Propriospinal afferent and efferent connections of the lateral and medial areas of the dorsal horn (laminae I-IV) in the rat lumbar spinal cord
Abstract
The different subdivisions along the mediolateral extent of the superficial dorsal horn of the spinal cord are generally regarded as identical structures that execute the function of sensory information processing without any significant communication with other regions of the spinal gray matter. In contrast to this standing, here we endeavor to show that neural assemblies along the mediolateral extent of laminae I-IV cannot be regarded as identical structures. After injecting Phaseolus vulgaris leucoagglutinin and biotinylated dextran amine into various areas of the superficial dorsal horn (laminae I-IV) at the level of the lumbar spinal cord in rats, we have demonstrated that the medial and lateral areas of the superficial dorsal horn show the following distinct features in their propriospinal afferent and efferent connections: 1) A 300- to 400-μm-long section of the medial aspects of laminae I-IV projects to and receives afferent fibers from a three segment long compartment of the spinal dorsal gray matter, whereas the same length of the lateral aspects of laminae I-IV projects to and receives afferent fibers from the entire rostrocaudal extent of the lumbar spinal cord. 2) The medial aspects of laminae I-IV project extensively to the lateral areas of the superficial dorsal horn. In contrast to this, the lateral areas of laminae I-IV, with the exception of a few fibers at the segmental level, do not project back to the medial territories. 3) There is a substantial direct commissural connection between the lateral aspects of laminae I-IV on the two sides of the lumbar spinal cord. The medial part of laminae I-IV, however, does not establish any direct connection with the gray matter on the opposite side. 4) The lateral aspects of laminae I-IV appear to be the primary source of fibers projecting to the ipsi- and contralateral ventral horns and supraspinal brain centers. Projecting fibers arise from the medial subdivision of laminae I-IV in a substantially lower number. The findings indicate that the medial and lateral areas of the superficial spinal dorsal horn of rats may play different roles in sensory information processing. J. Comp. Neurol. 422:312–325, 2000. © 2000 Wiley-Liss, Inc.
Several hypothetical neural circuits underlying sensory information processing in the dorsal horn of the spinal cord were constructed by various authors in the past few decades (Szentágothai, 1964a,b; Réthelyi and Szentágothai, 1969, 1973; Price, 1984; Réthelyi, 1984; Ruda et al., 1986). These models substantially differ from each other in many respects, but all of them are based on two common principles. The superficial dorsal horn (laminae I-IV) is regarded as a unit that executes the function of sensory information processing without any significant communication with other regions of the spinal gray matter. In addition to this, the models suggest that the different subdivisions along the mediolateral extent of laminae I-IV can be regarded as identical structures. However, a large body of experimental evidence has been accumulated in recent years that does not agree with these principles. On the one hand, it has been extensively demonstrated that the medial and lateral subdivisions of the superficial dorsal horn show many distinct features concerning cytoarchitectonic organization (Bice and Beal, 1997a), primary afferent inputs (Light and Perl, 1979; Perl, 1980; Fuji et al., 1983; Sugiura et al., 1988; Chung et al., 1989; Cruz et al., 1993), synaptic and neurochemical properties of interneurons (Gibson et al., 1981, 1984; Ren and Ruda, 1994; Tachibana et al., 1994), and densities of terminals arising from various brainstem nuclei (Marlier et al., 1991; Antal et al., 1996), as well as numbers of neurons with axons projecting to supraspinal brain centers (Willis et al., 1979; Menétrey et al., 1983, 1992; Granum, 1986; Apkarian and Hodge, 1989; Bice and Beal, 1997b). On the other hand, a steadily growing body of physiological evidence indicates that the activities of various regions of the ipsi- and contralateral spinal gray matter exert a substantial influence on the nociceptive information processing machineries of the superficial dorsal horn through propriospinal connections. It has been reported that propriospinal neurons originating from various areas of the spinal gray matter modulate background activity and noxious heat-evoked responses of neurons in the superficial dorsal horn (Sandkühler et al., 1993). Experimentally induced unilateral hindpaw inflammation produces bilateral changes in the expression of immediate early genes (Williams et al., 1990; Herdegen et al., 1991a,b; Ren and Ruda, 1996), substance P and CGRP immunoreactivities (Sluka et al., 1992; Mapp et al., 1993), and NADPH-diaphorase activity (Solodkin et al., 1992), as well as in dorsal horn postsynaptic currents (Colvin et al., 1996). It is also well established that unilateral noxious stimulation of the limb and tail of rats evokes changes in dorsal horn cell activity at both sides of the spinal cord (Fitzgerald, 1982). Increased bilateral mRNA expression of preprotachykinin and calcitonin gene-related peptide in dorsal root ganglia has also been observed in the initial period of adjuvant monoarthritis in the rat (Donaldson et al., 1992).
However, concerning the anatomic substantiation of the propriospinal interconnectivity of the superficial dorsal horn, we have to rely exclusively on the classic degeneration and Golgi-impregnation studies of Szentágothai (1951, 1964a,b), Scheibel and Scheibel (1968), and Réthelyi and Szentágothai (1973). Based on his degeneration studies, Szentágothai (1951) reported that degenerated “boutons-terminaux” were seen in the dorsal horn after lesions of the intermediate gray matter. In addition to this propriospinal projection from the intermediate gray matter to the dorsal horn, Golgi-impregnation and degeneration studies revealed also a considerable number of dorsal commissural fibers that originate and terminate in the dorsal horn (Szentágothai, 1964a,b; Scheibel and Scheibel, 1968; Réthelyi and Szentágothai, 1973). However, since these classic investigations, no advance can be recorded. There has been no attempt to make an accurate account concerning the propriospinal relations of the superficial laminae of the spinal dorsal gray matter with modern anterograde and retrograde neural tracing methods.
Accordingly, in the present experiment, we studied the propriospinal afferent and efferent connections of the superficial laminae of the dorsal horn (laminae I-IV) in the rat lumbar spinal cord by using the highly sensitive anterograde and retrograde neural tracers Phaseolus vulgaris leucoagglutinin (Gerfen and Sawchenko, 1984) and biotinylated dextran amine (Rajakumar et al., 1993). Preliminary observations from this experiment have been reported in abstract form (Antal and Petkó, 1996).
MATERIALS AND METHODS
Animals, injection of neural tracers, and preparation of tissue sections
Experiments were performed on adult male rats (Wistar-Kyoto, 250–300 g, Gödöllö, Hungary). All animal study protocols were approved by the Animal Care and Protection Committee at the University Medical School of Debrecen. Laminectomy was performed to expose the lumbar spinal cord under deep sodium pentobarbital anaesthesia (50 mg/kg i.p.), while the animals were held in a stereotaxic frame. Glass micropipettes with a tip diameter of 20–30 μm were filled with a 10% solution of biotinylated dextran amine (BDA; molecular weight, 10,000; Molecular Probes) or 2.5% solution of Phaseolus vulgaris leucoagglutinin (PHA-L, Vector Laboratories) dissolved in 0.1 M phosphate buffer (PB, pH 7.4). The tracers were injected into the lateral and medial parts of the superficial dorsal horn at the level of the L2-L4 segments of the lumbar spinal cord unilaterally by iontophoresis, by using positive direct current of 5 μA with a pulse duration of 7 seconds followed by 3-second intervals for a period of 20 minutes. Efforts were made to minimize animal suffering during surgical procedures.
After 1 week (in case of BDA injections) or 3 weeks (in case of PHA-L injections) postoperative survival period, the animals were reanaesthetised with an overdose of sodium pentobarbital (70 mg/kg, i.p.) and transcardially perfused first with Tyrode's solution (oxygenated with a mixture of 95% O2, 5% CO2), followed by a fixative containing 2.5% glutaraldehyde, 0.5% paraformaldehyde, and 0.2% picric acid in 0.1 M PB. The lower thoracic, lumbar, and upper sacral segments of the spinal cord were removed, postfixed in the same fixative for 1–2 hours, and immersed in 10% and 20% sucrose dissolved in 0.1 M PB until they sank. To enhance reagent penetration, the removed spinal cord was freeze-thawed in liquid nitrogen, sectioned at 60 μm on a Vibratome, and extensively washed in 0.1 M PB.
Histochemical detection of the tracers
Biotinylated dextran amine.
For histochemical detection of the injected BDA, free-floating sections of the spinal cord were incubated with avidin-biotinylated horseradish peroxidase complex (ABC, Vector Laboratories, diluted 1:100) for overnight at 4°C.
Phaseolus vulgaris leucoagglutinin.
To detect the injected PHA-L, free-floating sections of the spinal cord were first incubated with biotinylated anti–PHA-L made in goat (Vector Laboratories, diluted 1:1,000) for 2 days at 4°C. The sections were then transferred into a solution of avidin biotinylated-horseradish peroxidase complex (ABC, Vector Laboratories, diluted 1:100) for 4 hours at room temperature.
The histochemical reactions, in both cases, were completed with a nickel-intensified diaminobenzidine (3,3′-diaminobenzidine tetrahydrochloride; Sigma Co.) chromogen reaction (Hancock, 1984). The sections were then extensively washed, mounted on chrome alum-gelatin coated slides, counterstained with cresyl violet, and covered with Permount neutral medium.
RESULTS
Injection sites
The tracers were delivered into the lumbar spinal cord at the level of L2-L4 segments. The iontophoretic injections of both PHA-L and BDA yielded small and well defined injection sites that involved ovoid cross-sectional areas with a mediolateral extent of 100–300 μm and extended across laminae I-III with some involvement of lamina IV (Fig. 1). As detected by the histochemical procedure, many cells incorporated the tracer within the confines of the areas infiltrated by the tracers. The appearance of these cells, as well as the injection sites, were similar to those reported in previous studies (Gerfen and Sawchenko, 1984; Wouterlood and Groenewegen, 1985; Veenman et al., 1992; Rajakumar et al., 1993).
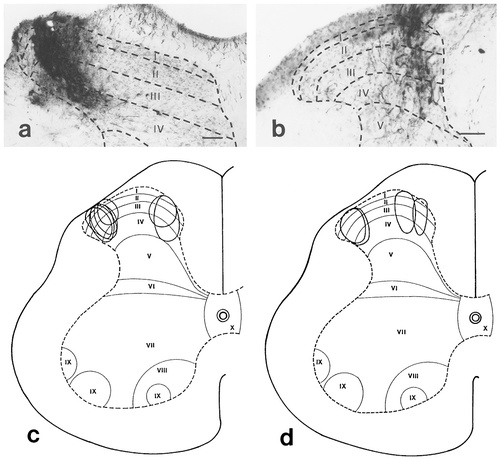
a,b: Photomicrographs that show an injection site of biotinylated dextran amine (BDA) in the lateral (a) and an injection site of Phaseolus vulgaris leucoagglutinin (PHA-L) in the medial (b) part of the dorsal horn. c,d: Camera lucida drawings of transverse sections of the lumbar spinal cord showing the sites and sizes of BDA (c) and PHA-L (d) injections into the lateral and medial aspects of the dorsal horn. The borders of the gray and white matters, and the cytoarchitectonical laminae of the gray matter are drawn by dashed lines and refer to Molander et al. (1984). Roman numerals indicate the Rexed's laminae of the gray matter. Scale bars = 100 μm in a,b.
The experiments were carried out on 21 animals. Because of inappropriate localization of the injection sites, seven animals had to be excluded from the final evaluation. Consequently, data presented in this study are based on the results obtained in 14 animals. On the basis of the applied tracer and the location of the injection site, the animals were divided into the following four experimental groups: (1) PHA-L injection into the lateral regions of laminae I-IV (three animals, Fig. 1d), (2) BDA injection into the lateral part of laminae I-IV (five animals, Fig. 1a,c), (3) PHA-L injection into the medial areas of laminae I-IV (three animals, Fig. 1b,d), (4) BDA injection into the medial aspect of laminae I-IV (three animals, Fig. 1c). In one animal in the third experimental group, the dorsal roots at the level of the injection site were cut three weeks before the PHA-L injection.
Injections of PHA-L into the medial areas of laminae I-IV
After injections of PHA-L into the medial areas of laminae I-IV, labelled axon terminals were revealed in a 2.0- to 2.5-mm-long section of the spinal dorsal horn ipsilateral to the injection site, and the stained terminals showed a specific laminar distribution (Figs. 2b,c, 3). The majority of the labelled terminals were found in a region of the medial aspect of the superficial dorsal horn that corresponded to the rostrocaudal elongation of the area in which the injection was located (Fig. 3). Most of the terminals at this location were revealed in laminae IIi, III, and IV. Terminals within the confines of laminae I-IIo were found exclusively at the level or close to the level of the injection site. In addition to the medial aspect of laminae I-IV, the lateral areas of laminae IIi, III, and IV were also densely covered by stained axon varicosities, and labelled terminals were also found in laminae V-VI. Labelled terminals in laminae V-VI were located exclusively at the level or very close to the level of the injection site, whereas the stained axon terminals in the lateral aspects of the dorsal horn were distributed along a 2.0- to 2.5-mm-long section of the spinal gray matter (Fig. 3). In addition to the dense labelling in the dorsal horn, stained terminals were occasionally also found in the ventral horn both ipsi- and contralateral to the injection site. Axons in a very limited number crossed the midline and terminated in the medial aspects of the dorsal horn contralateral to the injection site.
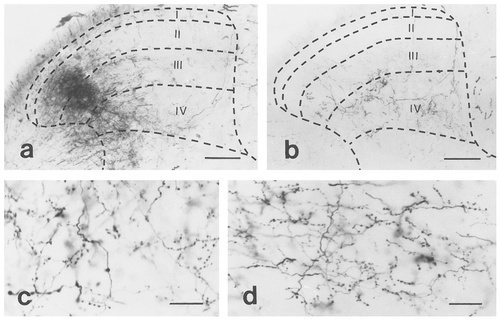
Photomicrographs showing Phaseolus vulgaris leucoagglutinin (PHA-L) -labelled fibers in the dorsal horn ipsilateral and one segment rostral to the injection site. a: A dense projection to the lateral aspect of laminae III-IV. Note that lamina I is almost free of labelling. PHA-L was injected into the lateral part of the dorsal horn. b: Axon terminals in laminae III and IV. PHA-L was injected into the medial part of the dorsal horn. c,d: Labelled fibers and axon terminals with higher magnification. The borders of the gray and white matters, and the cytoarchitectonical laminae of the gray matter are drawn by dashed lines and refer to Molander et al. (1984). Roman numerals indicate the Rexed's laminae of the gray matter. Scale bars = 100 μm in a,b; 20 μm in c,d.
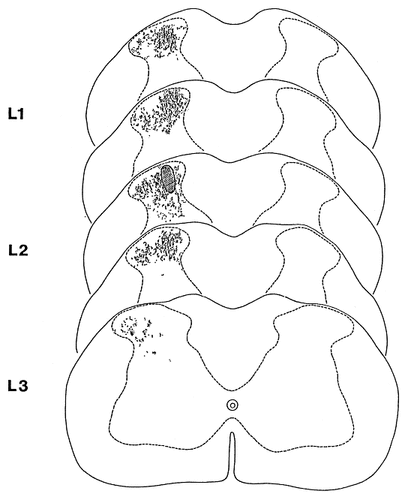
Schematic representation of the distribution of terminals of propriospinal fibers that were anterogradely labelled with Phaseolus vulgaris leucoagglutinin after injecting the tracer into a 100- to 300-μm-wide area of the medial aspect of laminae I-IV. Hatched area indicates the injection site of PHA-L. Dots represent varicosities of labelled propriospinal axons.
Although in very low numbers, labelled axons were revealed also in the white matter. These fibers, representing presumably ascending fibers to supraspinal brain centers, were located exclusively either in the dorsolateral funiculus or in the dorsal column ipsilateral to the injection site.
Injections of BDA into the medial areas of laminae I-IV
After injections of BDA into a 100- to 300-μm-wide area of the medial aspect of laminae I-IV, retrogradely labelled neurons were recovered in a 2.0- to 2.5-mm-long section of the spinal dorsal horn ipsilateral to the injection site (Fig. 4). A substantial proportion of the stained neurons was confined to the medial aspects of laminae I-IV, but several stained neurons were also observed in laminae V-VI. Neurons in laminae V-VI were located at the level or close to the level of the injection site and were scattered throughout the entire mediolateral extent of the dorsal horn (Fig. 4).
Schematic representation of the distribution of propriospinal neurons that were retrogradely labelled with biotinylated dextran amine (BDA) after injecting the tracer into a 100- to 300-μm-wide area of the medial aspect of laminae I-IV. Hatched areas indicate the injection sites of BDA. Dots represent the perikarya of retrogradely labelled neurons.
Injections of PHA-L into the lateral areas of laminae I-IV
After delivering PHA-L into a 100- to 300-μm-wide area of the lateral aspect of laminae I-IV, labelled axons and axon terminals were revealed throughout the entire length and on both sides of the lumbar spinal cord (Figs. 2a,d, 5). Ipsilateral to the injection site, the labelled terminals were concentrated mostly in the lateral areas of the dorsal horn, innervating the entire dorsoventral extent of the dorsal gray matter (laminae I-VI) (Fig. 5). However, the stained terminals were unevenly distributed in this territory. Labelled terminals were recovered only in moderate numbers in laminae I-IIo, whereas the density of stained terminals was substantially higher in deeper layers. In contrast to the dense labelling in the lateral areas, the medial regions of the dorsal horn ipsilateral to the injection site were poorly supplied with labelled terminals (Fig. 5). With the exception of a few terminals at the very close vicinity of the injection site, the medial dorsal horn was almost free of labelling.
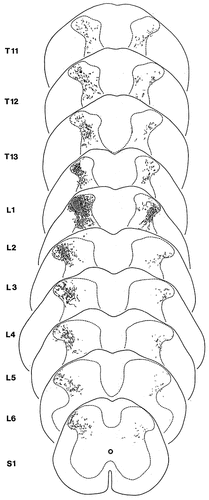
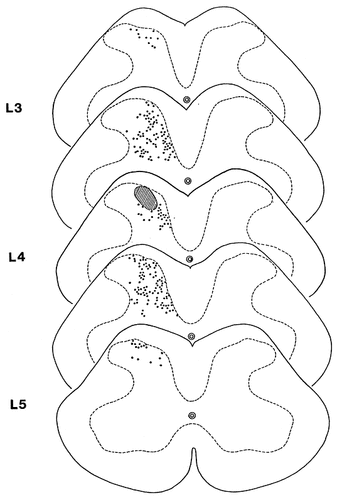
Schematic representation of the distribution of terminals of propriospinal fibers that were anterogradely labelled with Phaseolus vulgaris leucoagglutinin (PHA-L) after injecting the tracer into a 100- to 300-μm-wide area of the lateral aspect of laminae I-IV. The hatched area indicates the injection site of PHA-L. Dots represent varicosities of labelled propriospinal axons.
After injections of PHA-L into the lateral dorsal horn, extensive terminal labelling was observed in the lateral aspects of the contralateral dorsal horn (Figs. 5, 6, 9c). Labelled fibers ascended or descended for various distances from the injection site in substantial numbers, then they turned medially and crossed the midline dorsal to the central canal in small bundles, forming a ladder-like pattern along the rostrocaudal axis of the spinal cord (Fig. 6). On the contralateral side, the axons reached the lateral aspect of the dorsal horn and terminated there, forming a continuous termination field mostly in laminae III-IV (Figs. 5, 6).
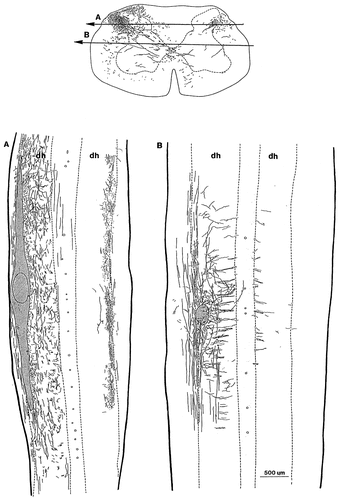
Camera lucida drawings representing the injection site of Phaseolus vulgaris leucoagglutinin (PHA-L) in a transverse and the distribution of labelled propriospinal fibers and axon terminals in both sides of the spinal cord in horizontal sections. Arrows A and B in the transverse section indicate the dorsoventral levels of the horizontal sections. The hatched areas represent the injection site of PHA-L. The borders of the gray and white matters are drawn by dashed lines. dh, dorsal horn.
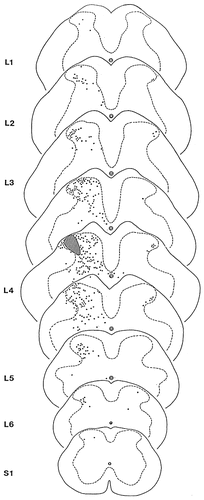
Schematic representation of the distribution of propriospinal neurons that were retrogradely labelled with biotinylated dextran amine (BDA) after injecting the tracer into a 100- to 300-μm-wide area of the lateral aspect of laminae I-IV. The hatched areas indicate the injection sites of BDA. Dots represent the perikarya of retrogradely labelled neurons.
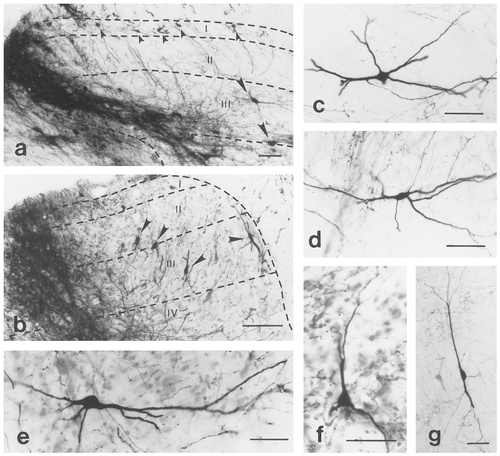
Photomicrographs showing retrogradely labelled neurons in lamina I (a), lamina II (b), lamina III-IV (a–d,f), and laminae IV-VI (e,g) ipsilateral to injections of biotinylated dextran amine (BDA) into the lateral aspect of the superficial dorsal horn. The darkly stained areas in a and b label the injection site of BDA. The borders of the gray and white matters, and the cytoarchitectonical laminae of the gray matter are drawn by dashed lines and refer to Molander et al. (1984). Roman numerals indicate the Rexed's laminae of the gray matter. Arrowheads in a and b point to perikarya of retrogradely labelled neurons. Scale bars = 100 μm in a,b; 50 μm in c–g.
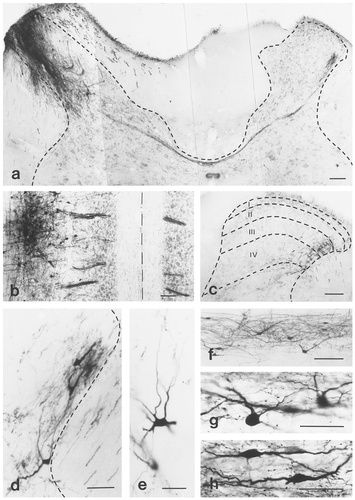
Photomicrographs showing retrogradely labelled neurons and anterogradely labelled fibers and axon terminals contralateral to the injection site. The darkly stained area in a labels an injection site of biotinylated dextran amine (BDA). a: A fiber bundle crossing the midline dorsal to the central canal. The fibers arise from the site of BDA injection and terminate in the lateral margin of laminae III-IV contralateral to the injection site. b: A horizontal section dorsal to the central canal. The darkly stained area at the left-upper corner of the micrograph represents a densely innervated area ventral to the injection site of BDA. Note that the fibers crossing the midline are arranged in small bundles. The midline is indicated by a dashed line. c: Phaseolus vulgaris leucoagglutinin-labelled fibers in the lateral margin of laminae III-IV contralateral to the injection site. d–h: Retrogradely labelled neurons in laminae III-IV contralateral to the site of BDA injection in transverse (d,e) and horizontal (f–h) sections. The borders of the gray and white matters (a,c,d), and the cytoarchitectonical laminae of the gray matter (c) are drawn by dashed lines and refer to Molander et al. (1984). Roman numerals in c indicate the Rexed's laminae of the gray matter. Scale bars = 100 μm in a–f; 50 μm in d,e,g,h.
In addition to the dense projections to the dorsal horn, stained axon terminals were also revealed in the ventral horn of the spinal gray matter both ipsi- and contralateral to the injection site (Fig. 6). In contrast to the wide rostrocaudal distribution of terminals in the dorsal horn, the projections to the ipsi- and contralateral ventral horns were confined to the segmental level of the injection site.
Stained axons, representing presumably ascending fibers to supraspinal brain centers, were revealed in the white matter in large numbers (Fig. 6). Most of these axons were located in the ipsilateral dorsal and lateral as well as in the contralateral anterior funiculi.
Injections of BDA into the lateral areas of laminae I-IV
After injections of BDA into a 100- to 300-μm-wide area of the lateral dorsal horn, retrogradely labelled neurons were revealed throughout the entire length and on both sides of the lumbar spinal cord (Figs. 7-9). Ipsilateral to the injection site, retrogradely labelled neurons were widely distributed in various areas of the dorsal horn. At the level of the injection site, in a 2.0- to 2.5-mm-long section of the spinal cord, stained cells were recovered in all laminae (laminae I-VI) and throughout the entire mediolateral extent of the dorsal horn (Fig. 7). Most of the neurons in laminae I-IV presented round cell bodies and thin slender dendrites that were difficult to recognize as they usually left the section within a few micrometers from the cell body (Fig. 8a,b). In other cases, three or four dendritic trunks originated from the cell body of labelled cells that endowed the neurons with a triangular or quadrangular appearance (Fig. 8c,d,f). In laminae V-VI, labelled neurons showed a high degree of morphologic variability. Most of them were multipolar (Fig. 8e), but neurons with spindle shaped cell bodies were also revealed (Fig. 8g). Beyond the limit of the injection site into both rostral and caudal directions, the number of stained neurons rapidly decreased, and the labelling became completely extinct in laminae I-II, V-VI and in the medial aspects of laminae III-IV (Fig. 7). However, in a region of laminae III-IV that corresponded to the rostrocaudal elongation of the area in which the injection was located, retrogradely labelled neurons were revealed throughout the entire rostrocaudal extent of the lumbar spinal cord (Fig. 7).
In addition to the ipsilateral cells, neurons were labelled also on the contralateral side of the dorsal horn. With a few exceptions, these cells were confined to the very lateral aspects of laminae III-IV (Figs. 7, 9d). They presented multipolar perikarya or fusiform cell bodies with two main dendrites oriented parallel to the rostrocaudal axis of the spinal cord (Fig. 9d–h). The axons of these cells crossed the midline within the posterior commissure, and terminated in areas of the contralateral dorsal horn that were identical to that in which their cells of origin were located (Fig. 9a,b,d).
DISCUSSION
Labelling of axons and axon terminals of propriospinal neurons in the spinal dorsal horn with Phaseolus vulgaris leucoagglutinin
To label axons and axon terminals of propriospinal neurons in the spinal dorsal horn, PHA-L, the highly sensitive anterograde tracer (Gerfen and Sawchenko, 1984, 1985; Wouterlood and Groenewegen, 1985) has been iontophoretically injected into laminae I-IV of the spinal gray matter. A long line of experimental evidence indicates that after extracellular iontophoretic application PHA-L is internalized almost exclusively by perikarya and dendrites of neurons located within the region of the injection site (Gerfen and Sawchenko, 1984, 1985; Wouterlood and Groenewegen, 1985; Grove et al., 1986; Wouterlood et al., 1987). Consequently, most of the labelled axons and axon terminals that we encountered in this study may represent axons and axon terminals of neurons that are located in the medial and lateral areas of laminae I-IV. However, it has also been reported that some local axon terminals and fibers that originate outside the injection site and run through the area infiltrated by the tracer can also take up and transport PHA-L (Gerfen and Sawchenko, 1984, 1985; Wouterlood and Groenewegen, 1985; Grove et al, 1986; Wouterlood et al., 1987; Cliffer and Giesler, 1988; Chen and Aston-Jones, 1998). Therefore, in principle, in addition to axons of local neurons, some nonpropriospinal axons such as primary afferents or descending fibers of supraspinal origin could also take up and transport the lectin, causing unspecific labelling in the spinal gray matter. However, it is highly probable that this nonspecific labelling was very weak in our experiments. First, the labelling of fibers of passage has always been found to be poor in the central nervous system of mammals (Gerfen and Sawchenko, 1984, 1985; Keller et al., 1985; Wouterlood and Groenewegen, 1985; Grove et al., 1986: Wouterlood et al., 1987). Second, in the animal in which we eliminated the primary afferents by cutting the segmental dorsal roots 3 weeks before the tracer application, the distribution and density of the labelled axons and axon terminals were identical with those findings that were obtained from animals with intact dorsal roots. Third, labelled neurons outside the confines of the injection site that could take up PHA-L by their local axon terminals and transport the tracer along their spinal axon collaterals (Chen and Aston-Jones, 1998), causing unspecific labelling, were observed in a minimal number. Therefore, it seems that the unspecific labelling of nonpropriospinal fibers does not interfere with the major findings of the present study, and it does not lead us to dubious conclusions concerning the distribution of the genuine population of propriospinal axons and axon terminals.
Labelling of perikarya of propriospinal neurons in the spinal dorsal horn with biotinylated dextran amine
It seems to be well established that BDA shows excellent properties for both anterograde and retrograde labelling (Veenman et al., 1992; Rajakumar et al., 1993). The tracer is taken up by axons, axon terminals, dendrites, and cell bodies and is transported both anterogradely and retrogradely to yield Golgi-like labelling of axon terminals and the somatodendritic compartment of neurons. After injecting BDA into the medial and lateral areas of laminae I-IV of the spinal dorsal horn, where various constituents of the spinal neural assembly (such as cell bodies, dendrites and axons of propriospinal neurons, axon terminals of primary afferents and descending fibers of supraspinal origin, and fibers of passage of supraspinally projecting spinal neurons) are located, we have obtained an extensive labelling of both axons and cell bodies. However, in BDA-injected animals, our attention have been exclusively focused on the distribution of the retrogradely labelled propriospinal neurons, whereas the anterogradely labelled axons and axon terminals were left out of consideration.
Neurons retrogradely labelled with BDA could take up the tracer by their axons, axon terminals, and dendrites. Therefore, in addition to spinal neurons with axon terminals in laminae I-IV, neurons with dendrites that extend into the superficial dorsal horn could also be labelled in our experiments. It is well documented that several neurons in the deep dorsal horn give rise to dendrites that extend into the superficial dorsal horn (for review see Willis, 1985), thus, a proportion of stained neurons in laminae V-VI that were observed at the level of the injection sites could be labelled through their dendrites. However, the rostrocaudal extent of the dendtritic arbors of dorsal horn neurons is less extensive. There is no account in the literature that they would extend longer than a few hundred micrometers. Thus, neurons in laminae I-IV that took up the tracer by their dendrites are presumably located within a few hundred micrometer wide rim around the injection site. Stained neurons outside this area could be labelled exclusively through their axons.
Injecting BDA into the lateral areas of the dorsal horn, fibers of passage that traverse the sites of injections, e.g., supraspinally projecting neurons that are located in the medial areas of laminae I-IV and send their ascending axons into the dorsolateral funiculus, could also take up and transport the tracer, causing retrograde labelling of nonpropriospinal neurons in the medial dorsal horn. However, it is highly probable that this mechanism did not play a significant role in our experiments. First, although it is generally assumed that BDA is taken up by fibers of passage (Veenman et al., 1992; Rajakumar et al., 1993; Dolleman-Van der Weel et al., 1994; Sidibé and Smith, 1996), it has also been demonstrated that the tracer can presumably be transported only by damaged fibers of passage and not by intact fibers (Veenman et al., 1992; Rajakumar et al., 1993; Sidibé and Smith, 1996). Several observations indicate that, if the tracer is delivered with iontophoresis, as it has been done in our experiments, the injection causes minimal tissue damage, and the uptake and subsequent transport of BDA by fibers of passage is negligible (Rajakumar et al., 1993; Sidibé and Smith, 1996). Second, a long line of experimental evidence indicates that the number of supraspinally projecting neurons with axons in the dorsolateral funiculus is very moderate in the medial regions of the superficial dorsal horn (Willis et al., 1979; Menétrey et al., 1983, 1992; Granum, 1986; Apkarian and Hodge, 1989). In agreement with these findings, injecting PHA-L into the medial dorsal horn, we have observed ascending fibers in the dorsolateral funiculus only occasionally. Consequently, although we cannot rule out the possibility that a small number of retrogradely labelled cells in the spinal gray matter were a result of transport of BDA along damaged fibers of passage, it seems likely that this nonspecific labelling was light; therefore, it does not interfere with the major conclusions of the present study.
Propriospinal relations of the medial and lateral areas of laminae I-IV in the rat lumbar spinal cord
It has been noted by several authors that the medial and lateral areas of the superficial dorsal horn of the spinal cord show many distinct features concerning the termination pattern of C and Aδ primary afferents (Light and Perl, 1979; Perl, 1980; Fuji et al., 1985; Sugiura et al., 1988; Chung et al., 1989; Cruz et al., 1993). As a conclusion of a thorough ultrastructural study of horseradish peroxidase labelled primary afferents, a possible segregation of thin afferents of different calibers has been suggested in the superficial dorsal horn of rats, with the Aδ fibers arborizing primarily medially and the C-fibers terminating preferentially in the lateral aspects of laminae I-II (Cruz et al., 1993). It has also been reported that different subunits of AMPA-selective glutamate receptors are unevenly distributed along the mediolateral extent of laminae I-II, with a higher density at the lateral territories (Tachibana et al., 1994). The consistent finding that neurons immunoreactive for the calcium binding protein parvalbumin are more numerous in the lateral than in the medial areas of lamina II (Antal et al., 1990; Ren and Ruda, 1994) indicate that the neurochemical properties of interneurons in the medial and lateral subdivisions of the superficial dorsal horn of the spinal cord may also show distinct features. Results obtained from retrograde labellings of supraspinally projecting spinal neurons also indicate disparity between the different subdivisions along the mediolateral extent of the superficial dorsal horn (Menétrey et al., 1983, 1992; Lima and Coimbra, 1988, 1989, 1990, 1991a,b; Bice and Beal, 1997b). It has been demonstrated that the major ascending spinal pathways that are supposed to conduct nociceptive signals toward supraspinal brain centers arise predominantly from the lateral aspects of the superficial and deep laminae of the dorsal horn (Willis et al., 1979; Menétrey et al., 1983, 1992; Granum, 1986; Apkarian and Hodge, 1989). In addition, axons descending from the rostral ventromedial medulla, that are believed to play a substantial role in the descending control of spinal nociceptive information processing (Basbaum and Fields, 1984; Fields et al., 1991), were found to project more densely to the lateral than to the medial aspects of laminae I-II (Marlier et al., 1991; Antal et al., 1996).
Here, we demonstrated that, in addition to the unequal distribution of primary afferents, supraspinally projecting neurons and descending fibers of brainstem origin, the medial and lateral subdivisions of the superficial dorsal horn show distinct characteristics also in their propriospinal relations. Neural assemblies along the mediolateral extent of laminae I-IV cannot be regarded as identical structures. The propriospinal afferent and efferent connections of the medial and lateral areas of the superficial dorsal horn (laminae I-IV) show the following substantial differences:
(1) A 300- to 400-μm-long section of the medial aspect of laminae I-IV projects to and receives afferent fibers from a three-segment long compartment of the lumbar spinal gray matter (Fig. 10a,b), whereas the same length of the lateral aspect of laminae I-IV projects to and receives afferent fibers from the entire rostrocaudal extent of the lumbar spinal gray matter (Fig. 10c,d). In agreement with previous reports, this finding indicates that there are at least two, a short and a long, propriospinal projection systems in the superficial dorsal horn of the rat lumbar spinal cord (Szentágothai, 1964a; Réthelyi and Szentágothai, 1973). The short propriospinal system may be functional both in the medial and lateral areas of the dorsal horn, whereas the long propriospinal system, as it is indicated by our results, exists exclusively in the lateral subdivisions of laminae I-IV. Short propriospinal axons may arise from all four laminae of the superficial dorsal horn (laminae I-IV), they extend 2.0–2.5 mm in the rostrocaudal direction and terminate mostly, if not exclusively, in laminae IIi, III, and IV. In contrast to this, long propriospinal axons may arise mostly from neurons in the lateral aspects of laminae III-IV, traverse the entire rostrocaudal length of the lumbar spinal cord and innervate all laminae of the gray matter in the lateral aspects of the dorsal horn from lamina I as deep as lamina VI.
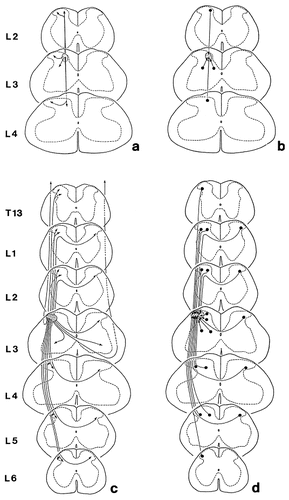
Schematic representation of the propriospinal efferent (a,c) and afferent (b,d) connections of the medial (a,b) and lateral (c,d) areas of the dorsal horn (laminae I-IV) in the rat lumbar spinal cord. Dots mark locations of perikarya and arrows label sites of axon terminals of propriospinal neurons. The hatched areas at segment L3 indicate regions from which the illustrated efferent fibers arise and in which the represented afferent fibers terminate.
(2) The medial aspect of laminae I-IV projects extensively to the lateral aspect of the superficial dorsal horn, but, with the exception of a few fibers at the segmental level, does not receive afferents from this area (Fig. 10a,b). We found that the cells of origin of the mediolaterally projecting propriospinal axons are located in the medial aspects of laminae I-IV, at the area where neurons with short propriospinal axons are also abundant. This spatial coincidence between these two populations of neurons make the notion possible that some neurons in the medial aspects of laminae I-IV may have double propriospinal projection, participating partly in the formation of the short propriospinal system and contributing also to the mediolateral projection. The unique termination pattern of the mediolaterally projecting axons (they terminate mostly, if not exclusively, in the lateral aspects of laminae III-IV where the cells of origin of long propriospinal axons are concentrated) suggests that this projection system may play a substantial role in the activation of neurons with long propriospinal axons. The experimental verification of these notions is in progress in our laboratory.
(3) There is a substantial direct commissural connection between the lateral aspects of laminae I-IV on the two sides of the lumbar spinal cord (Fig. 10c,d). However, the medial part of laminae I-IV does not establish any direct connection with the gray matter on the opposite side of the spinal cord. It is also interesting to note that the cells of origin of the commissural axons are distributed in a very restricted area of the lateral part of laminae III-IV and their axon terminals are concentrated also in the same territory on the opposite side. However, within this area the terminal arbors of the axons are surprisingly extensive, commissural neurons in a 300- to 400-μm-long section of the gray matter project to the entire rostrocaudal extent of the lumbar spinal cord. On the basis of the location of the perikarya and the extent of the axonal termination fields, one may get the impression that this commissural pathway resembles the long propriospinal system, or, in other words, the long propriospinal system is inhomogeneous in the sense that it consists of an ipsilateral and a commissural component.
Functional considerations
The present experiments provided additional evidence that an area of the superficial dorsal horn that receives sensory inputs from a delineated region of the body cannot be regarded as a unit that executes the function of sensory information processing without any significant communication with other regions of the spinal gray matter. On the contrary, our results suggest that the sensory information processing machineries of laminae I-IV are presumably deeply influenced by propriospinal systems. Sensory inputs conducted by thin and thick primary afferents activate local neural circuits either in the medial or the lateral areas of the superficial dorsal horn according to the somatotopic projection pattern of primary afferents. The embryonically ventral parts of the hindlimb, or flexor surface (posterior and plantar in the adult), are represented medially and the embryonically dorsal parts, or extensor surface, laterally in the dorsal horn of the lumbar spinal cord (Swett and Woolf, 1985; Molander and Grant, 1985, 1986; Woolf and Fitzgerald, 1986), whereas the most lateral areas of the dorsal horn are occupied by the projections of the dorsal rami of spinal nerves (Devor and Claman, 1980). If the local neural apparatus becomes excited either in the medial or lateral regions of the dorsal horn, short propriospinal neurons, which presumably represent an integral part of the segmental apparatus (Bice and Beal, 1997a) will activate a 2.0- to 2.5-mm-long rostrocaudally oriented cell column. If the excitation level in the activated cell column is strong enough, then the long propriospinal system, which is present exclusively in the lateral areas of the dorsal horn, can also be activated. Neural events in the lateral dorsal horn can activate the long propriospinal system directly, whereas the activities of the medial dorsal horn are presumably first transmitted to the segmental and short propriospinal systems of the lateral subdivisions by mediolaterally projecting propriospinal neurons. The long propriospinal system that involves the entire length of the lumbar cord might then process the sensory signals even further in close collaboration with the contralateral dorsal horn. After all, through the short and long propriospinal and commissural systems, the entire length and both sides of the lumbar spinal cord can participate in the processing and modulation of sensory signals. If, as the result of this complex information processing, the excitation level of the lateral dorsal horn reaches a threshold level, projection neurons can be activated that may then conduct volleys to the ipsi- and contralateral ventral horns, evoking segmental motor responses, whereas others may project to supraspinal brain centers where the sensory impulses are further processed and can finally generate various behaviours including pain sensation and other sensory modalities.
Acknowledgements
The authors are grateful to Mrs. J. Varga, Mrs. É. Medgyessi, and Mrs. D. Á. Miklós for technical assistance. M.A. was supported, in part, by an International Research Scholar's award from the Howard Hughes Medical Institute.




