa, S.; Ninomiya, Y.; Tsuge, O., Bull. Chem. Soc. Jpn. 1990, 63, 476. (b) Stern, A. J.; Swenton, J. S., Chem. Commun./J. Chem. Soc., Chem. Commun. 1988, 1255.
- 11 (a) Meyers, A. I.; Barner, B. A., J. Org. Chem. 1986, 51, 120. (b) Kundig, E. P.; Liu, R. G.; Ripa, A., Helv. Chim. Acta 1992, 75, 2657.
- 12 (a) Beak, P.; Worley, J. W., J. Am. Chem. Soc. 1970, 92, 4142. (b) Ohno, A.; Nakamura, K.; Uohama, M.; Oka, S., Chem. Lett. 1975, 983.
- 13 Schaumann, E.; Walter, W., Ber. Dtsch. Chem. Ges./Chem. Ber. 1974, 107, 3562.
- 14 Beak, P. Worley, J. W., J. Am. Chem. Soc. 1972, 94, 597.
- 15
(a) Overberger, C. G.;
Lombardino, J. G.;
Hiskey, R. G.,
J. Am. Chem. Soc.
1957,
79,
6430.
(b) Uno, H.;
Okada, S.;
Suzuki, H.,
J. Heterocycl. Chem.
1991,
28,
346.
10.1002/jhet.5570280225 Google Scholar(c) Nakagawa, M.; Kawate, T.; Yamazaki, H.; Hino, T., Chem. Commun./J. Chem. Soc., Chem. Commun. 1990, 991. (d) Uno, H.; Terakawa, T.; Suzuki, H., Chem. Lett. 1989, 1079. (e) Uno, H.; Terakawa, T.; Suzuki, H., Synlett 1991, 559.
- 16 (a) Itsuno, S.; Hachisuka, C.; Kitano, K.; Ito, K., Tetrahedron Lett. 1992, 33, 627. (b) Zimmerman, H. E.; Wright, C. W., J. Am. Chem. Soc. 1992, 114, 363. (c) Walborsky, H. M.; Niznik, G. E., J. Org. Chem. 1972, 37, 187.
- 17 Walborsky, H. M.; Periasamy, M. P., J. Org. Chem. 1974, 39, 611.
- 18 (a) Peterson, P. E.; Grant, G., J. Org. Chem. 1991, 56, 16. (b) Brown, H. C.; Rangaishenvi, M. V., Tetrahedron Lett. 1990, 31, 7115. (c) Merkel, D.; Köbrich, G., Ber. Dtsch. Chem. Ges./Chem. Ber. 1973, 106, 2040.
- 19 (a) Tomalia, D. A.; Falk, J. C., J. Heterocycl. Chem. 1972, 9, 891. (b) Kasatkin, A. N., Izv. Akad. Nauk SSSR, Ser. Khim. 1988, 2159.
- 20 (a) Cristol, S. J.; Douglass, J. R.; Meek, J. S., J. Am. Chem. Soc. 1951, 73, 816. (b) Aithie, G. C. M.; Miller, J. A., Tetrahedron Lett. 1975, 4419. (c) Rychnovsky, S. D.; Griesgraber, G.; Zeller, S.; Skalitzky, D. J., J. Org. Chem. 1991, 56, 5161. (d) Ukaji, Y.; Yoshida, A.; Fujisawa, T., Chem. Lett. 1990, 157.
- 21 (a) Searles, S., J. Am. Chem. Soc. 1951, 73, 125. (b) Eis, M. J.; Ganem, B., Tetrahedron Lett. 1985, 26, 1153.
- 22 (a) Reich, H. J. In Organoselenium Chemistry; D. Liotta, Ed.; Wiley: New York, 1987; p 243. (b) Dumont, W.; Bayet, P.; Krief, A., Angew. Chem., Int. Ed. Engl. 1974, 13, 243.
- 23
Tomoki, H.;
Kambe, N.;
Ogawa, A.;
Miyoshi, N.;
Murai, S.;
Sonoda, N.,
Angew. Chem., Int. Ed. Engl.
1987,
26,
1187.
10.1002/anie.198711871 Google Scholar
- 24 (a) Reich, H. J.; Phillips, N. H., Pure Appl. Chem. 1987, 59, 1021. (b) Seyferth, D.; Weiner, M. A., Org. Synth., Coll. Vol. 1973, 5, 452. (c) Seyferth, D.; Weiner, M. A., J. Am. Chem. Soc. 1961, 83, 3583. (d) Bristow, G. S., Aldrichim. Acta 1984, 17, 75. (e) Eisch, J. J., Org. Synth. 1981, 2, 92.
- 25 (a) Eaton, P. E.; Cunkle, G. T.; Marchioro, G.; Martin, R. M., J. Am. Chem. Soc. 1987, 109, 948. (b) Maercker, A.; Dujardin, R., Angew. Cheml 1984, 96, 222.
- 26 (a) Batalov, A. P.; Rostokin, G. A., J. Gen. Chem. USSR (Engl. Transl.) 1971, 41, 154, 1740, 1743. (b) Batalov, A. P.; Rostokin, G. A., J. Gen. Chem. USSR (Engl. Transl.) 1973, 43, 959. (c) Winkler, H. J. S.; Winkler, H., J. Am. Chem. Soc. 1966, 88, 964, 969.
- 27 (a) Gilman, H.; Towle, J. L.; Spatz, S. M., J. Am. Chem. Soc. 1946, 68, 2017. (b) Köbrich, G.; Buck, P., Ber. Dtsch. Chem. Ges./Chem. Ber. 1970, 103, 1412. (c) Lucchesini, F., Tetrahedron 1992, 48, 9951.
- 28 Tashiro, M.; Yamato, T., Chem. Lett. 1982, 61.
- 29 Frejd, T.; Karlsson, J. O.; Gronowitz, S., J. Org. Chem. 1981, 46, 3132.
- 30 (a) Lefloch, P.; Carmichael, D.; Mathey, F., Organometallics 1991, 10, 2432. (b) Lefloch, P.; Carmichael, D.; Ricard, L.; Mathey, F.; Jutand, A.; Amatore, C., Organometallics 1992, 11, 2475.
- 31 Fuchs, P. L.; Anderson, M. B., J. Org. Chem. 1990, 55, 337.
- 32 (a) Gilman, H.; Morton, J. W., Org. React. 1954, 8, 286. (b) Gschwend, H. W.; Rodriguez, H. R., Org. React. 1976, 26, 1. (c) Beak, P.; Snieckus, V., Acc. Chem. Res. 1982, 15, 306. (d) Beak, P.; Zajdel, W. J.; Reitz, D. B., Chem. Rev. 1984, 84, 471.
- 33 (a) Weinstock, J.; Boekelheide, V., Org. Synth., Coll. Vol. 1963, 4, 641. (b) Cason, J.; Sumrell, G.; Mitchell, R. S., J. Org. Chem. 1950, 15, 850.
- 34 (a) Catlin, E. R.; Hassell, C. H., J. Chem. Soc. (C) 1971, 460. (b) Lambooy, J. P., J. Am. Chem. Soc. 1956, 78, 771.
- 35 (a) Compagnon, P.-L.; Gasquez, F.; Kimny, T., Synthesis 1986, 948. (b) Lee, J. W.; Anderson, W. K., Synth. Commun. 1992, 22, 369.
- 36 (a) Huisgen, R.; Konig, H., Ber. Dtsch. Chem. Ges./Chem. Ber. 1959, 92, 203. (b) Iida, H.; Yuasa, Y.; Kibayashi, C., Synthesis 1977, 879.
- 37 Streitwieser, A., Jr.; Scannon, P. J.; Niemeyer, H. M., J. Am. Chem. Soc. 1972, 94, 7936.
- 38 Nicolaou, K. C.; Renaud, J.; Nantermet, P. G.; Couladouros, E. A.; Guy, R. K.; Wrasidlo, W., J. Am. Chem. Soc. 1995, 117, 2409–2420.
- 39 (a) Cimarelli, C.; Palmieri, G.; Volpini, E., Can. J. Chem. 2004, 82, 1314–1321. (b) Jones, S.; Norton, H. C., Synlett 2004, 338–340.
- 40 Lemoucheux, L.; Seitz, T.; Rouden, J.; Lasne, M. C., Org. Lett. 2004, 6, 3703–3706.
- 41 Smith, K.; El-Hiti, G. A.; Hamilton, A., J. Chem. Soc., Perkin Trans. 1 1998, 4041–4042.
- 42 Honda, M.; Ohkura, N.; Saisyo, S. I.; Segi, M.; Nakajima, T., Tetrahedron 2003, 59, 8203–8212.
- 43 Clayden, J.; Watson, D. W.; Chambers, M., Tetrahedron 2005, 61, 3195–3203.
- 44 (a) Takeda, K.; Tanaka, T., Synlett 1999, 705–708. (b) Takeda, K.; Haraguchi, H.; Okamoto, Y., Org. Lett. 2003, 5, 3705–3707.
- 45 (a) Reznikov, V. A.; Volodarsky, L. B., Tetrahedron Lett. 1994, 35, 2239–2240. (b) Shibata, T.; Uemae, K.; Yamamoto, Y., Tetrahedron: Asymmetry 2000, 11, 2339–2346. (c) Murga, J.; Portolés, R.; Falomir, E.; Carda, M.; Marco, J. A., Tetrahedron: Asymmetry 2005, 16, 1807–1816.
- 46 (a) Cogan, D. A.; Liu, G.; Ellman, J., Tetrahedron 1999, 55, 8883–8904. (b) Domínguez, E.; de Blas, J.; del Prado, M.; Aranda, M. T.; Carreño, M. C.; García Ruano, G. L., Tetrahedron Lett. 2000, 41, 9825–9828. (c) Shaw, A. W.; de Solms, S. J., Tetrahedron Lett. 2001, 42, 7173–7176. (d) Pflum, D. A.; Krishnamurthy, D.; Han, Z.; Wald, S. A.; Senanayake, C. H., Tetrahedron Lett. 2002, 43, 923–926. (e) Sun, X.; Wang, S.; Sun, S.; Zhu, J.; Deng, J., Synlett 2005, 2776–2780.
- 47 Richter, J.; Feiling, J.; Schmidt, H. G.; Noltemeyer, M.; Brüser, W.; Edelmann, F. T., Zeitschrift fuer Anorganische und Allgemeine Chemie 2004, 630, 1269–1275.
- 48 Bilke, J. L.; Dzuganova, M.; Fröchlich, R.; Würthwein, E. U., Org. Lett. 2005, 7, 3267–3270.
- 49 Kolotuchin, S. V.; Meyers, A. I., J. Org. Chem. 2000, 65, 3018–3026.
- 50 (a) Ichikawa, J.; Wada, Y.; Miyazaki, H.; Mori, T.; Kuroki, H., Org. Lett. 2003, 5, 1455–1458. (b) Kobayashi, K.; Shiokawa, T.; Morikawa, O.; Konishi, H., Chem. Lett. 2004, 33, 236–237.
- 51 Dolbier, W. R. Jr; Xu, Y. L., J. Fluorine Chem. 2003, 123, 71–73.
- 52 Afarinkia, K.; De Pascale, E., Synlett 2002, 990–992.
- 53
(a) Buchholz, M.;
Hiller, F.;
Reißig, H. U.,
Eur. J. Org. Chem.
2002,
16,
2838–2843.
10.1002/1099-0690(200208)2002:16<2838::AID-EJOC2838>3.0.CO;2-O Google Scholar(b) Buchholz, M.; Reissig, H. U., Synthesis 2002, 1412–1422.
- 54 Senge, M. O.; Feng, X., J. Chem. Soc., Perkin Trans. 1 2000, 3615–3621.
- 55 (a) Sperandio, D.; Hansen, H. J., Helv. Chim. Acta 1995, 78, 765–771. (b) Iwama, N.; Uchino, H.; Osano, Y. T.; Sugano, T., Organometallics 2004, 23, 3267–3269. (c) Iwama, N.; Osano, Y. T., Organometallics 2005, 24, 132–135.
- 56 Abe, N.; Tanaka, M.; Maeda, T.; Fujii, H.; Kakehi, A., Heterocycles 2005, 66, 229–240.
- 57 (a) Neipp, C. E.; Humphrey, J. M.; Martin, S. F., J. Org. Chem. 2001, 66, 531–537. (b) Suzuka, T.; Ogasawara, M.; Hayashi, T., J. Org. Chem. 2002, 67, 3355–3359.
- 58 (a) Bégué, J. P.; Bonnet-Delpon, D.; Bouvet, D.; Rock, M. H., J. Org. Chem. 1996, 61, 9111–9114. (b) Peters, J. G.; Seppi, M.; Fröhlich, R.; Wibbeling, B.; Hoppe, D., Synthesis 2002, 381–392. (c) Xue, S.; Han, K. Z.; He, L.; Guo, Q. X., Synlett 2003, 870–872. (d) Barluenga, J.; Fernández, A.; Álvarez-Rodrigo, L.; Rodríguez, F.; Fañanás, F. J., Synlett 2005, 2513–2515. (e) 2005, 2513–2515.
- 59 (a) Geier, J.; Exner, K.; Vögtle, M.; Kegel, M.; Yang, F.; Hunkler, D.; Cullmann, O.; Prinzbach, H., Tetrahedron 2002, 58, 1137–1145. (b) Lockard, J. V.; Zink, J. I.; Trieber, D. A. II; Konradsson, A. E.; Weaver, M. N.; Nelsen, S. F., J. Phys. Chem. A 2005, 109, 1205–1215.
- 60 Nelsen, S. F.; Trieber, D. A. II; Ismagilov, R. F.; Teki, Y., J. Am. Chem. Soc. 2001, 123, 5684–5694.
- 61 Faragó, J.; Novák, Z.; Schlosser, G.; Csámpai, A.; Kotschy, A., Tetrahedron 2004, 60, 1991–1996.
- 62 Kanaya, S.; Kozaki, M.; Shiomi, D.; Sato, K.; Takui, T.; Okada, K., Synthetic Metals 2001, 121, 1808–1809.
- 63 Yasui, E.; Wada, M.; Takamura, N., Tetrahedron Lett. 2006, 47, 743–746.
- 64 (a) Flinois, K.; Yuan, Y.; Bastide, C.; Harrison-Marchand, A.; Maddaluno, J., Tetrahedron 2002, 58, 4707–4716. (b) Rudolph, R.; Hermanns, N.; Bolm, C., J. Org. Chem. 2004, 69, 3997–4000.
- 65 Denmark, S. E.; Nakajima, N.; Nicaise, O. J. C., J. Am. Chem. Soc. 1994, 116, 8797–8798. (b) Cabello, N.; Kizirian, J. C.; Alexakis, A., Tetrahedron Lett. 2004, 45, 4639–4642. (c) Cabello, N.; Kizirian, J. C.; Gille, S.; Alexakis, A.; Bernardinelli, G.; Pinchard, L.; Caille, J. C., Eur. J. Org. Chem. 2005, 22, 4835–4842.
- 66 Cogan, D. A.; Ellman, J. A., J. Am. Chem. Soc. 1999, 121, 268–269.
- 67 Alexakis, A.; Amiot, F., Tetrahedron: Asymmetry 2002, 13, 2117–2122.
- 68 Chrzanowska, M.; Sokolowska, J., Tetrahedron: Asymmetry 2001, 12, 1435–1440.
- 69 Peña, D.; López, F.; Harutyunyan, S. R.; Minnaard, A. J.; Feringa, B. L., Chem Commun. 2004, 1836–1837.
- 70 (a) Asano, Y.; Iida, A.; Tomioka, K., Tetrahedron Lett. 1997, 38, 8973–8976. (b) Asano, Y.; Iida, A.; Tomioka, K., Chem. Pharm. Bull. 1998, 46, 184–186. (c) Asano, Y.; Yamashita, M.; Nagai, K.; Kuriyama, M.; Yamada, K. I.; Tomioka, K., Tetrahedron Lett. 2001, 42, 8493–8495.
- 71 Jones, C. A.; Jones, I. G.; North, M.; Pool, C. R., Tetrahedron Lett. 1995, 36, 7885–7888.
- 72 Yamashita, M.; Yamada, K. I.; Tomioka, K., Tetrahedron 2004, 60, 4237–4242.
- 73 (a) Uerdingen, M.; Krause, N., Tetrahedron 2000, 56, 2799–2804. (b) Hayashi, T.; Yamamoto, S.; Tokunaga, N., Angew. Chim. Int. Ed. 2005, 44, 4224–4227.
- 74 Lempereur, C.; Plé, N.; Turck, A.; Quéguiner, G.; Corbin, F.; Alayrac, C.; Metzner, P., Heterocycles 1998, 48, 2019–2034.
- 75 Boyd, D. R.; Sharma, N. D.; Haughey, S. A.; Kennedy, M. A.; Malone, J. F.; Shepherd, S. D.; Allen, C. C. R.; Dalton, H., Tetrahedron 2004, 60, 549–559.
- 76 Cogan, D. A.; Liu, G.; Kim, K.; Backes, B. J.; Ellman, J. A., J. Am. Chem. Soc. 1998, 120, 8011–8019.
- 77 Sisko, J.; Kassick, A. J.; Shetzline, S. B., Org. Lett. 2000, 2, 2877–2880.
- 78 Voets, M.; Smet, M.; Dehaen, W., J. Chem. Soc. Perkin Trans. 1 1999, 1473–1476.
- 79 Hill, B.; De Vleeschauwer, M.; Houde, K.; Belley, M., Synlett 1998, 407–410.
- 80 Wyatt, P.; Hudson, A., Org. Biomolec. Chem. 2004, 2, 1528–1530.
- 81 Gimisis, T.; Arsenyan, P.; Georganakis, D.; Leondiadis, L., Synlett 2003, 1451–1454.
- 82 Katritzky, A. R.; Fang, Y., Heterocycles 2000, 53, 1783–1788.
- 83 (a) Guintchin, B.; Bienz, S., Tetrahedron 2003, 59, 7527–7533. (b) Gansäuer, A.; Justicia, J.; Rosales, A.; Worgull, D.; Rinker, B.; Cuerva, J. M.; Oltra, J. E., Eur. J. Org. Chem. 2006, 18, 4115–4127.
- 84 Spino, C.; Beaulieu, C., J. Am. Chem. Soc. 1998, 120, 11832–11833.
- 85 (a) Bégué, J. P.; Bonnet-Delpon, D.; Rock, M. H., Tetrahedron Lett. 1995, 36, 5003–5006. (b) Ichikawa, J.; Ishibashi, Y.; Fukui, H., Tetrahedron Lett. 2003, 44, 707–710. (c) Ichikawa, J.; Fukui, H.; Ishibashi, Y., J. Org. Chem. 2003, 68, 7800–7805.
- 86 Dieter, R. K.; Guo, F., Org. Lett. 2006, 8, 4779–4782.
- 87 (a) Di Bussolo, V.; Caselli, M.; Pineschi, M.; Crotti, P., Org. Lett. 2003, 5, 2173–2176. (b) Di Bussolo, V.; Caselli, M.; Romano, M. R.; Pineschi, M.; Crotti, P., J. Org. Chem. 2004, 69, 7383–7386. (c) Ooyama, Y.; Yoshikawa, S.; Watanabe, S.; Yoshida, K., Org. Biomol. Chem. 2006, 4, 3406–3409.
- 88 (a) Couty, F.; Durrat, F.; Evano, G.; Prim, D., Tetrahedron Lett. 2004, 45, 7525–7528. (b) Couty, F.; Durrat, F.; Evano, G.; Marrot, J., Eur. J. Org. Chem. 2006, 18, 4214–4223.
- 89 Yamamoto, G.; Kobayashi, Y.; Ono, K.; Yano, E.; Minoura, M.; Mazaki, Y., Bull. Chem. Soc. Jpn. 2006, 79, 1293–1299.
- 90 Martin, R.; Fürstner, A., Angew. Chem. Int. Ed. 2004, 43, 3955–3957.
- 91 Bica, K.; Gaertner, P., Org. Lett. 2006, 8, 733–735.
- 92 (a) Eaton, P. E.; Tsanaktsidis, J., J. Am. Chem. Soc. 1990, 112, 876–878. (b) Eaton, P. E.; Pramod, K.; Emrick, T.; Gilardi, R., J. Am. Chem. Soc. 1999, 121, 4111–4123.
- 93 (a) Shimizu, M.; Fujimoto, T.; Liu, X.; Minezaki, H.; Hata, T.; Hiyama, T., Tetrahedron 2003, 59, 9811–9823. (b) Liu, X.; Shimizu, M.; Hiyama, T., Angew. Chim. Int. Ed. 2004, 43, 879–882.
- 94 (a) Müller, P.; Nury, P., Org. Lett. 2000, 2, 2845–2847. (b) Ooi, T.; Miura, T.; Itagaki, Y.; Ichikawa, H.; Maruoka, K., Synthesis 2002, 279–291.
- 95 (a) Baussanne, I.; Chiaroni, A.; Royer, J., Tetrahedron: Asymmetry 2001, 12, 1219–1224. (b) Amat, M.; Llor, N.; Hidalgo, J.; Escolano, C.; Bosch, J., J. Org. Chem. 2003, 68, 1919–1928. (c) Cimarelli, C.; Palmieri, G.; Volpini, E., Tetrahedron: Asymmetry 2002, 13, 2417–2426.
- 96 (a) Zeng, X.; Qian, M.; Hu, Q.; Negishi, E. I., Angew. Chem. Int. Ed. 2004, 43, 2259–2263. (b) Lloyd, D. G.; Hughes, R. B.; Zisterer, D. M.; Williams, D. C.; Fattorusso, C.; Catalanotti, B.; Campiani, G.; Meegan, M. J., J. Med. Chem. 2004, 47, 5612–5615.
- 97
Cossy, J.;
Pévet, I.;
Meyer, C.,
Eur. J. Org. Chem.
2001,
15,
2841–2850.
10.1002/1099-0690(200108)2001:15<2841::AID-EJOC2841>3.0.CO;2-W Google Scholar(b) Miura, K.; Hondo, T.; Okajima, S.; Nakagawa, T.; Takahashi, T.; Hosomi, A., J. Org. Chem. 2002, 67, 6082–6090. (c) Lee, P. H.; Lee, S. W.; Lee, K., Org. Lett. 2003, 5, 1103–1106.
- 98 (a) Veenstra, S. J.; Hauser, K.; Betschart, C., Bioorg. Med. Chem. Lett. 1997, 7, 347–350. (b) Varela, J. A.; Peña, D.; Goldfuss, B.; Polborn, K.; Knochel, P., Org. Lett. 2001, 3, 2395–2398. (c) Varela, J. A.; Peña, D.; Goldfuss, B.; Denisenko, D.; Kulhanek, J.; Polborn, K.; Knochel, P., Chem. Eur. J. 2004, 10, 4252–4264.
- 99 Tingoli, M.; Tiecco, M.; Testaferri, M.; Temperini, A.; Pelizzi, G.; Bacchi, A., Tetrahedron 1995, 51, 4691–4700.
- 100 Miller, J. A., Tetrahedron Lett. 2002, 43, 7111–7114.
- 101 (a) Palmgren, A.; Thorarensen, A.; Bäckvall, J. E., J. Org. Chem. 1998, 63, 3764–3768. (b) Son, E. C.; Tsuji, H.; Saeki, T.; Tamao, K., Bull. Chem Soc. Jpn. 2006, 79, 492–494.
- 102 (a) Hartung, C. G.; Tillack, A.; Trauthwein, H.; Beller, M., J. Org. Chem. 2001, 66, 6339–6343. (b) Garcia Castro, I.; Tillack, A.; Hartung, C. G.; Beller, M., Tetrahedron Lett. 2003, 44, 3217–3221. (c) Mukai, C.; Hirose, T.; Teramoto, S.; Kitagaki, S., Tetrahedron 2005, 61, 10983–10994. (d) Gorman, J. S. T.; Iacono, S. T.; Pagenkopf, B. L., Org. Lett. 2004, 6, 67–70.
- 103 (a) Monkowius, U.; Mitzel, N. W.; Schier, A.; Schmidbaur, H., J. Am. Chem. Soc. 2002, 124, 6126–6132. (b) Toffano, M.; Dobrota, C.; Fiaud, J. C., Eur. J. Org. Chem. 2006, 3, 650–656.
- 104 Kurita, J.; Ishii, M.; Yasuike, S.; Tsuchiya, T., Chem. Pharm. Bull. 1994, 42, 1437–1441.
- 105 (a) Kakusawa, N.; Ikeda, T.; Osada, A.; Kurita, J.; Tsuchiya, T., Synlett 2000, 1503–1505. (b) Kakusawa, N.; Kurita, J., Heterocycles 2006, 68, 1335–1348.
- 106 Sebahar, H. L.; Yoshida, K.; Hegedus, L. S., J. Org. Chem. 2002, 67, 3788–3795.
- 107 (a) Labrecque, D.; New, K. T.; Chan, T. H., Organometallics 1994, 13, 332–335. (b) Krempner, C.; Reinke, H.; Oehme, H., Chem. Ber. 1995, 128, 143–149. (c) Iwama, N.; Uchino, H.; Osano, Y. T.; Sugano, T., Organometallics 2004, 23, 3267–3269. (d) Allen, J. M.; Aprahamian, S. L.; Sans, E. A.; Shechter, H., J. Org. Chem. 2002, 67, 3561–3574. (e) Amrein, S.; Studer, A., Chem. Commun. 2002, 1592–1593. (f) Pietschnig, R.; Schäfer, S., Silicon Chemistry 2003, 2, 131–135. (g) Dimopoulos, P.; George, J.; Tocher, D. A.; Manaviazar, S.; Hale, K. J., Org. Letters 2005, 7, 5377–5380.
- 108 (a) Hirabayashi, K.; Ando, J. I.; Kawashima, J.; Nishihara, Y.; Mori, A.; Hiyama, T., Bull Chem. Soc. Jpn. 2000, 73, 1409–1417. (b) Barrett, A. G. M.; Beall, J. C.; Braddock, D. C.; Flack, K.; Gibson, V. C.; Salter, M. M., J. Org. Chem. 2000, 65, 6508–6514. (c) Ahmed, M.; Atkinson, C. E.; Barrett, A. G. M.; Malagu, K.; Procopiou, P. A., Org. Lett. 2003, 5, 669–672. (d) Sarkar, T. K.; Haque, S. A.; Basak, A., Angew. Chim. Int. Ed. 2004, 43, 1417–1419. (e) Terauchi, M.; Yahiro, Y.; Abe, H.; Ichikawa, S.; Tovey, S. C.; Dedos, S. G.; Taylor, C. W.; Potter, B. V. L.; Matsuda, A.; Shuto, S., Tetrahedron 2005, 61, 3697–3707.



