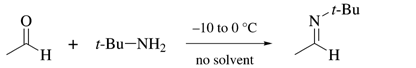Acetaldehyde N-tert-Butylimine
Abstract
[7020-80-6] (E) [62058-77-9] C6H13N (MW 99.17)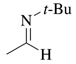
InChI = 1S/C6H13N/c1-5-7-6(2,3)4/h5H,1-4H3/b7-5+
InChIKey = RPVBCVQKDPOMII-FNORWQNLSA-N
InChI = 1/C6H13N/c1-5-7-6(2,3)4/h5H,1-4H3/b7-5+
InChIKey = RPVBCVQKDPOMII-FNORWQNLBY
(acetaldehyde equivalent; synthesis of α,β-unsaturated aldehydes)
Alternate Names: N-ethylidene-1,1-dimethylethylamine; N-(ethylidene)-tert-butylamine; N-ethylidene-2-methyl-2-propaneamine.
Physical Data: bp 25–28 °C/95 mmHg; nD19.2 1.4005.
Solubility: sol most organic solvents.
Form Supplied in: colorless liquid; not commercially available.
Analysis of Reagent Purity: 1H NMR; GC.
Preparative Methods: condensation of freshly distilled Acetaldehyde with
Handling, Storage, and Precautions: very sensitive to hydrolysis; store in closed vessels under an inert atmosphere in the refrigerator. Use of the freshly distilled reagent is recommended. Use in a fume hood.