Pulmonary ischemia/reperfusion injury: A quantitative study of structure and function in isolated heart-lungs of the rat
Abstract
Early graft dysfunction after lung transplantation is a significant and unpredictable problem. Our study aimed at a detailed investigation of structure-function correlations in a rat isolated heart-lung model of ischemia/reperfusion injury.
Variable degrees of injury were induced by preservation with potassium-modified Euro-Collins solutions, 2 hr of cold ischemia, and 40 min of reperfusion. Pulmonary artery pressure (Ppa), pulmonary vascular resistance (PVR), peak inspiratory pressure (PIP), and perfusate gases (ΔPO2, ΔPCO2) were recorded during reperfusion. Right lungs were used to calculate W/D-weight ratios. Nineteen experimental and six control left lungs were fixed for light and electron microscopy by vascular perfusion. Systematic random samples were analyzed by stereology to determine absolute and relative volumes of lung structures, the amount of interstitial and intraalveolar edema, and the extent of epithelial injury. Lectin- and immunohistochemistry using established epithelial cell markers were performed in three animals per group to reveal sites of severe focal damage.
Experimental lungs showed a wide range in severity of ischemia/reperfusion injury. Intraalveolar edema fluid amounted to 77–909 mm3 with a mean of 448±250 mm3 as compared with 22±22 mm3 in control lungs (P<0.001). Perfusate oxygenation (ΔPO2) decreased from 30.5±15.2 to 21.7±15.2 mm Hg (P=0.05) recorded after 5 and 40 minutes of reperfusion. In experimental lungs, a surface fraction of 1% to 58% of total type I pneumocyte surface was damaged. Intraalveolar edema per gas exchange region (Vv ape,P) and ΔPO2 were related according to ΔPO2 = 96 − 60 × log10(Vv ape,P) [mm Hg]. The extent of epithelial injury did not correlate with ΔPO2 nor with intraalveolar edema, but increased significantly with PVR. Lectin- and immunohistochemistry revealed focal severe damage to the alveolar epithelium at the border of perivascular cuffs.
We conclude that ischemia/reperfusion-associated respiratory compromise is a direct function of the amount of intraalveolar edema, however, it is not determined by the actual extent of diffuse alveolar epithelial damage at the air-blood-barrier but by the presence of focal severe epithelial damage at the perivascular/alveolar interface. Anat Rec 255:84–99, 1999. © 1999 Wiley-Liss, Inc.
Abbreviations used
A = alveolus, alveolar duct; AE = intraalveolar edema; Ar = artery; Br = bronchiole; C = capillary; E = capillary endothelium; P2 = alveolar type II pneumocyte; PS = peribronchovascular space; V = vessel.
Lung transplantation represents the therapy of choice for end-stage pulmonary diseases (Grover et al., 1997; Hosenpud et al., 1997). One of the most widely established procedures to preserve human donor lungs for clinical transplantation is the technique of flush perfusion of the organ via the pulmonary artery using modified Euro-Collins solution (ECS) (Haverich, 1995; Hopkinson et al., 1998). However, early graft dysfunction remains a significant and unpredictable problem. In its most severe form it may result in primary graft failure, which is characterized by formation of intraalveolar edema associated with diffuse alveolar damage and respiratory compromise (Tazelaar and Starnes, 1994). Although in some cases, preservation injury appeared to be the cause of primary graft failure, a common initial injury, which leads to diffuse alveolar damage and pulmonary edema, has not yet been established (Zenati et al., 1990). Various transplantation-related factors as for example, donor management, organ preservation and harvesting, ischemia, and reperfusion have been shown to contribute to the development of this reimplantation response (Hall et al., 1992; Mills et al., 1992; Prop et al., 1984). Nevertheless, even if almost identical clinical situations were noted, one graft presented without any complication while another one developed severe reimplantation injury (Zenati et al., 1990). Consequently, we looked at the cumulative effects induced by the whole sequence of transplantation-related events, i.e., preservation, cold ischemic storage, and reperfusion, rather than looking at the particular contribution of the individual factors. Therefore, a detailed stereological study of lung injury in relation to pulmonary function was performed to reveal potential sites of failure of the transplanted lung.
To reduce the high complexity inherently present in the in vivo situation, we have chosen an isolated organ preparation, which has been established recently (Fukuse et al., 1995, 1996b). Following flush perfusion of the isolated lung via the pulmonary artery with cold ECS and subsequent ischemic storage, functional parameters were recorded during a 40 min period of reperfusion. The lungs were then fixed by vascular perfusion with a mixture of paraformaldehyde/glutaraldehyde followed by osmication and uranyl acetate stabilization (Fehrenbach et al., 1995, 1998). Thus, accumulation of proteinaceous edema fluid could be visualized to be studied by means of light microscopy (LM), scanning electron microscopy (SEM), and transmission electron microscopy (TEM), and the degree of injury to the air-blood-barrier could be determined by TEM-based stereology. In addition, Bauhinia purpurea lectin- (BPL) histochemistry (Kasper et al., 1994) and immunohistochemistry using an epithelial cell-specific anti-cytokeratin antibody (Kasper and Singh, 1995) were used to look for frank disruptions of the epithelial barrier by LM. To ensure that the samples to be analyzed by standard stereological methods (Weibel, 1990) were representative of the whole organ, tissue blocks were collected according to the rules of systematic random sampling (Michel and Cruz-Orive, 1988). The stereological data were analyzed for correlation with hemodynamic and respiratory parameters. In particular, the hypothesis was tested to determine whether the degree of ischemia/reperfusion-related pulmonary edema and respiratory dysfunction depended on the overall extent of injury to the air-blood-barrier or on the presence of severe focal lesions.
MATERIALS AND METHODS
In 25 male Sprague-Dawley rats weighing 325–465 g (mean ± SD, 374 ± 37.5 g) anesthetization with pentobarbital (Nembutal 1 mg/kg body weight i.p.), heparinization via the caudal vena cava (100 IU), operation and excision of the heart-lung block were performed as described recently (Fukuse et al., 1995, 1996b). In 19 experimental animals, initial basic measurements of physiological parameters in the isolated heart-lung system (see below) were performed prior to perfusion of the lungs via the right atrium with 20 ml of ECS. Ventilation with room air at a tidal volume of 4 ml was continuously performed. Lungs were preserved with standard (115 mM K+), medium (40 mM K+) or low (10 mM K+) ECS (n=6, 6, 7, respectively), which are known to result in different degrees of ischemia/reperfusion injury (Fukuse et al., 1996a; Xiong et al., 1994). The hearts were preserved by coronary perfusion via the aorta with 10 ml of St. Thomas cardioplegic solution. The heart-lung block was then stored for 2 hours at +4°C in ECS with the trachea clamped after the lungs had been inflated with 5 ml room air. Then, the organ block was replaced into the perfusion system. Once steady beating of the heart reoccurred, reperfusion of the lungs was initiated to provoke the development of ischemia/reperfusion-induced pulmonary edema. Hemodynamic and respiratory parameters were continuously monitored during reperfusion. The period of reperfusion was limited to 40 min to ensure minimal effects by isolated perfusion per se and adequate heart function, a prerequisite in this working heart-lung system (Fukuse et al., 1995). At the end of reperfusion, the right lung was used for determination of the wet weight to dry weight-ratio (W/D-ratio). The left lung was fixed via the pulmonary artery, and processed for light and electron microscopy (see below). Any additional step of preperfusion with another cell-free buffer-system to remove red blood cells prior to vascular perfusion fixation was omitted to avoid any additional event that might result in or aggravate reperfusion-induced edema.
In addition, the left lungs of six anesthetized and heparinized rats weighing 355–390 g (mean ± SD, 369 ± 12 g) were fixed at identical settings. Fixation by vascular perfusion was performed immediately after excision, so that the lungs had neither been subjected to perfusion with ECS nor to experimental ischemia or reperfusion. However, as in the experimental animals, the time period required to remove the lungs and place the catheters resulted in a short ischemia of 3–5 min prior to fixation. Thus, any effect induced by this procedure-dependent ischemia alone could be seen in the control lungs as well. Preperfusion with any buffer to remove blood cells was omitted in order not to introduce any event that might mimic reperfusion in the control group. This further takes into account that reperfusion in the experimental lungs was performed with a KH-buffer containing red blood cells. The entire procedure of fixation to stereologic analysis of the control lungs was performed as described for the experimental group (see below).
Additionally, three control and three experimental lungs fixed by vascular perfusion were embedded in paraffin and processed for lectin- and immunohistochemistry (see below).
Rat Isolated Heart-Lung Model
The rat isolated-heart lung model has been described in detail elsewhere (Fukuse et al., 1995). Briefly, the heart-lung block was suspended in a warmed, humidified chamber at an angle of 45° in order to mimic the in situ position of the lungs relative to gravitation and to achieve homogeneous perfusion and ventilation. The temperature in the chamber was kept at 37°C by means of waterjacketed vessels connected to a warming pump (water thermostat type VTS 13c; Radiometer Ltd., Copenhagen, Denmark). The perfusate used was a Krebs-Henseleit buffer containing washed bovine red blood cells (KHrb) at a hematocrit of 38–40%, which has been shown to improve functional characteristics of isolated lungs, and to minimize perfusion dependent pulmonary edema in comparison with a cell-free KH-buffer (Fukuse et al., 1995). Leukocytes were removed by means of a leukocyte removal filter (RC100E, Pall Europe Ltd., Portsmouth, UK). Absence of leukocyte sequestration was confirmed histologically. The perfusate reaching the coronary arteries via the aorta was oxygenated with a mixture of 95% O2 and 5% CO2. The perfusate of the pulmonary circuit reached the lung via the right atrium after passage of a second oxygenator (Monolyth integrated membrane lung, Sorin Biomedical Ltd., Saluggia, Italy) gassed with 95% N2 and 5% O2 to obtain a deoxygenated pulmonary arterial inflow. From a preload pool, perfusion of the right atrium was performed at a hydrostatic pressure of 5 cm H2O, and the right ventricle of the working heart provided pulsatile flow of the perfusate through the lung. The opening of the left atrial cannula was positioned to ensure a constant left atrial pressure (Pla) of 2 cm H2O. The lungs were ventilated with room air at a tidal volume of 4 ml and a rate of 40 breaths per min. A positive end-expiratory pressure of 3 cm H2O was maintained.
Measurement of Hemodynamic and Respiratory Parameters
Pulmonary artery pressure (Ppa) was measured via a pressure transducer and monitor (Servomed, Hellige Ltd., Hamburg, Germany). Pla was constant at 2 cm H2O (see above). Cardiac output (CO) was determined by collecting pulmonary venous outflow. Ppa [mm Hg], Pla [cm H2O], and CO [ml× min−1] were used to calculate pulmonary vascular resistance (PVR) according to the standard equation PVR = (Ppa - Pla) × 80 / CO [dynes × sec × cm-5] (Fukuse et al., 1995). Blood gases were determined at intervals of 10 min. PO2 and PCO2 of the deoxygenated perfusate of the preload pool were defined as arterial PaO2 and PaCO2. PO2 and PCO2 of the perfusate collected from the left atrium as venous PvO2 and PvCO2. To assess the lungs' capability for gas exchange, ΔPO2 and ΔPCO2 were defined as PvO2 − PaO2 and PvCO2 − PaCO2, respectively. Peak inspiratory pressure (PIP) was measured by means of the small animal respirator (Mod. No. 4601, Rhema Corp., Hofheim, Germany) and registered every 10 min. After 40 min of reperfusion, left and right lungs were separated for determination of the W/D-ratio and for fixation and subsequent histological and ultrastructural analysis, respectively.
Fixation
In all the control and experimental animals, fixation of the left lung was performed by vascular perfusion via the pulmonary artery at a hydrostatic pressure of 15 cm H2O for only about 2 min in order to minimize potential fixation dependent fluid shifts. The quality of structural preservation did not differ from rat lungs, which were fixed by vascular perfusion for 10 min in the course of another study (Uhlig et al., 1995). During fixation, the airway pressure was adjusted to 12 cm H2O. The fixative used was a mixture of 1.5% glutaraldehyde and 1.5% paraformaldehyde in 0.1 M cacodylate buffer at pH 7.35. The osmolality of the cacodylate buffer, also used in any further step, was adjusted to isotonicity, i.e., to 300 mOsm/kg. This resulted in a total osmolality of the primary aldehyde-containing fixative of about 760 mOsm/kg. At the end of perfusion fixation, main left bronchus and pulmonary artery were tightly clamped to prevent any leakage of air or edema fluid and to allow stiffening of the lungs during storage in cold fixative for 18–24 hours prior to sampling. Thus, collapse of the lung due to disconnection of the trachea from the spirometer and deformation of tissue blocks during sampling, the only disadvantages of this type of fixation method (Bachofen et al., 1982: method II), could be avoided.
Lung Volume Determination
After cold storage in fixative, the volume of the fixed lung (V, L) was determined by fluid displacement in an isotonic NaCl solution containing 10% dextran (osmolality: 310 mOsm/kg; specific weight: 1.045g/ml). Volume determination was performed immediately before sampling of the tissue blocks as recommended (Michel and Cruz-Orive, 1988). Because storage of up to 5 days in the fixative did not result in a significant alteration of fixed lung volume (A. Fehrenbach, Göttingen, unpublished observations), we can exclude that differences observed in lung volumes were due to the duration of storage in the aldehyde fixative.
Sampling
Systematic random sampling was performed according to Michel and Cruz-Orive (1988). Briefly, the fixed left lung was embedded in 2% agar-agar dissolved in distilled water. By means of a tissue slicer, the organ was cut from apex to basis into 3 mm thick slices, which were laid down apical face up. Then, the collection of slices was covered with a transparent sheet with 11×11 10-mm spaced points. Tissue blocks of approximately 3×3×3 mm3 in size were only taken from those sites where a test point, which determined the lower left edge of any block, fell on the section area of a lung slice. Thus, 5–11 blocks were obtained from each lung for LM and TEM. From the remaining lung slices, samples were collected for SEM. From the three control and three experimental lungs fixed for subsequent lectin histochemistry, every third lung slice was collected in toto for embedding in paraffin.
Tissue Processing
Samples to be studied by SEM were processed according to a modified OTOTO (osmium tetroxide − thiocarbohydrazide − osmium tetroxide) method described earlier (Uhlig et al., 1995). Briefly, tissue blocks were rinsed in 0.1 M cacodylate buffer and postfixed for 2 hr with cacodylate buffered 1% osmium tetroxide. Then, they were rinsed in distilled water, transferred to 1% aqueous thiocarbohydrazide for 1 hr, washed with distilled water, treated with 1% aqueous osmium tetroxide for 2 hr, and rinsed again with distilled water. These steps were repeated twice. After dehydration through a graded series of ethanols, critical point drying was performed and the specimens could then directly be observed by means of a Zeiss DSM 960 without additional metal-sputtering.
For LM and TEM, the tissue samples were processed as described earlier (Fehrenbach et al., 1995). Processing was standardized using an automated tissue processor with temperature control and cooling device (Histomat, Bio-med, Theres, Germany). Briefly, after 6 rinses (5 min each) in 0.1 M cacodylate buffer (300 mOsm/kg), samples were post-fixed in 1% OsO4 in 0.1 M cacodylate buffer (300 mOsm/kg), washed again in the same buffer (4 rinses, 5 min each), rinsed in distilled water (2 rinses, 5 min each), transferred to half-saturated, aqueous uranyl acetate for en bloc-staining (overnight). All steps were performed at 8°C. After washing in distilled water, specimens were dehydrated through a graded series of ethanol (30%, 50%, 70%, 90%, 3 × 100%), transferred to Araldite via propylene oxide and a 1:1-mixture of propylene oxide/Araldite, embedded in Araldite, and polymerized at 60°C for 3 days. For histological studies, 5–10 semi-thin sections of three systematic random samples per lung were stained with Methylen blue and analyzed by means of LM (Laborlux 11; Leitz, Wetzlar, Germany). For ultrastructural studies, two or three tissue blocks per lung were selected from the sample collection by random numbers. From each block five ultrathin sections were cut, collected on Formvar-coated 1x2 mm slot grids, and counter stained with lead citrate by means of an Ultrostainer (Leica, Hamburg, Germany). From these five ultrathin sections the technically best one was chosen for subsequent stereological analysis. Qualitative TEM studies and systematic quadrate sub-sampling of micrographs for stereological analysis were performed by means of an EM 10 A (Zeiss, Oberkochen, Germany).
For lectin- and immunohistochemistry, lung slices were processed by routine paraffin embedding. The biotinylated form of the type I pneumocyte-specific marker BPL (Vector Laboratories, Burlingham, USA) and the mouse monoclonal epithelial cell-specific cytokeratin-antibody MNF116 (Dako, Hamburg, Germany) were demonstrated as described by Kasper and co-workers (1994). Briefly, deparaffinized sections were preincubated with 5% normal horse serum, blocked for endogenous peroxidase with hydrogen peroxide, incubated with biotinylated BPL (1:400 dilution) for 30 min followed by avidin-biotin-peroxidase complex (ABC) (Vector Laboratories). For immunohistochemistry, dewaxed sections were treated with 0.05% Pronase (15 min, 37°C) prior to incubation for 60 min at 37°C with MNF116 (1:1,500). An anti-mouse ABC-kit was used as detection system (Vector Laboratories). Sections were developed with 0.05% 3,3-diaminobenzidine tetrahydrochloride (Serva, Germany) and 0.03% hydrogen peroxide, and counterstained with haematoxylin. Phosphate buffered saline (PBS) was used as incubation and rinsing buffer. A control section, which was incubated with PBS only, was processed together with each lectin- or antibody-incubated section. The specificity of the lectin was tested by preincubation with the specific sugar residue N-acetyl galactosamine (EY Laboratories, San Mateo, USA) (Kasper et al., 1994).
Stereology
Stereological analysis was performed according to a cascade procedure to allow for the determination of the absolute volumes of the various compartments according to standard procedures (Weibel, 1990). Volume densities (Vv) of the structures of interest were recorded at four different levels (Table 1). All parameters were determined by means of point and intersection counting. Test fields and micrographs were collected according to systematic quadrate sub-sampling (Michel and Cruz-Orive, 1988).
| Level 0 | Level 1 | Level 2 | Level 3 | Level 4 |
|---|---|---|---|---|
| (Fluid displacement) | (LM, ×100) | (LM, ×160) | (LM, ×1,000) | (TEM, ×6,500) |
| Lung Volume (L) | Nonparenchyma (np) | Airways (aw) | ||
| —Wall (aww) | ||||
| —Lumen (awl) | ||||
| Peribronchovascular Space (ps) | ||||
| —Free of erythrocytes (psf) | ||||
| —Extravasated erythrocytes (pse) | ||||
| Vessels (vs) | ||||
| —Wall (vsw) | ||||
| —Lumen (vsl) | ||||
| Parenchyma (p) | Alveolar Space (ap) | |||
| —Air (apa) | ||||
| —Edema Fluid (ape) | ||||
| Alveolar Septum (as) | ||||
| —Capillaries (asc) | ||||
| —Tissue (ast) | Endothelium (en) | |||
| Interstitium (int) | ||||
| Epithelium (ep) | ||||
| —Type II Cells (p2) | ||||
| —Type I Cells (p1) | ||||
| —normal (np1) | ||||
| —>—swollen (sp1) | ||||
| —fragmented (fp1) |
Three blocks of tissue per lung were used for stereological analyses at levels 1–3. One technically good 0.5μm thick section per sample was examined according to the procedure of systematic quadrate sub-sampling (Michel and Cruz-Orive, 1988). Using a Leitz Laborlux 11 light microscope, the whole section was scanned by moving the object stage at uniform intervals of 0.5 mm along the axes. At each position, point counting was performed directly by means of an eye-piece containing an integration plate (100/25 points).
Two or three tissue blocks of each lung were used for stereological analysis by means of TEM (level 4). One technically good ultra-thin section per tissue block was examined according to the procedure of systematic quadrate sub-sampling (Michel and Cruz-Orive, 1988). The whole section was scanned by moving the object stage at a uniform distance determined by the stage coordinates. Whenever the center of the fluorescence screen of the microscope hit the alveolar septum, a micrograph was recorded at a primary magnification of 3,150 x. The final magnification after photographic reproduction was determined by means of a calibration grid to be 6,500 x. Calibration was performed during each photographic session. A multipurpose coherent test system was used for point and intersection counting (324 points, test line length d = 10 mm).
The following structural parameters were analyzed (Table 1). At the first level (LM, 100 x), a distinction was made between parenchyma and nonparenchyma. In the mean (± SD), 70.6 ± 23.3 fields of view per lung were analyzed, which resulted in a total count of 1,764 ± 583 points per lung with 224 ± 155 points falling on non-parenchyma. At the second level (LM, 160 x), non-parenchyma was studied to distinguish the lumen and the wall of airways (tunica mucosa and muscularis) and vessels (tunica intima and media), respectively, as well as their joint connective tissue sheet, the peribronchovascular space. A distinction was made between the peribronchovascular space occupied by extravasated erythrocytes and the fraction that was free of erythrocytes. In the mean, 54.2 fields of view per lung were analyzed, which resulted in a total count of 1,354 ± 928 points falling on non-parenchymal structures counted per lung. At the third level (LM, 1000 x), parenchyma was studied to distinguish the alveolar space including alveolar ducts (filled with air or with edema-fluid) and the alveolar septum (tissue and capillaries). In the mean, 45.5 ± 14.5 fields of view were analyzed per lung, which resulted in a total count of 1,129 ± 359 points per lung falling on parenchyma, with a mean of 262 ± 77 points falling on alveolar septum.
The collection of micrographs obtained by systematic quadrate sub-sampling was used to determine the arithmetic mean thickness of the total air-blood-barrier as well as of its components, epithelium, septal interstitium, and capillary endothelium at the fourth level (TEM, 6,500 x). In the mean, 20.0 ± 2.9 micrographs per lung were collected and analyzed. Using the multipurpose test point system described above, the mean arithmetic thicknesses (τb), which can be defined as the ratio of tissue volume per barrier surface, were determined by counting the points falling on the respective barrier (Pb) and the intersections (Ib) with its two bounding surfaces according to Weibel, 1990, page 224 (formula 38): τb = 2 × k1 × d × Pb/Ib with constant k1 = 0.5 and test line length d = 1.54 μm, characteristics of our test system at the given final magnification. In addition, the degree of cell damage to alveolar type I pneumocytes was quantitatively determined as the fraction of normal, swollen, and fragmented areas (covered with or free of edema-fluid, respectively) relative to the total surface of alveolar type I pneumocytes, i.e., the ratio of intersections with injured areas per total number of intersections counted (Velazquez et al., 1991). A mean of 249 ± 74 intersections was counted per lung. The different types of injury to the alveolar type I epithelium were defined as follows: normal, only if there were absolutely no alterations in fine structure; swollen, if there were small vacuoles or a clearing of the cytoplasmic ground substance with the cell membranes being continuous; fragmented, if the apical cell membrane was disrupted, the epithelial leaflet vesiculated, or the basal lamina completely free of any epithelial remnants.
Since ischemia/reperfusion-induced lung injury and edema formation involved several compartments of the lung at the same time, a stereological analysis based on the determination of volume densities alone could run into the “reference trap” (Braendgaard and Gundersen, 1986). For direct comparisons of experimental and control groups, we therefore followed Mayhew (1991), who pointed out that “emphasis is given to absolute quantities because these avoid dangers prevalent when comparing groups using relative data.” The absolute volumes were used to define compartments that actually responded by volume changes. The absolute volumes of the main pulmonary components were estimated by multiplication of the volume densities obtained at each level with the absolute volume of the left lung (V, L) determined by fluid displacement. Total interstitial edema volume was defined as the sum of the absolute volumes of alveolar septal interstitium (V int) and peribronchovascular space (V ps) minus the respective control mean volumes. The analysis of structure-function correlation was based on volumes relative to total lung volume, since comparisons of physiological variables are generally based on normalized rather than absolute values (Jones and Longworth, 1992).
Statistics
Data referring to individual lungs are given as discrete values obtained according to standard stereological formulas. Mean values are given ± SD unless stated otherwise. Differences between control and experimental animals were tested for significance by an unpaired t-test, given that normality and equal variance were not rejected at P< 0.05. Otherwise, the nonparametric Mann-Whitney rank sum test was performed. Correlation between variables was tested by means of the nonparametric Spearman rank order correlation. We point out that the multiple correlation tests were used in terms of “exploratory” rather than “decisive” data analysis. All statistical analyses and computation of regression curves were performed using the SigmaStat 2.0 and SigmaPlot 3.0 software programs (Jandel Scientific, Erkrath, Germany). P<0.05 was considered to be statistically significant.
RESULTS
The rat isolated heart-lungs subjected to preservation with different potassium-modified ECS, followed by 2 hr of cold ischemic storage and reperfusion for 40 min, developed pulmonary edema to variable degrees. This was associated with pulmonary dysfunction and structural alterations. Both functional and structural characteristics showed broad variability (Fig. 1), which is a typical feature of clinical pulmonary ischemia/reperfusion response.
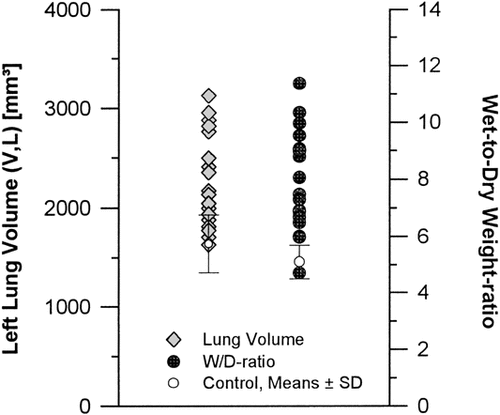
Fixed left lung volumes (V, L) and W/D-ratios of the individual experimental animals to display the wide range of variability within the experimental group subjected to ischemia/reperfusion stress.
Functional Parameters
Hemodynamic and respiratory parameters are given in Table 2. The W/D-ratio ranged between 4.7 and 11.4 as compared to 5.1 ± 0.6 in the control group (Fig. 1), values also reported by others for normal and edematous lungs (Fisher et al., 1980; Michel et al., 1986; Uhlig et al., 1995). In the mean, oxygenation (ΔPO2) was significantly decreased, total pulmonary vascular resistance (PVR) and PIP were significantly increased in comparison to baseline data. However, the individual lungs exhibited a broad range of data from normal to pathologic. Correlation analysis (Table 3) indicated significant negative relationships between oxygenation (Δ PO2) and W/D-ratio (P< 0.01), and ΔPO2 and PIP (r=-0.63; P<0.003), respectively. The virtual incongruity of a weak negative correlation between W/D-ratio and PVR (r=-0.49; P<0.05) will resolve in view of the stereological data.
| Functional | Baseline | Reperfusion | Level of |
|---|---|---|---|
| parameter | dataa | datab | significancec |
| W/D-ratio | 5.1 ± 0.6 | 8.0 ± 1.8 | P < 0.01 |
| ΔPO2 [mm Hg] | 30.5 ± 15.2 | 21.7 ± 18.3 | P = 0.05 |
| ΔPCO2 [mm Hg] | 8.15 ± 7.2 | 8.3 ± 7.2 | n.s. |
| Ppa [mm Hg] | 10.9 ± 2.2 | 9.8 ± 2.5 | n.s. |
| PVR [dyn × sec × cm−5] | 53 ± 17 | 594 ± 679 | P < 0.001 |
| PIP [cm H2O] | 17.9 ± 4.0 | 32.3 ± 5.6 | P < 0.001 |
- a Mean values ± S.D. of baseline data recorded at the onset of the respective experiments, i.e., 5 min after initiation of rebeating of the heart following 2 hr of cold ischemia. W/D-ratios were calculated from nonischemic control lungs perfused for 40 min.
- b Mean values ± S.D. of data recorded after 40 min of reperfusion following 2 hr of cold ischemia.
- c Levels of significance (P) were determined by paired t-test if normality was not rejected at P < 0.05, otherwise the nonparametric Wilcoxon signed rank test was used. Means of W/D-ratio were tested by the nonparametric Kruskal-Wallis rank sum test.
| ΔPO2 | ΔPCO2 | Ppa | PVR | PIP | |
|---|---|---|---|---|---|
| [mm Hg] | [mm Hg] | [mm Hg] | [dyn × sec × cm−5] | [cm H2O]> | |
| W/D-ratio | r = −0.601 | n.s. | n.s. | r = −0.494 | n.s. |
| P < 0.01 | P < 0.05 | ||||
| ΔPO2 [mm Hg] | r = 0.449 | n.s. | r = 0.446 | r = −0.633 | |
| P < 0.05 | P < 0.05 | P < 0.003 | |||
| ΔPCO2 [mm Hg] | n.s. | n.s. | n.s. | ||
| Ppa [mm Hg] | n.s. | n.s. | |||
| PVR [dyn × sec × cm−5] | n.s. |
- a Correlation coefficients (r) with levels of significance (P) determined according to Spearman's rank order correlation.
Structural Parameters
Fluid accumulation resulted in a significantly higher (P<0.05) mean volume of the fixed left lung in the experimental group (2,298 ± 452 mm3) as compared with the control group (1,640 ± 293 mm3). A corresponding increase in the volume of the fixed lung has been reported to occur in hydrostatic pulmonary edema induced in isolated rabbit lungs (Wu et al., 1995). Wide variations were seen by LM and EM with respect to pulmonary edema and to the degree of damage to the air-blood-barrier. Accumulation of edema fluid was observed in different compartments of the lung: i) alveolar space and alveolar ducts (Fig. 2), ii) peribronchovascular space (Fig. 3), and iii) interstitium of the alveolar septum (Figs. 2b, c). In severely affected lungs, intraalveolar edema was accompanied by various structural defects as were extensive alveolar (Fig. 2a) and perivascular hemorrhage (Fig. 3b), extended damage of alveolar type 1 pneumocytes (Fig. 4), and large gaps in the alveolar epithelial boundary of perivascular cuffs (Fig. 5d, f). Edema fluid did not accumulate in the lumen of airways proximal to alveolar ducts. The absolute and relative volumes of the various compartments analyzed by stereology are shown in Tables 4 and 5. Looking at the estimates of the various edema accumulations, however, one has to take into account that the chemical fixation procedure used, which is a prerequisite for the stereological approach, might result in an underestimation of edema volumes (see discussion).
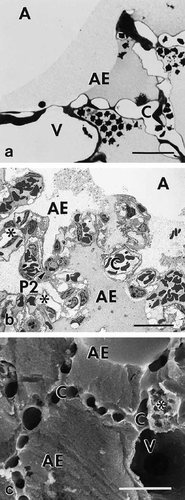
Pulmonary parenchyma of experimental rat lung showing intraalveolar edema. a) Photomicrograph displaying intraalveolar edema associated with alveolar hemorrhage. Note the differences in staining intensity of edema. b) Transmission electron micrograph showing intraalveolar edema and severe interstitial edema of alveolar septa (asterisks). Note again the differences in staining intensity of edema. c) Scanning electron micrograph showing alveoli filled with homogeneous edema material, and interstitial edema of alveolar septa (asterisk). Capillaries and vessel are open. Scale bars = 25μm.
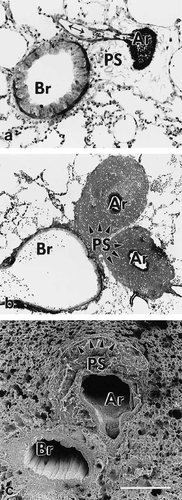
Pulmonary nonparenchyma of experimental rat lungs. a) Photomicrograph showing peribronchovascular space with interstitial edema that is most prominent around an artery, moderate around the originating arteriole (doublearrow), and absent around the bronchiole. b) Photomicrograph showing peribronchovascular space completely occupied by extravasated erythrocytes around the two arterial branches. c) Scanning electron micrograph. Peribronchovascular space around the artery is edematously swollen, and the adjacent parenchyma is characterized by compression of alveoli (arrowheads). Scale bar = (a) 100μm; (b) 200μm; (c) 250μm.
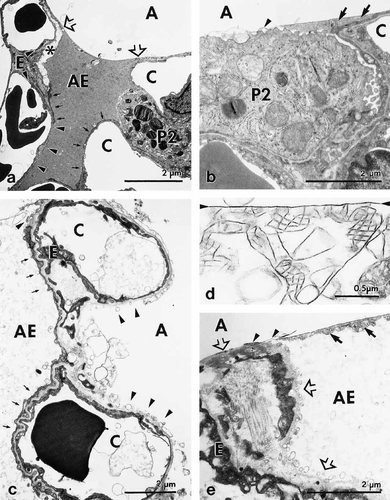
Lung injury and intraalveolar edema. Transmission electron micrographs showing the different types of damage to alveolar type I pneumocytes and surfactant. a) Control lung. Small pool of intraalveolar edema fluid that covers alveolar type I pneumocytes exhibiting areas of normal (arrows), swollen (asterisk), and fragmented cells (arrowheads). Edema fluid is delimited by surfactant lining layer (open arrows). b) Control lung. Surfactant lining layer (arrowhead) covering a type II pneumocytes, and regular tubular myelin (arrows) located in a small pool at the cell margin. c) Experimental lung. Left alveolus, which shows largely normal type I pneumocyte (arrows), is filled with intraalveolar edema fluid, while right alveolus showing severe fragmentation (arrowheads) of type I pneumocyte is free of edema. d) Experimental lung. Surfactant lining layer (arrowheads) is associated with irregular, disintegrated tubular myelin. e) Experimental lung. Alveolar septum with severe fragmentation of type I pneumocytes (open arrows), remnants of tubular myelin (arrows), and a surfactant lining layer (arrowheads) closing up the alveolar entrance (open arrow) and delimiting intraalveolar edema fluid.
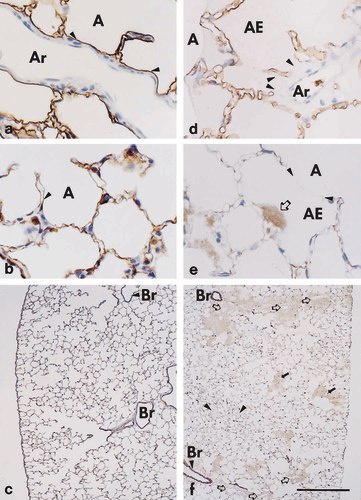
Lectin- and immunohistochemistry to detect epithelial injury in paraffin sections of ischemic/reperfused (d-f) versus control lungs (a-c). a, d) Bauhinia purpurea lectin staining reveals (a) continuous alveolar epithelium (arrowheads) lining the perivascular cuff of an artery of control lungs, whereas (d) in ischemic/reperfused lungs large epithelial gaps (arrowheads) are seen. Note the intact lining of alveolar septa and the presence of intraalveolar edema in adjacent alveoli. b, e) Immunohistochemical staining with the anti-cytokeratin antibody MNF116, an epithelial cell marker, reveals (b) discrete staining of the alveolar epithelium in control lungs (arrowhead), whereas (e) fluffy staining (open arrow) of disintegrated epithelium can focally be seen in edematous regions. Note that staining is clearly not a result of an unspecific staining of edema components because most of the region occupied by intraalveolar edema is unstained (meniscus of fluid is indicated by arrowheads). c, f) Immunohistochemical staining with the anti-cytokeratin antibody MNF116 reveals (f) that regions with disintegrated epithelium are most frequently seen in close vicinity to arterial (open arrows) and venous (solid arrows) branches of the vasculature, which were identified at higher magnification, while additional unstained, edematous alveoli (arrowheads) can be seen within the parenchyma. Control lungs (c) are unobtrusive. Scale bars = (a,b,d,e) 50μm; (c,f) 500μm.
| Stereological parameter | Control (n = 6) | Experimental (n = 19) | Level of significance | ||
|---|---|---|---|---|---|
| Mean ± SD | Median | Mean ± SD | Median | ||
| Absolute volumes (Volume densities)* | Absolute volumes (Volume densities) | ||||
| Lung Volume [mm3] | 1,640 ± 293 | 2,298 ± 452 | P < 0.003 | ||
| Parenchyma [mm3] | 1,504 ± 237 | 1,963 ± 504 | P < 0.05 | ||
| (92.1 ± 3.2) | (84.9 ± 8.8) | (n.s.) | |||
| Alveolar Space [mm3] | 1,191 ± 193 | 1,220 | 1,495 ± 434 | 1,444 | n.s. |
| (72.9 ± 4.1) | (73.1) | (64.3 ± 8.9) | (67.4) | (P < 0.03) | |
| Air [mm3] | 1,169 ± 196 | 1,046 ± 310 | n.s. | ||
| (71.5 ± 4.6) | (45.8 ± 8.4) | (P < 0.0001) | |||
| Alveolar Edema [mm3] | 22 ± 22 | 17 | 448 ± 250 | 353 | P < 0.001 |
| (1.4 ± 1.3) | (1.0) | (18.5 ± 8.6) | (16.2) | (P < 0.0001) | |
| Alveolar Septum [mm3] | 314 ± 71 | 469 ± 113 | P = 0.005 | ||
| (19.2 ± 2.9) | (20.6 ± 3.7) | (n.s.) | |||
| Capillaries [mm3] | 135 ± 37 | 188 ± 61 | n.s. | ||
| (8.3 ± 1.9) | (8.1 ± 2.3) | (n.s.) | |||
| Septal Tissue [mm3] | 179 ± 46 | 280 ± 74 | P = 0.005 | ||
| (10.9 ± 1.9) | (12.5 ± 2.7) | (n.s.) | |||
| τB [μm] | 2.13 ± 0.20 | 2.14 | 2.54 ± 0.59 | 2.49 | n.s. |
| Epithelium [mm3] | 28 ± 5 | 46 ± 17 | P < 0.05 | ||
| (1.7 ± 0.2) | (1.7) | (2.1 ± 0.8) | (2.0) | (n.s.) | |
| τepi [μm] | 0.35 ± 0.05 | 0.35 | 0.44 ± 0.34 | 0.36 | n.s. |
| Interstitium [mm3] | 104 ± 28 | 176 ± 54 | P = 0.005 | ||
| (6.4 ± 1.5) | (5.9) | (7.9 ± 1.8) | (7.4) | (n.s.) | |
| τint [μm] | 1.24 ± 0.17 | 1.60 ± 0.41 | P = 0.05 | ||
| Endothelium [mm3] | 47 ± 16 | 43 | 61 ± 20 | 56 | n.s. |
| (2.8 ± 0.5) | (2.6) | (2.7 ± 0.8) | (2.5) | (n.s.) | |
| τen [μm] | 0.54 ± 0.07 | 0.57 | 0.50 ± 0.06 | 0.50 | n.s. |
- * Volume densities refer to fixed left lung volume. Differences between groups were tested for significance (P) according to an unpaired t-test if normality and equal variance were not rejected at P < 0.05. Otherwise, the nonparametric Mann-Whitney rank sum test was performed, and the median values are given.
Intraalveolar edema
By means of LM and stereology (Table 4), the absolute volume of intraalveolar edema accumulation was determined to be in a range of 77–909 mm3 (mean ± SD, 448 ± 250 mm3) corresponding to 4.2–36.1% (mean ± SD, 18.5 ± 8.6 %) of the left lung volume (Vv ape,L). The surface fraction of alveoli and alveolar ducts covered by edema fluid (Ss ape,Ep) was 25.0–76.7% (mean ± SD, 52.4 ± 15.0 %) of the total alveolar epithelial surface. In the control lungs, intraalveolar fluid, comprising the small liquid pools regularly seen in the corners of alveoli, amounted to only 22 ± 22 mm3, which was equivalent to 1.4 ± 1.3 % of the left lung volume. In isolated control rabbit lungs, Wu and co-workers (1995) stereologically estimated this alveolar liquid pool to be 195 ± 197 mm3, which is equivalent to 0.25 ± 0.25 % of the lung volume of 79 ± 8.6 cm3. The higher amount of edema in our control lungs is probably an effect of the short period of ischemia between excision and fixation.
Peribronchovascular edema
A 2.5-fold increase in total volume of the nonparenchyma of experimental lungs was observed in comparison with the control lungs (Table 5). The peribronchovascular space was largely responsible for the volumetric increase in total nonparenchyma as shown by the highly significant correlation of V,np and V,ps (r=0.95; P <0.001). Accumulation of edema was most prominent around the vessels, while it was inconspicuous around the airways (Fig. 3). The volume of the peribronchovascular space amounted to 179 ± 106 mm3 in the experimental lungs as compared to 30 ± 11 mm3 in the control lungs. The peribronchovascular space free of erythrocytes amounted to 112 ± 73 mm3, while extravasated erythrocytes occupied a volume of 68 ± 56 mm3. The parameters characterizing the peribronchovascular space were significantly increased in the experimental lungs in comparison with the control lungs. While vessel lumen and wall were considerably, albeit insignificantly enlarged, the volumes of airway lumen and wall were not affected (Table 5).
| Stereological parameter | Control (n = 6) | Experimental (n = 19) | Level of significance | ||
|---|---|---|---|---|---|
| Mean ± SD | Median | Mean ± SD | Median | ||
| Absolute volumes (Volume densities)* | Absolute volumes (Volume densities)* | ||||
| Nonparenchyma [mm3] | 135 ± 71 | 335 ± 170 | P < 0.02 | ||
| (7.9 ± 3.2) | (15.1 ± 8.8) | (n.s.) | |||
| Airways [mm3] | 47 ± 31 | 40 | 65 ± 62 | 45 | n.s. |
| (2.8 ± 1.4) | (2.6) | (2.9 ± 3.2) | (1.9) | (n.s.) | |
| Airway lumen [mm3] | 32 ± 24 | 24 | 43 ± 47 | 28 | n.s. |
| (1.9 ± 1.1) | (1.4) | (1.9 ± 2.4) | (1.1) | (n.s.) | |
| Airway wall [mm3] | 15 ± 8 | 15 | 22 ± 19 | 16 | n.s. |
| (0.9 ± 0.4) | (0.9) | (1.0 ± 0.9) | (0.6) | (n.s.) | |
| Vessels [mm3] | 58 ± 37 | 91 ± 38 | n.s. | ||
| (3.4 ± 1.7) | (3.0) | (4.1 ± 1.9) | (3.3) | (n.s.) | |
| Vessel lumen [mm3] | 45 ± 30 | 32 | 67 ± 31 | 51 | n.s. |
| (2.6 ± 1.4) | (2.2) | (2.9 ± 1.5) | (2.2) | (n.s.) | |
| Vessel wall [mm3] | 12 ± 7 | 25 ± 14 | n.s. | ||
| (0.7 ± 0.4) | (1.1 ± 0.7) | (n.s.) | |||
| Peribronchovascular Space [mm3] | 30 ± 11 | 32 | 179 ± 106 | 140 | P < 0.001 |
| (1.8 ± 0.6) | (1.9) | (8.1 ± 5.2) | (6.6) | (P < 0.0001) | |
| Free of erythrocytes [mm3] | 30 ± 11 | 32 | 112 ± 73 | 82 | P < 0.002 |
| (1.8 ± 0.6) | (1.9) | (5.3 ± 3.8) | (3.7) | (P < 0.02) | |
| Extravasated erythrocytes [mm3] | 0.05 ± 0.08 | 0.00 | 68 ± 56 | 62 | P < 0.001 |
| (<0.1 ± 0.1) | (<0.01) | (2.7 ± 2.6) | (2.0) | (P < 0.0001) | |
- * Volume densities refer to fixed left lung volume. Differences between groups were tested for significance (P) according to an unpaired t-test if normality and equal variance were not rejected at P < 0.05. Otherwise, the nonparametric Mann-Whitney rank sum test was performed, and the median values are given.
Interstitial edema of alveolar walls
In terms of absolute volumes (Table 4), the interstitium of the alveolar septa was significantly higher in the experimental (176 ± 54 mm3) than in the control lungs (104 ± 27 mm3). Correspondingly, a significant increase in the arithmetic mean thickness of the interstitial space (τint) of the alveolar septa was observed in the experimental (mean ± SD, 1.60 ± 0.41μm) compared with the control group (1.24 ± 0.17μm).
Interstitial vs. intraalveolar edema
Intraalveolar edema was by far the most significant type of edema in the ischemic/reperfused lungs. Equivalent amounts of total interstitial edema were present in all lungs, while differences between individual lungs largely resulted from intraalveolar edema (Fig. 6). The volume of intraalveolar edema fluid (V,ape) correlated positively with the volume of septal interstitium (r=0.53; P<0.025). The unexpected negative correlation (r=-0.63; P=0.005) of V,ape with the volume of free peribronchovascular space (V,psf) most probably resulted from displacement of edema fluid from the peribronchovascular into the alveolar space by extravasated erythrocytes. This is supported by the lack of correlation between V,ape and the volume of total peribronchovascular space (V,ps). Notably, the peribronchovascular space was increased at the expense of air-filled alveolar space (Figs. 3, 7) indicating that the expanding peribronchovascular cuffs encroach on the more compliant parenchymal airspace.
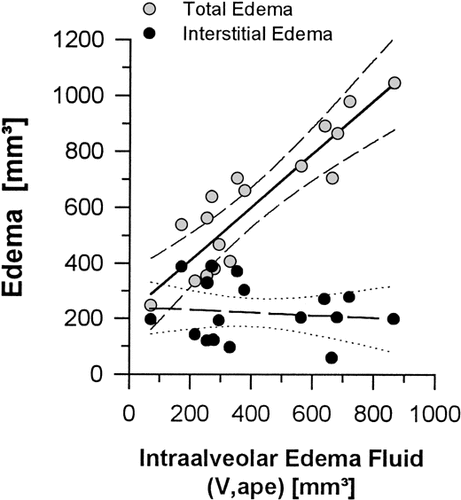
Stereologic data showing that in experimental lungs the total volume of interstitial edema, i.e., the absolute volumes of the interstitium of alveolar septa (V int) and of the peribronchovascular space (V ps) minus the respective control means, is independent of the volume of intraalveolar edema fluid (V ape). Total edema, i.e., total interstitial edema plus intraalveolar edema increased with alveolar edema fluid accumulation. The respective regression curves and 95% confidence intervals are shown.
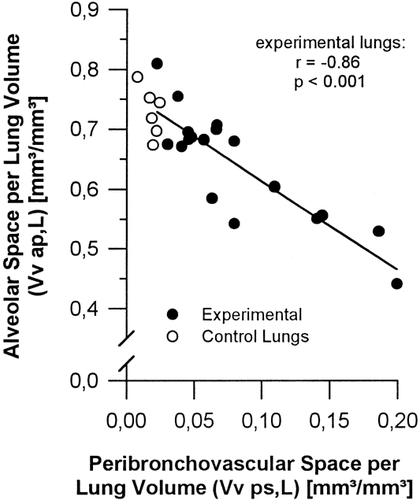
Stereologic data showing that in experimental lungs the increase in peribronchovascular space relative to total lung volume (Vv ps,L) is achieved at the expense of alveolar space (Vv ap,L).
Injury to alveolar air-blood-barrier
TEM revealed a wide variation in the structural integrity of alveolar type I pneumocytes. Normally structured and edematously swollen cells were seen in direct vicinity to areas of fragmented cells or even denuded basal lamina (Fig. 4a, c). The surface fractions of epithelial areas showing the respective category of integrity are given in Table 6. In the experimental lungs, regions with fragmented type I pneumocytes amounted from only 0.8% up to 58.0% of the total type I pneumocyte surface (mean ± SD, 23.1 ± 16.8%). In the control lungs, fragmentation was limited to 4.6 ± 4.8 % of total type I cell surface, which was significantly different from the experimental group (P<0.02). Notably, fragmentation of type I pneumocytes in experimental lungs did not necessarily coincide with the barrier being covered by edema fluid (Fig. 4c). This is supported by the stereological finding (Table 7) that in the experimental lungs equivalent degrees of cellular injury were determined for edematous and edema-free regions. Notably, the extent of epithelial fragmentation did not unambiguously predict the extent of edema coverage of the alveolar epithelium (Fig. 8). While even small areas of fragmentation could result in extensive edema-coverage, a high degree of fragmentation was always associated with wide surface areas being covered by edema fluid. This could be further elucidated by lectin-histochemistry of sections of complete left lung slices. By means of BPL, a specific marker for type I pneumocytes (Kasper et al., 1994), large gaps were seen in the alveolar epithelium separating alveoli and perivascular cuffs (Fig. 5a, d). By immunohistochemistry using an established epithelial cell-specific marker (Kasper and Singh, 1995) the disintegration of the alveolar epithelium with a loss of cytokeratin into the intraalveolar edema fluid could predominantly be seen in alveoli close to pulmonary vessels (Fig. 5b, c, e, f). Ultrastructural analysis of the liquid-gas interphase (Fig. 4b, e) indicated that the surface-active lining layer was capable of limiting the accumulation of intraalveolar edema fluid to preexisting pools. Notably, tubular myelin, the surface-active form of alveolar surfactant sub-types, frequently appeared irregular and disintegrated in experimental lungs (Fig. 4d).
| Stereological parameter | Control (n = 6) | Experimental (n = 19) | |||
|---|---|---|---|---|---|
| Mean ± SD | Median | Mean ± SD | Median | Level of significancec | |
| Ss ape, EP [%]a | 7.0 ± 5.0 | 7.2 | 52.4 ± 15.0 | 49.5 | P < 0.001 |
| Ss np1, P1 [%]b | 80.8 ± 13.5 | 56.8 ± 19.7 | P < 0.02 | ||
| Ss sp1, P1 [%]b | 14.6 ± 12.6 | 20.0 ± 10.9 | n.s. | ||
| Ss fp1, P1 [%]b | 4.6 ± 4.8 | 3.0 | 23.1 ± 16.8 | 23.5 | P < 0.02 |
- a Fraction of alveolar epithelial surface covered with edema (Ss ape, EP) was determined by LM at level 3.
- b Fractions of type I pneumocytes exhibiting normal (Ss np1, P1), swollen, (Ss sp1, P1) or fragmented (Ss fp1, P1) ultrastructure were determined by TEM at level 4.
- c Differences between groups were tested for significance (P) according to an unpaired t-test if normality and equal variance were not rejected at P < 0.05. Otherwise, the nonparametric Mann-Whitney rank sum test was performed, and the median values are given.
|
Stereological parametera |
Type I cells, free of edema Mean ± SD |
Type I cells, covered with edema Mean ± SD |
Level of significanceb |
|---|---|---|---|
| Control (n = 6) | |||
| Ss np1, P1 [%] | 84.1 ± 11.9 | 53.4 ± 34.8 | P = 0.068 |
| Ss sp1, P1 [%] | 11.5 ± 9.9 | 41.9 ± 34.5 | P = 0.065 |
| Ss fp1, P1 [%] | 4.4 ± 5.4 | 4.7 ± 6.9 | P = 0.943 |
| Experimental (n = 19) | |||
| Ss np1, P1 [%] | 62.0 ± 23.1 | 59.8 ± 23.9 | P = 0.771 |
| Ss sp1, P1 [%] | 17.6 ± 15.0 | 13.5 ± 10.5 | P = 0.331 |
| Ss fp1, P1 [%] | 20.4 ± 18.1 | 26.8 ± 19.6 | P = 0.305 |
- a Fractions of type I pneumocytes exhibiting normal (Ss np1, P1), swollen, (Ss sp1, P1) or fragmented (Ss fp1, P1) ultrastructure were determined by TEM at level 4.
- b Differences between groups were tested for significance (P) according to an unpaired t-test, because normality and equal variance were not rejected at P < 0.05.
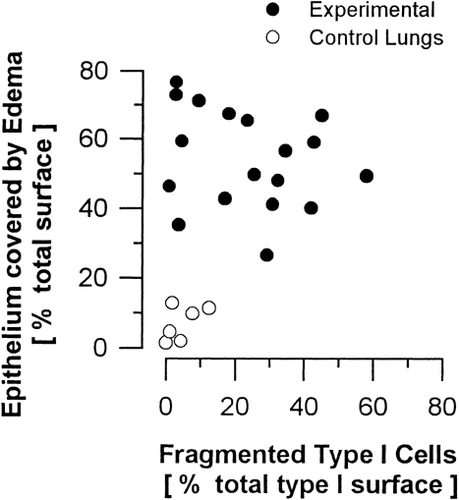
Stereologic data. Scatter plot of surface fraction of fragmented type I pneumocytes (Ss fp1,P1) versus surface fraction of alveolar and alveolar duct epithelium covered by edema fluid (Ss ape,EP). Even lungs with minor extension of type I cell fragmentation may show extended areas of epithelium covered with edema fluid. In contrast, control lungs showing a similar degree of epithelial injury did not develop equivalent intraalveolar edema. Experimental lungs showing a high degree of epithelial damage are always characterized by extended edema-coverage.
Explorative analysis of structure-function correlation
Significant relationships based on the analysis of the individual data within the ischemia/reperfusion group were seen (Table 8). Total pulmonary edema, i.e., the sum of intraalveolar (Vv ape,L), peribronchovascular (Vv psf,L), and interstitial edema of alveolar septa (Vv int/L) correlated significantly with the W/D-ratio (r=0.61, P<0.01). Only intraalveolar edema alone showed a significant correlation with the W/D-ratio. Intraalveolar edema further correlated highly significant with oxygenation, with the volume of intraalveolar edema relative to the gas exchange region (Vv ape,P) showing the strongest correlation with ΔPO2 (r=-0.82; P<0.001) (Fig. 9). Further, the more intraalveolar edema was present the higher was the PIP measured (r=0.65; P<0.005). The weak inverse relationship between PIP and peribronchovascular edema simply reflects the inverse relationship between peribronchovascular and intraalveolar edema. The same indirect relationship was seen with PVR, which showed a highly significant increase with peribronchovascular edema (r=0.64; P<0.005), but in turn showed a virtual decrease with increasing intraalveolar edema. This indirect relation was also reflected by the negative relationship of PVR with W/D-ratio (Table 2). While pulmonary gas exchange and intraalveolar edema did not correlate with epithelial injury, a negative correlation of alveolar epithelial integrity (Ss np1,P1) and PVR was noted (r=-0.58, P<0.01).
| Total pulmonary edema | Intraalveolar edema | Peribronchovascular edema | Alveolar septal edema | Cellular injury | ||||
|---|---|---|---|---|---|---|---|---|
| Vv ape, L + Vv psf, L | Vv ape, L | Ss ape, Ep | Vv psf, L | Vv int, L | τint | Ss np1, P1 | Ss fp1, P1 | |
| + Vv int, L [%] | [%] | [%] | [%] | [%] | [μm] | [%] | [%] | |
| W/D-ratio | r = 0.60 P < 0.01 | r = 0.56 P < 0.02 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| ΔPO2 [mm Hg] | r = −0.66 P = 0.002 | r = −0.72 P < 0.001 | r = −0.45 P < 0.05 | n.s. | n.s. | n.s. | n.s. | n.s. |
| ΔPCO2 [mm Hg] | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| PIP [cm H2O] | r = 0.56 P < 0.02 | r = 0.65 P < 0.005 | r = 0.54 P < 0.02 | r = −0.49 P < 0.05 | n.s. | n.s. | n.s. | n.s. |
| Ppa [mm Hg] | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| PVR [dyn × sec × cm−5] | n.s. | r = −0.55 P < 0.02 | n.s. | r = 0.64 P < 0.005 | n.s. | n.s. | r = −0.58 P < 0.01 | n.s. |
- a Correlation coefficients (r) with levels of significance (P) determined according to Spearman's rank order correlation.
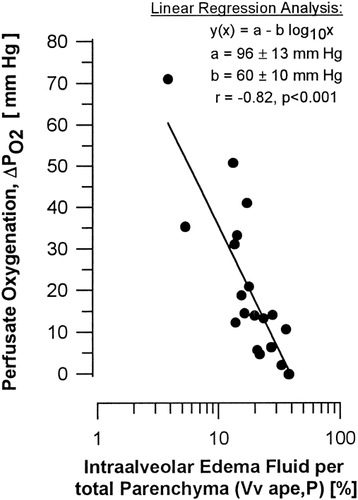
Semilogarithmic scatter plot of stereologic versus functional data illustrating the strong correlation between oxygenation and intraalveolar edema. Oxygenation was determined as ΔPO2 = PvO2 - PaO2 after 40 min of reperfusion, i.e., immediately prior to fixation for subsequent examination of histology and ultrastructure. Intraalveolar edema is given as volume density relative to total parenchymal volume (Vv ape,P).
DISCUSSION
On the basis of the physiological and quantitative stereological parameters recorded, our study of ischemia/reperfusion injury in the isolated perfused rat heart-lung model demonstrated the predominant role of intraalveolar over interstitial edema for the severity of respiratory compromise. Important new findings were: i) a logarithmic relationship could be developed to describe the relationship between the amount of intraalveolar edema per gas exchange region and the oxygenation potential of the lung (ΔPO2 = 96 - 60 × log10(Vv ape,P) [mm Hg]); ii) the extent of alveolar epithelial injury did not show any relationship with pulmonary oxygenation or intraalveolar edema in this severe situation, but increased significantly with rising PVR; iii) as indicated by lectin- and immunohistochemistry, alveolar flooding occurred through large gaps in the epithelium separating perivascular cuffs from the adjacent alveoli, irrespective of the integrity of the air-blood-barrier of the alveolar septae.
Methodological Aspects
The rat lungs examined showed a wide range of slight to severe pulmonary edema as reflected by the W/D-ratio. On the basis of semi-quantitative data, Montaner and co-workers (1986) showed that both interstitial and intraalveolar edema may result in equivalent W/D-ratios. Our stereologic approach allowed to estimate the volumes of the respective compartments to quantitate the particular contributions of interstitial and intraalveolar edema. Several studies clearly showed that, suitable fixation procedures given, LM and TEM are appropriate techniques to demonstrate and quantify edema accumulations in the peribronchovascular space (Michel et al., 1986; 1987; Zwikler et al., 1994) as well as in the alveolar space (Bachofen et al., 1993; Wu et al., 1995). There is no doubt that cryofixation is the best means to retain fluid in lung samples (Bastacky et al., 1995; Luchtel et al., 1991). However, cryofixation suitable for EM investigation is limited to the region just below the pleural surface (Bastacky et al., 1995). Stereological studies investigating organ function require that a representative collection of tissue blocks is obtained from the whole organ to guarantee an unbiased sampling protocol (Mayhew, 1991). Therefore, chemical fixation has to be performed to ensure adequate structural preservation of all parts of the lung. Adequate preservation of edema accumulations can be achieved by means of sequential vascular perfusion (Bachofen et al., 1982: method IV) or sequential processing, as in our study, using a glutaraldehyde-containing primary fixative, and osmium tetroxide and uranyl acetate as post-fixatives (Bachofen et al., 1982: method II). This approach permits studying the same lung by means of LM, SEM, and TEM with all samples being collected by unbiased systematic random sampling based on the entire organ. On this basis only, the volume of edema fluid accumulations and the extent of cell injury to the air-blood-barrier could be estimated for the same lung.
One may argue that the exclusion of leukocytes from the blood perfusate in our model might have resulted in less severe ischemia/reperfusion effects, because leukocytes may also contribute to reperfusion injury. However, the contribution of leukocytes to the development of reperfusion injury is controversially discussed (Deeb et al., 1990; Seibert et al., 1993).
Distribution of Edema and Functional Significance
The stereological estimates of the volumes of all three edema compartments, i. e. alveolar space, peribronchovascular space, and interstitium of alveolar septa, were significantly increased after preservation, ischemia and reperfusion as compared with control lungs. The mean volumetric increase over control values of the interstitial space of alveolar septa was nearly identical to the increase of the peribronchovascular space, i. e. about 72 mm3 versus 82 mm3. With a mean volume of 448 mm3, intraalveolar edema, however, clearly exceeded the rise in peribronchovascular and alveolar septal edema by about 5- to 6-fold. As a consequence, pulmonary function was governed by the amount of intraalveolar edema as indicated by its significant correlation with W/D-ratio, PIP, and ΔPO2. Our data not only support the general view of alveolar flooding being responsible for respiratory impairment (Michel et al., 1986; Montaner et al., 1986; Staub, 1983), but we could further develop a logarithmic relationship between intraalveolar edema and ΔPO2 following ΔPO2 = 96 - 60 × log10(Vv ape,P) [mm Hg] (Fig. 9). This agrees well with physiological data of 83.5 - 96 mm Hg reported for normal rats of 555–297 g body weight (Boggs, 1992). We may infer from the equation developed that if intraalveolar edema occupies about 10% of the lung's gas exchange region, pulmonary oxygenation will only reach about 40 mm Hg, while with an edema fraction of 40%, oxygenation will drop to zero.
Peribronchovascular edema showed a significant correlation with PVR only. This, however, could not be explained by compression of the nonparenchymal vasculature. The absolute volumes of the lumina of vessels and of airways of the experimental lungs did not differ significantly from control values. There was also no decrease in absolute or relative volumes of their lumina with increasing peribronchovascular space. These observations are consistent with the findings of Michel and co-workers (1987) that neither vessels nor airways are compressed by interstitial peribronchovascular edema. These authors suggested that the expanding peribronchovascular cuffs encroach on the more compliant parenchyma rather than on the lumina of vessels or airways. This is supported by our stereological finding that the increase in peribronchovascular space per lung volume was achieved at the expense of alveolar space (Fig. 7). Hence, the significant correlation between peribronchovascular edema and PVR may rely on an indirect effect of the expanding peribronchovascular space on the capillary bed. This is in line with findings obtained from isolated perfused dog lung lobes, which showed that vascular resistance raised and the number of open capillaries decreased only after interstitial edema had developed, but that the mere presence of liquid in the alveoli had no effect (Muir et al., 1975).
Cell Injury and Edema Formation
As a consequence of ischemia/reperfusion, a significant increase in alveolar epithelial cell injury was seen in the experimental group compared with the control group, while in turn the surface fraction of normal type I pneumocytes decreased. In parallel, the amount of intraalveolar edema was increased in experimental lungs. These findings are in line with other studies comparing different experimental groups (Bachofen et al., 1993; Montaner et al., 1986; Wu et al., 1995). Our analysis of the individual data, however, revealed that the extent of epithelial injury did not unambiguously determine the amount of intraalveolar edema. While highly injured experimental lungs always showed extended coverage of the epithelium with edema, some lungs with minor epithelial fragmentation also developed considerable edema (Fig. 8). This may be explained by our lectin- and immunohistochemical findings of large gaps in the alveolar epithelium lining the perivascular cuffs. These lesions likely represent the route along which excess interstitial fluid pours into and drains the encompassing alveoli, and may further proceed via pores of Kohn to adjacent alveoli, irrespective of the integrity of their air-blood-barrier. Alternatively, this unexpected phenomenon may be explained by the pulmonary surfactant being involved. The epithelial lining layer, which due to the surface tension created contributes to the pressure gradient across the air-blood-barrier (Clements, 1961), appeared to be capable of limiting the accumulation of intraalveolar edema fluid to the preexisting pools, which is in line with observations reported by Bachofen and co-workers (1993). With the surface tension being increased in fluid filled alveoles, probably in consequence of the disintegrated tubular myelin (Fig. 4d), a pressure gradient may be created across the air-blood-barrier along which edema fluid drains into a preexisting pool through small epithelial gaps, rather than flowing to the opposite side of the barrier despite the presence of a large area of fragmentation. Hence, studies of the relationship between intraalveolar edema accumulation and pulmonary surfactant alterations are needed to further enlighten this problem.
Acknowledgements
We gratefully acknowledge the expert technical assistance of B. Giere and M. Fathollahy (Hannover), Ch. Rühling, H. Hühn and A. Gerken (Göttingen), and I. Peterson (Dresden). We thank M. Ochs and A. Schmiedl (Göttingen) for valuable discussions, and M. Kasper (Dresden) for his advice in lectin- and immunohistochemistry. Cyrilla Maelicke, B.Sc. (Göttingen), kindly edited our “broken” English. Parts of this work were done in the course of the MD thesis of D. Schepelmann.




