Anaerobic and aerobic batch cultivations of Saccharomyces cerevisiae mutants impaired in glycerol synthesis
Abstract
Glycerol is formed as a by-product in production of ethanol and baker's yeast during fermentation of Saccharomyces cerevisiae under anaerobic and aerobic growth conditions, respectively. One physiological role of glycerol formation by yeast is to reoxidize NADH, formed in synthesis of biomass and secondary fermentation products, to NAD+. The objective of this study was to evaluate whether introduction of a new pathway for reoxidation of NADH, in a yeast strain where glycerol synthesis had been impaired, would result in elimination of glycerol production and lead to increased yields of ethanol and biomass under anaerobic and aerobic growth conditions, respectively. This was done by deletion of GPD1 and GPD2, encoding two isoenzymes of glycerol 3-phosphate dehydrogenase, and expression of a cytoplasmic transhydrogenase from Azotobacter vinelandii, encoded by cth. In anaerobic batch fermentations of strain TN5 (gpd2-Δ1), formation of glycerol was significantly impaired, which resulted in reduction of the maximum specific growth rate from 0.41/h in the wild-type to 0.08/h. Deletion of GPD2 also resulted in a reduced biomass yield, but did not affect formation of the remaining products. The modest effect of the GPD1 deletion under anaerobic conditions on the maximum specific growth rate and product yields clearly showed that Gdh2p is the important factor in glycerol formation during anaerobic growth. Strain TN6 (gpd1-Δ1 gpd2-Δ1) was unable to grow under anaerobic conditions due to the inability of the strain to reoxidize NADH to NAD+ by synthesis of glycerol. Also, strain TN23 (gpd1-Δ1 gpd2-Δ1 YEp24-PGKp-cth-PGKt) was unable to grow anaerobically, leading to the conclusion that the NAD+ pool became limiting in biomass synthesis before the nucleotide levels favoured a transhydrogenase reaction that could convert NADH and NADP+ to NADPH and NAD+. Deletion of either GPD1 or GPD2 in the wild-type resulted in a dramatic reduction of the glycerol yields in the aerobic batch cultivations of strains TN4 (gpd1-Δ1) and TN5 (gpd2-Δ1) without serious effects on the maximum specific growth rates or the biomass yields. Deletion of both GPD1 and GPD2 in strain TN6 (gpd1-Δ1 gpd2-Δ1) resulted in a dramatic reduction in the maximum specific growth rate and in biomass formation. Expression of the cytoplasmic transhydrogenase in the double mutant, resulting in TN23, gave a further decrease in μmax from 0.17/h in strain TN6 to 0.09/h in strain TN23, since the transhydrogenase reaction was in the direction from NADPH and NADP+ to NADH and NADP+. Thus, it was not possible to introduce an alternative pathway for reoxidation of NADH in the cytoplasm by expression of the transhydrogenase from A. vinelandii in a S. cerevisiae strain with a double deletion in GPD1 and GPD2. Copyright © 2000 John Wiley & Sons, Ltd.
INTRODUCTION
Glycerol is formed as a by-product in production of ethanol and baker's yeast during fermentation of Saccharomyces cerevisiae under anaerobic and aerobic growth conditions, respectively. In anaerobic batch cultivations approximately 5% of the carbon source is converted into glycerol (Oura, 1977), while less is formed in the respiratory–fermentative growth phase in aerobic batch cultivations. During anaerobic growth, glycerol formation counteracts depletion of the intracellular pool of NAD+, since synthesis of biomass and organic acids, e.g. succinic acid, acetic acid and pyruvic acid, results in a net reduction of NAD+ to NADH (Oura, 1977; van Dijken and Scheffers, 1986; Nissen et al., 1997). The respiratory chain does not function under anaerobic conditions. Instead, NADH is reoxidized to NAD+ by formation of glycerol, since synthesis of one mole of glycerol from glucose leads to reoxidation of 1 mole of NADH. During aerobic growth, glycerol is formed at high glucose concentrations, where respiration is repressed, reducing the capability of the organism to reoxidize surplus amounts of NADH formed in biosynthetic reactions. Furthermore, glycerol is formed and accumulated inside the cell during growth under osmotic stress conditions, where the compound functions as an efficient osmolyte protecting the cell against lysis (André et al., 1991; Larsson et al., 1993; Ansell et al., 1997).
Formation of glycerol occurs in two steps from dihydroxyacetone phosphate (DHAP), catalysed by glycerol 3-phosphate dehydrogenase and glycerol 3-phosphate phosphatase. Two genes, GPD1 and GPD2, encoding isoenzymes of glycerol 3-phosphate dehydrogenase, have been found in S. cerevisiae (Larsson et al., 1993; Eriksson et al., 1995). Attempts have been made to increase ethanol formation during anaerobic growth by eliminating glycerol synthesis through deletions of GPD1 and GPD2 (Björkqvist et al., 1997). No reduction in the glycerol yield was observed when GPD1 was deleted and the small reduction in the glycerol yield of the Δgpd2 deletion mutant was accompanied by a significant reduction in the maximum specific growth rate, resulting in a strain with a lower ethanol productivity than the wild-type. The Δgpd1Δgpd2 double deletion mutant did not grow under anaerobic conditions, due to accumulation of intracellular NADH. Furthermore, auxotrophic strains were used by Björkqvist et al. (1997). The exponential growth rates of these strains were reported to decrease before complete exhaustion of the carbon source, due to exhaustion of one of the medium compounds which might affect the product yields.
NADH+NADP+→NAD++NADPH
((Reaction 1))Earlier, we isolated a gene, encoding the cytoplasmic transhydrogenase from Azotobacter vinelandii (Nissen et al., submitted paper). Expression of the gene in a Δgpd1Δgpd2 double deletion mutant could potentially represent an alternative route for reoxidation of NADH to NAD+, enabling the strain to grow under anaerobic growth conditions. In this paper we present physiological studies of prototrophic strains impaired in the glycerol synthesis pathway and expressing the cytoplasmic transhydrogenase from A. vinelandii. The effects of the genetic changes were studied under both anaerobic and aerobic growth conditions.
MATERIALS AND METHODS
Micro-organisms and their maintenance
Escherichia coli DH5α (Gibco BRL, Gaithersburg, MD) was used for subcloning. All yeast strains were maintained at 4°C on selective plates, monthly prepared from a glycerol stock kept at −80°C.
Preparation of DNA
Plasmid DNA from E. coli and chromosomal DNA from S. cerevisiae were prepared as described earlier (Nissen et al., submitted paper). DNA primers were purchased from DNA Technology (Aarhus, Denmark).
Deletion of GPD1 and GPD2
Primers GPD1BamHI (5′-CGG GAT CCC GTT CAC ATA TCG TCT TTG GCC TCT CTT G-3′), containing a synthetic site for the restriction enzyme BamHI and nucleotides 574–548 upstream of GPD1, and GPD1XbaI (5′-GCT CTA GAG CAA GTA GTT ATG AGA AAT GAC ATA ATG C-3′), containing a synthetic site for the restriction enzyme XbaI and the complementary strand of nucleotides 76–102 downstream of the GPD1 stop codon, were used to isolate a 1851 bp PCR fragment, using the Pfu DNA polymerase (Strategene) with proofreading. The fragment was digested with SspI and XbaI, and two fragments, corresponding to nucleotides 366 to 15 upstream of GPD1 and nucleotides 317 upstream of the GPD1 stop codon to 100 bp downstream of GPD1, respectively, were isolated. The fragments were inserted in two steps into the SmaI and SpeI digestion sites of plasmid pFA6A–kanMX3 (Wach et al., 1994), respectively. The desired orientation of each insert was verified by PCR. The resulting plasmid, pGPD1del, was digested with BamHI and SacII prior to transformation and a 3300 bp fragment, containing G418r, encoding resistance towards geneticin, flanked by two short direct repeats and the two inserts, was isolated.
Primers GPD2BamHI (5′-CGG GAT CCC GTC AGT CAT CAT CAT TAC CGA GTT TGT T-3′), containing a synthetic site for the restriction enzyme BamHI and nucleotides 598 to 572 upstream of GPD2, and GPD2EcoRI (5′-CGG AAT TCC GTG AAA AGC TCG AAG AAA CAG CTT TAA G-3′), containing a synthetic site for the restriction enzyme EcoRI and the complementary strand of nucleotides 321 to 347 downstream of the GPD2 stop codon, were used to isolate a 2100 bp PCR fragment, using the Pfu DNA polymerase. The fragment was digested by BamHI and EcoRI, and two fragments, encoding nucleotides 596 to 51 upstream of GPD2 and nucleotides 56 upstream of the GPD2 stop codon to 345 downstream of GPD2, respectively, were isolated. The fragments were ligated into plasmid pFA6A–kanMX3, digested with BamHI/BglII and EcoRI, respectively, in two steps. The desired orientation of each insert was verified by PCR. The resulting plasmid, pGPD2del, was digested with BanI and SpeI prior to transformation and a 3477 bp fragment, containing G418r, encoding resistance towards geneticin, flanked by two short direct repeats and the two inserts, was isolated.
Transformation of E. coli and S. cerevisiae
E. coli DH5α and S. cerevisiae cells were transformed as described earlier (Nissen et al., submitted paper). S. cerevisiae transformants were suspended in YPD at 109 per ml at 30°C for 24 h prior to plating on YPD containing 150 mg/l geneticin in order to allow expression of the G418 resistance gene. Loop-out of G418r by homologous recombination of the two direct repeats in the insert (Wach et al., 1994) was obtained (with a frequency of 10−4 colonies) after cultivation of a transformant in non-selective YPD medium for 30 generations.
Strain construction
The haploid wild-type strain TN1 (Nissen et al., submitted paper) was transformed with the 3300 bp BamHI/SacII fragment, and correct insertion of the fragment into the GPD1 locus was verified by PCR. Loop-out of G418r was obtained as described above, resulting in strain TN4, containing a deletion in GPD1. GPD2 was deleted in strain TN4 by transformation with the 3500 BanI/SpeI fragment, resulting in strain TN6. Correct insertion of the fragment into the GPD2 locus was verified by PCR. Deletion of URA3 in strain TN6 was obtained as described earlier (Nissen et al., submitted paper), resulting in strain TN7, and expression of cth, encoding the cytoplasmic transhydrogenase from A. vinelandii, was obtained as described earlier (Nissen et al., submitted paper) by introducing plasmid YEp24–PGK–CTH into strain TN7, resulting in strain TN23. Furthermore, GPD2 was deleted in strain TN1 by transformation with the 3500 BanI/SpeI fragment, resulting in strain TN5. Correct insertion of the fragment into the GPD2 locus was verified by PCR.
Medium for the controlled batch cultivations
The strains of S. cerevisiae were cultivated in a mineral medium prepared according to Verduyn et al. (1990). Vitamins were added by sterile filtration following heat sterilization of the medium. Initial concentrations of glucose and (NH4)2SO4 were 25 g/l and 3.75 g/l, respectively, in the anaerobic batch cultivations of strains TN1, TN4, TN5, TN6 and TN23 and in the aerobic batch cultivations of strains TN6 and TN23. In the aerobic batch cultivations of strains TN1, TN4 and TN5 the glucose concentration was 20 g/l, while the concentrations of the remaining medium components were as described above. Growth of S. cerevisiae under anaerobic conditions requires supplementation of the medium with ergosterol and unsaturated fatty acids, typically in the form of Tween 80 (Andreasen and Stier, 1953). Ergosterol and Tween 80 were dissolved in hot 96% (v/v) ethanol. The final concentrations of ergosterol and Tween 80 were 4.2 mg/g dry weight and 175 mg/g dry weight, respectively. Antifoam (Sigma A-5551) was added at 75 µl/l. Ergosterol and Tween 80 were not added to the medium in the aerobic batch cultivations.
Experimental set-up for the batch cultivations
Anaerobic batch cultivations were performed at 30°C and at a stirring speed of 600 rpm in in-house manufactured bioreactors (Schulze, 1995). The working volume of the batch reactors was 4.5 l. pH was kept constant at 5.00 by addition of 2 M KOH. The bioreactors were continuously sparged (aerated with nitrogen gas) containing less than 5 ppm O2, obtained by passing N2 of a technical quality (AGA 3.8), containing less than 100 ppm O2, through a column (250×30 mm) filled with copper flakes and heated to 400°C. The column was regenerated daily by sparging with H2 (AGA 3.6). A mass flow controller (Bronkhorst HiTec F201C) was used to keep the gas flow into the bioreactors constant at 0.40 l/min nitrogen. Norprene tubing (Cole-Parmer Instruments) was used throughout in order to minimize diffusion of oxygen into the bioreactors. The bioreactors were inoculated to an initial biomass concentration of 1 mg/l with precultures grown in unbaffled shake flasks at 30°C and 100 rpm for 24 h. The aerobic batch cultivations were carried out as described for the anaerobic cultivations with the exception that the bioreactors were sparged with 1.0 l/min air. Ethanol evaporation from the bioreactors was minimized by exhaust gas condensers cooled to 2°C.
Analysis of product formation and determination of dry weight
The content of glucose, ethanol, glycerol, acetic acid, pyruvic acid, succinic acid and 2-oxoglutarate in filtered samples withdrawn from the batch cultivations was determined by HPLC, as described earlier (Nissen et al., submitted paper). The CO2 concentration in the exhaust gas condenser was determined using a Brüel and Kjær 1308 acoustic gas analyser (RSD=0.02%) (Christensen et al., 1995). The biomass concentration in the medium was measured gravimetrically as described earlier (Nissen et al., 1997).
Measurement of enzyme activities
The total protein pool was extracted from cell samples withdrawn from the batch cultivations, as described earlier (Nissen et al., submitted paper). Glycerol 3-phosphate dehydrogenase activity in the extracts was determined according to André et al. (1991). Transhydrogenase activity was assayed as described by Voordouw et al. (1979). Reaction rates, corrected for endogenous rates, were proportional to the amount of extract added. Enzyme activities are expressed as M/min substrate converted per mg total cellular protein, as determined by the Lowry method.
RESULTS
Anaerobic batch cultivations
The effects on the cellular physiology of the introduced genetic changes in strain TN1 (wild-type), resulting in strains TN4 (Δgpd1), TN5 (Δgpd2), TN6 (Δgpd1 Δgpd2) and TN23 (Δgpd1 Δgpd2 [YEp24-PGK-CTH]) were first studied under anaerobic growth conditions.
The specific activity of glycerol 3-phosphate dehydrogenase in cell extracts of the wild-type, the Δgpd1 strain and the Δgpd2 strain withdrawn from the bioreactors during exponential growth was 13 mU/mg total cellular protein, 2 mU/mg total cellular protein and 16 mU/mg total cellular protein, respectively (Table 1). Thus, deletion of GPD1 had a significant effect on the measurable amount of the enzyme in the cell, while deletion of GPD2 actually resulted in a small increase in the expression level of glycerol 3-phosphate dehydrogenase. This observation was in contrast to the effect of the genetic changes on the maximum specific growth rates, μmax, of the three strains (Table 1), where only a small decrease was measured when GPD1 was deleted, while deletion of GPD2 resulted in a five-fold reduction in μmax. Accordingly, fermentation of 25 g/l glucose lasted approximately 13–14 h in the anaerobic cultivations of strains TN1 and TN4, while the total fermentation time was 24–25 h when strain TN5 was cultivated anaerobically (Figures 1 and 2).
| TN1 | TN4 | TN5 | |
|---|---|---|---|
| (wild-type) | (Δgpd1) | (Δgpd2) | |
| Glycerol 3-phosphate DH activity | 0.013 | 0.002 | 0.016 |
| Maximum specific growth rate | 0.41 | 0.38 | 0.08 |

Consumption of glucose (◊, ⧫) and production of ethanol (□, ▪) and glycerol (○, •) in anaerobic batch cultivations of strains TN4 (Δgpd1, open symbols) and TN5 (Δgpd2, filled symbols).
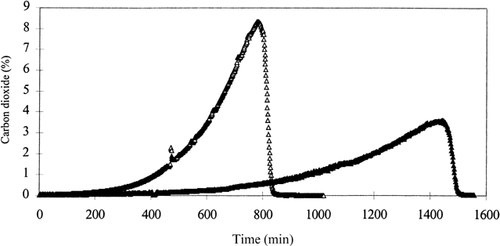
Production of carbon dioxide (▵, ▴) in anaerobic batch cultivations of strains TN4 (Δgpd1, open symbols) and TN5 (Δgpd2, filled symbols).
The product yields in the anaerobic batch cultivations of the same strains are listed in Table 2. Again, deletion of GPD1 only resulted in minor changes in the yields of ethanol and glycerol, while formation of biomass and the three organic acids were unaffected by the deletion. Deletion of GPD2 resulted in a marked reduction of approximately 4%, 31% and 13% in the yields of ethanol, glycerol and biomass, respectively, while formation of carbon dioxide was significantly increased, by 20%. Also formation of pyruvate and acetate were slightly increased by deletion of GPD2. Strain TN6, containing a double deletion of GPD1 and GPD2, and strain TN23, also containing the double deletion and expressing the cytoplasmic transhydrogenase from Azotobacter vinelandii, did not grow under anaerobic growth conditions.
| Metabolite | TN1 | TN4 | TN5 |
|---|---|---|---|
| (wild-type) | (Δgpd1) | (Δgpd2) | |
| Ethanol | 0.491 | 0.505 | 0.470 |
| Glycerol | 0.105 | 0.098 | 0.073 |
| Pyruvate | 0.004 | 0.004 | 0.004 |
| Acetate | 0.004 | 0.004 | 0.005 |
| Succinate | 0.003 | 0.003 | 0.007 |
| Carbon dioxide | 0.271 | 0.271 | 0.329 |
| Biomass | 0.112 | 0.113 | 0.098 |
| Total | 0.990 | 0.998 | 0.986 |
Aerobic batch cultivations
Aerobic batch cultivations of strains TN1 (wild-type), TN4 (Δgpd1), TN5 (Δgpd2), TN6 (Δgpd1 Δgpd2) and TN23 (Δgpd1 Δgpd2 [YEp24-PGK-CTH]) were carried out to analyse the role of glycerol formation under these conditions and to evaluate the effect on the aerobic physiology of the transhydrogenase expression in the Δgpd1Δgpd2 double mutant. The aerobic batch cultivations of strains TN1 (Figure 3), TN4 and TN5 could be divided into five growth phases. Phase 1 consisted of the exponential growth phase, where the three strains grew at their maximum specific growth rate. Due to the Crabtree effect, ethanol, acetate, pyruvate and succinate were formed as metabolic products and the remainder of the carbon source was converted into biomass and glycerol. In phase 2, glucose was depleted from the medium and pyruvate, secreted in phase 1, was consumed by respiration. Also, consumption of glycerol, formed during phase 1, was initiated. The growth phase was characterized by a steep decrease in the carbon dioxide content in the exhaust gas, caused by the depletion of glucose, followed by a small increase when pyruvate was metabolized. Phase 3 started after depletion of pyruvate from the medium, and consisted of respiration of the acetate that was formed in phase 1. The depletion of pyruvate resulted in a small drop in the carbon dioxide content in the exhaust gas, while consumption of acetate led to another increase. Phases 2 and 3 are commonly considered as a lag phase during which the cell adapts itself to the new environmental conditions. This is somewhat misleading, since dynamic changes, both intracellularly and extracellularly, take place during this phase in aerobic batch cultivations. Phase 4 was characterized by the simultaneous consumption of ethanol and glycerol, formed in phase 1. In all cultivations of strains TN1, TN4 and TN5, acetate was formed immediately after depletion of glycerol from the medium during this phase. Phase 5 started after depletion of ethanol from the medium and consisted of consumption of the acetate, formed in phase 4. This gave rise to a characteristic peak in the carbon dioxide content in the exhaust gas. The small amounts of succinate that were formed throughout the cultivations (data not shown), were not consumed.
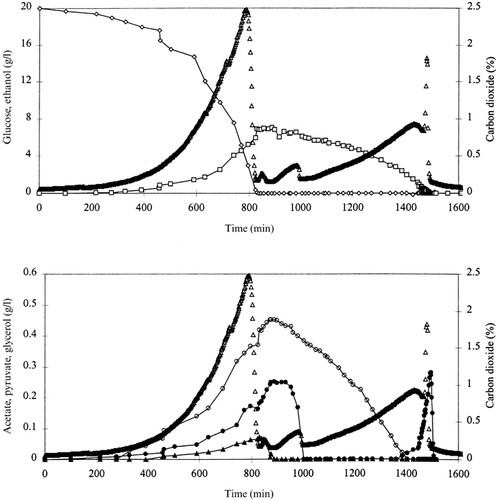
The concentrations of glucose (◊), ethanol (□), glycerol (○), acetate (•) and pyruvate (▴) (g/l), and of carbon dioxide (%) (▵) (% of exhaust gas) during an aerobic batch cultivation of strain TN1 (wild-type).
The aerobic batch cultivations of strains TN6 (Δgpd1 Δgpd2) and TN23 (Δgpd1 Δgpd2 [YEp24-PGK-CTH]) differed from the cultivations of the three other strains (Figures 4 and 5). The duration of growth phase 1 was significantly longer, due to a dramatic reduction in the maximum specific growth rates of the two strains (Table 3). No glycerol formation could be detected from the two strains, confirming that deletion of both GPD1 and GPD2 results in a total shut-down of the glycerol synthesis pathway. The absence of glycerol synthesis had a significant influence on the course of growth phases 2–5 in the two strains. In strain TN6 the formation of pyruvate in phase 1 was reduced to less than half of what was measured in the aerobic cultivations of strains TN1, TN4 and TN5, and thus growth phase 2 lasted less than 50 min. Instead, growth phase 3 was significantly elongated. Thus, consumption of the acetate that was formed in growth phase 1 lasted more than 400 min, to be compared with 100, 140 and 120 min in strains TN1, TN4 and TN5, respectively. Furthermore, the consumption of acetate occurred under simultaneous consumption of the ethanol secreted in growth phase 1. Thus, growth phases 3 and 4 in strains TN1, TN4 and TN5 were converted into a single growth phase in strain TN6. After depletion of acetate from the medium, the formation rate of carbon dioxide and the consumption rate of ethanol were reduced. Finally, growth phase 5 was absent in the aerobic cultivation of strain TN6. In general, strain TN23, expressing the cytoplasmic transhydrogenase at a level of 4.2 U/mg total cellular protein in a Δgpd1Δgpd2 genetic background, grew significantly slower than the four other strains, resulting in elongation of all growth phases. Formation of 2-oxoglutarate was observed in growth phases 1–3. This was also the case in an earlier study of the anaerobic physiology of a wild-type strain expressing the transhydrogenase (Nissen et al., submitted paper). After depletion of pyruvate in growth phase 2, acetate and ethanol were consumed simultaneously, as observed in the aerobic cultivation of strain TN6. Following depletion of acetate, a lag phase was observed during which the consumption rate of ethanol and the formation rate of carbon dioxide were significantly reduced. In this phase the strain probably readjusted its metabolism towards simultaneous consumption of 2-oxoglutarate and ethanol, which lasted until depletion of the two compounds from the medium. Again, growth phase 5 in the cultivations of TN1, TN4 and TN5 was not observed in the aerobic cultivation of TN23.
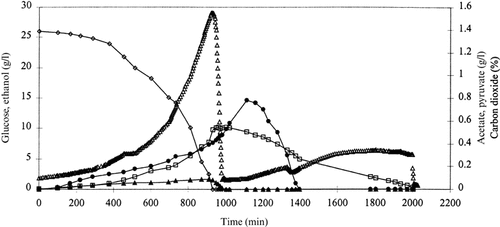
The concentrations of glucose (◊), ethanol (□), acetate (•) and pyruvate (▴) (G/l) and of carbon dioxide (▵) (% of exhaust gas) during an aerobic batch cultivation of strain TN6 (Δgpd1Δgpd2).

The concentrations of glucose (◊), ethanol (□), 2-oxoglutarate (▪), acetate (•) and pyruvate (▴) (g/l) and of carbon dioxide (▵) (% of exhaust gas) during an aerobic batch cultivation of strain TN23 (Δgpd1Δgpd2 [YEp24–PGK–CTH]).
| Metabolite | TN1 | TN4 | TN5 | TN6 | TN23 |
|---|---|---|---|---|---|
| Ethanol | 0.450 | 0.460 | 0.465 | 0.507 | 0.364 |
| Glycerol | 0.024 | 0.008 | 0.008 | n.d. | n.d. |
| Pyruvate | 0.004 | 0.007 | 0.008 | 0.003 | 0.003 |
| Acetate | 0.009 | 0.023 | 0.017 | 0.016 | 0.020 |
| Succinate | 0.002 | 0.002 | 0.002 | 0.003 | 0.007 |
| 2-Oxoglutarate | n.d. | n.d. | n.d. | n.d. | 0.053 |
| Carbon dioxide | 0.330 | 0.345 | 0.332 | 0.340 | 0.413 |
| Biomass | 0.170 | 0.149 | 0.160 | 0.121 | 0.110 |
| Total | 0.989 | 0.994 | 0.992 | 0.990 | 0.970 |
| μmax | 0.41 | 0.38 | 0.41 | 0.17 | 0.09 |
- n.d.=not detectable.
The maximum specific growth rates, μmax, and the product yields after growth phase 1 (the respiratory–fermentative growth phase) in the aerobic batch cultivations of strains TN1 (wild-type), TN4 (Δgpd1), TN5 (Δgpd2), TN6 (Δgpd1 Δgpd2) and TN23 (Δgpd1 Δgpd2 [YEp24-PGK-CTH]) are listed in Table 3. The wild-type strain had the same maximum specific growth rate under anaerobic and aerobic growth conditions, and thus absence of oxygen does not necessarily limit growth of S. cerevisiae. Also TN4, containing a deletion of GPD1, grew with the same specific rate in the presence and absence of oxygen, while TN5, containing a deletion of GPD2, grew five times faster under aerobic conditions than observed under anaerobic conditions. Deletion of both genes, encoding the isoenzymes of glycerol 3-phosphate dehydrogenase, resulted in a dramatic drop in μmax from approximately 0.4/h in the wild-type and the single deletion strains to 0.17/h strain TN6. Finally, expression of the cytoplasmic transhydrogenase in the double deletion strain impaired growth even more. Here, the maximum specific growth rate was measured to be 0.09/h, resulting in a total cultivation time of more than 86 h.
Deletion of either GPD1 or GPD2 resulted in large changes in formation of glycerol, pyruvate and acetate. In TN4 and TN5, formation of glycerol was reduced to one-third as compared with the wild-type, while formation of pyruvate and acetate were increased by 75% and 150%, respectively, in TN4, and by 100% and 90%, respectively, in TN5. Also, minor changes in formation of ethanol and biomass were observed. Thus, a reduction in the biomass yield by 12% was measured in the cultivations of strain TN4 (Δgpd1). As described above, formation of glycerol was absent in strain TN6, containing a deletion in both GPD1 and GPD2. Furthermore, the double deletion resulted in a significant increase of 12% and 77% in the ethanol yield and the acetate yield, respectively, and a decrease in the biomass yield by almost 30%, compared with the wild-type. In the cultivations of strain TN23, containing the cytoplasmic transhydrogenase and the double deletion of the GPD genes, more than 5% of the consumed glucose was converted to 2-oxoglutarate in the respiratory-fermentative growth phase. Furthermore, expression of the transhydrogenase resulted in significant changes in the yields of all other products. Thus, compared with strain TN6 the yields of carbon dioxide, acetate and succinate in TN23 were increased by 21%, 25% and 130%, respectively, while the yields of ethanol and biomass were decreased by 28% and 9%, respectively.
DISCUSSION
Anaerobic batch cultivations
The measured values for the maximum specific growth rates and product yields of strains TN4 and TN5 are the results of the first quantitative studies of the cellular physiology of S. cerevisiae strains with deletions in either GPD1 and GPD2 under fully anaerobic growth conditions. Earlier studies of such strains under anaerobiosis have concentrated on the effect of the deletions on glycerol formation and, furthermore, these earlier studies relied on the use of auxotrophic markers and corresponding supplementation to the medium, which probably have affected the reported yields (Björkqvist et al., 1997; Ansell et al., 1997). Supplementation of the medium with leucine, histidine, tryptophan, uracil and adenine will reduce the need for de novo biomass precursor synthesis, resulting in a smaller production of NADH in the biosynthetic reactions, and hence in a lower glycerol yield. Thus, the glycerol yields measured in the anaerobic cultivations in this study were 8–10% higher than those reported earlier (Björkqvist et al., 1997). Furthermore, insufficient addition of these compounds to the growth medium results in growth limitations of the auxotrophic strains during the exponential growth phase, while high concentrations of the compounds can lead to product inhibition of enzymes in the pathways where these compounds are synthesized. Both effects lead to difficulties in interpretation of the measured values for the maximum specific growth rate.
The modest effect of the GPD1 deletion on the maximum specific growth rate and product yields and, in contrast, the significant changes in the anaerobic physiology, imposed on the wild-type by deletion of GPD2, clearly supports earlier observations suggesting that the isoenzyme of glycerol 3-phosphate dehydrogenase, encoded by GPD2, is the important factor in glycerol formation during anaerobic conditions (Eriksson et al., 1995). Deletion of GPD1 resulted in a large decrease in the specific activity of glycerol 3-phosphate dehydrogenase in protein extracts from TN4. It has been demonstrated earlier that almost only the activity of Gpd1p is measured by the applied enzyme assay, while measuring the Gpd2p activity is extremely difficult, probably due to instability of the enzyme in the protein extracts (Björkqvist et al., 1997). Thus, the measured drop in the glycerol 3-phosphate dehydrogenase activity in TN4 as compared with the wild-type confirms that GPD1 has been deleted in the mutant, while no information about the expression levels of Gpd2p in the two strains can be extracted from the data.
In strain TN5, containing a deletion in GPD2, formation of glycerol was significantly impaired, which resulted in reduction of the maximum specific growth rate from 0.41/h in the wild-type to 0.08/h. The specific activity of glycerol 3-phosphate dehydrogenase increased to 16 mU/mg total cellular protein, indicating that the expression of Gpd1p was slightly induced. Thus, Gpd1p can substitute the physiological role of Gpd2p during anaerobic growth. The slow growth of the Δgpd2 mutant indicated, however, that this substitution is very inefficient, which might be due to a to low expression level of Gpd1p, resulting in a reduced glycerol formation rate. Thus, the low μmax of strain TN5 is probably caused by a limitation in the rate of reoxidation of NADH to NAD+ through formation of glycerol. This limitation affected the efficiency of biomass synthesis, whereby the total biomass yield was reduced by 12%, compared with the wild-type. The carbon and redox balances did not balance as well in the cultivations of TN5, as observed in the cultivations of TN1 and TN4. Whereas the balances close within 1–2% when TN1 and TN4 were cultivated, approximately 4% and 8%, respectively, of the added carbon and redox equivalents could not be found in the measured products in the cultivations of TN5. The degree of reduction of the missing carbon was 7.6 which indicated that not all ethanol was captured. Due to the low specific growth rate of TN5, the cultivations of this strain took much more time than TN1 and TN4 and this could lead to an increased loss of ethanol. If it is assumed that the missing 4.4% carbon in the cultivations of TN5 was evaporated ethanol, the redox balance would close within 2%, as observed in the other experiments. This would mean that the correct ethanol yield of TN5 would be 0.514 C-moles per C-mole glucose, an increase of 4.7% compared with the wild-type. The observed increase in the carbon dioxide yield in the cultivations of TN5 might be another sign of an unobserved) increase in ethanol formation.
As observed earlier, strain TN6, containing a double deletion in GPD1 and GPD2, was unable to grow under anaerobic conditions (Björkqvist et al., 1997). The strain is unable to reoxidize NADH to NAD+ by synthesis of glycerol and thus, the surplus formation of the reduced co-factor in biomass synthesis and in formation of organic acids probably results in depletion of the NAD+ pool in the cell under anaerobic growth conditions whereby growth is completely impaired. The direction of reaction 1, catalysed by the cytoplasmic transhydrogenase from A. vinelandii, is determined by the cytoplasmic concentrations of the four nucleotides, since ΔG0 of the reaction is close to zero. It would be possible for this enzyme to reoxidize NADH to NAD+ with simultaneous formation of NADPH from NADP+, if the ratio ([NADH][NADP+])/([NAD+][NADPH]) were to increase beyond a value of 1. It is conceivable that this could occur in anaerobic cultivations of Δgpd1Δgpd2 strains prior to cell death caused by a limited availability of NAD+ for the biosynthetic reactions. Thus, we considered the possibility that expression of the cytoplasmic transhydrogenase in the Δgpd1Δgpd2 strain could constitute a system that would enable a S. cerevisiae strain to grow under anaerobic conditions without formation of glycerol. Unfortunately, strain TN23 was unable to grow anaerobically, leading to the conclusion that the NAD+ pool became limiting in biomass synthesis before the nucleotide levels favoured a transhydrogenase reaction that could convert NADH and NADP+ to NADPH and NAD+.
Aerobic batch cultivations
Deletion of either GPD1 or GPD2 in the wild-type resulted in a dramatic reduction of the glycerol yields in the aerobic cultivations of strains TN4 and TN5 without serious effects on the maximum specific growth rates or the biomass yields. Thus, it can be concluded that the physiological role of a part of the glycerol, that was secreted by the wild-type, could be substituted by other reactions in the metabolism without impairment of growth.
Formation of glycerol and formation of the organic acids acetate and pyruvate seem to be linked during aerobic growth. First, a significant increase in formation of the two latter compounds were observed during the respiratory–fermentative growth phase of strains TN4 and TN5. Formation of both pyruvate and acetate from glucose results in a net reduction of NAD+ to NADH in the cell, and thus the link between the lower glycerol yields in the respiratory-fermentative growth phase of strains TN4, TN5, TN6 and TN23 and the increase in formation of pyruvate and acetate is probably independent of changes in the intracellular levels of NAD+ and NADH, that might occur due to the introduced genetic changes in these strains. Instead, the increase in secretion of pyruvate and acetate could be caused by higher leakage from the cells, since a reduction in the synthesis rate of glycerol 3-phosphate, a precursor in lipid synthesis, might affect the composition of the plasma membrane of the cells.
Second, depletion of glycerol in growth phase 4, where a simultaneous consumption of glycerol and ethanol occurred, resulted in immediate secretion of acetate in the aerobic batch cultivations of strains TN1, TN4 and TN5. This phenomenon was not observed in strains TN6 and TN23, where glycerol formation was absent. It indicated that consumption of ethanol either requires the simultaneous consumption of glycerol or production of acetate in order to be efficient. Formation of acetate from ethanol probably occurs through conversion of ethanol to acetaldehyde, catalysed by alcohol dehydrogenase II (Ciriacy, 1979), and further conversion of acetaldehyde to acetate, catalysed by the cytoplasmic acetaldehyde dehydrogenase I or VI (Wang et al., 1998; Meaden et al., 1997). This results in net formation of one NADH and one NADPH in the cytoplasm each time one molecule of ethanol is converted to acetate. Conversion of glycerol to dihydroxyacetone phosphate by Gut1p (Pavlik et al., 1993) and Gut2p (Rønnow and Kielland-Brandt, 1993), and further into pyruvate through the EMP pathway followed by degradation to carbon dioxide in the TCA cycle, results in net formation of FADH2 in the mitochondria and of NADH in the mitochondria and the cytoplasm. Thus, the common physiological effect of acetate formation and glycerol consumption during respiration of ethanol is reduction of NAD+ to NADH in the cytoplasm. We find it likely that this function is important for an efficient conversion of ethanol into biomass under aerobic conditions.
Deletion of both GPD1 and GPD2 in strain TN6 resulted in a dramatic reduction in the maximum specific growth rate, compared with strains TN1, TN4 and TN5, and also a significant decrease in biomass formation was observed. Thus, formation of glycerol or glycerol 3-phosphate under aerobic conditions is important for optimal growth of S. cerevisiae. The cells were not subjected to osmotic stress conditions in this study, and hence it is obvious to consider the aerobic formation of glycerol in parallel with its role during anaerobic growth. In the exponential growth phase, glucose is consumed through a large glycolytic flux towards pyruvate. The Crabtree effect describes the limited ability of the cell to direct this flux through the TCA cycle and further into full respiration of the carbon source. Thus, the capacity of the respiratory pathway to reoxidize surplus NADH, formed in the cytoplasm in biosynthetic reactions, probably is limited. Instead, NADH is converted to NAD+ in this compartment by secretion of glycerol.
As discussed above, the limited ability of the double mutant to reoxidize NADH to NAD+ in this compartment probably resulted in the low μmax of strain TN6. Expression of the cytoplasmic transhydrogenase in the Δgpd1Δgpd2 double mutant did not result in an increase in the maximum specific growth rate, which could be expected, if the enzyme were to convert NADH and NADP+ to NAD+ and NADPH in the cytoplasm. Instead, the transhydrogenase catalysed the opposite reaction, whereby NADPH was consumed and even larger amounts of surplus NADH was formed in the cytoplasm, which resulted in a further decrease in μmax from 0.17/h in strain TN6 to 0.09/h in strain TN23. Thus, it was not possible to introduce an alternative pathway for reoxidation of NADH in the cytoplasm by expression of the transhydrogenase from A. vinelandii in a S. cerevisiae strain with a double deletion in GPD1 and GPD2.




