Allenylboronic Acid
Abstract
[83816-41-5] C3H5BO2 (MW 83.88)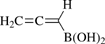
InChI = 1S/C3H5BO2/c1-2-3-4(5)6/h3,5-6H,1H2
InChIKey = UWYKUBJFERKTAF-UHFFFAOYSA-N
(propargylating reagent for carbonyl groups; chiral allenylboronic ester as a reagent for enantioselective alkylation;1 1,3-asymmetric induction in propargylation of β-hydroxy ketones2)
Physical Data: mp 150 °C (dec).
Solubility: sol ether, alcohol.
Form Supplied in: white solid; widely available.
Analysis of Reagent Purity: 1H NMR (CDCl3–Me2SO-d6) δ 4.48 (d, J = 5.8 Hz, 1H), 4.51 (d, J = 7.2 Hz, 1H), 4.83 (dd, J = 5.8 and 7.2 Hz, 1H), 6.52 (br s, 2H).
Preparative Method: from Propargylmagnesium Bromide and Trimethyl Borate.1
Handling, Storage, and Precautions: allenylboronic acid can be stored at −20 °C under argon. In air, it catches fire within several minutes. It should only be used in a fume hood.



