Identification of a putative DEAD-box RNA helicase and a zinc-finger protein in Candida albicans by functional complementation of the S. cerevisiae rok1 mutation
Abstract
We identified two novel genes, CHR1 and CSR1, of the fungal pathogen Candida albicans, by functional complementation of the Saccharomyces cerevisiae rok1 mutation. The Rok1 protein is a member of the DEAD protein family of ATP-dependent RNA helicases. ROK1 is required for cell cycle progression and also for rRNA processing. The CHR1 gene product of 578 amino acids is highly homologous to the Rok1 protein (54% identity) and is considered to be a putative DEAD-box RNA helicase. We predict that the CSR1 gene encodes a 73 kDa protein of 612 amino acids with five zinc-finger motifs at the C-terminal region. CHR1 or CSR1 on a high-copy number plasmid showed a slow-growth phenotype in a condition where the ROK1 expression is turned on from the GAL1 promoter. This result is consistent with the lethality caused by the ROK1 overexpression. We conclude that CHR1 encodes a functional homologue of Rok1 protein and CSR1 is a heterologous suppressor of the rok1 mutation. The GenBank Accession Nos for the CHR1 and CSR1 sequences are AF140505 and AF140504, respectively. Copyright © 2000 John Wiley & Sons, Ltd.
INTRODUCTION
Candida albicans is a leading cause of invasive fungal disease in human patients (Scherer and Magee, 1990; Shepherd et al., 1985). It is an opportunistic pathogen and causes serious medical problems in individuals with impaired immunity, such as AIDS patients or transplant recipients. In patients undergoing antibiotic treatment or cancer therapy, Candida infections are also frequently encountered. C. albicans is capable of a yeast-to-hyphal-phase transition and this switch is thought to be important for Candida's pathogenicity (Cutler, 1991; Lo et al., 1997; Madhani and Fink, 1998). The hyphal growth form appears to be most suited for adherence and penetration of epithelial or endothelial cell layers and also for exit from ingesting macrophages. Because Candida is a diploid and has no known sexual cycle, direct genetic elucidation of Candida pathogenesis has been difficult (Fonzi and Irwin, 1993; Shepherd et al., 1985). An alternative approach might be to employ Saccharomyces cerevisiae that is genetically more amenable and has a significant similarity at the molecular level with C. albicans. The C. albicans filamentation regulatory genes have been identified through their effects in S. cerevisiae (Mitchell, 1998; Liu et al., 1994).
The DEAD protein family of ATP-dependent RNA helicases is characterized by a central region of eight consensus motifs, which include the characteristic DEAD (Asp-Glu-Ala-Asp) box (Schmid and Linder, 1992). Members of this family show strong sequence homology to the prototype RNA helicase, the mammalian translation initiation factor eIF-4A. Some of these members (mammalian eIF-4A, human p68, Xenopus An3, and Drosophila vasa) have been shown to possess both ATP hydrolysis and RNA helicase activities in vitro (Fuller-Pace, 1994; Gururajan and Weeks, 1997; Rozen et al., 1990). The DEAD protein family includes about 60 proteins that originate from a wide range of organisms from bacteria to humans. These proteins are implicated in a variety of cellular processes, including translation, pre-mRNA splicing, RNA transport, rRNA processing, ribosome assembly and maternal RNA targeting (Fuller-Pace, 1994; Schmid and Linder, 1992). Since a variety of cellular processes require modulation of RNA secondary structures or RNA–protein interactions, it is interesting to identify new members of the RNA helicase family and characterize their functions.
The Rok1 protein of S. cerevisiae is a member of the DEAD-box RNA helicase family (Schmid and Linder, 1992; Song et al., 1995). The amino acid sequence of Rok1p contains eight highly conserved domains found in the DEAD protein family and shows a striking similarity to a number of proteins included in this family. Amino acid substitution mutations introduced in the conserved domains of ROK1 abolish both in vivo ROK1 function and in vitro biochemical activity (Jeong et al., 1996; Oh and Kim, 1999). The ROK1 gene has been initially identified as a high-copy suppressor of the kem1 null mutation and implicated in microtubule-mediated functions (Kim and Kim, 1992). Disruption or overexpression of ROK1 causes cell cycle arrest at the unbudded stage, implying that ROK1 is required for cell cycle progression at the G1/S stage (Jeong et al., 1998; Song et al., 1995). A subsequent report has suggested that Rok1p is required for rRNA processing (Venema et al.,1997). Depletion analysis of Rok1p demonstrates that it is required for the pre-rRNA cleavages which generate 18S rRNA.
Western analysis using polyclonal antibodies raised against Rok1p have detected cross-reacting antigens in protein extracts of Candida albicans (Rhee et al., 1998). These results imply that the immunocross-reactive proteins in C. albicans might be homologues of Rok1p. To search for the RNA helicase gene in C. albicans, which is homologous to ROK1, we used a genetic complementation method. By screening the Candida genomic library, we have isolated two independent clones which complement the lethality of the S. cerevisiae rok1 null mutation. We predict that the novel CHR1 (Candida homologue of ROK1) gene of 1734 bp ORF encodes a putative DEAD-box RNA helicase with 54% similarity with Rok1p. The CSR1 (Candida suppressor of ROK1) gene of 1836 bp ORF appears to encode a novel protein with zinc-finger motifs.
MATERIALS AND METHODS
Strains and growth conditions
Escherichia coli strain JM109 (recA supE44 endA1 hsdR17 gyrA96 relA1 thiΔ (lac-proAB) [F′ traD36 proAB+ lacIq lacZΔ M15]) was used to amplify plasmid DNA. S. cerevisiae strain JK348 (MATα ura3–52 leu2–3,112 trp1–289 can1 cyh2 Gal+ rok1::LEU2 [pJI299–TRP1 CEN1 GAL1::ROK1]) is an PGAL–ROK1 conditional strain and was used to test the complementation of the rok1 mutation. This strain expresses ROK1 in the presence of galactose in the media (Oh and Kim, 1999). The preparation of E. coli and yeast media was done by established procedures (Adams et al., 1997; Sambrook et al., 1989). Galactose medium contains 2% filter-sterilized galactose instead of glucose as the sole carbon source. 5-FOA (5-fluoro-orotic acid) medium was made of 0.67% Yeast Nitrogen Base without amino acids, 2% dextrose, 0.005% uracil and 0.1% 5-FOA.
Transformation and DNA manipulation
E. coli transformation was performed by the method of Mandel and Higa (1970). Yeast transformation was carried out according to the lithium acetate method of Ito et al. (1983). Plasmid DNA was isolated from E. coli by the modified alkaline lysis method (Sambrook et al., 1989). Restriction enzyme analysis and agarose gel electrophoresis were processed by methods of Sambrook et al. (1989). Enzymes were purchased from Boerhinger-Mannheim and New England Biolab.
Isolation of CHR1 and CSR1 genes
To isolate functional homologues of ROK1 in C. albicans, we transformed a Candida genomic library constructed on plasmid pRS202 (2 µ, URA3) into S. cerevisiae strain JK348 (Candida genomic library was kindly provided by H. Liu) (Liu et al., 1994). Twenty transformants with the complementing activity were selected directly on SC–Trp–Leu–Ura/glucose media after incubating for 2 days at 30°C. Each candidate transformant was patched on 5-FOA/glucose plates to screen for non-growth colonies. The transformants that do not form colonies on 5-FOA/glucose plates have a tendency to maintain the URA3-marked library plasmid on glucose media. Plasmid DNA prepared from each candidate was retransformed to JK348 and complementation phenotypes were examined on SC–Trp–Leu–Ura/glucose and SC–Trp–Leu–Ura/galactose media. Two Candida clones carrying a 3.6 kb or 2.6 kb insert, respectively, have been identified.
DNA sequence analysis
The nucleotide sequences of 3.6 kb (CHR1) and 2.6 kb (CSR1) DNA inserts have been analysed using an Automated DNA Sequencer (ABI PRISMTM 377; Perkin-Elmer). We started to read sequences with universal primers in the vector. Based upon the read sequences, subsequent primers were synthesized. Sequence analyses were carried out on both strands. Parts of the sequence data were aligned with those appearing in the Candida genome database of Stanford University.
Subcloning of CHR1 or CSR1 onto a centromere-based plasmid pRS316
To subclone CHR1 or CSR1 onto a centromere-based plasmid pRS316 (Sikorski and Hieter, 1989), we isolated a 3.6 kb KpnI–SacI fragment carrying CHR1 or a 2.6 kb XhoI–ClaI fragment carrying CSR1 from the initial Candida clone carried on pRS202. These DNA fragments were ligated into KpnI–SacI or XhoI–ClaI sites of pRS316, respectively, resulting in plasmids pRS316H (pRS316–CHR1) and pRS316S (pRS316–CSR1).
RESULTS AND DISCUSSION
Isolation of Candida genes that complement S. cerevisiae rok1 mutation
To isolate C. albicans genes that are functionally homologous to ROK1, a DEAD-box RNA helicase gene in S. cerevisiae, we screened a Candida genomic library by the genetic complementation method (Liu et al., 1994). We looked for the clones that complement the lethal phenotype of the rok1 null mutation of S. cerevisiae. Since ROK1 had been shown to be essential for cell viability, a PGAL–ROK1 conditional system was used for the complementation assay (Oh and Kim, 1999; Song et al., 1995). In the conditional strain, JK348, the chromosomal copy of ROK1 was disrupted with the LEU2 gene and a plasmid copy of ROK1 was expressed under the control of a galactose-inducible promoter, GAL1–10. This strain grows normally in galactose medium, but is unable to form colonies in glucose medium. The lethal phenotype of this strain in glucose medium was restored when a second plasmid carrying a wild-type ROK1 gene was introduced into the cell.
The Candida genomic library was transformed into the PGAL–ROK1 conditional strain and the transformants with complementing activity were selected directly on glucose medium. We tested the plasmid origin of the complementing activity by plating the transformants on 5-FOA/glucose plates. Three out of 20 candidate transformants showed no growth on these plates, indicating that these three transformants need to maintain the URA3-marked plasmid on glucose medium. The complementing activities of these clones were retested by plasmid DNA isolation and a retransformation procedure. Two independent clones, named CHR1 (Candida homologue of ROK1) and CSR1 (Candida suppressor of ROK1, see below), respectively, have been finally identified (Figure 1). The CHR1 and CSR1 clones have 3.6 kb and 2.6 kb inserts, respectively. Southern blot analysis of C. albicans genomic DNA with an internal DNA fragment of CHR1 or CSR1 as a probe produced a strong hybridization band of the expected size (data not shown).
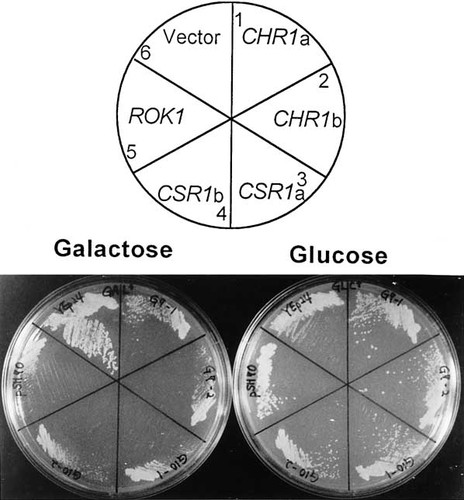
Complementation of the lethality of the rok1 null mutation by C. albicans genes CHR1 and CSR1. The PGAL–ROK1 conditional strain JK348 was transformed with YEp24, pJI70 (ROK1), pRS202CHR1 or pRS202CSR1 and tested for its growth on either galactose or glucose plates. Plates were incubated at 30°C for 3 days. The growth (or non-growth) on these plates were scored by examining a single colony formation.
As shown in Figure 1, newly isolated C. albicans clones, CHR1 and CSR1, restore the viability of the PGAL–ROK1 conditional strain on glucose medium but show slow-growth phenotypes on galactose medium, a ROK1-expressing condition. Previously, we have shown that overexpression of ROK1 on a high-copy number plasmid using a strong GAL1 promoter is lethal, leading cells to arrest at the unbudded stage (Jeong et al., 1998). In the PGAL–ROK1 conditional strain, the GAL–ROK1 construct is on centromere-based plasmid YCplac22. Therefore, the lethal overexpression of ROK1 was avoided. Since the Candida library was constructed on a 2µ-based high-copy number plasmid, pRS202, the presence of Candida clones CHR1 or CSR1 in a ROK1-expressing condition caused an effect similar to that of ROK1 overexpression. In good agreement with these results, the common cloning strategy, which is to select Ura+ transformants on SC–Ura/galactose plates first and to screen the transformants with complementing activity on glucose plates, has not been successful (data not shown).
Sequence analysis of Candida CHR1 and CSR1 genes
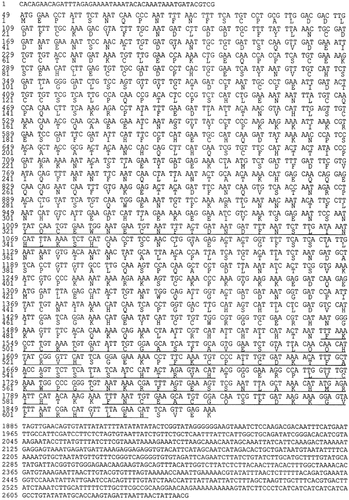
The nucleotide sequence of the CHR1 clone and the deduced amino acid sequence revealed that the open reading frame contains 1734 bp, which encodes a protein of 578 amino acids (Figure 2). The putative gene product had all eight consensus motifs found in members of the DEAD protein family of ATP-dependent RNA helicase. Thirty out of 34 amino acids in the consensus motifs are conserved in Chr1p. Four remaining amino acids are mostly conservative substitutions (Arg→Asn in one case; Gly→Leu in two; Gly→Ser in one). The amino acid sequence of Chr1p showed a striking similarity to Rok1p (54% identity in a 561 amino acid overlap). Especially, three of four divergent amino acids in the consensus motifs of Chr1p are the same in Rok1p (Figure 3). The putative ATP-dependent RNA helicases closely related to CHR1 are YA88 (Schizosaccharomyces pombe, 43% identity in a 440 amino acid overlap), VASA (Drosophila melanogaster, 33% identity in a 398 amino acid overlap), and eIF-4A (Leishmania brailiensis, 32% identity in a 356 amino acid overlap).

Nucleotide sequence of the C. albicans CHR1 gene and its predicted amino acid sequences. The amino acid sequences corresponding to the conserved domains of the DEAD-box RNA helicase family are underlined. The GeneBank Accession No. is AF140505.

Amino acid sequence alignment of C. albicans CHR1 and S. cerevisiae ROK1 gene products. Identical amino acids bewteen two proteins are indicated with *. Underlined sequences are the conserved domains of the DEAD-box RNA helicase family.
The nucleotide sequence of the CSR1 clone and the deduced amino acid sequence revealed that the open reading frame contains 1836 bp, which encodes a novel protein of 612 amino acids (Figure 4). It is interesting that the putative CSR1 gene product did not show any similarities in amino acid sequence to Rok1p. The sequence alignment in BLAST search showed a number of C2H2-type zinc-finger proteins (Figure 5). Csr1p has five zinc-finger motifs located in central (321–345) and C-terminal regions (499–524, 530–552, 558–582, 586–608). From these features, we expect that Csr1p could act as a DNA binding protein. CSR1 appeared to restore the viability of the PGAL–ROK1 conditional strain on glucose medium by not just activating transcription of the PGAL–ROK1 construct, because the complementing activity persists after the curing of the PGAL–ROK1 plasmid (data not shown). We expect that CSR1 is a heterologous suppressor of the rok1 mutation.
Nucleotide sequence of the C. albicans CSR1 gene and its predicted amino acid sequences. The amino acid sequences corresponding to the zinc-finger motifs are underlined. The GeneBank Accession No. is AF140505.
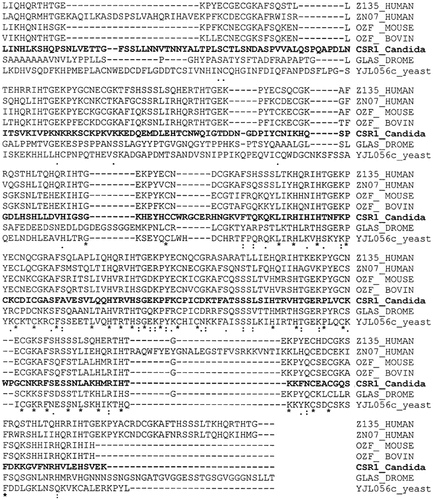
Amino acid sequence alignment of C. albicans CSR1 gene product with the conserved regions of zinc-finger proteins.
Complementation and suppression activity of CHR1 and CSR1 on a CEN vector
To ask whether CHR1 and CSR1 retain the complementing activity on a low copy number vector, these genes were subcloned onto the centromere-based plasmid, pRS316, and transformed into the PGAL–ROK1 conditional strain. As shown in Figure 6, transformants harbouring CHR1 or CSR1 on a low-copy vector grow normally on glucose medium, indicating that either CHR1 or CSR1 can functionally replace the ROK1 gene. Furthermore, the slow-growth phenotype caused by the presence of a high copy number of either CHR1 or CSR1 in a ROK1-expressing condition (galactose medium) disappeared when the copy number of CHR1 or CSR1 was lowered (Figure 6).
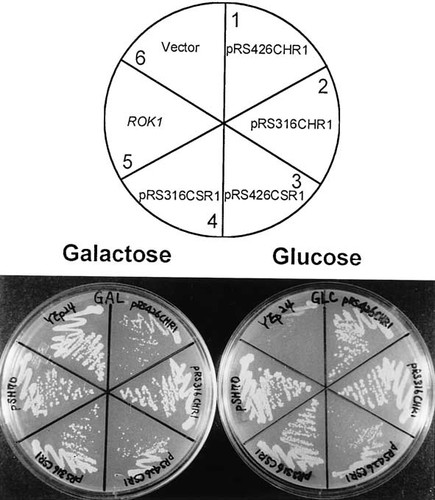
Complementation and suppression of the rok1 mutation by CHR1 and CSR1 on a centromere-based vector. The strain JK348 was transformed with each plasmid and tested for its growth on either galactose or glucose plates.
The sequence identity (54% in amino acid residues) between CHR1 and ROK1 and the complementation result clearly demonstrate that Chr1p is the functional homologue of Rok1p. However, the CSR1 gene lacks any sequence homology with ROK1 and is considered to be a zinc-finger protein. CSR1 also restores the lethality of the rok1 null mutation on a low copy plasmid. We are currently investigating how the CSR1 gene suppresses the rok1 null mutation. The Rok1p, a DEAD-box RNA helicase, has been reported to be involved in cell cycle progression at the G1/S stage or to play a critical role in pre-rRNA processing (Jeong et al., 1998; Song et al., 1995; Venema et al., 1997). We speculate that Rok1p, along with Kem1p, a cytoplasmic exoribonuclease, may participate in gene expression regulation, either by altering the level of mRNA or by affecting the translation efficiency (Jeong et al., 1998). The suppression mechanism of the newly isolated CSR1 gene could give us an explanation for the in vivo ROK1 function.
Acknowledgements
We are grateful to Dr Haoping Liu for the Candida genomic library. We thank Dr Doo-Il Chung for nucleotide sequence analysis. This work was supported by the 1997 research fund programme and by the 1998 international co-work programme from Korea Research Foundation.




