Effect of glucagon on carbohydrate-mediated secretion of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (7–36 amide) (GLP-1)
Abstract
Background
The insulinotropic hormones, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (7–36 amide) (GLP-1), regulate insulin secretion to nutrient intake and constitute the endocrine arm of the entero-insular axis. Glucagon has been implicated in the pathophysiology of conditions characterised by abnormal glucose tolerance such as obesity and diabetes mellitus although its effect on the entero-insular axis is not fully understood.
Materials and methods
We investigated the effect of exogenous glucagon on the entero-insular axis and its relation to gastric emptying in six healthy men aged [mean (±S.E.M.)] 23.6 (0.9) years with a body mass index of 24.0 (1.5) kg/m2. Plasma glucose, GIP, GLP-1, insulin and paracetamol concentrations were measured before and after a 100 g oral carhohydrate load containing 1.5 g of paracetamol for 6 h during intravenous infusion of either glucagon or saline.
Results
When compared to the saline infusion, peak and integrated insulin and glucose concentrations were higher (p<0.05) following glucagon infusion. After 60 min paracetamol concentrations were lower (p<0.05) following glucagon infusion. Integrated responses for GIP and GLP-1 were markedly reduced following glucagon infusion.
Conclusions
Exogenous glucagon in addition to its well-documented action of increasing glucose and insulin concentrations and delaying gastric emptying also markedly reduces GIP and GLP-1 secretion. The inhibition of GLP-1 soon after commencement of glucagon infusion supports a direct effect of glucagon on intestinal L-cells. We speculate that the marked inhibition of postprandial GLP-1 secretion by glucagon may be of importance in the pathogenesis of relative insulinopenia in Type 2 diabetes and in the development of reduced satiety in obesity and diabetes. Copyright © 1999 John Wiley & Sons, Ltd.
Introduction
Gastric inhibitory polypeptide (GIP) and glucagon-like peptide-1 (7–36 amide) (GLP-1) are intestinal hormones which potentiate glucose-induced insulin secretion and are considered to be the major enteral components of the entero-insular axis1-3.
Hyperglucagonaemia and glucose intolerance are features of obesity4, Type 2 diabetes5 and the glucagonoma syndrome. Since exogenous glucagon has been reported as inhibiting GIP6 secretion, it is possible that changes in carbohydrate metabolism in glucose intolerant states may be mediated by an effect of glucagon on the entero-insular axis. Markedly reduced post-carbohydrate GIP concentrations have been described in pancreatic glucagonomas with the glucagonoma syndrome7. The effect of glucagon on GLP-1 secretion has not been studied before.
We undertook this pilot study to investigate whether the administration of exogenous glucagon had any effect on the entero-insular axis. We measured glucose, insulin, GIP, GLP-1 and gastric emptying for 6 h during a 100 g oral glucose load with or without a glucagon infusion in six healthy men.
Subjects, materials and method
Subjects
Six healthy non-obese young men aged [mean (±S.E.M.)] 23.6 (0.9) years with a body mass index of 24.0 (1.5) kg/m2 who were non-smokers were recruited for study. Volunteers gave written consent to participate in the study which had been approved by the Ethics Committees of South West Surrey District and the University of Surrey.
Experimental procedures
Subjects were investigated on two randomised and single blind occasions within two weeks, following an overnight fast of 12 h, having abstained from alcohol for 24 h prior to the study.
During each visit, two intravenous cannulae were sited in each forearm, one for venous blood sample collection and the other for the continuous infusion of glucagon (study day) or saline (control day). Subjects were then given 100 g carbohydrate as HycalTM (Smith Kline Beecham, Herts, UK) made up to a volume of 300 ml with water containing 1.5 g soluble paracetamol (PanadolTM). An intravenous infusion of either glucagon or saline was simultaneously started (at time 0 min). During one visit, subjects were given glucagon [1.5 mg in 50 ml isotonic saline containing 1 g human albumin (SigmaTM Chemical Laboratories Ltd, Poole, UK) and aprotinin 500KIU (TrasylolTM, Bayer AG, Germany)] by continuous infusion over 6 h. The glucagon that was infused was biosynthetic glucagon identical in structure to human glucagon (Eli LillyTM, Hants RG21 5SY, UK). During the control visit, isotonic saline solution containing albumin and aprotinin but not glucagon was similarly infused. Venous blood samples were collected via the cannula before (−15 and 0 min) and at 15, 30, 45, 60, 90, 120, 150, 180, 210, 240, 270, 300, 330 and 360 min following the administration of HycalTM and soluble paracetamol.
Blood samples
Blood samples were taken into heparinised tubes containing aprotinin (SigmaTM Chemical Laboratories Ltd, Poole, UK) (1000 KIU/ml blood) for glucagon, insulin, GIP and GLP-1; into tubes containing fluoride/oxalate for glucose; and into plain glass bottles for paracetamol measurement. Samples collected for glucose and hormones were centrifuged immediately at 1200×g for 5 min; plasma was separated and frozen at −20°C in aliquots until analysis. Blood collected into plain glass bottles for paracetamol was centrifuged 30 min after collection at 1200×g for 5 min, and serum was separated and stored at −20°C until analysis.
Laboratory assays
Glucose was measured by an enzymatic method with an inter-assay coefficient of variation (CV) of <5%. Paracetamol was measured by an enzymatic method with an inter-assay CV of 1.4 and 0.9% at concentrations of 0.05 and 0.1 mmol/l. GIP and GLP-1 were measured by radioimmunoassay using antisera raised in the School of Biological Sciences, University of Surrey, Guildford, UK and are described in greater detail in the following sections8, 9. Insulin and glucagon were measured by immunoassay (Abbott Laboratories, IMx System Insulin Assay, 1992, Assay kit Manual; Euro-DiagnosticaTM glucagon kits, Ideon, SE-205, 12 Malmo, Sweden, Cat no RB310). The inter-assay CV for hormone measurements were: for insulin, 4.2 and 9.0% at 50 and 500 µU/ml, respectively; and for glucagon, 4.8 and 8.9% at 51.2 and 114.4 pmol/l, respectively.
The GIP assays employed in this study have been described previously8, 9; synthetic human GIP was used for the preparation of the standards and iodinated tracer (purified immediately prior to use by affinity chromatography) while the antiserum was raised in rabbits against natural porcine GIP; a PEG (polyethylene glycol 6000, BDH Laboratory supplies, Poole, UK) accelerated double antibody phase separation stage was employed. The detection limit of the assay was 16 pM and the inter-assay CV at 300 and 497 pM was 8.6 and 8.9%, respectively. The antiserum exhibits no cross-reactivity with GLP-1 (7–36) amide, glucagon, VIP, somatostatin, secretin, motilin, and GIP fragments 1–11, 19–25 as well as 19–30.
The GLP-1 assay employed in our assay has been described in detail previously8, 9; synthetic GLP-1 (7–36) amide was used for the preparation of the standards and iodinated tracer (purified immediately prior to use by affinity chromatography) while the antiserum was raised in rabbits against synthetic GLP-1 (7–36) amide; a double antibody phase separation stage employing a coating of donkey anti-rabbit second antibody on cellulose beads was used. The detection limit of the assay was 10 pM and the inter-assay CV was 17.8 and 15.4% at 22.6 and 64.5 pmol/l, respectively. The antiserum exhibits no cross-reactivity with GLP-2, glucagon, VIP, secretin, motilin, and GIP. The GLP-1 assay is directed against the C-terminal amide.
All samples were measured at the same time to minimise different assay conditions between different runs. Both our GIP and GLP-1 assays are homogenous assays not requiring extraction steps thereby minimising artefactual reduction of GLP-1 by processing.
Statistical analysis
Integrated responses were calculated using the linear trapezoidal rule. Paired student's t-test was used to measure the significance of differences between study and control. Values of p<0.05 were considered significant.
Results
All subjects experienced nausea beginning at 2.5–3 h following commencement of the study resulting in the discontinuation of the glucagon infusion in one of the subjects at 180 min although blood sampling continued. The data on the subject whose glucagon infusion was discontinued were included in the statistical analysis since its deletion did not alter the outcome qualitatively or quantitatively.
Plasma glucose, paracetamol, glucagon, insulin, GIP and GLP-1 results are shown in Figures 1-3 and in Table 1. In summary, during the glucagon infusion peak glucose and insulin were higher (p<0.01 and p<0.03, respectively) and integrated glucose and insulin responses were greater (p<0.05 for both) than during the saline infusion. GIP and GLP-1 integrated responses were markedly lower (p<0.05 for both) following the glucagon infusion when compared to saline infusion. Paracetamol, glucose and insulin concentrations were similar during both infusions for the first 45–60 min. Thereafter, peak and integrated paracetamol responses were lower (p<0.02 and <0.05, respectively) following glucagon infusion in comparison to the saline infusion.
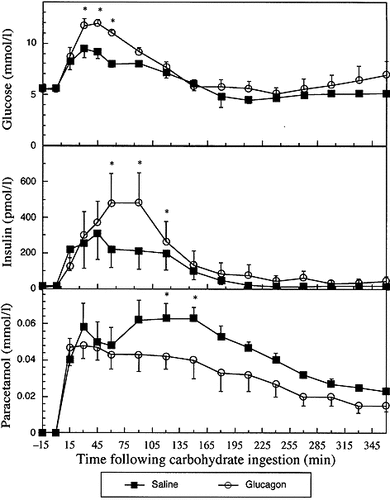
Plasma glucose, insulin and paracetomol responses (mean±S.E.M.) in six subjects following infusion of saline or glucagon. Saline and glucagon infusions commenced at time 0 min. *Paired Student's t-test p<0.05
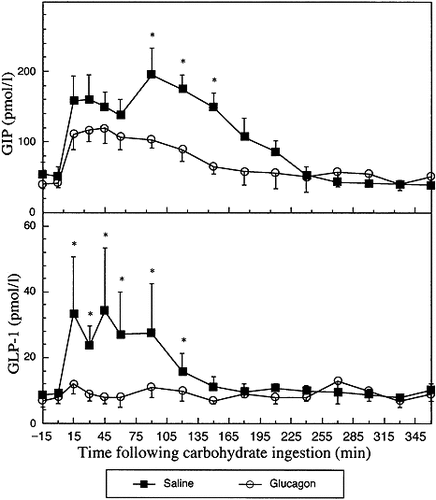
Plasma GIP and GLP-1 responses (mean±S.E.M.) in six subjects following infusion of saline or glucagon. Saline and glucagon infusions commenced at time 0 min. *Paired Student's t-test p<0.05
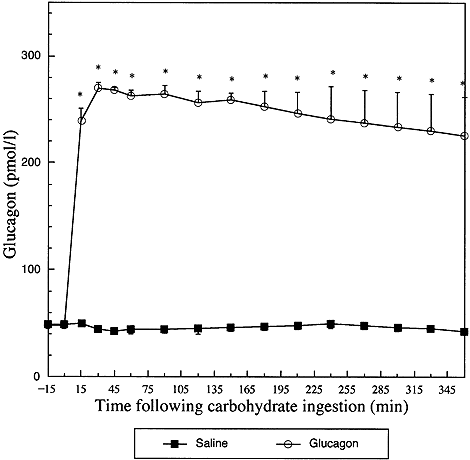
Circulating glucagon responses (mean±S.E.M.) in six subjects following infusion of saline or glucagon. Saline and glucagon infusions commenced at time 0 min. *Paired Student's t-test p<0.05
| Saline infusion | Glucagon infusion | |
|---|---|---|
| Total integrated responses (TAUC) | ||
| Serum paracetamol (mg/min) | 16.1 (1.4)* | 11.5 (2.2) |
| Plasma glucose (mmol/min) | 2210 (44)* | 2592 (111) |
| Plasma insulin (µU/ml/min) | 35 561 (16 355)* | 59 783 (24 380) |
| Plasma GIP (pmol/min) | 37 736 (3807)* | 25 871 (3725) |
| Plasma GLP-1 (pmol/min) | 5538 (1790)* | 3261 (796) |
| Incremental integrated responses (IAUC) | ||
| Plasma glucose (mmol/min) | 200 (56)* | 609 (105) |
| Plasma Insulin (µU/ml/min) | 30 095 (14 760)* | 56 243 (23 321) |
| Plasma GIP (pmol/min) | 18 768 (5762)* | 10 988 (3363) |
| Plasma GLP-1 (pmol/min) | 2336 (1549)* | 509 (231) |
- Saline infusion significantly different from glucagon infusion.
- * p<0.05.
- Values expressed as mean (±S.E.M.)
Discussion
The increase in circulating glucose and insulin levels following glucagon administration in our study is similar to those previously described10. All subjects experienced nausea during the glucagon infusion and this is a well-recognised side-effect of glucagon. Serum paracetamol concentrations were significantly lower during the glucagon infusion confirming that glucagon slows gastric emptying. Paracetamol responses, however, were similar during the saline and glucagon infusions for the first 60 min suggesting that inhibition of gastric emptying cannot be directly attributable to hyperglucagonaemia. Hyperglycaemia11, 12 and hyperinsulinaemia13 have both been shown to inhibit gastric emptying; the glucagon-induced relative hyperglycaemia and hyperinsulinaemia may explain the delayed reduction in circulating paracetamol levels.
GIP responses to oral carbohydrate were markedly reduced The increase in circulating glucose and insulin levels following following glucagon administration and is similar to those reported previously6. The inhibition of GLP-1 secretion was profound, immediate and sustained. The reduction in GIP and GLP-1 is likely to be due to reduced secretion rather than increased clearance.
The inhibition of GLP-1 secretion up to 45 min post-glucose during glucagon infusion occured at a time when there were no significant differences in circulating paracetamol, glucose or insulin levels. It is therefore likely that inhibition of GLP-1 secretion post-glucagon at these times is due to direct (rather than indirect) effects of glucagon on the release of GLP-1 from the intestinal L-cells.
Hyperglycaemia and hyperinsulinaemia exhibit feedback inhibition on GLP-114; higher glucose and insulin levels were seen post-glucagon after 45 min and this may contribute to reduced incretin responses at this time. GLP-1 secretion is influenced by the rate of nutrient delivery into the small intestine15, 16 and any reduction in gastric emptying would be expected to reduce the post-prandial GLP-1 response; this is supported by the reduction in paracetamol responses post-glucagon after 45 min.
In health, glucagon levels are higher in the postabsorptive states and suppressed following feeding unlike GLP-1 which is secreted in response to feeding and suppressed in the postabsoprtive state. The post-prandial period in subjects with obesity and Type 2 diabetes is, however, characterised by inappropriately high glucagon concentrations4, 5, 17. Reduced GLP-1 secretion has been demonstrated in obesity18, 19. Differences in assay specificity have produced conflicting GLP-1 results in Type 2 diabetes; some studies reported elevated fasting levels and post-glucose responses of GLP-1 in Type 2 diabetes20, 21 although others have reported reduced levels22. We therefore speculate that the attenuated post-prandial GLP-1 secretion in obesity and Type 2 diabetes may be attributable, at least in part, to the inappropriate post-prandial hyperglucagonaemia. In addition to stimulating insulin, GLP-1 may also have an important role in satiety23, 24. It is therefore possible that the glucagon-mediated inhibition of GLP-1 may play a part firstly in the relative insulinopenia in diabetes and secondly the reduced satiety in obesity and diabetes.
In summary, exogenous glucagon administration to produce supraphysiological concentrations of circulating glucagon reduced post-prandial GLP-1 and GIP secretion in healthy subjects immediately following the commencement of the infusion; the normal paracetamol, glucose and insulin levels up to 45 min post glucagon suggests that the inhibition of GLP-1 (and possibly GIP) may be due to a direct effect on GLP-1 and GIP producing cells. Since this inhibition was sustained for the duration of the glucagon infusion period, an additional inhibitory effect due to glucagon induced hyperglycaemia, hyperinsulinaemia and reduced paracetamol levels (delayed gastric emptying) in addition to a direct glucagon effect on intestinal L-cells cannot be excluded. Further experiments examining the effect of physiological concentrations of circulating glucagon on GLP-1 (and GIP) as well as the dose–response relationships should be undertaken.
Acknowledgements
We thank Dr Schaper (Institut Dr Schaper, Dresden, Germany) for the measurement of plasma insulin.




